Glomerular Hypertrophy and Splenic Red Pulp Degeneration Concurrent with Oxidative Stress in 3xTg-AD Mice Model for Alzheimer’s Disease and Its Exacerbation with Sex and Social Isolation
Abstract
:1. Introduction
2. Results
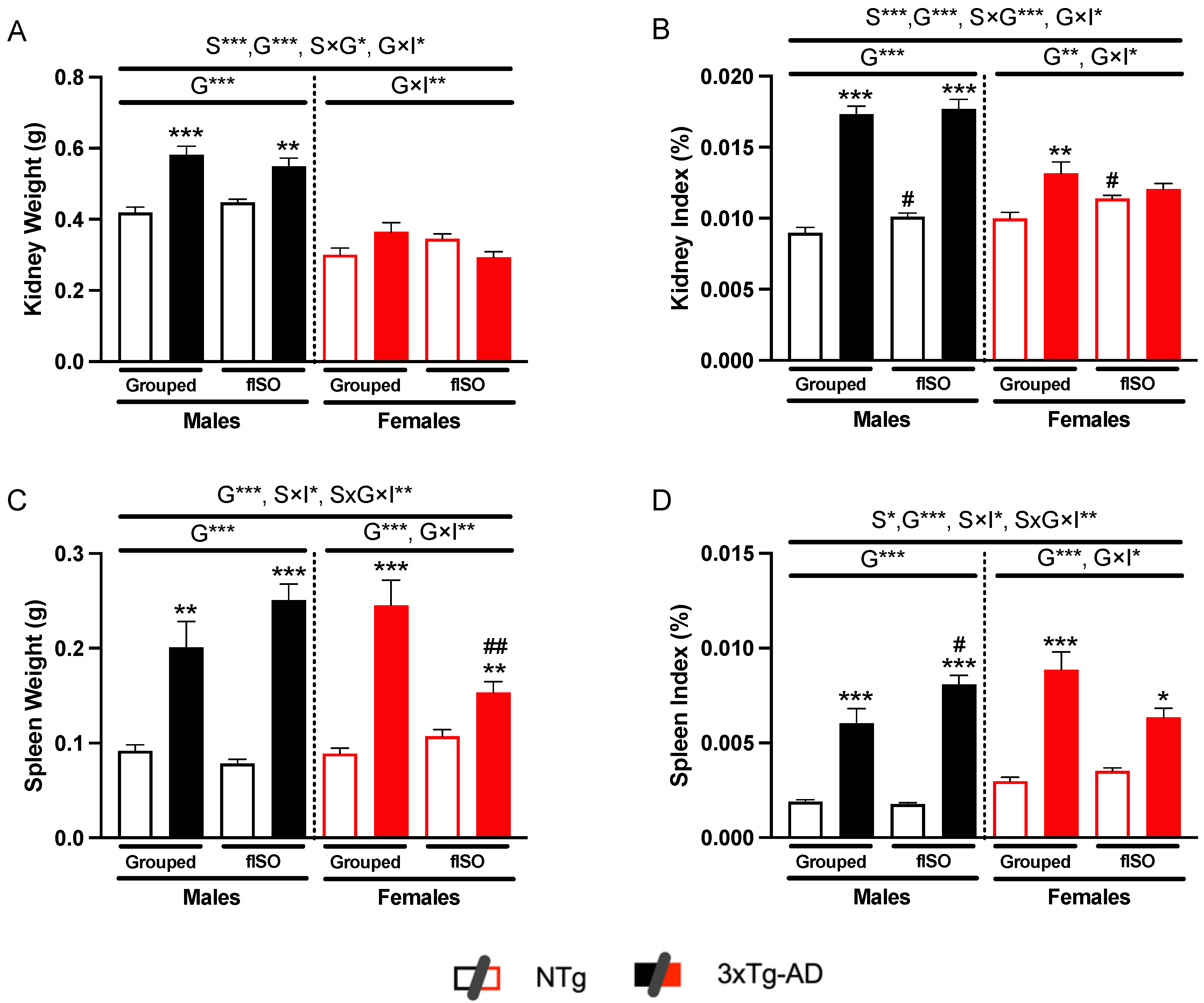
2.1. Physical Metrics of Kidney and Spleen
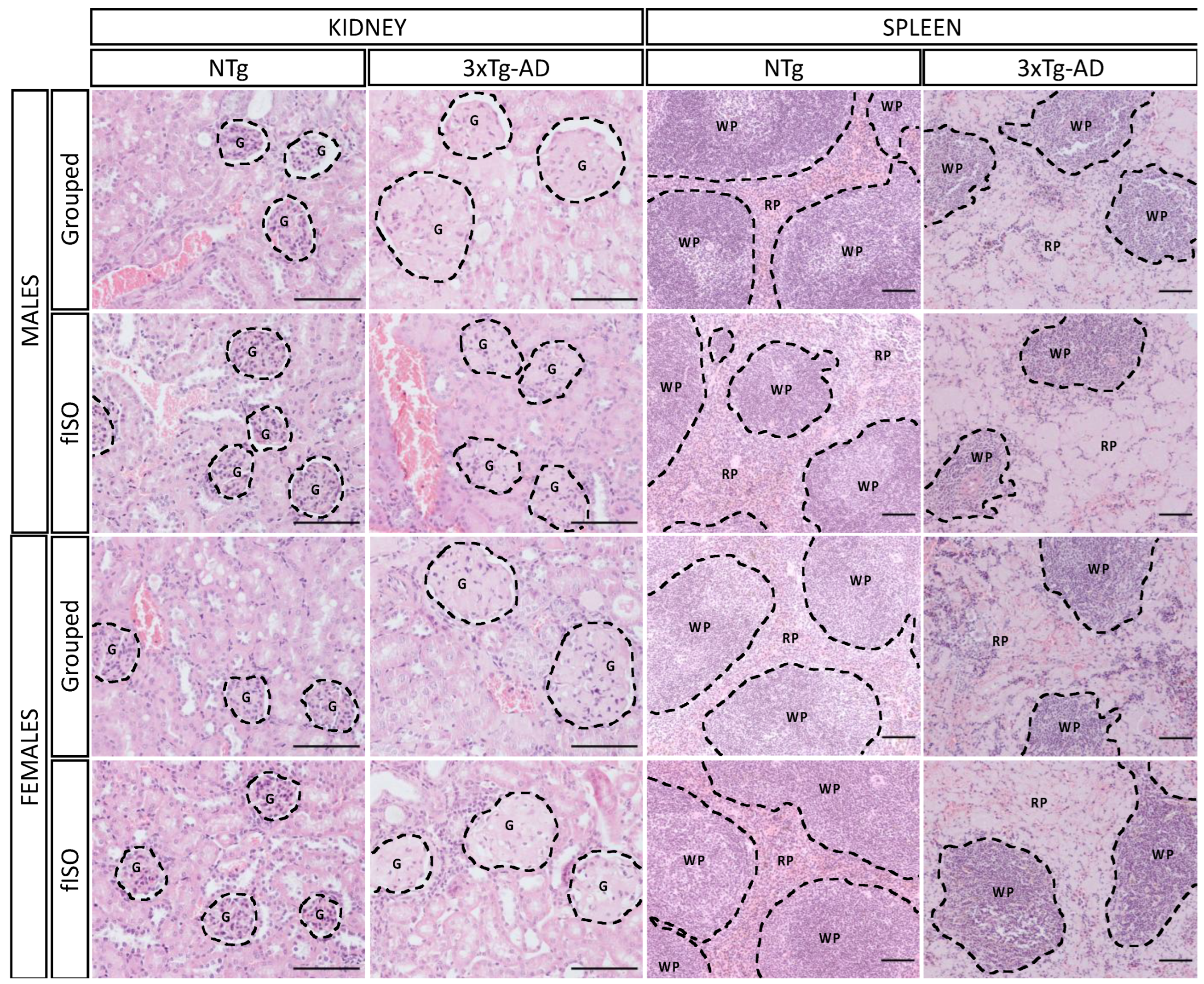
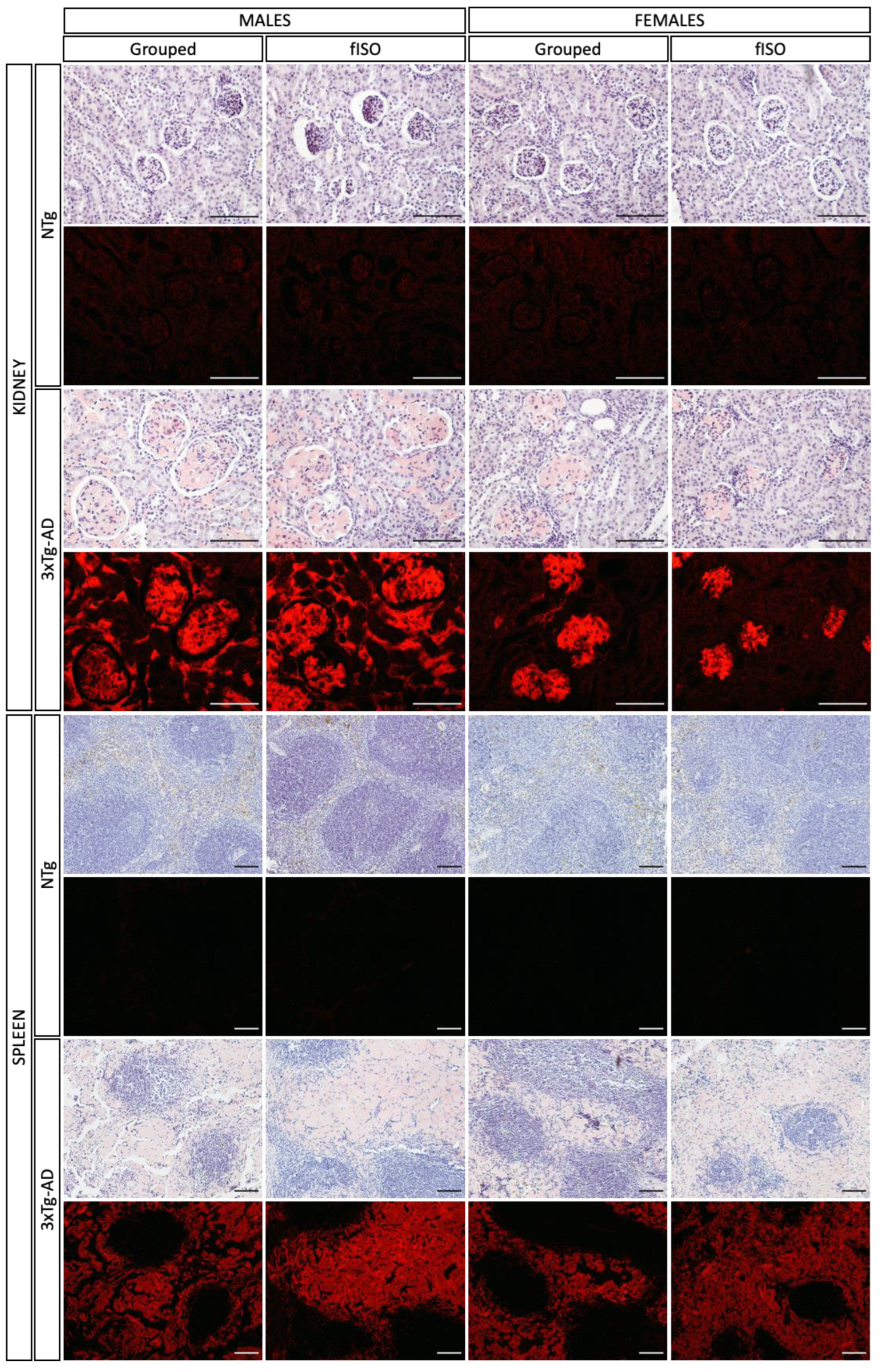
2.2. Histopathological Assessment: H&E and Congo Red Staining
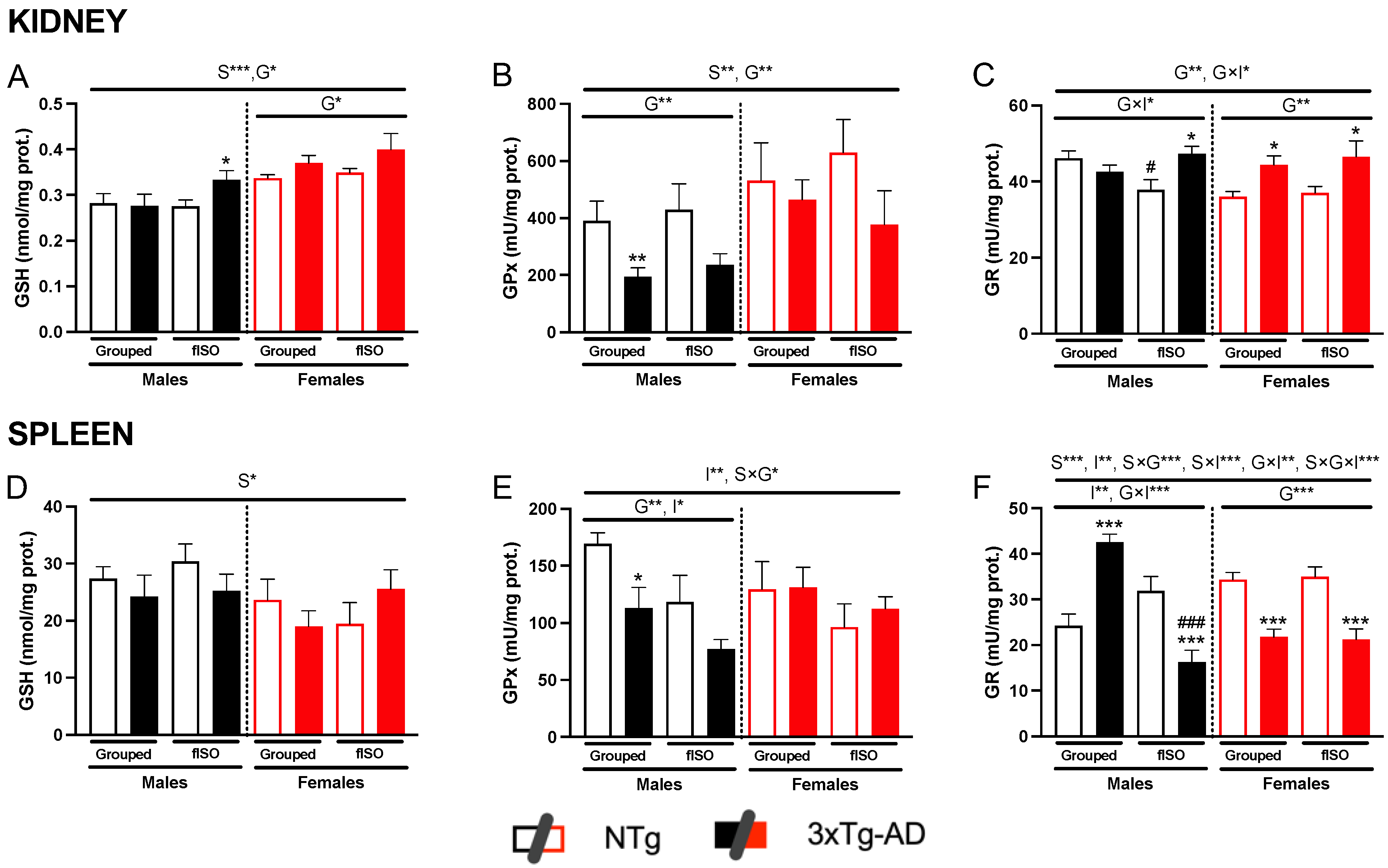
2.3. Oxidative Stress Status
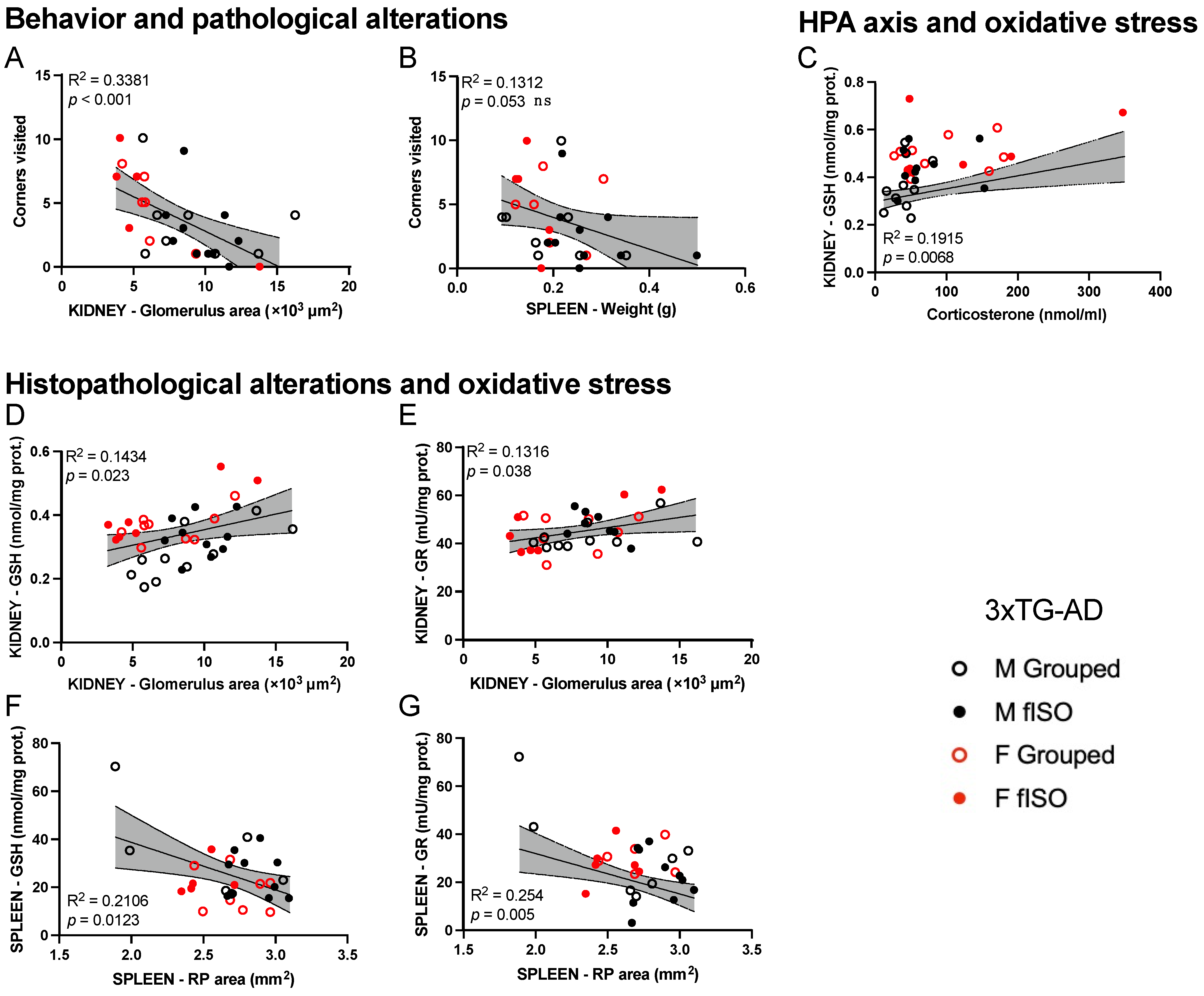
2.4. Statistical Correlation between the Studied Variables
3. Discussion
3.1. Physical and Histopathological Alterations of Kidney and Spleen
3.2. Kidney and Spleen Oxidative Stress
3.3. Correlations between Behavior, Histopathology, HPA Axis and Peripheral Oxidative Stress
3.4. Study Limitations
3.5. Future Perspective of Peripheral Organs in AD
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Physical Status
4.4. Behavioral Phenotype
4.5. Histopathological Assessment
4.5.1. Morphological and Structural Analysis with H&E
4.5.2. Congo Red Staining for Amyloidosis
4.6. Oxidative Stress Analysis
4.7. Functional Correlates
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef]
- Angiulli, F.; Conti, E.; Zoia, C.P.; Da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Blood-Based Biomarkers of Neuroinflammation in Alzheimer’s Disease: A Central Role for Periphery? Diagnostics 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zheng, H. Peripheral Immune System in Aging and Alzheimer’s Disease. Mol. Neurodegener. 2018, 13. [Google Scholar]
- Giménez-Llort, L.; Maté, I.; Manassra, R.; Vida, C.; De la Fuente, M. Peripheral Immune System and Neuroimmune Communication Impairment in a Mouse Model of Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2012, 1262, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tian, D.Y.; Wang, Y.J. Peripheral Clearance of Brain-Derived Aβ in Alzheimer’s Disease: Pathophysiology and Therapeutic Perspectives. Transl. Neurodegener. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Fraile-Ramos, J.; Garrit, A.; Reig-Vilallonga, J.; Giménez-Llort, L. Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity. Cells 2023, 12, 1517. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Crosstalk of Alzheimer’s Disease-Phenotype, HPA Axis, Splenic Oxidative Stress and Frailty in Late-Stages of Dementia, with Special Concerns on the Effects of Social Isolation: A Translational Neuroscience Approach. Front. Aging. Neurosci. 2022, 14. [Google Scholar] [CrossRef]
- Estrada, L.D.; Ahumada, P.; Cabrera, D.; Arab, J.P. Liver Dysfunction as a Novel Player in Alzheimer’s Progression: Looking Outside the Brain. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef]
- Joo Kim, M.; Baek, D.; Truong, L.; Ro, Y.J. Pathologic Findings of Amyloidosis: Recent Advances. In Amyloid Diseases; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Etgen, T.; Chonchol, M.; Frstl, H.; Sander, D. Chronic Kidney Disease and Cognitive Impairment: A Systematic Review and Meta-Analysis. Am. J. Nephrol. 2012, 35, 474–482. [Google Scholar] [CrossRef]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.M.; Choukroun, G.; Massy, Z.A. Cognitive Disorders and Dementia in CKD: The Neglected Kidney-Brain Axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Xiang, Y.; Wang, Y.R.; Jiao, S.S.; Wang, Q.H.; Bu, X.L.; Zhu, C.; Yao, X.Q.; Giunta, B.; Tan, J.; et al. Association Between Serum Amyloid-Beta and Renal Functions: Implications for Roles of Kidney in Amyloid-Beta Clearance. Mol. Neurobiol. 2015, 52, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.S.; Shen, L.L.; Bu, X.L.; Zhang, W.W.; Chen, S.H.; Huang, Z.L.; Xiong, J.X.; Gao, C.Y.; Dong, Z.; He, Y.N.; et al. Peritoneal Dialysis Reduces Amyloid-Beta Plasma Levels in Humans and Attenuates Alzheimer-Associated Phenotypes in an APP/PS1 Mouse Model. Acta Neuropathol. 2017, 134, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, T.; Liao, L.; Fan, X.; Chang, L.; Hashimoto, K. Brain-Spleen Axis in Health and Diseases: A Review and Future Perspective. Brain Res. Bull 2022, 182, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Kim, J.; Lee, M.J.; Kim, Y. Abnormalities of Plasma Cytokines and Spleen in Senile APP/PS1/Tau Transgenic Mouse Model. Sci. Rep. 2015, 5, 15703. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. Conversion of Psychological Stress into Cellular Stress Response: Roles of the Sigma-1 Receptor in the Process. Psychiatry Clin. Neurosci. 2015, 69, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, T.G.; Edwards, L. The Stress Response System Chronic Stress and the HPA Axis: Clinical Assessment and Therapeutic Considerations; The Point Institute of Nutraceutical Research: Stevens Point, WI, USA, 2010. [Google Scholar]
- Machado, A.; Herrera, A.J.; de Pablos, R.M.; Espinosa-Oliva, A.M.; Sarmiento, M.; Ayala, A.; Venero, J.L.; Santiago, M.; Villarán, R.F.; Delgado-Cortés, M.J.; et al. Chronic Stress as a Risk Factor for Alzheimer’s Disease. Rev. Neurosci. 2014, 25. [Google Scholar] [CrossRef]
- Drinkwater, E.; Davies, C.; Spires-Jones, T.L. Potential Neurobiological Links between Social Isolation and Alzheimer’s Disease Risk. Eur. J. Neurosci. 2022, 56, 5397–5412. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Giménez-Llort, L. Impact of Social Isolation on the Behavioral, Functional Profiles, and Hippocampal Atrophy Asymmetry in Dementia in Times of Coronavirus Pandemic (COVID-19): A Translational Neuroscience Approach. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Javonillo, D.I.; Tran, K.M.; Phan, J.; Hingco, E.; Kramár, E.A.; da Cunha, C.; Forner, S.; Kawauchi, S.; Milinkeviciute, G.; Gomez-Arboledas, A.; et al. Systematic Phenotyping and Characterization of the 3xTg-AD Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2022, 15. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; Johansson, B.; Oddo, S.; Tobeña, A.; LaFerla, F.M.; Fernández-Teruel, A. Modeling Behavioral and Neuronal Symptoms of Alzheimer’s Disease in Mice: A Role for Intraneuronal Amyloid. Neurosci. Biobehav. Rev. 2007, 31, 125–147. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; Rosa, R.; Vivó, M.; Oddo, S.; Navarro, X.; LaFerla, F.M.; Johansson, B.; Tobeña, A. Modeling Neuropsychiatric Symptoms of Alzheimer’s Disease Dementia in 3xTg-AD Mice. In Alzheimer’s Disease: New Advances; Medimond SRL: Pianoro, Italy, 2006; pp. 513–516. [Google Scholar]
- Baeta-Corral, R.; Giménez-Llort, L. Bizarre Behaviors and Risk Assessment in 3xTg-AD Mice at Early Stages of the Disease. Behav. Brain Res. 2014, 258, 97–105. [Google Scholar] [CrossRef]
- Manna, J.; Dunbar, G.L.; Maiti, P. Curcugreen Treatment Prevented Splenomegaly and Other Peripheral Organ Abnormalities in 3xTg and 5xFAD Mouse Models of Alzheimer’s Disease. Antioxidants 2021, 10, 899. [Google Scholar] [CrossRef]
- Tian, D.-Y.; Cheng, Y.; Zhuang, Z.-Q.; He, C.-Y.; Pan, Q.-G.; Tang, M.-Z.; Hu, X.-L.; Shen, Y.-Y.; Wang, Y.-R.; Chen, S.-H.; et al. Physiological Clearance of Amyloid-Beta by the Kidney and Its Therapeutic Potential for Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 6074–6082. [Google Scholar] [CrossRef]
- Tangde, A.; Sonwane, B.; Bindu, R. A Clinicohematological Profile of Splenomegaly. Int. J. Res. Med. Sci. 2019, 7, 1934. [Google Scholar] [CrossRef]
- Gibson, P.R.; Gibson, R.N.; Ditchfield, M.R.; Donlan, J.D. Splenomegaly-an Insensitive Sign of Portal Hypertension. Aust. New Zealand J. Med. 1990, 20, 771–774. [Google Scholar] [CrossRef]
- Marchese, M.; Cowan, D.; Head, E.; Ma, D.; Karimi, K.; Ashthorpe, V.; Kapadia, M.; Zhao, H.; Davis, P.; Sakic, B. Autoimmune Manifestations in the 3xTg-AD Model of Alzheimer’s Disease. JJ. Alzheimer’s Dis. 2014, 39, 191–210. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, D.; Tan, C.; Zeng, G.; He, C.; Wang, J.; Bu, X.; Wang, Y. Physiological Clearance of Aβ by Spleen and Splenectomy Aggravates Alzheimer-type Pathogenesis. Aging Cell 2022, 21. [Google Scholar] [CrossRef]
- Hawkins, P.N. Hereditary Systemic Amyloidosis with Renal Involvement. J. Nephrol. 2003, 3, 443–448. [Google Scholar]
- Xiang, Y.; Bu, X.L.; Liu, Y.H.; Zhu, C.; Shen, L.L.; Jiao, S.S.; Zhu, X.Y.; Giunta, B.; Tan, J.; Song, W.H.; et al. Physiological Amyloid-Beta Clearance in the Periphery and Its Therapeutic Potential for Alzheimer’s Disease. Acta Neuropathol. 2015, 130, 487–499. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Burgaletto, C.; Carta, A.R.; Saccone, S.; Lempereur, L.; Mulas, G.; Loreto, C.; Bernardini, R.; Cantarella, G. Beneficial Effects of Curtailing Immune Susceptibility in an Alzheimer’s Disease Model. J. Neuroinflammation 2019, 16, 166. [Google Scholar] [CrossRef]
- Gonzalez-Marrero, I.; Gimenez-Llort, L.; Johanson, C.E.; Carmona-Calero, E.M.; Castañera-Ruiz, L.; Brito-Armas, J.M.; Castañeyra-Perdomo, A.; Castro-Fuentes, R. Choroid Plexus Dysfunction Impairs Beta-Amyloid Clearance in a Triple Transgenic Mouse Model of Alzheimer’s Disease. Front. Cell Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar]
- Presnell, C.E.; Bhatti, G.; Numan, L.S.; Lerche, M.; Alkhateeb, S.K.; Ghalib, M.; Shammaa, M.; Kavdia, M. Computational Insights into the Role of Glutathione in Oxidative Stress. Curr. Neurovasc. Res. 2013, 10, 185–194. [Google Scholar] [CrossRef]
- Dorval, J. Role of Glutathione Redox Cycle and Catalase in Defense against Oxidative Stress Induced by Endosulfan in Adrenocortical Cells of Rainbow Trout (Oncorhynchus Mykiss). Toxicol. Appl. Pharmacol. 2003, 192, 191–200. [Google Scholar] [CrossRef]
- Iane Garlet, Q.; Vaitsa Losh Haskel, M.; Picada Pereira, R.; Cláudio Francisco Nunes Da Silva, W.; Batista Teixeira Da Rocha, J.; Sirlene Oliveira, C.; Sartori Bonini, J. Δ-Aminolevulinate Dehydratase and Glutathione Peroxidase Activity in Alzheimer’s Disease: A Case-Control Study. EXCLI J. 2019, 18, 866–875. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Parrado-Fernández, C.; Alvarez-Montón, I.; Frontiñán-Rubio, J.; Durán-Prado, M.; Peinado, J.R.; Johansson, B.; Alcaín, F.J.; Giménez-Llort, L. Neophobia, NQO1 and SIRT1 as Premorbid and Prodromal Indicators of AD in 3xTg-AD Mice. Behav. Brain Res. 2014, 271, 140–146. [Google Scholar] [CrossRef]
- España, J.; Giménez-Llort, L.; Valero, J.; Miñano, A.; Rábano, A.; Rodriguez-Alvarez, J.; LaFerla, F.M.; Saura, C.A. Intraneuronal β-Amyloid Accumulation in the Amygdala Enhances Fear and Anxiety in Alzheimer’s Disease Transgenic Mice. Biol. Psychiatry 2010, 67, 513–521. [Google Scholar] [CrossRef]
- Roda, A.R.; Esquerda-Canals, G.; Martí-Clúa, J.; Villegas, S. Cognitive Impairment in the 3xTg-AD Mouse Model of Alzheimer’s Disease Is Affected by Aβ-ImmunoTherapy and Cognitive Stimulation. Pharmaceutics 2020, 12, 944. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of Oxidative Stress in Kidney. Int. J. Nephrol. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Tamay-Cach, F.; Quintana-Pérez, J.C.; Trujillo-Ferrara, J.G.; Cuevas-Hernández, R.I.; Del Valle-Mondragón, L.; García-Trejo, E.M.; Arellano-Mendoza, M.G. A Review of the Impact of Oxidative Stress and Some Antioxidant Therapies on Renal Damage. Ren. Fail 2016, 38, 171–175. [Google Scholar] [CrossRef]
- Guerville, F.; De Souto Barreto, P.; Coley, N.; Andrieu, S.; Mangin, J.; Chupin, M.; Payoux, P.; Ousset, P.; Rolland, Y.; Vellas, B. Kidney Function and Cognitive Decline in Older Adults: Examining the Role of Neurodegeneration. J. Am. Geriatr. Soc. 2021, 69, 651–659. [Google Scholar] [CrossRef]
- Nava Catorce, M.; Acero, G.; Gevorkian, G. Age- and Sex-Dependent Alterations in the Peripheral Immune System in the 3xTg-AD Mouse Model of Alzheimer’s Disease: Increased Proportion of CD3+CD4-CD8- Double-Negative T Cells in the Blood. J. Neuroimmunol. 2021, 360, 577720. [Google Scholar] [CrossRef]
- Justice, N.J. The Relationship between Stress and Alzheimer’s Disease. Neurobiol. Stress 2018, 8, 127–133. [Google Scholar] [CrossRef]
- Møller, P.; Wallin, H.; Knudsen, L.E. Oxidative Stress Associated with Exercise, Psychological Stress and Life-Style Factors. Chem. Biol. Interact. 1996, 102, 17–36. [Google Scholar] [CrossRef]
- Ghezzi, P.; Floridi, L.; Boraschi, D.; Cuadrado, A.; Manda, G.; Levic, S.; D’Acquisto, F.; Hamilton, A.; Athersuch, T.J.; Selley, L. Oxidative Stress and Inflammation Induced by Environmental and Psychological Stressors: A Biomarker Perspective. Antioxid. Redox Signal. 2018, 28, 852–872. [Google Scholar] [CrossRef]
- Sagmeister, M.S.; Harper, L.; Hardy, R.S. Cortisol Excess in Chronic Kidney Disease – A Review of Changes and Impact on Mortality. Front. Endocrinol. 2023, 13. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11. [Google Scholar] [CrossRef]
- Zafir, A.; Banu, N. Modulation of in Vivo Oxidative Status by Exogenous Corticosterone and Restraint Stress in Rats. Stress 2009, 12, 167–177. [Google Scholar] [CrossRef]
- Peterman, J.L.; White, J.D.; Calcagno, A.; Hagen, C.; Quiring, M.; Paulhus, K.; Gurney, T.; Eimerbrink, M.J.; Curtis, M.; Boehm, G.W.; et al. Prolonged Isolation Stress Accelerates the Onset of Alzheimer’s Disease-Related Pathology in 5xFAD Mice despite Running Wheels and Environmental Enrichment. Behav. Brain Res. 2020, 379, 112366. [Google Scholar] [CrossRef]
- Ávila-Villanueva, M.; Marcos Dolado, A.; Gómez-Ramírez, J.; Fernández-Blázquez, M. Brain Structural and Functional Changes in Cognitive Impairment Due to Alzheimer’s Disease. Front. Psychol. 2022, 13. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef]
- Ardanaz, C.G.; Ramírez, M.J.; Solas, M. Brain Metabolic Alterations in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3785. [Google Scholar] [CrossRef]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the Role of Systemic Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef]
- Gimenez-Llort, L.; Torres-Lista, V.; Fuente, M. Crosstalk between Behavior and Immune System During the Prodromal Stages of Alzheimer’s Disease. Curr. Pharm. Des. 2014, 20, 4723–4732. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; De la Fuente, M.; Giménez-Llort, L. Sex-Dependent Worsening of NMDA-Induced Responses, Anxiety, Hypercortisolemia, and Organometry of Early Peripheral Immunoendocrine Impairment in Adult 3xTg-AD Mice and Their Long-Lasting Ontogenic Modulation by Neonatal Handling. Behav. Brain Res. 2023, 438, 114189. [Google Scholar] [CrossRef]
- Takata, M.; Nakashima, M.; Takehara, T.; Baba, H.; Machida, K.; Akitake, Y.; Ono, K.; Hosokawa, M.; Takahashi, M. Detection of Amyloid β Protein in the Urine of Alzheimer’s Disease Patients and Healthy Individuals. Neurosci. Lett. 2008, 435, 126–130. [Google Scholar] [CrossRef]
- Wang, M.J.; Yi, S.; Han, J.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric Forms of Amyloid-β Protein in Plasma as a Potential Blood-Based Biomarker for Alzheimer’s Disease. Alzheimers Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef]
- Nabers, A.; Perna, L.; Lange, J.; Mons, U.; Schartner, J.; Güldenhaupt, J.; Saum, K.; Janelidze, S.; Holleczek, B.; Rujescu, D.; et al. Amyloid Blood Biomarker Detects Alzheimer’s Disease. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into Structural and Functional Organization of the Brain: Evidence from Neuroimaging and Non-Invasive Brain Stimulation Techniques. Front. Psychiatry 2023, 14. [Google Scholar] [CrossRef]
- Khan, T.K.; Alkon, D.L. Peripheral Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 44, 729–744. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; Laferla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular A and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Baeta-Corral, R.; Giménez-Llort, L. Persistent Hyperactivity and Distinctive Strategy Features in the Morris Water Maze in 3xTg-AD Mice at Advanced Stages of Disease. Behav. Neurosci. 2015, 129, 129–137. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The Arrive Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Massey, V.; Williams, C.H. On the Reaction Mechanism of Yeast Glutathione Reductase. J. Biol. Chem. 1965, 240, 4470–4480. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Alvarado, C.; Álvarez, P.; Jiménez, L.; De la Fuente, M. Oxidative Stress in Leukocytes from Young Prematurely Aging Mice Is Reversed by Supplementation with Biscuits Rich in Antioxidants. Dev. Comp. Immunol. 2006, 30, 1168–1180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraile-Ramos, J.; Reig-Vilallonga, J.; Giménez-Llort, L. Glomerular Hypertrophy and Splenic Red Pulp Degeneration Concurrent with Oxidative Stress in 3xTg-AD Mice Model for Alzheimer’s Disease and Its Exacerbation with Sex and Social Isolation. Int. J. Mol. Sci. 2024, 25, 6112. https://doi.org/10.3390/ijms25116112
Fraile-Ramos J, Reig-Vilallonga J, Giménez-Llort L. Glomerular Hypertrophy and Splenic Red Pulp Degeneration Concurrent with Oxidative Stress in 3xTg-AD Mice Model for Alzheimer’s Disease and Its Exacerbation with Sex and Social Isolation. International Journal of Molecular Sciences. 2024; 25(11):6112. https://doi.org/10.3390/ijms25116112
Chicago/Turabian StyleFraile-Ramos, Juan, Josep Reig-Vilallonga, and Lydia Giménez-Llort. 2024. "Glomerular Hypertrophy and Splenic Red Pulp Degeneration Concurrent with Oxidative Stress in 3xTg-AD Mice Model for Alzheimer’s Disease and Its Exacerbation with Sex and Social Isolation" International Journal of Molecular Sciences 25, no. 11: 6112. https://doi.org/10.3390/ijms25116112






