Abstract
We have previously performed preclinical studies with the oxidized mannan-conjugated peptide MOG35–55 (OM-MOG35–55) in vivo (EAE mouse model) and in vitro (human peripheral blood) and demonstrated that OM-MOG35–55 suppresses antigen-specific T cell responses associated with autoimmune demyelination. Based on these results, we developed different types of dendritic cells (DCs) from the peripheral blood monocytes of patients with multiple sclerosis (MS) or healthy controls presenting OM-MOG35–55 or MOG-35–55 to autologous T cells to investigate the tolerogenic potential of OM-MOG35–55 for its possible use in MS therapy. To this end, monocytes were differentiated into different DC types in the presence of IL-4+GM-CSF ± dexamethasone (DEXA) ± vitamin D3 (VITD3). At the end of their differentiation, the DCs were loaded with peptides and co-cultured with T cells +IL-2 for 4 antigen presentation cycles. The phenotypes of the DC and T cell populations were analyzed using flow cytometry and the secreted cytokines using flow cytometry or ELISA. On day 8, the monocytes had converted into DCs expressing the typical markers of mature or immature phenotypes. Co-culture of T cells with all DC types for 4 antigen presentation cycles resulted in an increase in memory CD4+ T cells compared to memory CD8+ T cells and a suppressive shift in secreted cytokines, mainly due to increased TGF-β1 levels. The best tolerogenic effect was obtained when patient CD4+ T cells were co-cultured with VITD3-DCs presenting OM-MOG35–55, resulting in the highest levels of CD4+PD-1+ T cells and CD4+CD25+Foxp3+ Τ cells. In conclusion, the tolerance induction protocols presented in this work demonstrate that OM-MOG35–55 could form the basis for the development of personalized therapeutic vaccines or immunomodulatory treatments for MS.
Keywords:
peptides; MOG35–55; mannan; vitamin D; dendritic cells; cytokines; regulatory T cells; immunomodulation; human 1. Introduction
MS is a chronic demyelinating disease of the central nervous system (CNS) with an inflammatory and autoimmune etiology. A reduced number or dysfunctional regulatory cells, especially CD4+CD25+Foxp3+ T regulatory cells (Tregs), overactive effector CD4+ helper T (Th) cells, especially Th1 and Th17, CD8+ cytotoxic T cells, autoantibody production and activated antigen-presenting cells (APCs), including dendritic cells (DCs), play a critical role in mediating an inflammatory milieu that leads to an autoimmune attack on intrinsic protein components within the myelin sheath. This leads to axonal damage and neurodegeneration []. The best-characterized autoantigens in MS are myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG) [].
A promising approach to combat autoimmune diseases such as MS is immunotherapies aimed at restoring tolerance and avoiding the use of non-specific immunosuppressive drugs or biological agents such as monoclonal antibodies. These include cyclic peptides based on MBP, PLP and MOG, as well as altered peptide ligands (APL), which are closely related to native peptides (agonists or wild-type) and have 1–2 substituted amino acid residues that interact with the T cell receptor (TCR) but retain their binding ability to the human leukocyte antigen (HLA) [,,,].
Human clinical trials (phase I, II or III) conducted with MBP peptides, agonists or APLs yielded unsatisfactory results, either because of a lack of efficacy or because, although they were effective in blocking or switching autoreactive clones, they caused side effects such as the development of immediate-type hypersensitivity reactions, the formation of antibodies that cross-reacted with native MBP or poor tolerability [,,,]. Therefore, further extensive preclinical testing is required, and new peptides must be used with an appropriate carrier that induces tolerance or alters the resulting immune response.
Mannan, a poly-mannose isolated from the wall of yeast cells, has been shown to bind to the mannose receptor on DCs and is a ligand for Toll-like receptor 4 []. Mannan conjugated to the cancer protein MUC1 elicits an immune and protective response in mice, and its translation into human clinical trials has shown both immunologic and clinical efficacy [,]. Due to the immunomodulatory properties of mannan, its effect as a carrier for MS peptides is being investigated by our group [].
Mannan in oxidized or reduced form conjugated to the immunodominant agonist MOG35–55 peptide protected mice in prophylactic and therapeutic protocols against experimental autoimmune encephalomyelitis (EAE, an animal model for MS), with oxidized mannan-conjugated MOG35–55 (OM-MOG35–55) yielding the best results. Protection was peptide-specific and was associated with reduced antigen-specific T cell proliferation but not with changes in Th1, Th17 and T regulatory cell (Treg) differentiation or T cell apoptosis compared to EAE in controls []. Furthermore, humanized HLA-DR2 transgenic mice immunized with OM-MOG35–55 were protected against EAE in both prophylactic and therapeutic protocols [].
In a previous study [], in which peripheral blood from patients with relapsing-remitting MS (RRMS, n = 83) and healthy controls (n = 45) was used to investigate the types of regulatory cells and how they are affected by disease activity and type of treatment, we were able to show that in patients in the acute phase of the disease without therapy, the concentration of CD4+CD25+Foxp3+ T regulatory cells (Tregs) was significantly reduced compared to healthy controls and that Tregs responded to various peptides mapping to myelin antigens in culture with proliferation and cytokine secretion, with the OM-MOG35–55 peptide having the best tolerogenic effect. In addition, the stability and integrity of OM-MOG35–55 were confirmed using analytical and enzymatic methods [,].
To further these studies, we developed different types of DCs from peripheral blood monocytes of MS patients presenting OM-MOG35–55 or MOG-35–55 to autologous T cells to investigate the tolerogenic potential of OM-MOG35–55. Our working hypothesis is that the OM-MOG35–55 conjugate is a strong candidate for a therapeutic vaccine or immunomodulatory treatment of MS in the context of personalized medicine.
2. Results
2.1. Development of DCs from Peripheral Blood Monocytes
Monocytes isolated from PBMCs of RRMS patients and controls were differentiated into different DC types in the presence of IL-4 and GM-CSF with or without the addition of DEXA or VITD3 [,]. At the end of their differentiation, DCs were loaded with OM-MOG35–55 or MOG35–55 and received the LPS maturation signal []. They were then co-cultured with autologous, non-adherent PBMCs in the presence of IL-2 for 4 antigen presentation cycles (Figure 1).
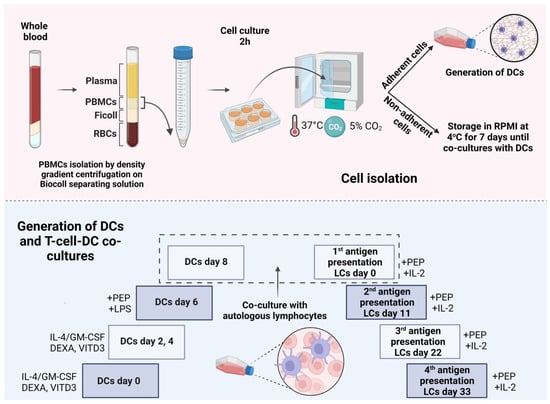
Figure 1.
Protocol for in vitro differentiation of peripheral blood monocytes into different types of DCs presenting peptides to T cells. PEP, peptide; LCs, lymphocytes. This image was created with BioRender (https://biorender.com, accessed on 28 November 2023).
Phenotypic analysis of adherent PBMCs on day 0 of culture showed that they were 100% CD14+ monocytes. On day 8 of culture, the cells had the morphology of DCs, as determined using light microscopy. Phenotypic analysis of the cells showed that they expressed all markers typical of DCs, i.e., HLA-DR, CD40, CD80, CD83 and CD86 (Figure 2). DCs generated with VITD3 or VITD3+DEXA showed the most typical semi-mature phenotype with low expression of CD80 [,]. The results were similar between patient and control DCs.
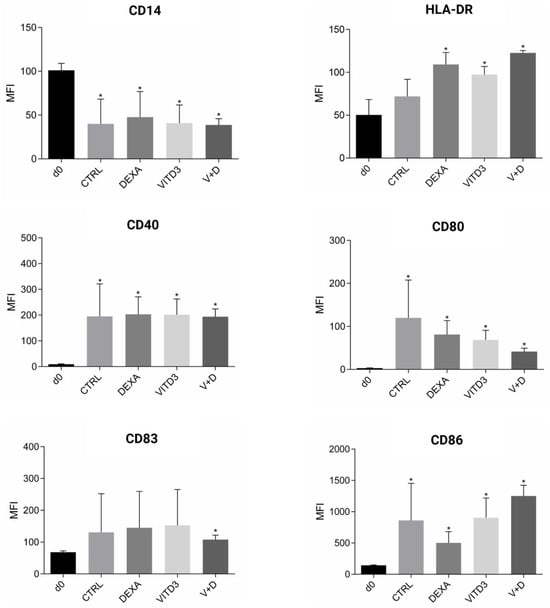
Figure 2.
Phenotypic markers on the surface of cultured monocytes on day 0 and DCs on day 8. The results shown are from patient-derived cells. Data are presented as mean (SD). Asterisks indicate statistically significant differences between markers expressed on monocytes on day 0 and DCs on day 8 (* p < 0.05). MFI, mean fluorescence intensity; CTRL, control DCs; DEXA, DEXA-DCs; VITD3, VITD3-DCs; V+D, VITD3+DEXA; d0, day 0.
2.2. Cytokines Secreted by the Different DC Types
DCs secreted the pro-inflammatory (type-1) cytokines IL-1, IL-6, IL-8, IL-12 and TNF-α and the anti-inflammatory (type-2) cytokines IL-10 and TGF-β. Overall, the highest secretion rates were observed for IL-8 and TGF-β. Compared to control-derived DCs, patient-derived CTRL-DCs secreted significantly higher amounts of IL-1, IL-6, IL-8, IL-12 and TNF-α. Patient-derived VITD3-DCs secreted significantly lower amounts of IL-1, IL-10 and IL-12 (Figure 3).
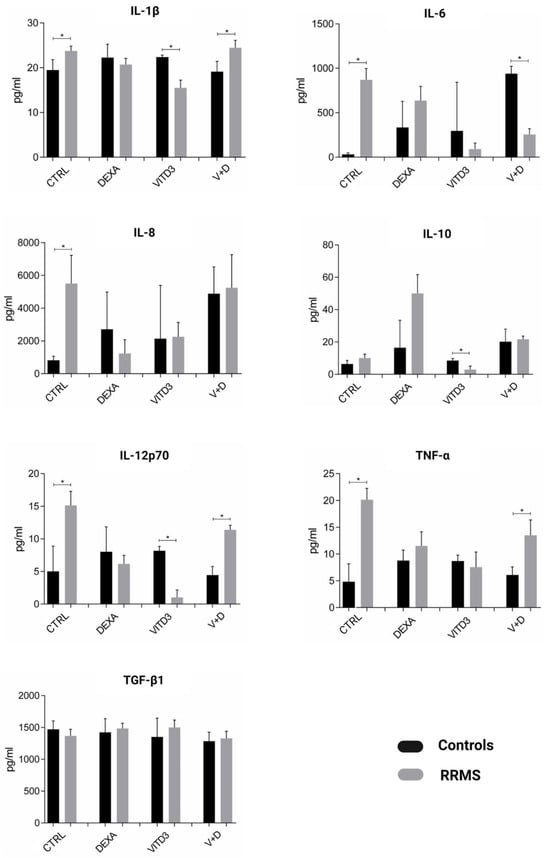
Figure 3.
Cytokines secreted by DCs generated from controls or RRMS patients under different culture conditions on day 8 of culture. Data are presented as mean (SD). Asterisks indicate statistically significant differences between cytokine levels secreted by patient- and control-derived DCs (* p < 0.05).
The ratio of type-2/type-1 (anti-inflammatory/pro-inflammatory) cytokines in the different DC cultures, reflecting an effector or suppressor shift in the overall cytokine profiles, shows a strong suppressor shift in the cytokines secreted by patient-derived DCs generated from monocytes in the presence of VITD3 or DEXA. A suppressor shift was not observed in control-derived DCs generated from monocytes in the presence of DEXA, VITD3 or DEXA+VITD3; control-derived VITD3-DCs had the highest suppressor cytokine profile, but the mean difference from the suppressor cytokine profile of CTRL-DCs did not reach statistical significance (Figure 4).
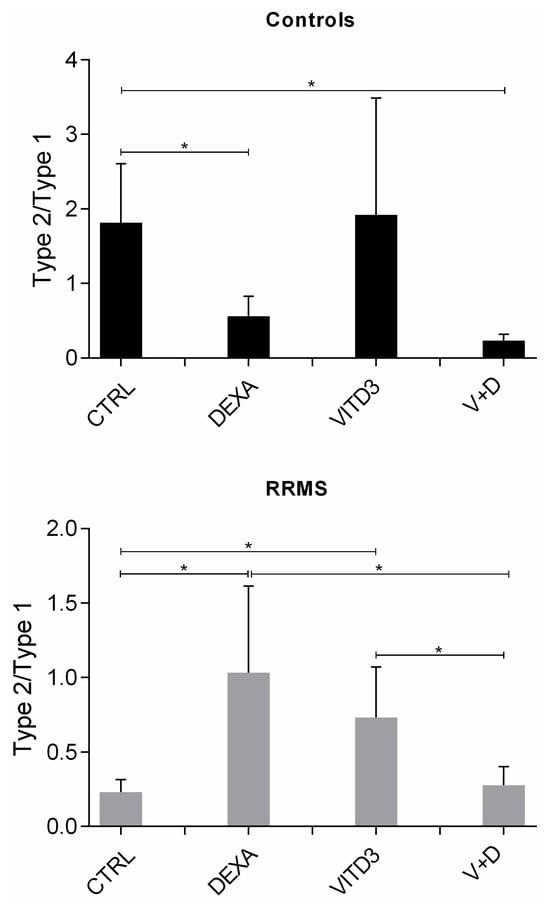
Figure 4.
Ratio of type-2/type-1 cytokines in DC cultures on day 8. Data are presented as mean (SD). Asterisks indicate statistically significant differences between the ratios of type-2/type-1 cytokines (* p < 0.05). Type-2/type-1: [IL-10+TGF-β]:[IL-1+IL-6+IL-8+IL-12+TNF-α].
2.3. Effect of Antigen Presentation by DCs to Autologous T Cells
2.3.1. Beginning of Cultures
The viability of the stored lymphocytes was estimated to be over 70% before the cells were added to the DC cultures (Supplementary Figure S1).
Phenotypic analysis of lymphocytes (Figure 5A) showed significant differences in the concentrations of CD4+ T cells and B cells; specifically, patients had a 10% lower concentration of CD4+ T cells and twice the concentration of B cells than the control group. In addition, patients had a significantly lower concentration of naive (CD45RA) CD4+ and CD8+ T cells and a significantly higher concentration of memory (CD45RO) CD4+ and CD8+ T cells than controls (Figure 5B,C).
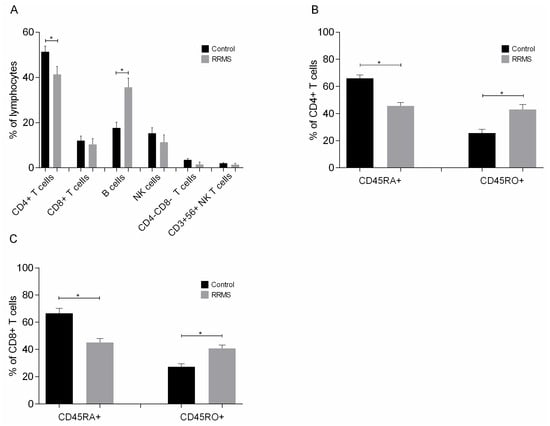
Figure 5.
Phenotypic analysis of (A) lymphocytes, (B) naive and memory CD4+ T cells and (C) naive and memory CD8+ T cells added to DC cultures on day 8. Data are presented as mean (SD). Asterisks indicate statistically significant differences between cell concentrations in patients and controls (* p < 0.05). CD45RA+, naive cells; CD45RO+, memory cells.
2.3.2. End of Cultures
- Cells
At the end of the culture and after four rounds of antigen presentation, the lymphocytes in the cultures consisted exclusively of T cells (>99.5%). As determined using light microscopy, DCs were still present in the cultures. In the cultures with DEXA+VITD3-DCs, there were very few viable cells that could not be characterized via phenotypic analysis. In the remaining cultures, all T cells were viable. Phenotypic analysis performed on days 33 and 36 of culture showed that the majority of T cells derived from both patients and controls and cultured with the different types of DCs presenting OM-MOG35–55 or MOG35–55 consisted of CD4+ T cells (Figure 6A). T cells cultured with CTRL-DCs presenting MOG35–55 contained the highest proportion of CD8+ T cells (Figure 6B). T cells cultured with CTRL-DCs presenting MOG35–55 or OM-MOG35–55 contained the highest proportion of CD4-CD8-T cells (Figure 6C).
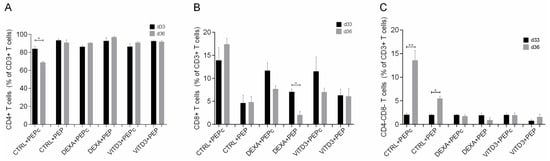
Figure 6.
Phenotypic analysis of (A) CD4+ T cells, (B) CD8+ T cells and (C) CD4-CD8-T cells on days 33 and 36 of culture with DCs presenting the peptide OM-MOG35–55 (PEP) or the peptide MOG-35–55 (PEPc). Data are presented as mean (SD). Asterisks indicate statistically significant differences between cell levels (* p < 0.05, ** p < 0.01).
Phenotypic analysis of CD4+ and CD8+ T cells showed that culture with all types of DCs mainly promoted the generation of memory CD4+ T cells derived from both patients and controls (Figure 7A,B), and to a much lower extent, the generation of memory CD8+ T cells (Figure 7C,D).
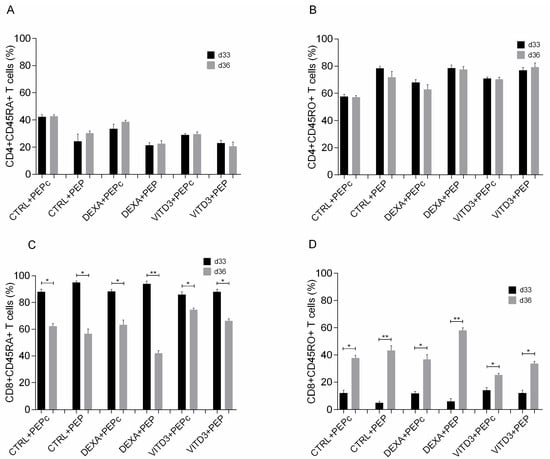
Figure 7.
Phenotypic analysis of (A) naive CD4+ T cells, (B) memory CD4+ T cells, (C) naive CD8+ T cells and (D) memory CD8+ T cells on days 33 and 36 of culture with DCs presenting the peptide OM-MOG35–55 (PEP) or the peptide MOG-35–55 (PEPc). Data are presented as mean (SD). Asterisks indicate statistically significant differences between cell levels (* p < 0.05, ** p < 0.01). (%), data are presented as % of total CD4+ or CD8+ T cell populations.
OM-MOG35–55-specific CD4+ T cells derived from the patients exhibited significantly increased PD-1 expression compared to control-derived CD4+ T cells when cultured for 33 days with DEXA-DCs and VITD3-DCs and on day 36 under all culture conditions, reaching a maximum of over 80% when cultured with VITD3-DCs (Figure 8A). In addition, the proportion of OM-MOG35–55-specific Tregs derived from the patients was significantly higher than that of Tregs from the control group, reaching a maximum of over 30% when cultured with VITD3-DCs on day 36 (Figure 8B).
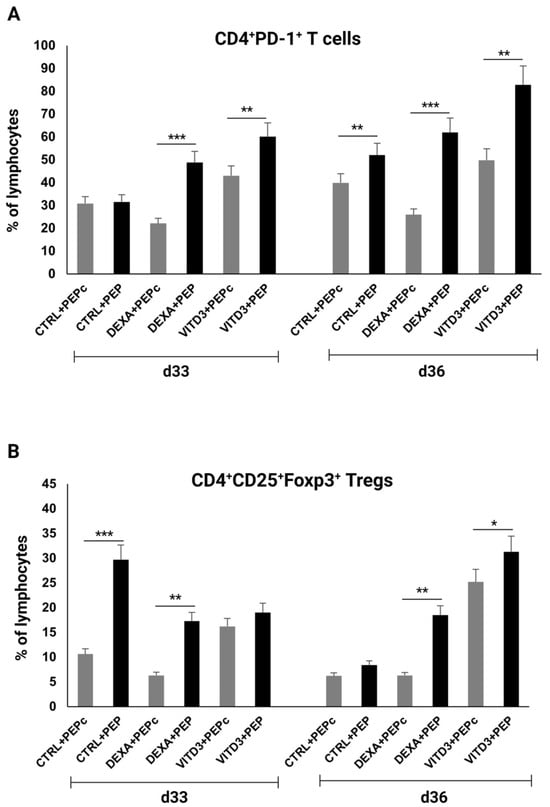
Figure 8.
Percentage of (A) CD4+PD-1+ T cells and (B) CD4+CD25+Foxp3+ Tregs on days 33 and 36 of culture with DCs presenting peptide OM-MOG35–55 (PEP) or peptide MOG-35–55 (PEPc). Asterisks indicate statistically significant differences between CD4+PD-1+ T cell or Treg levels in cultures with control-derived T cells (gray bars) and patient-derived T cells (black bars). * p < 0.05; ** p < 0.01; *** p < 0.001.
- Cytokines
At the end of the 36-day culture and after four rounds of antigen presentation, the results in the cultures with DCs derived from patients or controls were different in terms of cytokine secretion. In the cultures with DCs presenting OM-MOG35–55, the secreted cytokines were IL-4, IL-6, IL-10, IFN-γ, TNF-α and TGF-β, with TGF-β having the highest concentration; IL-17 was not detected in any of the cultures (Figure 9).
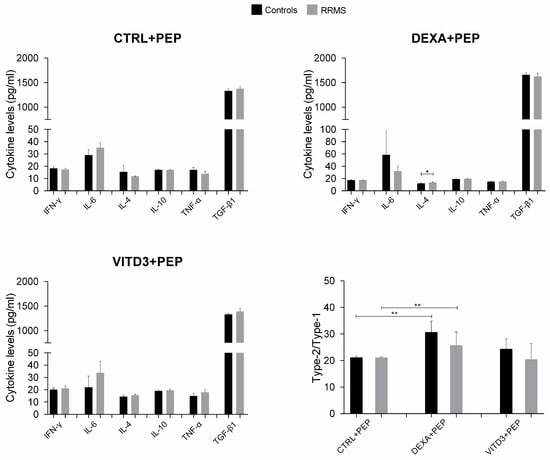
Figure 9.
Cytokine concentration in supernatants of 36-day cultures of DCs and T cells derived from RRMS patients and controls. Cytokine concentrations are shown as mean (SD). Type-2/type-1 cytokine ratio: [IL-4+IL-10+TGF-β1]: [IFN-γ+IL-6+TNF-α]. * p < 0.05, ** p < 0.01. PEP, OM-MOG35–55.
In the cultures of DCs with MOG35–55, the secreted cytokines were IL-6, IL-10, IFN-γ, TNF-α and TGF-β, with TGF-β having the highest concentration; IL-4 and IL-17 were not detected in any of the cultures (Figure 10).
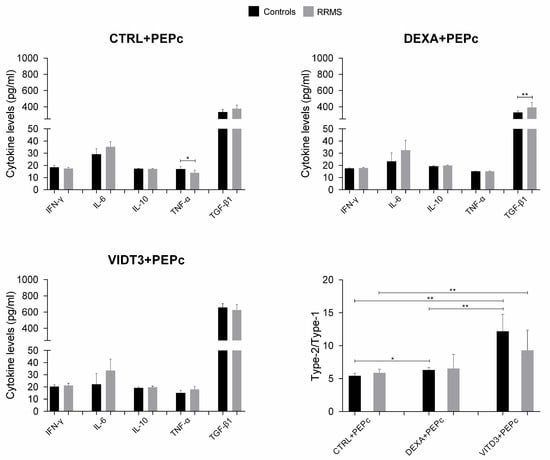
Figure 10.
Cytokine concentration in supernatants of 36-day cultures of DCs and T cells derived from RRMS patients and controls. Cytokine concentrations are shown as mean (SD). Type-2/type-1 cytokine ratio: [IL-10+TGF-β1]: [IFN-γ+IL-6+TNF-α]. * p < 0.05, ** p < 0.01. PEPc, MOG35–55.
The cultures with the MOG35–55 peptide contained significantly lower TGF-β levels compared to the cultures with OM-MOG35–55. In the cultures of DCs presenting OM-MOG35–55, the strongest suppressor cytokine shift was observed in the cultures with DEXA-DCs, whereas in the cultures of DCs presenting MOG35–55, the strongest suppressor cytokine shift was observed in the cultures with VITD3-DCs (Figure 9 and Figure 10).
3. Discussion
Our data suggest that OM-MOG35–55-presenting DCs induce T cell tolerance that is maintained via the administration of free OM-MOG35–55 peptides at 11-day intervals. OM-MOG35–55 peptides induced IL-4 secretion, whereas MOG35–55 did not, and furthermore, OM-MOG35–55 induced much higher TGF-β secretion, suggesting that this was due to mannan. Comparing the results of DC cultures with OM-MOG35–55 or MOG35–55 peptides, it is clear that the type of DCs is the main factor influencing the outcome of MOG35–55 presentation to T cells, while conjugation of the peptide to mannan appears to be the key factor for tolerance induction and overrides the maturation state of DCs. These results confirm our previous finding that the peptide OM-MOG35–55 elicits the best tolerogenic effect when added to PBMC cultures from RRMS patients [].
Our data also emphasize the importance of dexamethasone and vitamin D in the creation of tolerogenic DCs [,,]. Our results show that vitamin D has a better effect in this regard on DCs derived from healthy individuals. Moreover, the use of vitamin D or dexamethasone alone in the differentiation of monocytes into tolerogenic DCs resulted in the maintenance of viable T cells in long-term cultures compared to the joint use of vitamin D and dexamethasone. This is an interesting point that could be useful in experimental studies on the differentiation of human monocytes into DCs and the use of peptides in vaccination protocols. Importantly, presentation of OM-MOG35–55 by DCs generated in the presence of vitamin D resulted in the highest levels of CD4+PD-1+ and CD4+CD25+Foxp3+ T cells, which are significantly reduced in MS patients [,]. Considering that carefully monitored vitamin D supplementation has been shown to improve MS and other inflammatory conditions [,,], the administration of OM-MOG35–55 to patients together with vitamin D supplementation should also be considered.
The antigen presentation system developed by our research team to test the induction of tolerance of host T cells to MS antigens needs to be thoroughly evaluated with a larger number of peripheral blood samples from MS patients to determine the phenotype of the resulting T cell clones, their TCR repertoire, their function, their proliferation potential, their phenotypic stability over time and over several cycles of antigen challenge, and finally their suppressive potential against autologous effector T cells isolated from fresh blood samples from the same patients. In addition, peripheral blood samples from patients with RRMS with relatively low EDSS were used in this study. It would be interesting to test the effect of OM-MOG35–55 in other forms of MS and a broader EDSS spectrum.
A prerequisite for the clinical testing of a potential vaccine or immunomodulatory treatment with conjugated peptides in humans is the efficacy and stability of the peptides and conjugates. We have already conducted preclinical and clinical studies with mannan conjugated to a cancer protein and demonstrated its efficacy and safety [,]. We have also performed preclinical studies with mannan-conjugated peptides mapping to myelin epitopes in the EAE mouse model [,,] and in human peripheral blood [] and demonstrated that oxidized mannan gave the best results, suggesting that OM-peptides may be useful for suppressing antigen-specific CD4+ T cell responses associated with autoimmune CNS demyelination. We have also demonstrated the stability and integrity of OM-MOG35–55 [,].
More and more studies are looking at the manipulation of the immune system as a means of controlling or curing various diseases, from malignancies to autoimmune diseases. Harnessing the body’s own processes of antigen presentation is a promising tactic with a limited number of side effects and a broad spectrum of activity [,].
Our group is working on the development of peptide-based treatments for MS. The present work completes a cycle of preclinical testing (EAE → short-term testing in human PBMC cultures → long-term testing in antigen presentation cultures of DCs and T cells derived from human PBMCs) and demonstrates that the OM-MOG35–55 conjugate is the best candidate for human clinical trials to test whether it can be used as a therapeutic vaccine or as an immunomodulatory treatment for MS in the context of personalized medicine.
4. Materials and Methods
4.1. Study Subjects
Ten patients diagnosed with RRMS [] and 10 healthy control subjects participated in the study (Table 1). All study participants donated peripheral blood, which was used for preliminary experiments to establish the experimental protocols described in this study. Additional blood samples from 5 RRMS patients and 5 controls from the original pool of patients and control subjects were used for the experiments presented in the results.

Table 1.
Data of the study subjects.
Ethics: This study was approved by the Scientific Review Boards and Ethics Committees of Patras University Hospital (Reg# 451/17.10.2008) and Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece (Reg# 560/30.07.2018) in the context of applications for studies on the pathogenesis of multiple sclerosis involving the use of clinical data from study participants (without disclosure of their names) and blood samples for in vitro experiments described in publications. Both hospitals adhere to the Declaration of Helsinki on the ethical principles of medical research involving human subjects.
4.2. Cells and Cultures
Peripheral blood samples (10–20 mL) were collected from RRMS patients and controls in heparinized BD vacutainers (Becton Dickinson, BD, Franklin Lakes, NJ, USA). The percentage of peripheral blood lymphocytes and monocytes in the blood samples is shown in Table 1. PBMCs were isolated via density gradient centrifugation with Ficoll (Biochrom, Feucht/Nuremberg, Germany) as described []. Cells were cultured in RPMI1640 medium (Gibco-BRL, Thermo Fisher Scientific Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Gibco-BRL), 1% penicillin/streptomycin and 6 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) (culture medium, CM) at a concentration of 2 × 106 cells/mL for 2 h. At the end of the culture, non-adherent cells were collected and stored at 4 °C.
For the differentiation of monocytes into different DC types, adherent cells (monocyte-enriched fraction) were cultured in CM containing GM-CSF and IL-4 (PeproTech, Thermo Fisher Scientific Inc.) at a concentration of 1000 IU/mL, as described in [], in the presence or absence of 1 nM calcitriol (active form of vitamin D3, VITD3) or 10−6 M dexamethasone (DEXA) (Tocris Bioscience, Bristol, UK) or both for 6 days [,]. The CM with cytokines ± VITD3, DEXA or VITD3+DEXA was renewed every 2 days. At the end of their differentiation (day 6 of culture), the DCs were loaded with OM-MOG35–55 or MOG35–55 peptides that were added to the cultures at a concentration of 10 μg/mL. After 2 h, lipopolysaccharide (LPS, Sigma-Aldrich) was added to the cultures at a concentration of 0.25 μg/mL [].
Two days later (day 8 of culture), the DCs were phenotyped using flow cytometry (see below) and co-cultured with non-adherent PBMCs that were added to the cultures together with IL-2 (PeproTech) at a concentration of 25 IU/mL. OM-MOG35–55 or MOG35–55 was added to the cultures at a concentration of 10 μg/mL every 11 days for a total of 4 antigen presentation cycles.
4.3. Flow Cytometry
Cells harvested from the cultures at different time points were analyzed on a BD FACSCanto™ II flow cytometer with fluorescently labeled monoclonal antibodies against CD3, CD4, CD8, CD14, CD25, CD40, CD45RA, CD45RO, CD56, CD69, CD80, CD83, CD86, CD279 (PD-1), HLA-DR and Foxp3 for phenotypic analysis (Table 2). Cell viability was determined using the PE Annexin V Apoptosis Detection Kit I (BD). At least 10,000 events were recorded for extracellular or intracellular staining. All measurements were performed in triplicate. Data analysis was performed using FlowJo V10.8 software (Tree Star Inc., San Carlos, CA, USA).

Table 2.
Antibodies used for phenotypic analysis.
4.4. Measurement of Cytokines
The concentration of the cytokines IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IFN-γ and TNF-α in the culture supernatant was measured using flow cytometry on a BD FACSArray™ Bioanalyzer using the CBA Human Inflammatory Cytokine Kit (BD) and the CBA Human Th1/Th2/Th17 Kit (BD). The concentration of TGF-β1 was measured using ELISA (R&D Systems, Minneapolis, MN, USA). All measurements were performed in triplicate. Data analysis was performed using FlowJo V10.8 software and GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA).
4.5. Statistical Analysis
Data are presented as median or mean (SD) of at least three independent experiments. The Kolmogorov–Smirnov test was performed to determine distribution normality. Differences between two groups were analyzed using the unpaired Student’s t-test. A significance level of p < 0.05 was considered statistically significant. Data analysis and graphical representation were performed using GraphPad Prism 8.0 (GraphPad Software Inc.).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25116092/s1.
Author Contributions
Designed the study, M.R., A.-L.d.L. and A.M.; performed the experiments, M.R. and A.-L.d.L.; analyzed the data, M.R., A.-L.d.L., I.P., I.A. and A.M.; provided the peptides and detailed information on peptide chemistry, K.K. and J.M.; wrote the paper, A.M., M.R., A.-L.d.L., I.P., J.M. and V.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this manuscript was supported by the Greek General Secretariat for Research and Technology “Cooperation” with grant 09SYN-21-609 (O. P. Competitiveness & Entrepreneurship, EPAN ΙΙ) to A.M. and J.M.
Institutional Review Board Statement
This study was approved by the Scientific Review Boards and Ethics Committees of Patras University Hospital (Reg# 451/17.10.2008) and Eginition Hospital, National and Kapodistrian University of Athens, Athens, Greece (Reg# 560/30.07.2018) in the context of applications for studies on the pathogenesis of multiple sclerosis involving the use of clinical data from study participants (without disclosure of their names) and blood samples for in vitro experiments described in publications.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the neurologists, first from Patras University Hospital and later from Eginition Hospital, for the selection of patients and the collection of blood samples, as well as the patients and healthy volunteers who were kind enough to donate their blood and take an active interest in our research. It should be acknowledged that Maria Rodi, Anne-Lise de Lastic and Ioannis Panagoulias contributed equally to this manuscript and should be recognized as first authors.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Gakis, G.; Angelopoulos, I.; Panagoulias, I.; Mouzaki, A. Current knowledge on multiple sclerosis pathophysiology, disability progression assessment and treatment options, and the role of autologous hematopoietic stem cell transplantation. Autoimmun. Rev. 2023, 23, 103480. [Google Scholar] [CrossRef] [PubMed]
- Seil, F.J. Myelin Antigens and Antimyelin Antibodies. In Advances in Clinical Immunology, Medical Microbiology, COVID-19, and Big Data 2021; Jenny Stanford Publishing: New York, NY, USA, 2021; Chapter 5. [Google Scholar] [CrossRef]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87–99 cyclic analogue: Enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Lourbopoulos, A.; Deraos, G.; Matsoukas, M.T.; Touloumi, O.; Giannakopoulou, A.; Kalbacher, H.; Grigoriadis, N.; Apostolopoulos, V.; Matsoukas, J. Cyclic MOG35–55 ameliorates clinical and neuropathological features of experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2017, 25, 4163–4174. [Google Scholar] [CrossRef] [PubMed]
- Lourbopoulos, A.; Matsoukas, M.T.; Katsara, M.; Deraos, G.; Giannakopoulou, A.; Lagoudaki, R.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Cyclization of PLP139–151 peptide reduces its encephalitogenic potential in experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2018, 26, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Kritsi, E.; Matsoukas, M.T.; Christopoulou, K.; Kalbacher, H.; Zoumpoulakis, P.; Apostolopoulos, V.; Matsoukas, J. Design of Linear and Cyclic Mutant Analogues of Dirucotide Peptide (MBP82–98) against Multiple Sclerosis: Conformational and Binding Studies to MHC Class II. Brain Sci. 2018, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Comi, G.; Panitch, H.; Oger, J.; Antel, J.; Conlon, P.; Steinman, L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat. Med. 2000, 6, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B.; Goodwin, B.; Richert, N.; Cortese, I.; Kondo, T.; Afshar, G.; Gran, B.; Eaton, J.; Antel, J.; Frank, J.A.; et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 2000, 6, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.G.; Catz, I.; Ferenczi, L.Z.; Krantz, M.J. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA Class II-defined cohort of patients with progressive multiple sclerosis: Results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 2006, 13, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Bar-Or, A.; Oger, J.; Traboulsee, A.; Patry, D.; Young, C.; Olsson, T.; Li, D.; Hartung, H.P.; Krantz, M.; et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology 2011, 77, 1551–1560. [Google Scholar] [CrossRef]
- Sheng, K.C.; Kalkanidis, M.; Pouniotis, D.S.; Wright, M.D.; Pietersz, G.A.; Apostolopoulos, V. The adjuvanticity of a mannosylated antigen reveals TLR4 functionality essential for subset specialization and functional maturation of mouse dendritic cells. J. Immunol. 2008, 181, 2455–2464. [Google Scholar] [CrossRef]
- Vassilaros, S.; Tsibanis, A.; Tsikkinis, A.; Pietersz, G.A.; McKenzie, I.F.; Apostolopoulos, V. Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy 2013, 5, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Stojanovska, L.; McKenzie, I.F.; Vassilaros, S. Dendritic cell immunotherapy: Clinical outcomes. Clin. Transl. Immunol. 2014, 3, e21. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Tselios, T.; Androutsou, M.E.; Tapeinou, A.; Frilligou, I.; Stojanovska, L.; Matsoukas, J.; Apostolopoulos, V. Mannosylated Linear and Cyclic Single Amino Acid Mutant Peptides Using a Small 10 Amino Acid Linker Constitute Promising Candidates Against Multiple Sclerosis. Front. Immunol. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Tseveleki, V.; Tselios, T.; Kanistras, I.; Koutsoni, O.; Karamita, M.; Vamvakas, S.S.; Apostolopoulos, V.; Dotsika, E.; Matsoukas, J.; Lassmann, H.; et al. Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis. Exp. Neurol. 2015, 267, 254–267. [Google Scholar] [CrossRef]
- Dagkonaki, A.; Avloniti, M.; Evangelidou, M.; Papazian, I.; Kanistras, I.; Tseveleki, V.; Lampros, F.; Tselios, T.; Jensen, L.T.; Möbius, W.; et al. Mannan-MOG35–55 Reverses Experimental Autoimmune Encephalomyelitis, Inducing a Peripheral Type 2 Myeloid Response, Reducing CNS Inflammation, and Preserving Axons in Spinal Cord Lesions. Front. Immunol. 2020, 11, 575451. [Google Scholar] [CrossRef] [PubMed]
- Rodi, M.; Dimisianos, N.; de Lastic, A.L.; Sakellaraki, P.; Deraos, G.; Matsoukas, J.; Papathanasopoulos, P.; Mouzaki, A. Regulatory Cell Populations in Relapsing-Remitting Multiple Sclerosis (RRMS) Patients: Effect of Disease Activity and Treatment Regimens. Int. J. Mol. Sci. 2016, 17, 1398. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.; Deraos, G.; Kelaidonis, K.; Hossain, M.K.; Feehan, J.; Tzakos, A.G.; Matsoukas, E.; Topoglidis, E.; Apostolopoulos, V. Myelin Peptide-Mannan Conjugate Multiple Sclerosis Vaccines: Conjugation Efficacy and Stability of Vaccine Ingredient. Vaccines 2021, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Gkika, A.; Androutsou, M.E.; Aletras, A.J.; Tselios, T. Competitive ELISA for the identification of 35–55 myelin oligodendrocyte glycoprotein immunodominant epitope conjugated with mannan. J. Pept. Sci. 2023, 29, e3493. [Google Scholar] [CrossRef]
- Raϊch-Regué, D.; Grau-López, L.; Naranjo-Gómez, M.; Ramo-Tello, C.; Pujol-Borrell, R.; Martínez-Cáceres, E.; Borràs, F.E. Stable antigen-specific T-cell hyporesponsiveness induced by tolerogenic dendritic cells from multiple sclerosis patients. Eur. J. Immunol. 2012, 42, 771–782. [Google Scholar] [CrossRef]
- Anderson, A.E.; Swan, D.J.; Wong, O.Y.; Buck, M.; Eltherington, O.; Harry, R.A.; Patterson, A.M.; Pratt, A.G.; Reynolds, G.; Doran, J.P.; et al. Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-β1. Clin. Exp. Immunol. 2017, 187, 113–123. [Google Scholar] [CrossRef]
- Ardeshna, K.M.; Pizzey, A.R.; Devereux, S.; Khwaja, A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 2000, 96, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Hubo, M.; Trinschek, B.; Kryczanowsky, F.; Tuettenberg, A.; Steinbrink, K.; Jonuleit, H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front. Immunol. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.S.; Eelen, G.; Ferreira, G.B.; Ghesquière, B.; Cook, D.P.; Nikolic, T.; Roep, B.; Carmeliet, P.; Telang, S.; Mathieu, C.; et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J. Steroid Biochem. Mol. Biol. 2019, 187, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Rad, I.A.; Ahmadi-Salmasi, B. CTLA-4, PD-1 and TIM-3 expression predominantly downregulated in MS patients. J. Neuroimmunol. 2018, 323, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef] [PubMed]
- Triantos, C.; Aggeletopoulou, I.; Thomopoulos, K.; Mouzaki, A. Vitamin D-Liver Disease Association: Biological Basis and Mechanisms of Action. Hepatology 2021, 74, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular basis of vitamin D action in inflammatory bowel disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Chavda, V.P.; Feehan, J. Targeting dendritic cells for antigen delivery in vaccine design. In Advanced Vaccination Technologies for Infectious and Chronic Diseases; Academic Press: Cambridge, MA, USA, 2024; pp. 153–165. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- How to Isolate PBMCs from Whole Blood Using Density Gradient Centrifugation (Ficoll™ or Lymphoprep™). Available online: https://www.stemcell.com/how-to-isolate-mononuclear-cells-from-whole-blood-by-density-gradient-centrifugation.html (accessed on 27 May 2024).
- De Lastic, A.L.; Rodi, M.; Mouzaki, A. Effect of dendritic cell state and antigen-presentation conditions on resulting T-cell phenotypes and Th cytokine profiles. Immunobiology 2016, 221, 862–870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).