Particulate Matter-Induced Neurotoxicity: Unveiling the Role of NOX4-Mediated ROS Production and Mitochondrial Dysfunction in Neuronal Apoptosis
Abstract
1. Introduction
2. Results
2.1. PM-Induced Neurotoxicity Causes Neuronal Cell Apoptosis
2.2. PM Increases NOX4 Activity without Altering Protein and mRNA Expression in Neuro-2A Cells
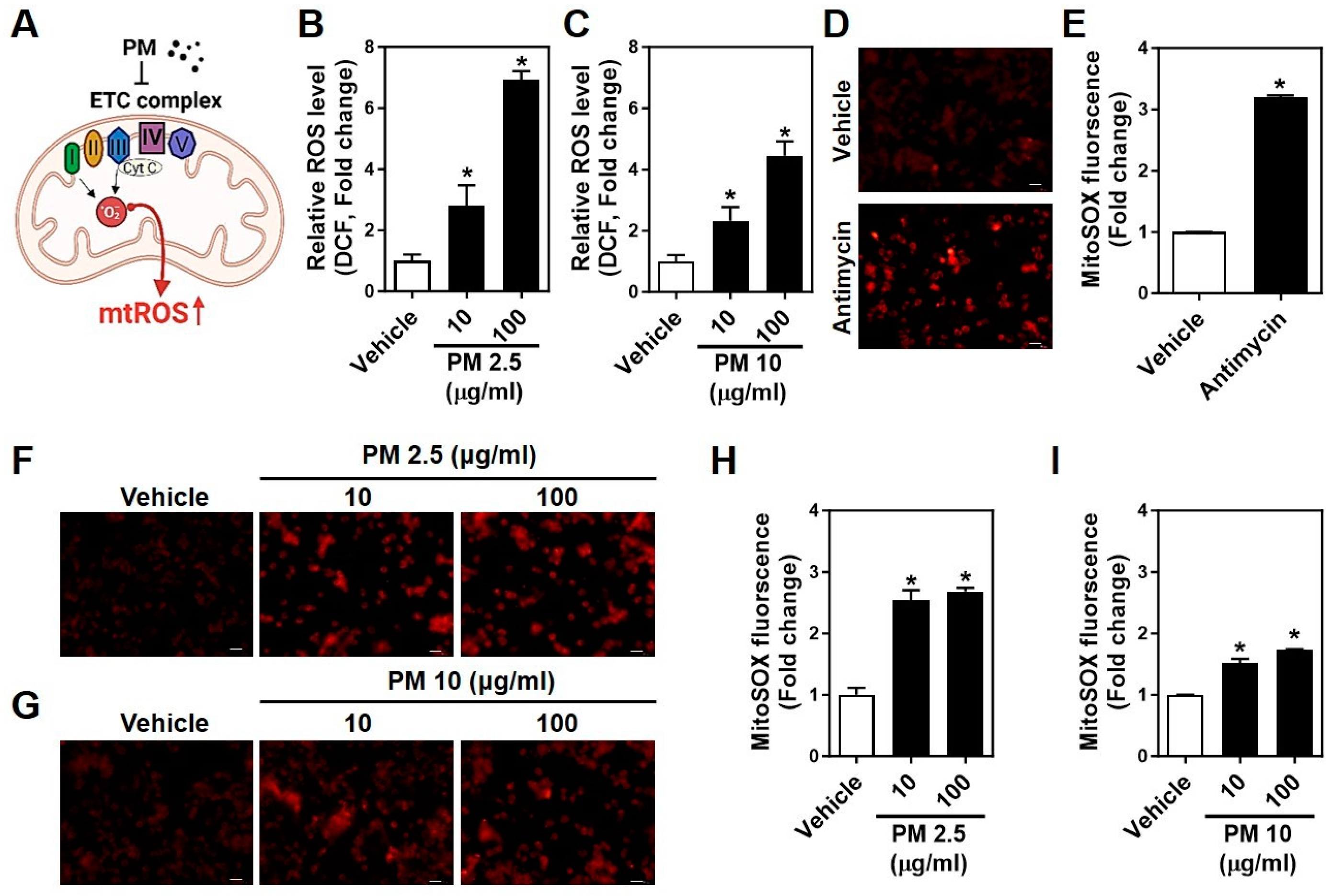
2.3. PMs Facilitate Cytosolic and Mitochondrial ROS Generation in Neuro-2A Cells
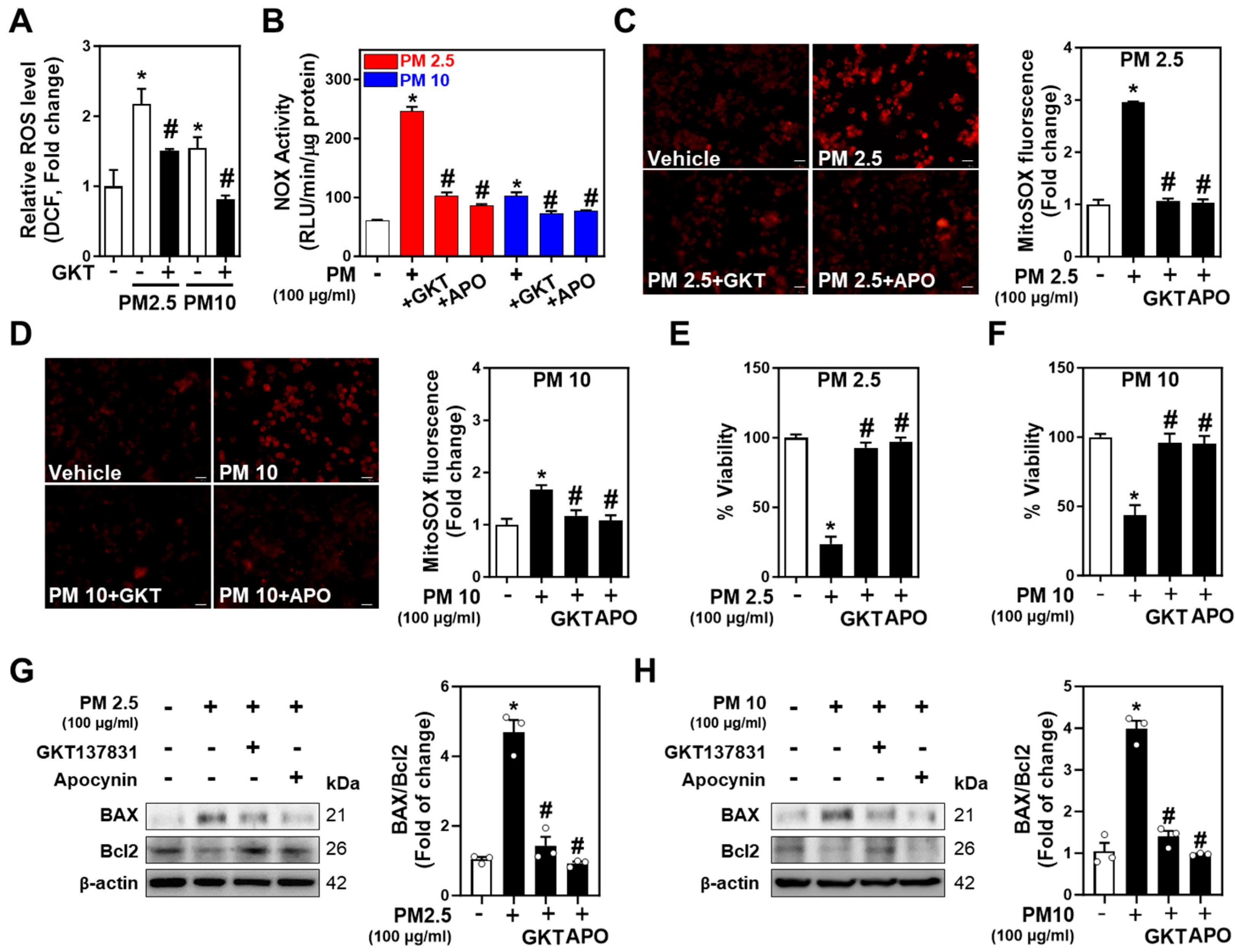
2.4. Inhibition of NOXs Mitigates PM-Induced Neurotoxicity and Neuronal Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Materials
4.3. MTT Assay
4.4. Measurement of Reactive Oxygen Species (ROS)
4.5. NOX Activity Assay
4.6. RT- and Real-Time qPCR
4.7. Western Blot
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, G.; Zuo, L.; Xiong, P.; Chen, L.; Liang, X.; Jing, C. Associations of PM2.5 and road traffic noise with mental health: Evidence from UK biobank. Environ. Res. 2022, 207, 112221. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, R.; Ye, T.; Abramson, M.J.; Morawska, L.; Jalaludin, B.; Johnston, F.H.; Henderson, S.B.; Knibbs, L.D.; Morgan, G.G.; et al. Estimates of global mortality burden associated with short-term exposure to fine particulate matter (PM2.5). Lancet. Planet. Health 2024, 8, e146–e155. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Casadei, B.; Harrington, R.A.; Kovacs, R.; Sliwa, K.; The WHF Air Pollution Expert Group. Taking a stand against air pollution-the impact on cardiovascular disease: A joint opinion from the world heart federation, American college of cardiology, American heart association, and the European society of cardiology. Circulation 2021, 143, e800–e804. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Hu, C.; Chang, Q.; Deng, Q.; Yang, X.; Wu, Y. Study of the neurotoxicity of indoor airborne nanoparticles based on a 3d human blood-brain barrier chip. Environ. Int. 2020, 143, 105598. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Wong, R.M.S.; Yung, K.K.L.; Li, R. Fine particulate matter induces endoplasmic reticulum stress-mediated apoptosis in human SH-SY5Y cells. Neurotoxicology 2022, 88, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, R.; Guo, J.; Li, Y.; Cheng, W.; Pang, Y.; Zheng, Y.; Zhang, R.; Tang, J. Olfactory bulb microglia activation mediated neuronal death in real-ambient particulate matter exposure mice with depression-like behaviors. Sci. Total Environ. 2022, 821, 153456. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, B.; Sang, N. Particulate matter (PM2.5) exposure season-dependently induces neuronal apoptosis and synaptic injuries. J. Environ. Sci. 2017, 54, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Tian, X.; Xu, C.; Ma, B.; Liu, W.; Sun, B.; Ru, Q.; Shu, X. PM2.5 exposure-induced ferroptosis in neuronal cells via inhibiting ERK/CREB pathway. Environ. Toxicol. 2022, 37, 2201–2213. [Google Scholar] [CrossRef]
- Ku, T.; Ji, X.; Zhang, Y.; Li, G.; Sang, N. PM2.5, SO2 and NO2 co-exposure impairs neurobehavior and induces mitochondrial injuries in the mouse brain. Chemosphere 2016, 163, 27–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Ma, J.; Zhao, Y. PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat. Environ. Pollut. 2018, 242, 994–1001. [Google Scholar] [CrossRef]
- Liang, Y.; Chu, P.H.; Tian, L.; Ho, K.F.; Ip, M.S.M.; Mak, J.C.W. Targeting mitochondrial permeability transition pore ameliorates PM2.5-induced mitochondrial dysfunction in airway epithelial cells. Environ. Pollut. 2022, 295, 118720. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Weerasinghe-Mudiyanselage, P.D.E.; Kim, B.; Kang, S.; Kim, J.S.; Moon, C. Particulate matter exposure and neurodegenerative diseases: A comprehensive update on toxicity and mechanisms. Ecotoxicol. Environ. Saf. 2023, 266, 115565. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Ho, Y.S.; Chang, R.C. The pathogenic effects of particulate matter on neurodegeneration: A review. J. Biomed. Sci. 2022, 29, 15. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xue, B.; Zhou, Q.; Su, R.; Li, Z. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J. Toxicol. Sci. 2018, 43, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wang, J.; Sun, J.; Xin, L. PM2.5 induces inflammatory responses via oxidative stress-mediated mitophagy in human bronchial epithelial cells. Toxicol. Res. 2022, 11, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, Q.; Zhang, X.; Wu, S.; Wang, S.; Chen, R.; Li, X. Role of astrocyte activation in fine particulate matter-enhancement of existing ischemic stroke in sprague-dawley male rats. J. Toxicol. Environ. Health. Part A 2016, 79, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. Nox4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Han, K.; Wang, Y.; Qu, R.; Liu, Y.; Wang, S.; Wang, Y.; An, Z.; Li, J.; Wu, H.; et al. Microglial activation and oxidative stress in PM2.5-induced neurodegenerative disorders. Antioxidants 2022, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Chen, Y.; Wang, S.; Dong, Y.; Ju, G.; Chen, B. The association between PM2.5 and depression in China. Dose Response 2020, 18, 1559325820942699. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef]
- Cuevas, A.K.; Niu, J.; Zhong, M.; Liberda, E.N.; Ghio, A.; Qu, Q.; Chen, L.C. Metal rich particulate matter impairs acetylcholine-mediated vasorelaxation of microvessels in mice. Part Fibre Toxicol. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, J. The involvement of NOX4 in fine particulate matter exposure-induced cardiac injury in mice. J. Toxicol. Sci. 2018, 43, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Fang, M.; Ma, Y.; Liu, N.; Yan, X.; Zhou, J.; Li, F. The kidney injury induced by short-term PM2.5 exposure and the prophylactic treatment of essential oils in BALB/c mice. Oxid. Med. Cell. Longev. 2018, 2018, 9098627. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFkappab by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Fan, X.; Dong, T.; Yan, K.; Ci, X.; Peng, L. PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 2023, 59, 102587. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. Nox4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef] [PubMed]
- Soberanes, S.; Misharin, A.V.; Jairaman, A.; Morales-Nebreda, L.; McQuattie-Pimentel, A.C.; Cho, T.; Hamanaka, R.B.; Meliton, A.Y.; Reyfman, P.A.; Walter, J.M.; et al. Metformin targets mitochondrial electron transport to reduce air-pollution-induced thrombosis. Cell Metab. 2019, 29, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, M.; Zollo, C.; Esposito, G.; Ammendola, R.; Cattaneo, F. Nox2-dependent reactive oxygen species regulate formyl-peptide receptor 1-mediated TrkA transactivation in SH-SY5Y cells. Oxidative Med. Cell. Longev. 2019, 2019, 2051235. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, W.; Seth, D.M.; Nair, A.R.; Francis, J.; Feng, Y. (pro)renin receptor mediates both angiotensin ii-dependent and -independent oxidative stress in neuronal cells. PLoS ONE 2013, 8, e58339. [Google Scholar] [CrossRef]
- Ehsanifar, M.; Yavari, Z.; Rafati, M. Exposure to urban air pollution particulate matter: Neurobehavioral alteration and hippocampal inflammation. Environ. Sci. Pollut. Res. Int. 2022, 29, 50856–50866. [Google Scholar] [CrossRef]
- Lin, C.H.; Nicol, C.J.B.; Wan, C.; Chen, S.J.; Huang, R.N.; Chiang, M.C. Exposure to PM2.5 induces neurotoxicity, mitochondrial dysfunction, oxidative stress and inflammation in human SH-SY5Y neuronal cells. Neurotoxicology 2022, 88, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Wang, D.; Matsuoka, H.; Morita, K.; Yasuda, H.; Yatera, K.; Kanazawa, T.; Yoshida, Y. Endocytosis of particulate matter induces cytokine production by neutrophil via toll-like receptor 4. Int. Immunopharmacol. 2018, 57, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Nayernia, Z.; Jaquet, V.; Krause, K.H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014, 20, 2815–2837. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.T.; Hoeffer, C.A.; Hu, D.; Pao, M.; Holland, S.M.; Klann, E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol. Cell. Biol. 2006, 26, 5908–5920. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, H.Y.; Cui, S.J.; Han, B.; Zhou, L.X.; Zhang, N.; Su, X.; Niu, Y.J.; Chen, W.; Chen, R.; et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard. Mater. 2019, 369, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; McBride, S.L.; Harper, M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013, 38, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Shimizu, S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000, 7, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Xu, Z.M.; Lyu, Y.; Li, X.H.; Li, Z.F.; He, H.; Tian, F.J.; Zheng, J.P. PM2.5 induces autophagy-dependent ferroptosis by endoplasmic reticulum stress in SH-SY5Y cells. J. Appl. Toxicol. 2023, 43, 1013–1025. [Google Scholar] [CrossRef]
- Yang, Q.; Li, K.; Li, D.; Zhang, Y.; Liu, X.; Wu, K. Effects of fine particulate matter on the ocular surface: An in vitro and in vivo study. Biomed. Pharmacother. 2019, 117, 109177. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-kappab, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Quan, X.; Hwang, K.H.; Xu, S.; Das, R.; Choi, S.K.; Wiederkehr, A.; Wollheim, C.B.; Cha, S.K.; Park, K.S. Mitochondrial oxidative stress mediates high-phosphate-induced secretory defects and apoptosis in insulin-secreting cells. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E933–E941. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Xu, S.; Nguyen, T.T.; Quan, X.; Choi, S.K.; Kim, S.J.; Lee, E.Y.; Cha, S.K.; Park, K.S. Transforming growth factor beta1-induced apoptosis in podocytes via the extracellular signal-regulated kinase-mammalian target of rapamycin complex 1-NADPH oxidase 4 Axis. J. Biol. Chem. 2015, 290, 30830–30842. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hwang, K.H.; Dang, B.T.N.; Eom, M.; Kong, I.D.; Gwack, Y.; Yu, S.; Gee, H.Y.; Birnbaumer, L.; Park, K.S.; et al. Insulin-activated store-operated Ca2+ entry via orai1 induces podocyte actin remodeling and causes proteinuria. Nat. Commun. 2021, 12, 6537. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Hwang, K.-H.; Kim, S.-H.; Kim, H.-J.; Kim, J.-M.; Lee, M.-Y.; Cha, S.-K.; Lee, J. Particulate Matter-Induced Neurotoxicity: Unveiling the Role of NOX4-Mediated ROS Production and Mitochondrial Dysfunction in Neuronal Apoptosis. Int. J. Mol. Sci. 2024, 25, 6116. https://doi.org/10.3390/ijms25116116
Kim J-H, Hwang K-H, Kim S-H, Kim H-J, Kim J-M, Lee M-Y, Cha S-K, Lee J. Particulate Matter-Induced Neurotoxicity: Unveiling the Role of NOX4-Mediated ROS Production and Mitochondrial Dysfunction in Neuronal Apoptosis. International Journal of Molecular Sciences. 2024; 25(11):6116. https://doi.org/10.3390/ijms25116116
Chicago/Turabian StyleKim, Ji-Hee, Kyu-Hee Hwang, Seong-Heon Kim, Hi-Ju Kim, Jung-Min Kim, Mi-Young Lee, Seung-Kuy Cha, and Jinhee Lee. 2024. "Particulate Matter-Induced Neurotoxicity: Unveiling the Role of NOX4-Mediated ROS Production and Mitochondrial Dysfunction in Neuronal Apoptosis" International Journal of Molecular Sciences 25, no. 11: 6116. https://doi.org/10.3390/ijms25116116
APA StyleKim, J.-H., Hwang, K.-H., Kim, S.-H., Kim, H.-J., Kim, J.-M., Lee, M.-Y., Cha, S.-K., & Lee, J. (2024). Particulate Matter-Induced Neurotoxicity: Unveiling the Role of NOX4-Mediated ROS Production and Mitochondrial Dysfunction in Neuronal Apoptosis. International Journal of Molecular Sciences, 25(11), 6116. https://doi.org/10.3390/ijms25116116




