Untargeted Metabolome Analyses Revealed Potential Metabolic Mechanisms of Leymus chinensis in Response to Simulated Animal Feeding
Abstract
:1. Introduction
2. Results
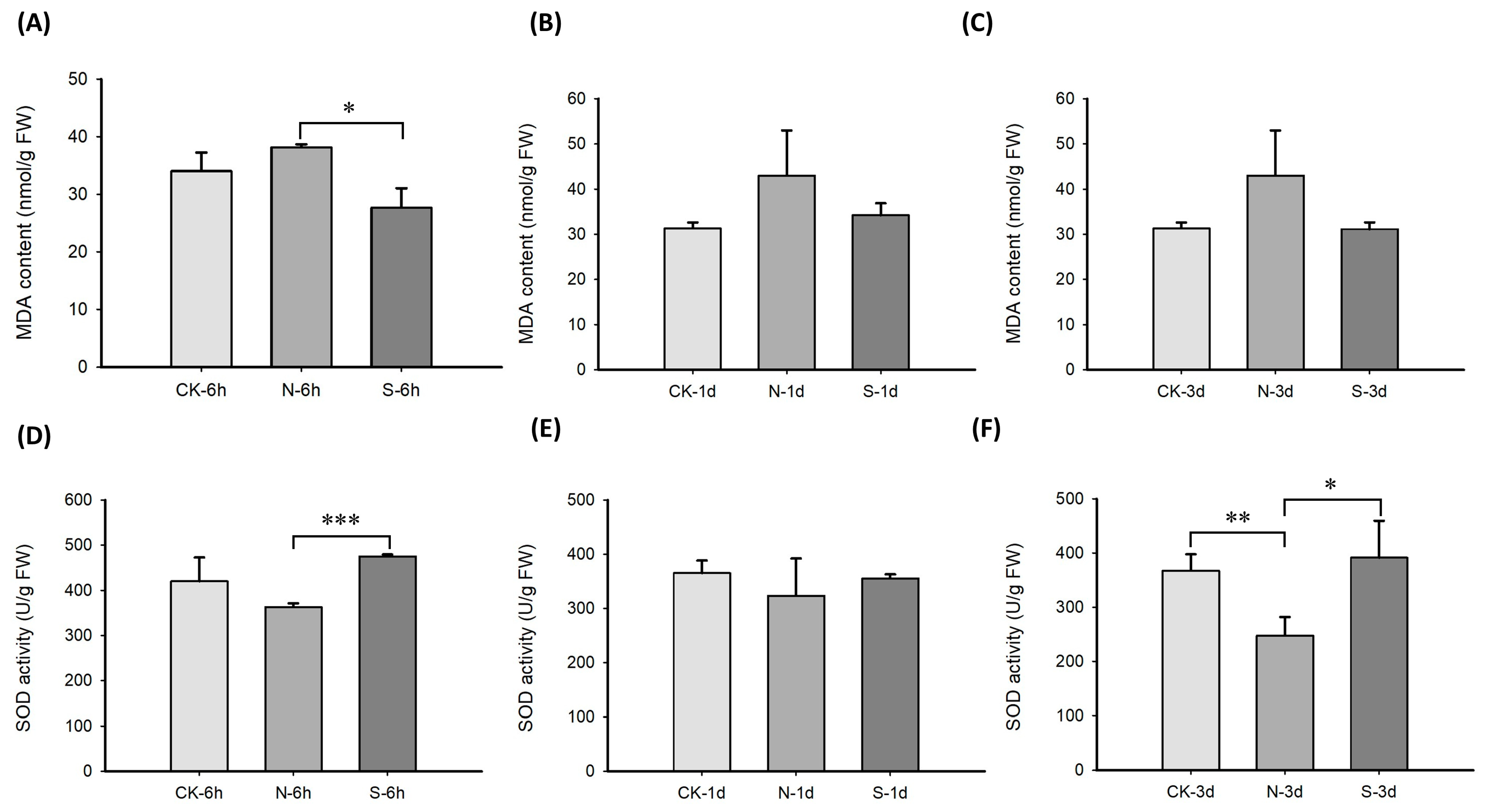
2.1. Accumulation of Oxidative-Stress-Related Factors in Leymus chinensis Experiencing Simulated Animal Feeding
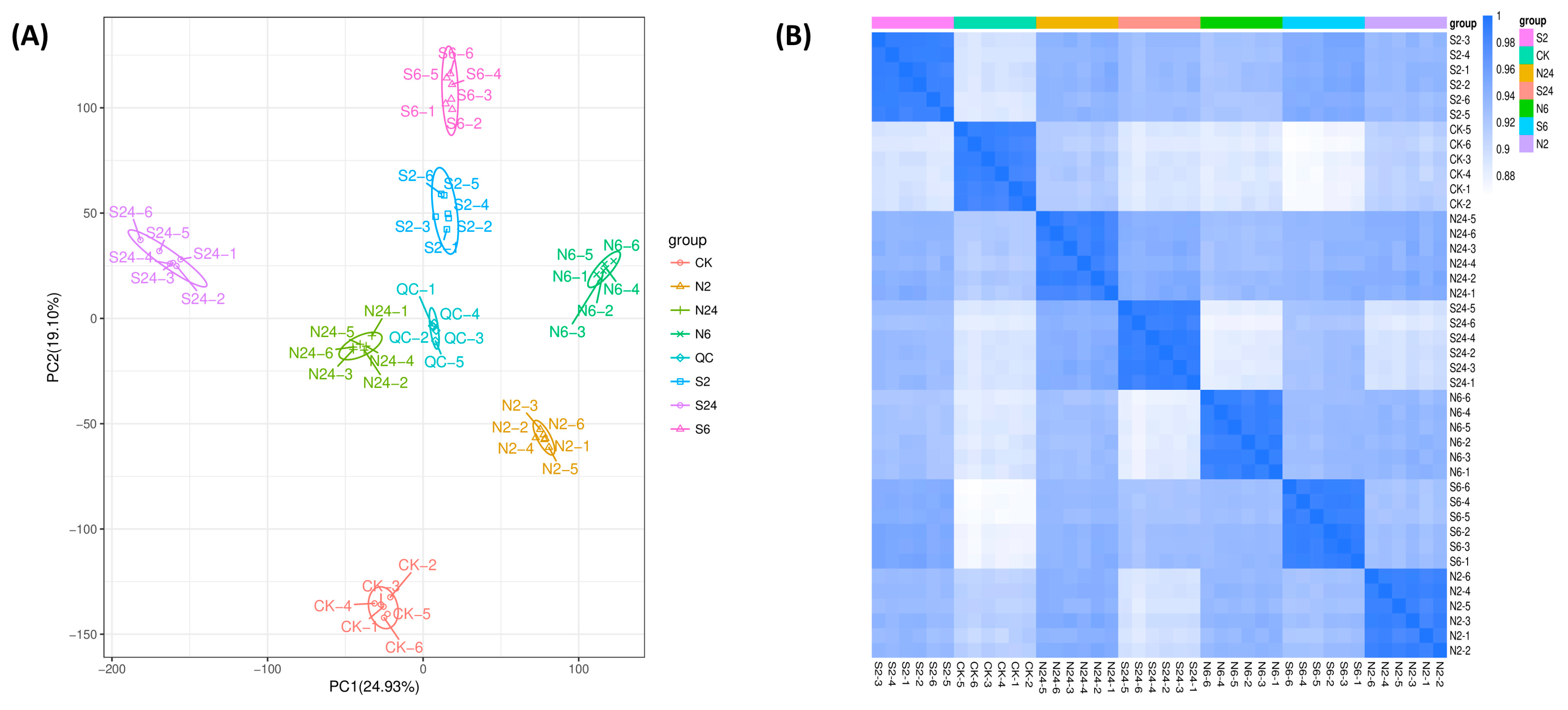
2.2. Overall Assessment of Untargeted Metabolomic Data in Response to Simulated Animal Feeding in L. chinensis
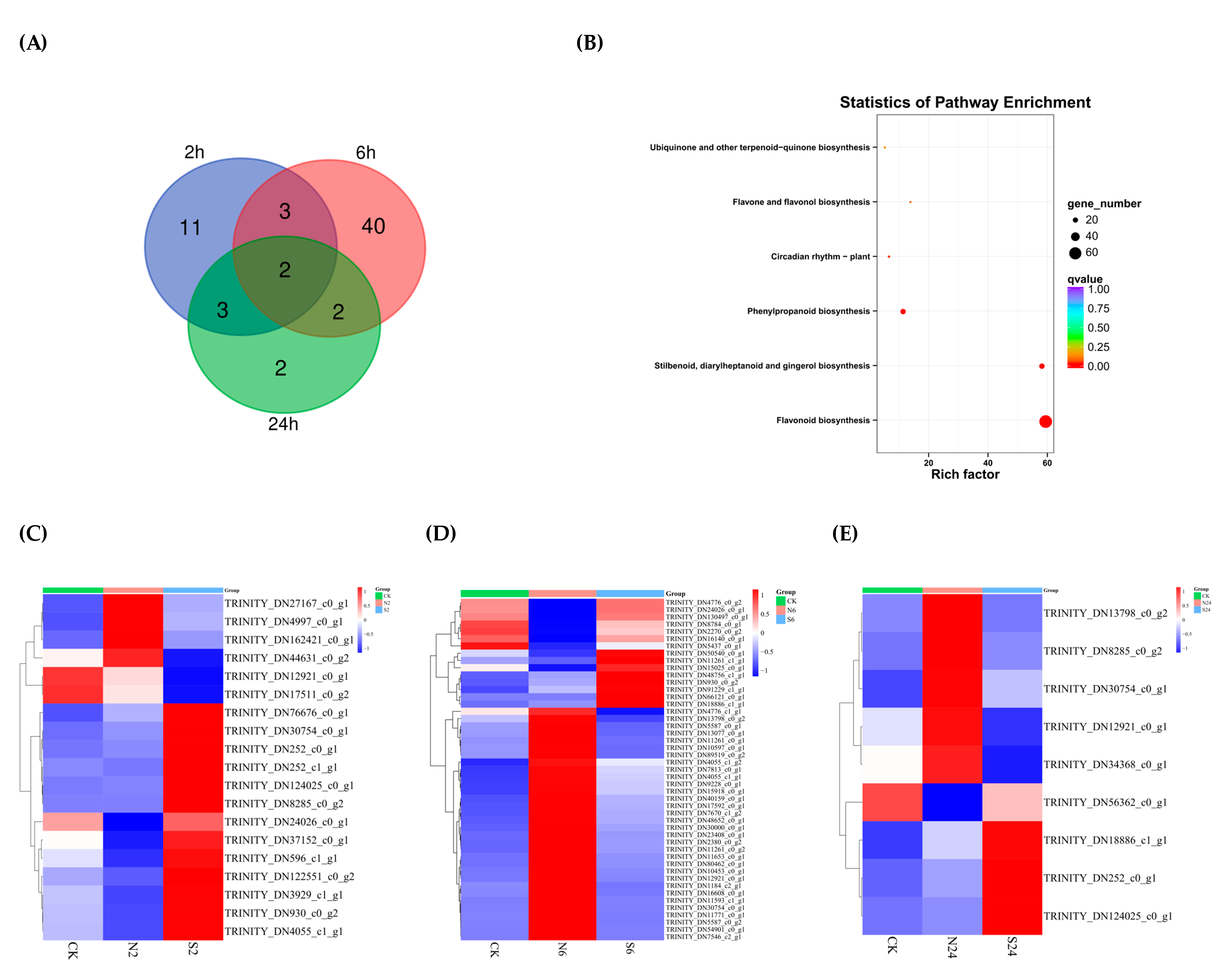
2.3. Analysis of Two Situations Resulting from the Action of Animal Saliva
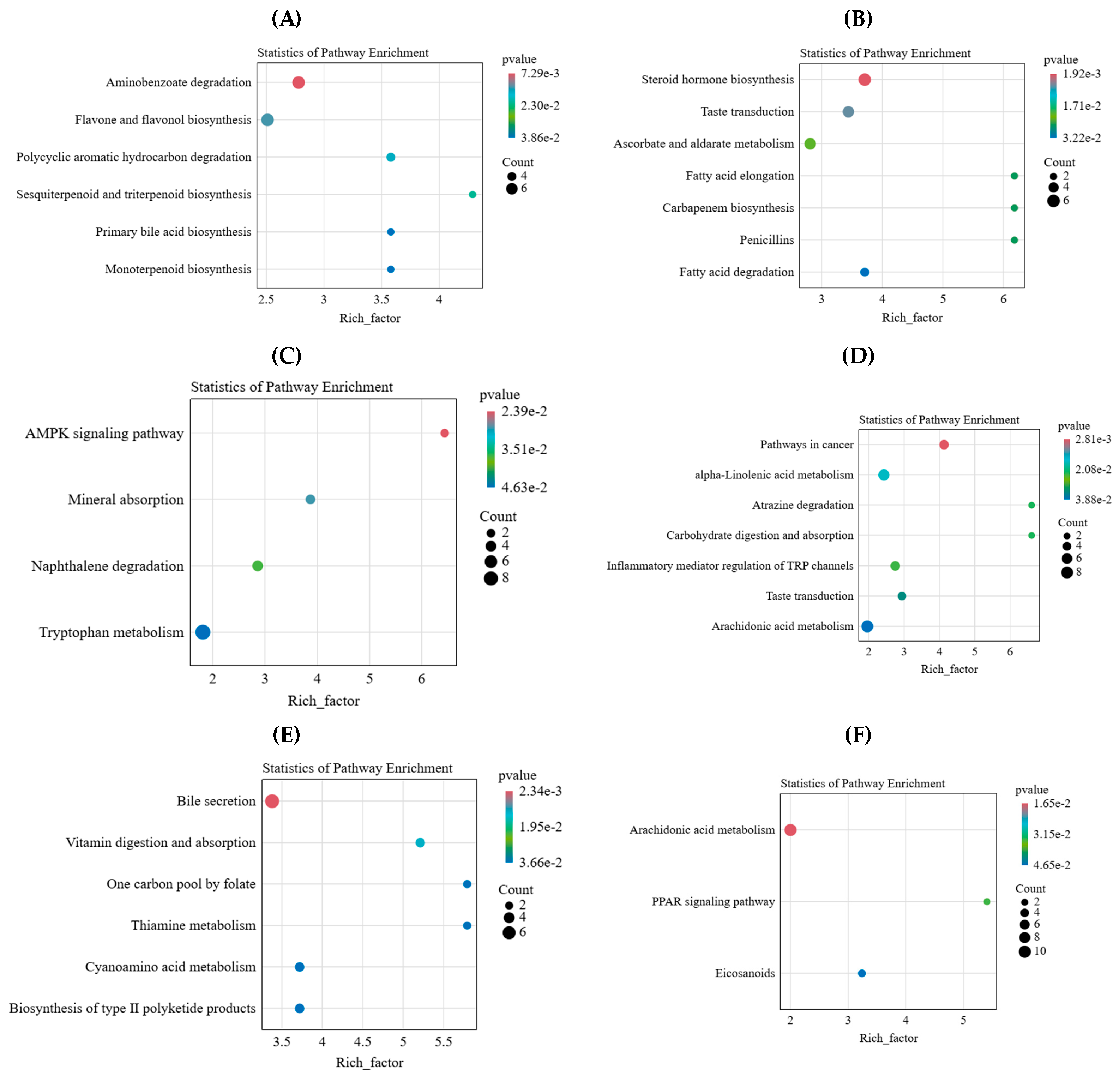
2.4. Key Differential Metabolites and Metabolic Pathways of L. chinensis in Response to Simulated Animal Feeding
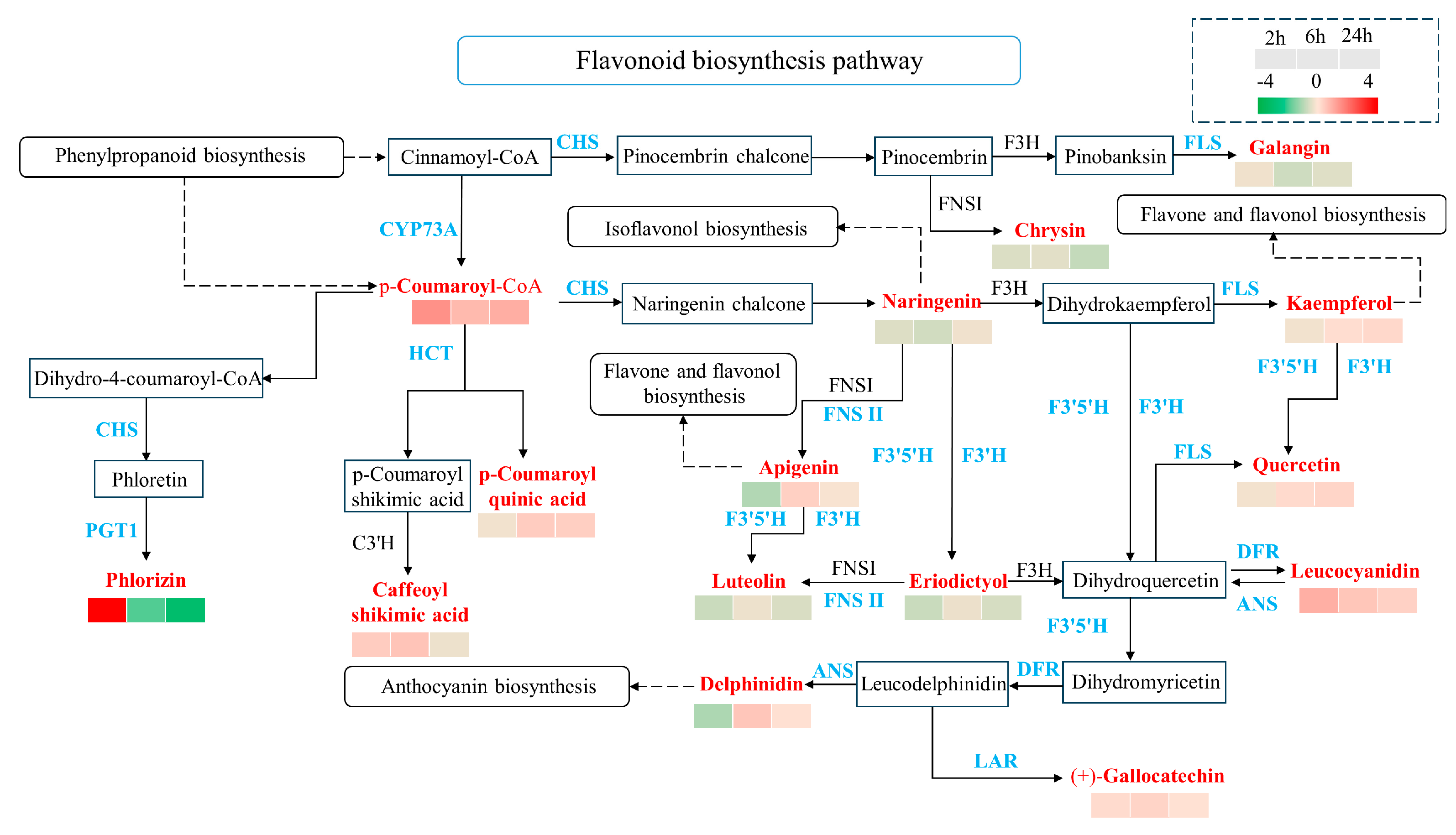
2.5. The Differentially Expressed Genes (DEGs) Are Enriched in the Flavonoid Biosynthesis Pathway in Response to Simulated Animal Feeding in L. chinensis
3. Discussion
4. Materials and Methods
4.1. Leymus chinensis Plants
4.2. Collection of Animal Saliva
4.3. Treatment of L. chinensis by Simulated Animal Grazing
4.4. Physiological Assays Related to Cellular Oxidation of L. chinensis
4.5. Widely Untargeted Metabonomic Analysis
4.6. Transcriptome and Metabolome Analysis of Flavonoid Biosynthesis Pathway in L. chinensis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Zhang, P.; Wang, F.; Li, R.; Liu, J.; Wang, Q.; Liu, W.; Wang, B.; Hu, G. Integrated Analysis of the Transcriptome and Metabolome Revealed Candidate Genes Involved in GA3-Induced Dormancy Release in Leymus chinensis Seeds. Int. J. Mol. Sci. 2021, 22, 4161. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gong, J.R.; Yang, B.; Ding, Y.; Zhang, Z.H.; Wang, B.; Zhu, C.C.; Hou, X.Y. Differences in the photosynthetic and physiological responses of Leymus chinensis to different levels of grazing intensity. BMC Plant Biol. 2019, 19, 558. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Gong, J.R.; Wang, B.; Li, X.B.; Ding, Y.; Yang, B.; Zhu, C.C.; Liu, M.; Zhang, W. Regrowth strategies of Leymus chinensis in response to different grazing intensities. Ecol. Appl. 2020, 30, e02113. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Y.; Pan, Y.; Gong, J.R.; Li, X.B.; Liu, M.; Yang, B.; Zhang, Z.H.; Baoyin, T. Physiology of Leymus chinensis under seasonal grazing: Implications for the development of sustainable grazing in a temperate grassland of Inner Mongolia. J. Environ. Manage. 2020, 271, 110984. [Google Scholar] [CrossRef] [PubMed]
- Wininger, K.; Rank, N. Evolutionary dynamics of interactions between plants and their enemies: Comparison of herbivorous insects and pathogens. Ann. N. Y. Acad. Sci. 2017, 1408, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Martínez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708. [Google Scholar] [CrossRef]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant Responses to Herbivory, Wounding, and Infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef]
- Zu, P.; Boege, K.; Del-Val, E.; Schuman, M.C.; Stevenson, P.C.; Zaldivar-Riverón, A.; Saavedra, S. Information arms race explains plant-herbivore chemical communication in ecological communities. Science 2020, 368, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Erb, M. Plant Defenses against Herbivory: Closing the Fitness Gap. Trends Plant Sci. 2018, 23, 187–194. [Google Scholar] [CrossRef]
- Gandhi, A.; Kariyat, R.; Harikishore, A.; Ayati, M.; Bhunia, A.; Sahoo, N. Deciphering the Role of Ion Channels in Early Defense Signaling against Herbivorous Insects. Cells 2021, 10, 2219. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J. The essential role of jasmonic acid in plant-herbivore interactions--using the wild tobacco Nicotiana attenuata as a model. J. Genet. Genomics 2013, 40, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, R.; Heise, A.M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef]
- Sedio, B.E. Recent advances in understanding the role of secondary metabolites in species-rich multitrophic networks. Curr. Opin. Insect Sci. 2019, 32, 124–130. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Li, E.Q.; Liu, J.S.; Li, X.F.; Xiang, H.Y.; Yu, J.P.; Wang, D.L. Animal saliva has stronger effects on plant growth than salivary components. Grass Forage Sci. 2014, 69, 153–159. [Google Scholar] [CrossRef]
- Ohse, B.; Hammerbacher, A.; Seele, C.; Meldau, S.; Reichelt, M.; Ortmann, S.; Wirth, C. Salivary cues: Simulated roe deer browsing induces systemic changes in phytohormones and defence chemistry in wild-grown maple and beech saplings. Functional Ecology 2017, 31, 340–349. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, X.; Bai, C.; Zhao, C.; Lu, G.; Xu, G. LC-MS-based metabonomics analysis. J. Chromatogr. B 2008, 866, 64–76. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.J.; Li, Z.P.; Zhao, Y.; Yang, H.L.; Zha, L.; Yu, C.X.; Wu, Y.J.; Song, X.X. The Response of Volvariella volvacea to Low-Temperature Stress Based on Metabonomics. Front. Microbiol. 2020, 11, 1787. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Zuo, W.T.; Gao, Y.R.; Li, R.Z.; Yu, C.X.; Liu, Z.Y.; Zheng, Y.; Shen, Y.Y.; Duan, L.S. Integration of Metabolome and Transcriptome Studies Reveals Flavonoids, Abscisic Acid, and Nitric Oxide Comodulating the Freezing Tolerance in Liriope spicata. Front. Plant Sci. 2022, 12, 764625. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Baldwin, I.T. The ecogenetics and ecogenomics of plant-herbivore interactions: Rapid progress on a slippery road. Curr. Opin. Genet. Dev. 2007, 17, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.B.; Hou, X.Y.; Wu, Z.N.; Kong, L.Q.; Guo, H.Q.; Hu, N.N.; Wan, D.L.; Zhang, J.Z. De novo transcriptomic profiling of the clonal Leymus chinensis response to long-term overgrazinginduced memory. Sci. Rep. 2018, 8, 17912. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.L.; Wan, Y.Q.; Ren, W.B.; Yu, L.Q.; Ding, Y.; Li, G.J.; Li, F.; Hou, X.Y. Common response of dominant plants in typical grassland of Inner Mongolia to long-term overgrazing revealed by transcriptome analysis. Grassland Sci. 2021, 67, 352–368. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Ferdinando, M.D.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Feng, Y.; Yu, S.H.; Fan, Z.Q.; Li, X.L.; Li, J.Y.; Yin, H.F. The Flavonoid Biosynthesis Network in Plants. I Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The Origin and Evolution of Plant Flavonoid Metabolism. Front. Plant Sci. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Fan, R.N.; Sun, G.L.; Sun, T.; Fan, Y.T.; Bai, S.L.; Guo, S.Y.; Huang, S.Q.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil. 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Si, C.; Dong, W.; da Silva, J.A.T.; He, C.M.; Yu, Z.M.; Zhang, M.Z.; Huang, L.; Zhao, C.H.; Zeng, D.Q.; Li, C.M.; et al. Functional analysis of flavanone 3-hydroxylase (F3H) from Dendrobium officinale, which confers abiotic stress tolerance. Hortic. Plant J. 2023, 9, 356–364. [Google Scholar] [CrossRef]
- Ochoa-Jiménez, V.A.; Berumen-Varela, G.; Pérez-Ramírez, I.F.; Balois-Morales, R.; Rubio-Melgarejo, A.; Bautista-Rosales, P.U. Metabolomics approach for phenolic compounds profiling of soursop (Annona muricata L.) fruit during postharvest storage. Metabolomics 2024, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Lal, S.K.; Lakra, N.; Mehta, S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 253, 45. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wen, H.; Xu, P.; Chen, J.; Xue, M.; Li, J.; Wang, M.; Song, C.; Li, H. PPAR Signaling Maintains Metabolic Homeostasis under Hypothermia in Freshwater Drum (Aplodinotus grunniens). Metabolites 2023, 13, 102. [Google Scholar] [CrossRef]
- Aubert, L.; Decamps, C.; Jacquemin, G.; Quinet, M. Comparison of Plant Morphology, Yield and Nutritional Quality of Fagopyrum esculentum and Fagopyrum tataricum Grown under Field Conditions in Belgium. Plants 2021, 10, 258. [Google Scholar] [CrossRef]



| S2 vs. N2 | S6 vs. N6 | S24 vs. N24 | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene Annotation | EC Number | Abbreviation | Up | Down | Up | Down | Up | Down |
| shikimate O-hydroxycinnamoyl transferase | EC:2.3.1.133 | HCT | 7 | 3 | 4 | 4 | 3 | 1 |
| flavonol synthase | EC:1.14.20.6 | FLS | 2 | 1 | 1 | 2 | ||
| leucoanthocyanidin reductase | EC:1.17.1.3 | LAR | 1 | 1 | 1 | |||
| chalcone synthase | EC:2.3.1.74 | CHS | 2 | 2 | 1 | |||
| trans-cinnamate 4-monooxygenase | EC:1.14.14.91 | CYP73A | 1 | 2 | ||||
| anthocyanidin synthase | EC:1.14.20.4 | ANS | 4 | 1 | ||||
| bifunctional dihydroflavonol 4-reductase/flavanone 4-reductase | EC:1.1.1.219/ 1.1.1.234 | DFR | 1 | |||||
| flavone synthase II | EC:1.14.19.76 | FNSⅡ | 1 | 1 | ||||
| flavanoid 3′,5′-hydroxylase | EC:1.14.14.81 | F3′5′H | 1 | |||||
| flavonoid 3′-hydroxylase | EC:1.14.14.82 | F3′H | 1 | |||||
| phlorizin synthase | EC:2.4.1.357 | PGT1 | 2 | 1 | 1 | 22 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhao, R.; Wang, H.; Liu, B.; Yu, Y.; Jiang, L. Untargeted Metabolome Analyses Revealed Potential Metabolic Mechanisms of Leymus chinensis in Response to Simulated Animal Feeding. Int. J. Mol. Sci. 2024, 25, 6110. https://doi.org/10.3390/ijms25116110
Zhou C, Zhao R, Wang H, Liu B, Yu Y, Jiang L. Untargeted Metabolome Analyses Revealed Potential Metabolic Mechanisms of Leymus chinensis in Response to Simulated Animal Feeding. International Journal of Molecular Sciences. 2024; 25(11):6110. https://doi.org/10.3390/ijms25116110
Chicago/Turabian StyleZhou, Chunxu, Ruiqi Zhao, Han Wang, Bao Liu, Yingjie Yu, and Lili Jiang. 2024. "Untargeted Metabolome Analyses Revealed Potential Metabolic Mechanisms of Leymus chinensis in Response to Simulated Animal Feeding" International Journal of Molecular Sciences 25, no. 11: 6110. https://doi.org/10.3390/ijms25116110






