Na+/K+-ATPase: More than an Electrogenic Pump
Abstract
1. Introduction
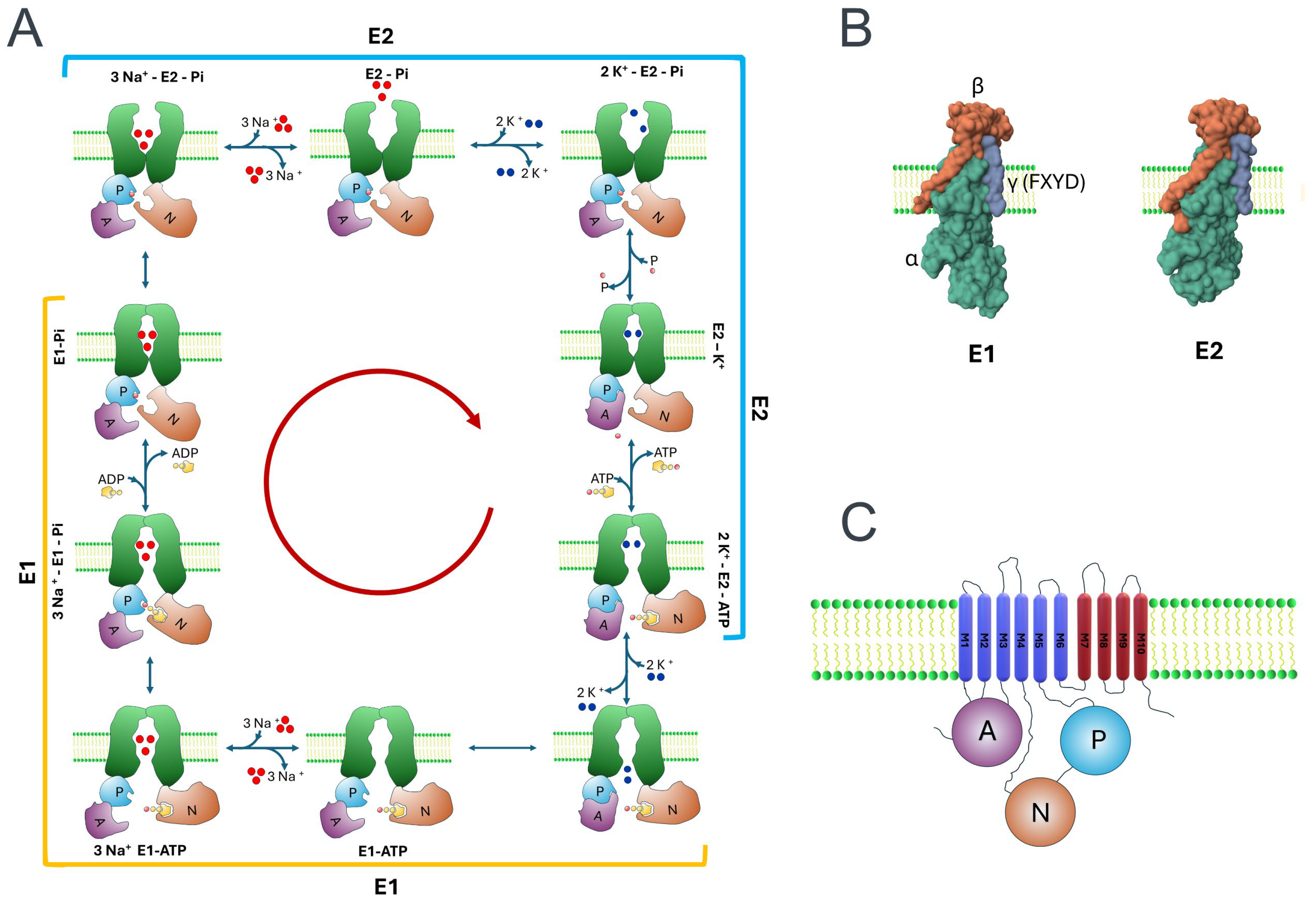
2. NKA’s Role as an Electrogenic Pump
2.1. α Subunits
2.2. β Subunits
2.2.1. The β Subunit’s Structure and Diversity
2.2.2. β Subunit Influences NKA Assembly and Pumping Function
2.2.3. β Subunit’s Role in Cell–Cell Adhesion
2.3. FXYD Subunits
2.3.1. FXYD Diversity and Tissue-Specific Expression
2.3.2. FXYD Functions
2.3.3. FXYD Expression as a Biomarker of Cancer Prognosis
2.4. Production and Analysis of Crystals Unveil Insights into the Functioning of NKA’s Pumping Mechanism
| PDB ID | Year | Source Organism | Refs. |
|---|---|---|---|
| 8K1L | 2023 | Artemia salina | [140] |
| 8JFZ, 8JBK, 8JBL, 8JBM | 2023 | Squalus acanthias | [138] |
| 8JBK | 2023 | Sus scrofa | [138] |
| 7WYS-7WYZ, 7WZ0, | 2022 | Squalus acanthias | [79] |
| 7Y45, 7Y46 | 2022 | Squalus acanthias | [79] |
| 7E1Z, 7E20, 7E21 | 2022 | Homo sapiens | [137] |
| 7YZR, 7Z04, 7QTV | 2022 | Sus scrofa | [141] |
| 8D3U-8D3Y | 2022 | Homo sapiens | [89] |
| 7X20-7X24 | 2022 | Rattus norvegicus | [142] |
| 7D91-7D94, 7DDF-7DDL | 2021 | Sus scrofa | [139] |
| 4XE5 | 2016 | Bos taurus | [143] |
| 4RET, 4RES | 2015 | Sus scrofa | [144] |
| 5AVX-5AVZ, 5AW1-5AW3, | 2015 | Squalus acanthias | [145] |
| 5AVQ-5AVW, 5AW4-5AW0 | |||
| 4HYT | 2013 | Sus scrofa | [146] |
| 4HQJ | 2013 | Sus scrofa | [147] |
| 3WGU, 3WGV | 2013 | Sus scrofa | [78] |
| 3N23 | 2011 | Sus scrofa | [148] |
| 2ZXE | 2009 | Squalus acanthias | [149] |
| 3A3Y | 2009 | Squalus acanthias | [150] |
| 2JO1 | 2007 | Homo sapiens | [151] |
| 3B8E, 3KDP | 2007 | Sus scrofa | [152] |
| 1XHH | 2005 | Sus scrofa | [153] |
| 1MO7, 1MO8 | 2003 | Rattus norvegicus | [154] |
| 1Q3I | 2003 | Sus scrofa | [155] |
| 1BG5 | 1998 | Rattus norvegicus | [156] |
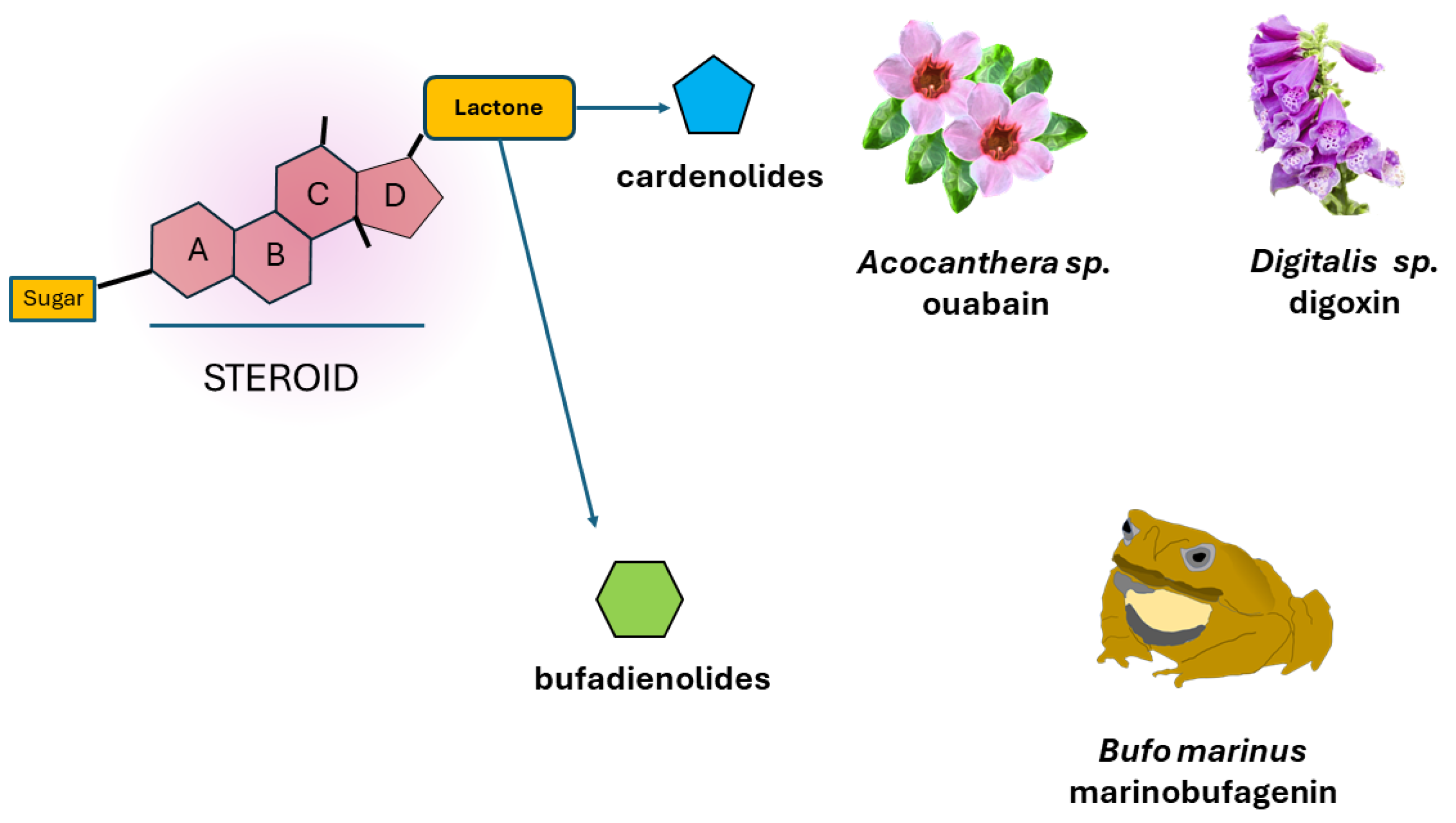
3. Cardiac Glycosides
3.1. CG´s Chemical Structure and Activity
3.2. Endogenous Cardiac Glycosides
3.3. Novel Cardiac Glycosides and Novel Therapeutic Properties
4. NKA’s Role as a Signal-Transducing Receptor
4.1. Signaling Pathways Resulting from Sequential Activation of Adjacent Proteins Assembled in Multiprotein Complexes (Signalosomes), Triggered by the Binding of CGs to NKA
4.2. Signaling Resulted from [Ca2+]i Oscillations Provoked by CGs Binding to NKA
4.3. Signaling Produced after Binding of Reactive Oxygen Species (ROS) to NKA
Influence of ROS Amplification Loop in Multiple Pathologies
4.4. Signaling Due to Changes in the [Na+]i/[K+]i Ratio
5. NKA in Epithelial Physiology
5.1. MDCK Cells as an Epithelial Model
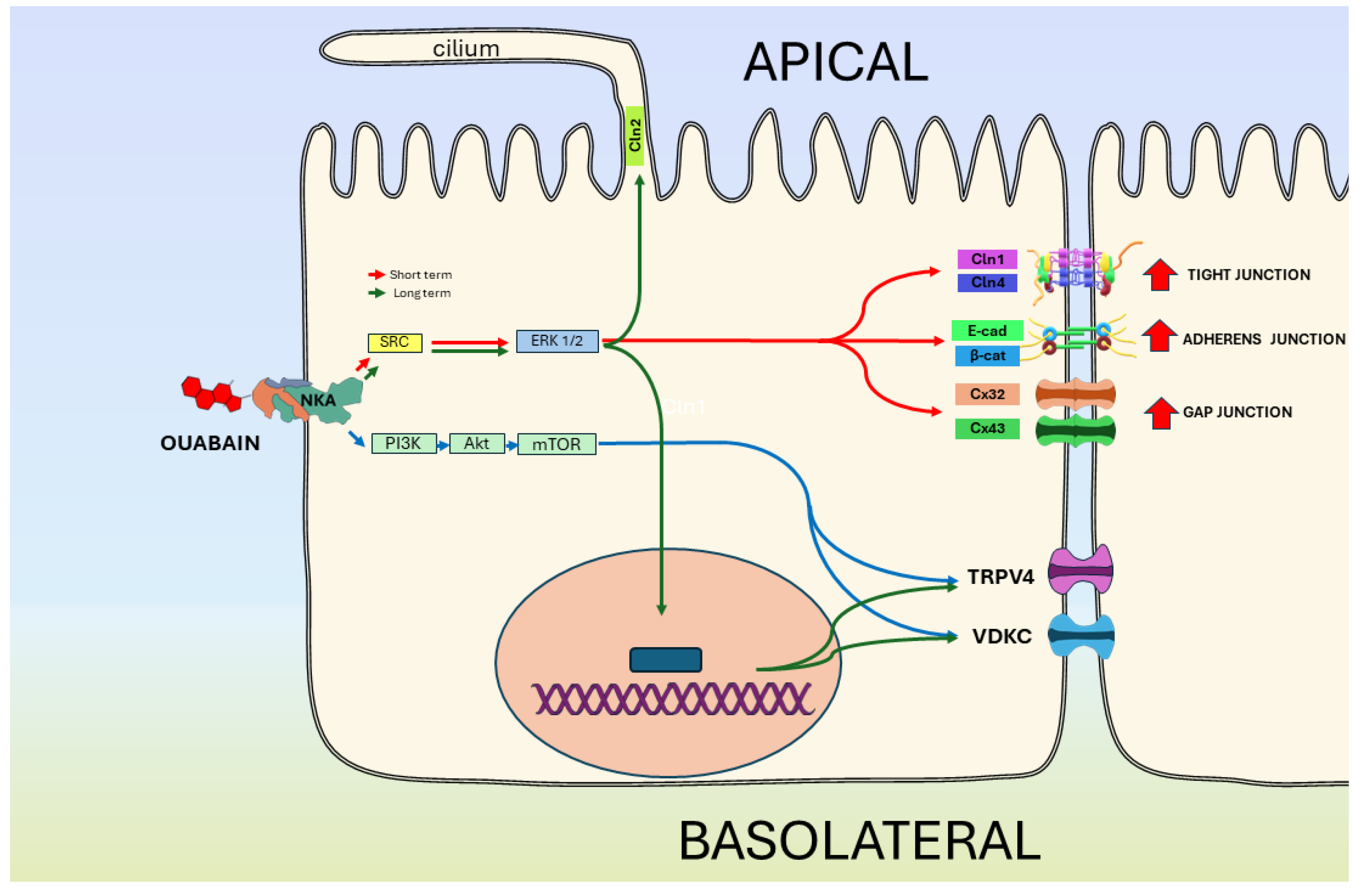
5.2. Influence of NKA as a Pump on Cell–Cell and Adhesion Contacts
5.3. NKA Is a Receptor of CGs That Regulates the Epithelial Phenotype
6. Novel Factors Affecting the Activity of NKA
7. Discussion and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Barrett, A.J. Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Enzyme Nomenclature. Recommendations 1992. Supplement 2: Corrections and Additions (1994). Eur. J. Biochem. 1995, 232, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 Update. Nucleic Acids Res. 2021, 49, D461–D467. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Na,K-ATPase. Curr. Opin. Nephrol. Hypertens. 1997, 6, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C.; Esmann, M. The Na,K-ATPase. J. Bioenerg. Biomembr. 1992, 24, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Lopina, O.; Bukach, O.; Sidorenko, S.; Klimanova, E. Na+,K+-ATPase As a Polyfunctional Protein. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2022, 16, 207–216. [Google Scholar] [CrossRef]
- Robinson, J.D. Moving Questions: A History of Membrane Transport and Bioenergetics; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-7600-9. [Google Scholar]
- Apell, H.-J. Finding Na,K-ATPase I-From Cell to Molecule. Substantia 2018, 2, 17–28. [Google Scholar] [CrossRef]
- Glynn, I.M. Annual Review Prize Lecture. “All Hands to the Sodium Pump”. J. Physiol. 1993, 462, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C. The Influence of Some Cations on an Adenosine Triphosphatase from Peripheral Nerves. Biochim. Biophys. Acta 1957, 23, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Shull, G.E.; Schwartz, A.; Lingrel, J.B. Amino-Acid Sequence of the Catalytic Subunit of the (Na+ + K+)ATPase Deduced from a Complementary DNA. Nature 1985, 316, 691–695. [Google Scholar] [CrossRef]
- Shull, G.E.; Lingrel, J.B. Isolation and Characterization of a cDNA for the Catalytic Subunit of the (Na+ + K+)-ATPase. Soc. Gen. Physiol. Ser. 1987, 41, 301–321. [Google Scholar]
- Lingrel, J.B.; Croyle, M.L.; Woo, A.L.; Argüello, J.M. Ligand Binding Sites of Na,K-ATPase. Acta Physiol. Scand. Suppl. 1998, 643, 69–77. [Google Scholar]
- Fedosova, N.U.; Habeck, M.; Nissen, P. Structure and Function of Na,K-ATPase-The Sodium-Potassium Pump. Compr. Physiol. 2021, 12, 2659–2679. [Google Scholar] [CrossRef] [PubMed]
- Horisberger, J.D.; Lemas, V.; Kraehenbühl, J.P.; Rossier, B.C. Structure-Function Relationship of Na,K-ATPase. Annu. Rev. Physiol. 1991, 53, 565–584. [Google Scholar] [CrossRef]
- Hobbs, A.S.; Albers, R.W. The Structure of Proteins Involved in Active Membrane Transport. Annu. Rev. Biophys. Bioeng. 1980, 9, 259–291. [Google Scholar] [CrossRef] [PubMed]
- Lopina, O.D. Na+,K+-ATPase: Structure, Mechanism, and Regulation. Membr. Cell Biol. 2000, 13, 721–744. [Google Scholar] [PubMed]
- Lingrel, J.B.; Kuntzweiler, T. Na+,K(+)-ATPase. J. Biol. Chem. 1994, 269, 19659–19662. [Google Scholar] [CrossRef]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef]
- Lingrel, J.B. Na,K-ATPase: Isoform Structure, Function, and Expression. J. Bioenerg. Biomembr. 1992, 24, 263–270. [Google Scholar] [CrossRef]
- Schatzmann, H.J. Cardiac glycosides as inhibitors of active potassium and sodium transport by erythrocyte membrane. Helv. Physiol. Pharmacol. Acta 1953, 11, 346–354. [Google Scholar]
- Schatzmann, H.J. The role of NA+ and K+ in the ouabain-inhibition of the NA+ + K+-activated membrane adenosine triphosphatase. Biochim. Biophys. Acta 1965, 94, 89–96. [Google Scholar] [CrossRef]
- Schatzmann, H.J. Effect of Cardiac Glycosides on Active Na-K-Transport. Protoplasma 1967, 63, 136–142. [Google Scholar] [CrossRef]
- Deepak, D.; Srivastava, S.; Khare, N.K.; Khare, A. Cardiac Glycosides. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1996; Volume 69, pp. 71–155. [Google Scholar] [CrossRef]
- Arnaud, M. Sur La Mattiere Cristallisee Active de Fleches Empoisonnee Des Somalis Extradite Du Bois d’Ovabio. CR Hebd. Seances Acad Sci. Paris 1888, 106, 1011–1162. [Google Scholar]
- Hauptman, P.J.; Kelly, R.A. Digitalis. Circulation 1999, 99, 1265–1270. [Google Scholar] [CrossRef]
- Kelly, R.A. Cardiac Glycosides and Congestive Heart Failure. Am. J. Cardiol. 1990, 65, 10E–16E; discussion 22E–23E. [Google Scholar] [CrossRef]
- Orlov, S.N.; Tverskoi, A.M.; Sidorenko, S.V.; Smolyaninova, L.V.; Lopina, O.D.; Dulin, N.O.; Klimanova, E.A. Na,K-ATPase as a Target for Endogenous Cardiotonic Steroids: What’s the Evidence? Genes Dis. 2020, 8, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.-Y.; Xie, Z.-J. Binding of Src to Na+/K+-ATPase Forms a Functional Signaling Complex. Mol. Biol. Cell 2006, 17, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, Z. The Na/K-ATPase/Src Complex and Cardiotonic Steroid-Activated Protein Kinase Cascades. Pflug. Arch. 2009, 457, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Ouabain, Endogenous Ouabain and Ouabain-like Factors: The Na+ Pump/Ouabain Receptor, Its Linkage to NCX, and Its Myriad Functions. Cell Calcium 2020, 86, 102159. [Google Scholar] [CrossRef]
- Rocha, S.C.; Pessoa, M.T.C.; Neves, L.D.R.; Alves, S.L.G.; Silva, L.M.; Santos, H.L.; Oliveira, S.M.F.; Taranto, A.G.; Comar, M.; Gomes, I.V.; et al. 21-Benzylidene Digoxin: A Proapoptotic Cardenolide of Cancer Cells That up-Regulates Na,K-ATPase and Epithelial Tight Junctions. PLoS ONE 2014, 9, e108776. [Google Scholar] [CrossRef]
- Schoner, W. Endogenous Cardiotonic Steroids. Cell. Mol. Biol. Noisy-Gd. Fr. 2001, 47, 273–280. [Google Scholar]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and Exogenous Cardiac Glycosides and Their Mechanisms of Action. Am. J. Cardiovasc. Drugs Drugs Devices Interv. 2007, 7, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous Cardiotonic Steroids: Physiology, Pharmacology, and Novel Therapeutic Targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M.; Blaustein, M.P. Endogenous Ouabain: Recent Advances and Controversies. Hypertens. Dallas Tex 1979 2016, 68, 526–532. [Google Scholar] [CrossRef]
- Słabiak-Błaż, N.; Piecha, G. Endogenous Mammalian Cardiotonic Steroids—A New Cardiovascular Risk Factor?—A Mini-Review. Life 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cai, T. Na+-K+--ATPase-Mediated Signal Transduction: From Protein Interaction to Cellular Function. Mol. Interv. 2003, 3, 157–168. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Z. Protein Interaction and Na/K-ATPase-Mediated Signal Transduction. Molecules 2017, 22, 990. [Google Scholar] [CrossRef]
- Harris, J.E. The Influence of the Metabolism of Human Erythrocytes on Their Potassium Content. J. Biol. Chem. 1941, 141, 579–595. [Google Scholar] [CrossRef]
- Danowski, T.S. The Transfer of Potassium across the Human Blood Cell Membrane. J. Biol. Chem. 1941, 139, 693–705. [Google Scholar] [CrossRef]
- Tosteson, D.C.; Hoffman, J.F. Regulation of Cell Volume by Active Cation Transport in High and Low Potassium Sheep Red Cells. J. Gen. Physiol. 1960, 44, 169–194. [Google Scholar] [CrossRef]
- Kay, A.R.; Blaustein, M.P. Evolution of Our Understanding of Cell Volume Regulation by the Pump-Leak Mechanism. J. Gen. Physiol. 2019, 151, 407–416. [Google Scholar] [CrossRef]
- Clausen, T. The Sodium Pump Keeps Us Going. Ann. N. Y. Acad. Sci. 2003, 986, 595–602. [Google Scholar] [CrossRef]
- Gagnon, K.B.; Delpire, E. Sodium Transporters in Human Health and Disease. Front. Physiol. 2020, 11, 588664. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Lederer, W.J. Sodium/Calcium Exchange: Its Physiological Implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef]
- Skou, J.C. The Sodium, Potassium-Pump. Scand. J. Clin. Lab. Investig. Suppl. 1986, 180, 11–23. [Google Scholar]
- Pavlov, K.V.; Sokolov, V.S. Electrogenic Ion Transport by Na+,K+-ATPase. Membr. Cell Biol. 2000, 13, 745–788. [Google Scholar]
- Apell, H.J.; Karlish, S.J. Functional Properties of Na,K-ATPase, and Their Structural Implications, as Detected with Biophysical Techniques. J. Membr. Biol. 2001, 180, 1–9. [Google Scholar] [CrossRef]
- Moreno, C.; Yano, S.; Bezanilla, F.; Latorre, R.; Holmgren, M. Transient Electrical Currents Mediated by the Na+/K+-ATPase: A Tour from Basic Biophysics to Human Diseases. Biophys. J. 2020, 119, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Albers, R.W. Biochemical Aspects of Active Transport. Annu. Rev. Biochem. 1967, 36, 727–756. [Google Scholar] [CrossRef]
- Lubin, M. Intracellular Potassium and Macromolecular Synthesis in Mammalian Cells. Nature 1967, 213, 451–453. [Google Scholar] [CrossRef]
- Jorgensen, P.L.; Hakansson, K.O.; Karlish, S.J.D. Structure and Mechanism of Na,K-ATPase: Functional Sites and Their Interactions. Annu. Rev. Physiol. 2003, 65, 817–849. [Google Scholar] [CrossRef] [PubMed]
- Post, R.L.; Kume, S. Evidence for an Aspartyl Phosphate Residue at the Active Site of Sodium and Potassium Ion Transport Adenosine Triphosphatase. J. Biol. Chem. 1973, 248, 6993–7000. [Google Scholar] [CrossRef] [PubMed]
- Post, R.L.; Hegyvary, C.; Kume, S. Activation by Adenosine Triphosphate in the Phosphorylation Kinetics of Sodium and Potassium Ion Transport Adenosine Triphosphatase. J. Biol. Chem. 1972, 247, 6530–6540. [Google Scholar] [CrossRef] [PubMed]
- Kresge, N.; Simoni, R.D.; Hill, R.L. Na,K-ATPase and the Post-Albers Cycle: The Work of Robert L. Post. J. Biol. Chem. 2006, 281, e2–e3. [Google Scholar] [CrossRef]
- Post, R.L. Seeds of Sodium, Potassium ATPase. Annu. Rev. Physiol. 1989, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Läuger, P. A Channel Mechanism for Electrogenic Ion Pumps. Biochim. Biophys. Acta 1979, 552, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Efthymiadis, A.; Schwarz, W. Conditions for a Backward-Running Na+/K+ Pump in Xenopus Oocytes. Biochim. Biophys. Acta BBA-Biomembr. 1991, 1068, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Garrahan, P.J.; Glynn, I.M. Driving the Sodium Pump Backwards to Form Adenosine Triphosphate. Nature 1966, 211, 1414–1415. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M. P-Type ATPases: Many More Enigmas Left to Solve. J. Biol. Chem. 2023, 299, 105352. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Nissen, P. P-Type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef]
- Stock, C.; Heger, T.; Basse Hansen, S.; Thirup Larsen, S.; Habeck, M.; Dieudonné, T.; Driller, R.; Nissen, P. Fast-Forward on P-Type ATPases: Recent Advances on Structure and Function. Biochem. Soc. Trans. 2023, 51, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Dyla, M.; Kjærgaard, M.; Poulsen, H.; Nissen, P. Structure and Mechanism of P-Type ATPase Ion Pumps. Annu. Rev. Biochem. 2020, 89, 583–603. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.H.; Fabbro, D.; Kelly, E.; Mathie, A.A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Davies, J.A.; et al. The Concise Guide to PHARMACOLOGY 2023/24: Transporters. Br. J. Pharmacol. 2023, 180 (Suppl. S2), S374–S469. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Béguin, P.; Hasler, U.; Beggah, A.; Geering, K. Regulation of Expression and Function by Subunits of Oligomeric P-Type ATPases. Acta Physiol. Scand. Suppl. 1998, 643, 283–287. [Google Scholar] [PubMed]
- Geering, K. Functional Roles of Na,K-ATPase Subunits. Curr. Opin. Nephrol. Hypertens. 2008, 17, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Thönges, D.; Hamer, E.; Antolovic, R.; Buxbaum, E.; Willeke, M.; Serpersu, E.H.; Scheiner-Bobis, G. Is the Sodium Pump a Functional Dimer? In The Sodium Pump: Structure Mechanism, Hormonal Control and Its Role in Disease; Bamberg, E., Schoner, W., Eds.; Steinkopff: Heidelberg, Germany, 1994; pp. 332–341. ISBN 978-3-642-72511-1. [Google Scholar]
- Linnertz, H.; Urbanova, P.; Obsil, T.; Herman, P.; Amler, E.; Schoner, W. Molecular Distance Measurements Reveal an (Aβ)2Dimeric Structure of Na+/K+-ATPase. J. Biol. Chem. 1998, 273, 28813–28821. [Google Scholar] [CrossRef] [PubMed]
- Farley, R.A.; Eakle, K.A.; Scheiner-Bobis, G.; Wang, K. Expression of Functional Na+/K+-ATPase in Yeast. In The Sodium Pump: Structure Mechanism, Hormonal Control and Its Role in Disease; Bamberg, E., Schoner, W., Eds.; Steinkopff: Heidelberg, Germany, 1994; pp. 11–20. ISBN 978-3-642-72511-1. [Google Scholar]
- Cornelius, F. The Sodium PUMP. In Biomembranes: A Multi-Volume Treatise; Lee, A.G., Ed.; ATPases; JAI: Greenwich, CT, USA, 1996; Volume 5, pp. 133–184. [Google Scholar]
- Fambrough, D.M. The Sodium Pump Becomes a Family. Trends Neurosci. 1988, 11, 325–328. [Google Scholar] [CrossRef]
- Blanco, G. Na,K-ATPase Subunit Heterogeneity as a Mechanism for Tissue-Specific Ion Regulation. Semin. Nephrol. 2005, 25, 292–303. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in Structure, Diversity in Function. Am. J. Physiol.-Ren. Physiol. 1998, 275, F633–F650. [Google Scholar] [CrossRef]
- Sweadner, K.J. Overview: Subunit Diversity in the Na,K-ATPase. Soc. Gen. Physiol. Ser. 1991, 46, 63–76. [Google Scholar] [PubMed]
- Matchkov, V.V.; Krivoi, I.I. Specialized Functional Diversity and Interactions of the Na,K-ATPase. Front. Physiol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Sweadner, K.J.; Rael, E. The FXYD Gene Family of Small Ion Transport Regulators or Channels: cDNA Sequence, Protein Signature Sequence, and Expression. Genomics 2000, 68, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Ogawa, H.; Vilsen, B.; Cornelius, F.; Toyoshima, C. Crystal Structure of a Na+-Bound Na+,K+-ATPase Preceding the E1P State. Nature 2013, 502, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Cornelius, F.; Vilsen, B.; Toyoshima, C. Cryoelectron Microscopy of Na+,K+-ATPase in the Two E2P States with and without Cardiotonic Steroids. Proc. Natl. Acad. Sci. USA 2022, 119, e2123226119. [Google Scholar] [CrossRef] [PubMed]
- Pressley, T.A. Structure and Function of the Na,K Pump: Ten Years of Molecular Biology. Miner. Electrolyte Metab. 1996, 22, 264–271. [Google Scholar] [PubMed]
- Lutsenko, S.; Kaplan, J.H. Organization of P-Type ATPases: Significance of Structural Diversity. Biochemistry 1995, 34, 15607–15613. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Biology, Structure and Mechanism of P-Type ATPases. Nat. Rev. Mol. Cell Biol. 2004, 5, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Schuurmans Stekhoven, F.M.; Swarts, H.G.; de Pont, J.J.; Bonting, S.L. Na+-like Effect of Imidazole on the Phosphorylation of (Na+ + K+)-ATPase. Biochim. Biophys. Acta 1985, 815, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Reyes, N.; Artigas, P.; Gadsby, D.C. The Ion Pathway through the Opened Na(+),K(+)-ATPase Pump. Nature 2008, 456, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W. From a Pump to a Pore: How Palytoxin Opens the Gates. Proc. Natl. Acad. Sci. USA 2003, 100, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Ygberg, S.; Akkuratov, E.E.; Howard, R.J.; Taylan, F.; Jans, D.C.; Mahato, D.R.; Katz, A.; Kinoshita, P.F.; Portal, B.; Nennesmo, I.; et al. A Missense Mutation Converts the Na+,K+-ATPase into an Ion Channel and Causes Therapy-Resistant Epilepsy. J. Biol. Chem. 2021, 297, 101355. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Deisl, C.; Fine, M.; Tippetts, T.S.; Uchikawa, E.; Bai, X.-C.; Levine, B. Structural Basis for Gating Mechanism of the Human Sodium-Potassium Pump. Nat. Commun. 2022, 13, 5293. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. The Functional Role of Beta Subunits in Oligomeric P-Type ATPases. J. Bioenerg. Biomembr. 2001, 33, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Hilbers, F.; Kopec, W.; Isaksen, T.J.; Holm, T.H.; Lykke-Hartmann, K.; Nissen, P.; Khandelia, H.; Poulsen, H. Tuning of the Na,K-ATPase by the Beta Subunit. Sci. Rep. 2016, 6, 20442. [Google Scholar] [CrossRef] [PubMed]
- Jaunin, P.; Jaisser, F.; Beggah, A.T.; Takeyasu, K.; Mangeat, P.; Rossier, B.C.; Horisberger, J.D.; Geering, K. Role of the Transmembrane and Extracytoplasmic Domain of Beta Subunits in Subunit Assembly, Intracellular Transport, and Functional Expression of Na,K-Pumps. J. Cell Biol. 1993, 123, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Vagin, O.; Dada, L.A.; Tokhtaeva, E.; Sachs, G. The Na-K-ATPase A₁β₁ Heterodimer as a Cell Adhesion Molecule in Epithelia. Am. J. Physiol. Cell Physiol. 2012, 302, C1271–C1281. [Google Scholar] [CrossRef]
- Cereijido, M.; Contreras, R.G.; Shoshani, L.; Larre, I. The Na+-K+-ATPase as Self-Adhesion Molecule and Hormone Receptor. Am. J. Physiol. Cell Physiol. 2012, 302, C473–C481. [Google Scholar] [CrossRef]
- Shoshani, L.; Contreras, R.G.; Roldán, M.L.; Moreno, J.; Lázaro, A.; Balda, M.S.; Matter, K.; Cereijido, M. The Polarized Expression of Na+,K+-ATPase in Epithelia Depends on the Association between Beta-Subunits Located in Neighboring Cells. Mol. Biol. Cell 2005, 16, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Vagin, O.; Tokhtaeva, E.; Sachs, G. The Role of the Beta1 Subunit of the Na,K-ATPase and Its Glycosylation in Cell-Cell Adhesion. J. Biol. Chem. 2006, 281, 39573–39587. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Benavides, T.; Roldán, M.L.; Larre, I.; Flores-Benitez, D.; Villegas-Sepúlveda, N.; Contreras, R.G.; Cereijido, M.; Shoshani, L. The Polarized Distribution of Na+,K+-ATPase: Role of the Interaction between {beta} Subunits. Mol. Biol. Cell 2010, 21, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Páez, O.; Martínez-Archundia, M.; Villegas-Sepúlveda, N.; Roldan, M.L.; Correa-Basurto, J.; Shoshani, L. A Model for the Homotypic Interaction between Na(+),K(+)-ATPase β(1) Subunits Reveals the Role of Extracellular Residues 221-229 in Its Ig-Like Domain. Int. J. Mol. Sci. 2019, 20, 4538. [Google Scholar] [CrossRef] [PubMed]
- Tokhtaeva, E.; Sachs, G.; Souda, P.; Bassilian, S.; Whitelegge, J.P.; Shoshani, L.; Vagin, O. Epithelial Junctions Depend on Intercellular Trans-Interactions between the Na,K-ATPase Β₁ Subunits. J. Biol. Chem. 2011, 286, 25801–25812. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.A.; Palmer, L.G.; Moon, S.Y.; Peralta Soler, A.; Apodaca, G.L.; Harper, J.F.; Zheng, Y.; Rajasekaran, A.K. Na,K-ATPase Activity Is Required for Formation of Tight Junctions, Desmosomes, and Induction of Polarity in Epithelial Cells. Mol. Biol. Cell 2001, 12, 3717–3732. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.G.; Shoshani, L.; Flores-Maldonado, C.; Lázaro, A.; Cereijido, M. Relationship between Na(+),K(+)-ATPase and Cell Attachment. J. Cell Sci. 1999, 112 Pt 23, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Nestor, C.A.; Roldán, M.L.; Leonardi, A.; Navea, J.G.; Padilla-Benavides, T.; Shoshani, L. Ouabain Enhances Cell-Cell Adhesion Mediated by β(1) Subunits of the Na(+),K(+)-ATPase in CHO Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2111. [Google Scholar] [CrossRef] [PubMed]
- Lobato-Álvarez, J.A.; Roldán, M.L.; López-Murillo, T.D.C.; González-Ramírez, R.; Bonilla-Delgado, J.; Shoshani, L. The Apical Localization of Na(+), K(+)-ATPase in Cultured Human Retinal Pigment Epithelial Cells Depends on Expression of the β(2) Subunit. Front. Physiol. 2016, 7, 450. [Google Scholar] [CrossRef]
- Gloor, S.; Antonicek, H.; Sweadner, K.J.; Pagliusi, S.; Frank, R.; Moos, M.; Schachner, M. The Adhesion Molecule on Glia (AMOG) Is a Homologue of the Beta Subunit of the Na,K-ATPase. J. Cell Biol. 1990, 110, 165–174. [Google Scholar] [CrossRef]
- Antonicek, H.; Schachner, M. The Adhesion Molecule on Glia (AMOG) Incorporated into Lipid Vesicles Binds to Subpopulations of Neurons. J. Neurosci. Off. J. Soc. Neurosci. 1988, 8, 2961–2966. [Google Scholar] [CrossRef] [PubMed]
- Müller-Husmann, G.; Gloor, S.; Schachner, M. Functional Characterization of Beta Isoforms of Murine Na,K-ATPase. The Adhesion Molecule on Glia (AMOG/Beta 2), but Not Beta 1, Promotes Neurite Outgrowth. J. Biol. Chem. 1993, 268, 26260–26267. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.L.; Ramírez-Salinas, G.L.; Martinez-Archundia, M.; Cuellar-Perez, F.; Vilchis-Nestor, C.A.; Cancino-Diaz, J.C.; Shoshani, L. The β(2)-Subunit (AMOG) of Human Na(+), K(+)-ATPase Is a Homophilic Adhesion Molecule. Int. J. Mol. Sci. 2022, 23, 7753. [Google Scholar] [CrossRef] [PubMed]
- Antonicek, H.; Persohn, E.; Schachner, M. Biochemical and Functional Characterization of a Novel Neuron-Glia Adhesion Molecule That Is Involved in Neuronal Migration. J. Cell Biol. 1987, 104, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Forbush, B.; Kaplan, J.H.; Hoffman, J.F. Characterization of a New Photoaffinity Derivative of Ouabain: Labeling of the Large Polypeptide and of a Proteolipid Component of the Na, K-ATPase. Biochemistry 1978, 17, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Béguin, P.; Wang, X.; Firsov, D.; Puoti, A.; Claeys, D.; Horisberger, J.D.; Geering, K. The Gamma Subunit Is a Specific Component of the Na,K-ATPase and Modulates Its Transport Function. EMBO J. 1997, 16, 4250–4260. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.Q.; Seflova, J.; Sweazey, R.; Artigas, P.; Robia, S.L. FXYD Proteins and Sodium Pump Regulatory Mechanisms. J. Gen. Physiol. 2021, 153, e202012633. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. FXYD Proteins: New Regulators of Na-K-ATPase. Am. J. Physiol. Renal Physiol. 2006, 290, F241–F250. [Google Scholar] [CrossRef] [PubMed]
- Delprat, B.; Bibert, S.; Geering, K. FXYD proteins: Novel regulators of Na,K-ATPase. Med. Sci. MS 2006, 22, 633–638. [Google Scholar] [CrossRef][Green Version]
- Tipsmark, C.K. Identification of FXYD Protein Genes in a Teleost: Tissue-Specific Expression and Response to Salinity Change. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1367–R1378. [Google Scholar] [CrossRef]
- Crambert, G.; Geering, K. FXYD Proteins: New Tissue-Specific Regulators of the Ubiquitous Na,K-ATPase. Sci. STKE Signal Transduct. Knowl. Environ. 2003, 2003, RE1. [Google Scholar] [CrossRef] [PubMed]
- Garty, H.; Karlish, S.J.D. Role of FXYD Proteins in Ion Transport. Annu. Rev. Physiol. 2006, 68, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Lo, C.F.; Numann, R.; Cuddy, M. Characterization of the Human and Rat Phospholemman (PLM) cDNAs and Localization of the Human PLM Gene to Chromosome 19q13.1. Genomics 1997, 41, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Fuller, W.; Shattock, M.J. Novel Regulation of Cardiac Na Pump via Phospholemman. J. Mol. Cell. Cardiol. 2013, 61, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.J.; Bijlani, S.; de Sautu, M.; Spontarelli, K.; Young, V.C.; Gatto, C.; Artigas, P. FXYD Protein Isoforms Differentially Modulate Human Na/K Pump Function. J. Gen. Physiol. 2020, 152, e202012660. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.W.; Moorman, J.R.; Kowdley, G.C.; Kobayashi, Y.M.; Jones, L.R.; Leder, P. Mat-8, a Novel Phospholemman-like Protein Expressed in Human Breast Tumors, Induces a Chloride Conductance in Xenopus Oocytes. J. Biol. Chem. 1995, 270, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Bibert, S.; Roy, S.; Schaer, D.; Felley-Bosco, E.; Geering, K. Structural and Functional Properties of Two Human FXYD3 (Mat-8) Isoforms. J. Biol. Chem. 2006, 281, 39142–39151. [Google Scholar] [CrossRef] [PubMed]
- Béguin, P.; Crambert, G.; Guennoun, S.; Garty, H.; Horisberger, J.D.; Geering, K. CHIF, a Member of the FXYD Protein Family, Is a Regulator of Na,K-ATPase Distinct from the Gamma-Subunit. EMBO J. 2001, 20, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteomics MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Ino, Y.; Gotoh, M.; Sakamoto, M.; Tsukagoshi, K.; Hirohashi, S. Dysadherin, a Cancer-Associated Cell Membrane Glycoprotein, down-Regulates E-Cadherin and Promotes Metastasis. Proc. Natl. Acad. Sci. USA 2002, 99, 365–370. [Google Scholar] [CrossRef]
- Hou, W.; Cai, J.; Shen, P.; Zhang, S.; Xiao, S.; You, P.; Tong, Y.; Li, K.; Qi, Z.; Luo, H. Identification of FXYD6 as the Novel Biomarker for Glioma Based on Differential Expression and DNA Methylation. Cancer Med. 2023, 12, 22170–22184. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, K.; Sugimoto, K.; Yamaguchi, F.; Song, T.; Watanabe, Y.; Singh, K.; Tokuda, M. Phosphohippolin Expression in the Rat Central Nervous System. Brain Res. Mol. Brain Res. 2004, 125, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Function of FXYD Proteins, Regulators of Na, K-ATPase. J. Bioenerg. Biomembr. 2005, 37, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kim, Y.J.; Teh, R.; Garcia, A.; Hamilton, E.J.; Cornelius, F.; Baxter, R.C.; Rasmussen, H.H. Displacement of Native FXYD Protein From Na+/K+-ATPase With Novel FXYD Peptide Derivatives: Effects on Doxorubicin Cytotoxicity. Front. Oncol. 2022, 12, 859216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Gao, K.; Xiong, J.; Liu, Z.; Chen, Y.; Yi, L. The Roles of FXYD Family Members in Ovarian Cancer: An Integrated Analysis by Mining TCGA and GEO Databases and Functional Validations. J. Cancer Res. Clin. Oncol. 2023, 149, 17269–17284. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhang, H.; Yang, J.; Zheng, Z.; Liu, K. Expression Mode and Prognostic Value of FXYD Family Members in Colon Cancer. Aging 2021, 13, 18404–18422. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, S.; Ueda, K. FXYD3 Expression Predicts Poor Prognosis in Renal Cell Carcinoma with Immunosuppressive Tumor Microenvironment. Cancers 2022, 14, 3596. [Google Scholar] [CrossRef] [PubMed]
- Tassi, R.A.; Gambino, A.; Ardighieri, L.; Bignotti, E.; Todeschini, P.; Romani, C.; Zanotti, L.; Bugatti, M.; Borella, F.; Katsaros, D.; et al. FXYD5 (Dysadherin) Upregulation Predicts Shorter Survival and Reveals Platinum Resistance in High-Grade Serous Ovarian Cancer Patients. Br. J. Cancer 2019, 121, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, N.; Jorgensen, P.L.; Maunsbach, A.B. Ultrastructure of the Sodium Pump. Comparison of Thin Sectioning, Negative Staining, and Freeze-Fracture of Purified, Membrane-Bound (Na+,K+)-ATPase. J. Cell Biol. 1977, 75, 619–634. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J. How Cryo-electron Microscopy and X-ray Crystallography Complement Each Other. Protein Sci. Publ. Protein Soc. 2017, 26, 32–39. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An Overview of Scoring Functions Used for Protein-Ligand Interactions in Molecular Docking. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.P.D. RCSB PDB: Homepage. Available online: https://www.rcsb.org/ (accessed on 20 March 2024).
- Guo, Y.; Zhang, Y.; Yan, R.; Huang, B.; Ye, F.; Wu, L.; Chi, X.; Shi, Y.; Zhou, Q. Cryo-EM Structures of Recombinant Human Sodium-Potassium Pump Determined in Three Different States. Nat. Commun. 2022, 13, 3957. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Vilsen, B.; Cornelius, F.; Toyoshima, C. Crystal Structures of Na+, K+ -ATPase Reveal the Mechanism That Converts the K+ -Bound Form to Na+ -Bound Form and Opens and Closes the Cytoplasmic Gate. FEBS Lett. 2023, 597, 1957–1976. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Cornelius, F.; Ogawa, H.; Motoyama, K.; Vilsen, B.; Toyoshima, C. Binding of Cardiotonic Steroids to Na+,K+-ATPase in the E2P State. Proc. Natl. Acad. Sci. USA 2021, 118, e2020438118. [Google Scholar] [CrossRef] [PubMed]
- Artigas, P.; Meyer, D.J.; Young, V.C.; Spontarelli, K.; Eastman, J.; Strandquist, E.; Rui, H.; Roux, B.; Birk, M.A.; Nakanishi, H.; et al. A Na Pump with Reduced Stoichiometry Is Up-Regulated by Brine Shrimp in Extreme Salinities. Proc. Natl. Acad. Sci. USA 2023, 120, e2313999120. [Google Scholar] [CrossRef] [PubMed]
- Fruergaard, M.U.; Dach, I.; Andersen, J.L.; Ozol, M.; Shahsavar, A.; Quistgaard, E.M.; Poulsen, H.; Fedosova, N.U.; Nissen, P. The Na+,K+-ATPase in Complex with Beryllium Fluoride Mimics an ATPase Phosphorylated State. J. Biol. Chem. 2022, 298, 102317. [Google Scholar] [CrossRef] [PubMed]
- Young, V.C.; Nakanishi, H.; Meyer, D.J.; Nishizawa, T.; Oshima, A.; Artigas, P.; Abe, K. Structure and Function of H+/K+ Pump Mutants Reveal Na+/K+ Pump Mechanisms. Nat. Commun. 2022, 13, 5270. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, J.L.; Mattle, D.; Fedosova, N.U.; Nissen, P.; Reinhard, L. Isolation, Crystallization and Crystal Structure Determination of Bovine Kidney Na(+),K(+)-ATPase. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and Characterization of Digoxin- and Bufalin-Bound Na+,K+-ATPase Compared with the Ouabain-Bound Complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Cornelius, F.; Hirata, A.; Toyoshima, C. Sequential Substitution of K(+) Bound to Na(+),K(+)-ATPase Visualized by X-Ray Crystallography. Nat. Commun. 2015, 6, 8004. [Google Scholar] [CrossRef]
- Laursen, M.; Yatime, L.; Nissen, P.; Fedosova, N.U. Crystal Structure of the High-Affinity Na+K+-ATPase-Ouabain Complex with Mg2+ Bound in the Cation Binding Site. Proc. Natl. Acad. Sci. USA 2013, 110, 10958–10963. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, M.; Poulsen, H.; Gourdon, P.; Reinhard, L.; Andersson, M.; Lindahl, E.; Fedosova, N.; Nissen, P. Crystal Structure of Na+, K(+)-ATPase in the Na(+)-Bound State. Science 2013, 342, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Yatime, L.; Laursen, M.; Morth, J.P.; Esmann, M.; Nissen, P.; Fedosova, N.U. Structural Insights into the High Affinity Binding of Cardiotonic Steroids to the Na+,K+-ATPase. J. Struct. Biol. 2011, 174, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, T.; Ogawa, H.; Cornelius, F.; Toyoshima, C. Crystal Structure of the Sodium-Potassium Pump at 2.4 A Resolution. Nature 2009, 459, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Shinoda, T.; Cornelius, F.; Toyoshima, C. Crystal Structure of the Sodium-Potassium Pump (Na+,K+-ATPase) with Bound Potassium and Ouabain. Proc. Natl. Acad. Sci. USA 2009, 106, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Teriete, P.; Franzin, C.M.; Choi, J.; Marassi, F.M. Structure of the Na,K-ATPase Regulatory Protein FXYD1 in Micelles. Biochemistry 2007, 46, 6774–6783. [Google Scholar] [CrossRef] [PubMed]
- Morth, J.P.; Pedersen, B.P.; Toustrup-Jensen, M.S.; Sørensen, T.L.-M.; Petersen, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. Crystal Structure of the Sodium-Potassium Pump. Nature 2007, 450, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; Lou, Y.-C.; Wu, K.-P.; Wu, S.-H.; Chang, W.-C.; Chen, C. Novel Solution Structure of Porcine Beta-Microseminoprotein. J. Mol. Biol. 2005, 346, 1071–1082. [Google Scholar] [CrossRef]
- Hilge, M.; Siegal, G.; Vuister, G.W.; Güntert, P.; Gloor, S.M.; Abrahams, J.P. ATP-Induced Conformational Changes of the Nucleotide-Binding Domain of Na,K-ATPase. Nat. Struct. Biol. 2003, 10, 468–474. [Google Scholar] [CrossRef]
- Håkansson, K.O. The Crystallographic Structure of Na,K-ATPase N-Domain at 2.6A Resolution. J. Mol. Biol. 2003, 332, 1175–1182. [Google Scholar] [CrossRef]
- Zhang, Z.; Devarajan, P.; Dorfman, A.L.; Morrow, J.S. Structure of the Ankyrin-Binding Domain of Alpha-Na,K-ATPase. J. Biol. Chem. 1998, 273, 18681–18684. [Google Scholar] [CrossRef] [PubMed]
- Petschenka, G.; Züst, T.; Hastings, A.P.; Agrawal, A.A.; Jander, G. Quantification of Plant Cardenolides by HPLC, Measurement of Na+/K+-ATPase Inhibition Activity, and Characterization of Target Enzymes. In Methods in Enzymology; Jez, J., Ed.; Biochemical Pathways and Environmental Responses in Plants: Part B; Academic Press: Cambridge, MA, USA, 2023; Volume 680, pp. 275–302. [Google Scholar]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A Review of Cardiac Glycosides: Structure, Toxicokinetics, Clinical Signs, Diagnosis and Antineoplastic Potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Diamandis, E.P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Soto-Blanco, B. Cardiac Glycosides. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 410–414. ISBN 978-3-030-57401-7. [Google Scholar]
- Mannem, R.R.; Thoti, N.; Aidhen, I.S. Chapter Three-Bioactive C-Glycosides Inspired from Natural Products towards Therapeutics. In Carbohydrates in Drug Discovery and Development; Tiwari, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–153. ISBN 978-0-12-816675-8. [Google Scholar]
- Jha, S. Bufadienolides*. In Phytochemicals in Plant Cell Cultures; Constabel, F., Vasil, I.K., Eds.; Academic Press: Cambridge, MA, USA, 1988; pp. 179–191. ISBN 978-0-12-715005-5. [Google Scholar]
- Wilkins, M.R.; Kendall, M.J.; Wade, O.L. William Withering and Digitalis, 1785 to 1985. Br. Med. J. Clin. Res. Ed 1985, 290, 7–8. [Google Scholar] [CrossRef] [PubMed]
- de Micheli Serra, A.; Pastelín Hernández, G. A tribute to the memory of the illustrious maestro and academic Dr. Rafael Méndez Martínez, pioneer in the pharmacological studies of digitalis and digitalis glycosides. Gac. Med. Mex. 2015, 151, 660–665. [Google Scholar] [PubMed]
- Blaustein, M.P.; Zhang, J.; Chen, L.; Hamilton, B.P. How Does Salt Retention Raise Blood Pressure? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R514–R523. [Google Scholar] [CrossRef] [PubMed]
- Azalim, P.; do Monte, F.M.; Rendeiro, M.M.; Liu, X.; O’Doherty, G.A.; Fontes, C.F.; Leitão, S.G.; Quintas, L.E.M.; Noël, F. Conformational States of the Pig Kidney Na+/K+-ATPase Differently Affect Bufadienolides and Cardenolides: A Directed Structure-Activity and Structure-Kinetics Study. Biochem. Pharmacol. 2020, 171, 113679. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P. Sodium Ions, Calcium Ions, Blood Pressure Regulation, and Hypertension: A Reassessment and a Hypothesis. Am. J. Physiol. 1977, 232, C165–C173. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M.; Ringel, R.; Schaeffer, J.; Levinson, P.D.; Hamilton, B.P.; Kowarski, A.A.; Blaustein, M.P. A Circulating Inhibitor of (Na+ + K+)ATPase Associated with Essential Hypertension. Nature 1982, 300, 650–652. [Google Scholar] [CrossRef]
- Hamlyn, J.M.; Lu, Z.R.; Manunta, P.; Ludens, J.H.; Kimura, K.; Shah, J.R.; Laredo, J.; Hamilton, J.P.; Hamilton, M.J.; Hamilton, B.P. Observations on the Nature, Biosynthesis, Secretion and Significance of Endogenous Ouabain. Clin. Exp. Hypertens. N. Y. N 1993 1998, 20, 523–533. [Google Scholar] [CrossRef]
- Goto, A.; Ishiguro, T.; Yamada, K.; Ishii, M.; Yoshioka, M.; Eguchi, C.; Shimora, M.; Sugimoto, T. Isolation of a Urinary Digitalis-like Factor Indistinguishable from Digoxin. Biochem. Biophys. Res. Commun. 1990, 173, 1093–1101. [Google Scholar] [CrossRef]
- Komiyama, Y.; Dong, X.H.; Nishimura, N.; Masaki, H.; Yoshika, M.; Masuda, M.; Takahashi, H. A Novel Endogenous Digitalis, Telocinobufagin, Exhibits Elevated Plasma Levels in Patients with Terminal Renal Failure. Clin. Biochem. 2005, 38, 36–45. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and Exogenous Cardiac Glycosides: Their Roles in Hypertension, Salt Metabolism, and Cell Growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Zhu, W.; Yan, Y.; Jiang, X.; Xie, Z.; Feng, F.; Zhang, J. Bioassay-Guided Fractionation and Biological Activity of Cardenolides from Streptocaulon Juventas. Planta Med. 2023, 89, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Meneses-Sagrero, S.E.; Rascón-Valenzuela, L.A.; García-Ramos, J.C.; Vilegas, W.; Arvizu-Flores, A.A.; Sotelo-Mundo, R.R.; Robles-Zepeda, R.E. Calotropin and Corotoxigenin 3-O-Glucopyranoside from the Desert Milkweed Asclepias Subulata Inhibit the Na+/K+-ATPase Activity. PeerJ 2022, 10, e13524. [Google Scholar] [CrossRef]
- Hafner, S.; Schmiech, M.; Lang, S.J. The Cardenolide Glycoside Acovenoside A Interferes with Epidermal Growth Factor Receptor Trafficking in Non-Small Cell Lung Cancer Cells. Front. Pharmacol. 2021, 12, 611657. [Google Scholar] [CrossRef]
- Zhai, J.; Dong, X.; Yan, F.; Guo, H.; Yang, J. Oleandrin: A Systematic Review of Its Natural Sources, Structural Properties, Detection Methods, Pharmacokinetics and Toxicology. Front. Pharmacol. 2022, 13, 822726. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, J.; Chen, S.; Meng, F.-D.; Ning, J.; Sun, S.-L. Oleandrin, a Cardiac Glycoside, Induces Immunogenic Cell Death via the PERK/elF2α/ATF4/CHOP Pathway in Breast Cancer. Cell Death Dis. 2021, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Bernhem, K.; Fontana, J.M.; Svensson, D.; Zhang, L.; Nilsson, L.M.; Scott, L.; Blom, H.; Brismar, H.; Aperia, A. Super-Resolution Microscopy Reveals That Na+/K+-ATPase Signaling Protects against Glucose-Induced Apoptosis by Deactivating Bad. Cell Death Dis. 2021, 12, 739. [Google Scholar] [CrossRef]
- Asrorov, A.M.; Kayumov, M.; Mukhamedov, N.; Yashinov, A.; Mirakhmetova, Z.; Huang, Y.; Yili, A.; Aisa, H.A.; Tashmukhamedov, M.; Salikhov, S.; et al. Toad Venom Bufadienolides and Bufotoxins: An Updated Review. Drug Dev. Res. 2023, 84, 815–838. [Google Scholar] [CrossRef]
- de Sousa, L.Q.; da Conceição Machado, K.; de Carvalho Oliveira, S.F.; da Silva Araújo, L.; Monção-Filho, E.D.S.; de Carvalho Melo-Cavalcante, A.A.; Vieira-Júnior, G.M.; Ferreira, P.M.P. Bufadienolides from Amphibians: A Promising Source of Anticancer Prototypes for Radical Innovation, Apoptosis Triggering and Na+/K+-ATPase Inhibition. Toxicon Off. J. Int. Soc. Toxinol. 2017, 127, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.-J.; Li, Y.; Qi, M.; Liu, J.-S.; Wang, S.; Hu, L.-J.; Lei, Y.-H.; Jiang, R.-W.; Chen, W.-M.; Qi, Q.; et al. Molecular Mechanisms of Bufadienolides and Their Novel Strategies for Cancer Treatment. Eur. J. Pharmacol. 2020, 887, 173379. [Google Scholar] [CrossRef] [PubMed]
- Hirasaki, Y.; Okabe, A.; Fukuyo, M.; Rahmutulla, B.; Mano, Y.; Seki, M.; Hoshii, T.; Namiki, T.; Kaneda, A. Cinobufagin Inhibits Proliferation of Acute Myeloid Leukaemia Cells by Repressing C-Myc Pathway-Associated Genes. Chem. Biol. Interact. 2022, 360, 109936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jian, B. Resibufogenin: An Emerging Therapeutic Compound with Multifaceted Pharmacological Effects—A Comprehensive Review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2024, 30, e942783. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fu, J.-L.; Hao, H.-F.; Jiao, Y.-N.; Li, P.-P.; Han, S.-Y. Metabolic Reprogramming by Traditional Chinese Medicine and Its Role in Effective Cancer Therapy. Pharmacol. Res. 2021, 170, 105728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, Z.; Xue, Z.; Wang, S.; Liu, X.; Hu, Y.; Zhang, Y.; Wang, J.; Li, X.; Chen, A. Resibufogenin Targets the ATP1A1 Signaling Cascade to Induce G2/M Phase Arrest and Inhibit Invasion in Glioma. Front. Pharmacol. 2022, 13, 855626. [Google Scholar] [CrossRef] [PubMed]
- Plakhova, V.B.; Penniyaynen, V.A.; Rogachevskii, I.V.; Podzorova, S.A.; Khalisov, M.M.; Ankudinov, A.V.; Krylov, B.V. Dual Mechanism of Modulation of NaV1.8 Sodium Channels by Ouabain. Can. J. Physiol. Pharmacol. 2020, 98, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-L.; Demirev, A.V.; Kim, N.-Y.; Kim, D.-H.; Yoon, S.-Y. Ouabain Activates Transcription Factor EB and Exerts Neuroprotection in Models of Alzheimer’s Disease. Mol. Cell. Neurosci. 2019, 95, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Amarelle, L.; Lecuona, E. The Antiviral Effects of Na,K-ATPase Inhibition: A Minireview. Int. J. Mol. Sci. 2018, 19, 2154. [Google Scholar] [CrossRef]
- Yang, C.-W.; Hsu, H.-Y.; Chang, H.-Y.; Lee, Y.-Z.; Lee, S.-J. Natural Cardenolides Suppress Coronaviral Replication by Downregulating JAK1 via a Na+/K+-ATPase Independent Proteolysis. Biochem. Pharmacol. 2020, 180, 114122. [Google Scholar] [CrossRef]
- Souza E Souza, K.F.C.; Moraes, B.P.T.; de Palmer Paixão, I.C.N.; Burth, P.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Na+/K+-ATPase as a Target of Cardiac Glycosides for the Treatment of SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 624704. [Google Scholar] [CrossRef] [PubMed]
- Kryvenko, V.; Vadász, I. Molecular Mechanisms of Na,K-ATPase Dysregulation Driving Alveolar Epithelial Barrier Failure in Severe COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L1186–L1193. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jia, X.; Liu, Y.; Wang, S.; Cao, J.; Zhang, B.; Xiao, G.; Wang, W. Inhibition of Na+/K+ ATPase Blocks Zika Virus Infection in Mice. Commun. Biol. 2020, 3, 380. [Google Scholar] [CrossRef] [PubMed]
- Amarelle, L.; Katzen, J.; Shigemura, M.; Welch, L.C.; Cajigas, H.; Peteranderl, C.; Celli, D.; Herold, S.; Lecuona, E.; Sznajder, J.I. Cardiac Glycosides Decrease Influenza Virus Replication by Inhibiting Cell Protein Translational Machinery. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1094–L1106. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.; Kumavath, R.; Barh, D.; Azevedo, V.; Ghosh, P. Anticancer and Antiviral Properties of Cardiac Glycosides: A Review to Explore the Mechanism of Actions. Molecules 2020, 25, 3596. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Bejček, J.; Pavlíčková, V.S.; Rimpelová, S. Repurposing Cardiac Glycosides: Drugs for Heart Failure Surmounting Viruses. Molecules 2021, 26, 5627. [Google Scholar] [CrossRef]
- Askari, A. The Other Functions of the Sodium Pump. Cell Calcium 2019, 84, 102105. [Google Scholar] [CrossRef]
- Pierre, S.V.; Blanco, G. Na/K-ATPase Ion Transport and Receptor-Mediated Signaling Pathways. J. Membr. Biol. 2021, 254, 443–446. [Google Scholar] [CrossRef]
- Aperia, A.; Akkuratov, E.E.; Fontana, J.M.; Brismar, H. Na+-K+-ATPase, a New Class of Plasma Membrane Receptors. Am. J. Physiol. Cell Physiol. 2016, 310, C491–C495. [Google Scholar] [CrossRef]
- Xie, Z. Ouabain Interaction with Cardiac Na/K-ATPase Reveals That the Enzyme Can Act as a Pump and as a Signal Transducer. Cell. Mol. Biol. Noisy-Gd. Fr. 2001, 47, 383–390. [Google Scholar]
- Peng, M.; Huang, L.; Xie, Z.; Huang, W.H.; Askari, A. Partial Inhibition of Na+/K+-ATPase by Ouabain Induces the Ca2+-Dependent Expressions of Early-Response Genes in Cardiac Myocytes. J. Biol. Chem. 1996, 271, 10372–10378. [Google Scholar] [CrossRef]
- Xie, Z.; Kometiani, P.; Liu, J.; Li, J.; Shapiro, J.I.; Askari, A. Intracellular Reactive Oxygen Species Mediate the Linkage of Na+/K+-ATPase to Hypertrophy and Its Marker Genes in Cardiac Myocytes. J. Biol. Chem. 1999, 274, 19323–19328. [Google Scholar] [CrossRef]
- Xie, Z. Molecular Mechanisms of Na/K-ATPase-Mediated Signal Transduction. Ann. N. Y. Acad. Sci. 2003, 986, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kometiani, P.; Li, J.; Gnudi, L.; Kahn, B.B.; Askari, A.; Xie, Z. Multiple Signal Transduction Pathways Link Na+/K+-ATPase to Growth-Related Genes in Cardiac Myocytes. The Roles of Ras and Mitogen-Activated Protein Kinases. J. Biol. Chem. 1998, 273, 15249–15256. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.; Xie, Z. Ouabain-Induced Hypertrophy in Cultured Cardiac Myocytes Is Accompanied by Changes in Expression of Several Late Response Genes. J. Mol. Cell. Cardiol. 1997, 29, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Kometiani, P.; Xie, Z.; Askari, A. Role of Protein Kinase C in the Signal Pathways That Link Na+/K+-ATPase to ERK1/2. J. Biol. Chem. 2001, 276, 42050–42056. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, J.; Haas, M.; Shapiro, J.I.; Askari, A.; Xie, Z. Ouabain Interaction with Cardiac Na+/K+-ATPase Initiates Signal Cascades Independent of Changes in Intracellular Na+ and Ca2+ Concentrations. J. Biol. Chem. 2000, 275, 27838–27844. [Google Scholar] [CrossRef]
- Pratt, R.D.; Brickman, C.R.; Cottrill, C.L.; Shapiro, J.I.; Liu, J. The Na/K-ATPase Signaling: From Specific Ligands to General Reactive Oxygen Species. Int. J. Mol. Sci. 2018, 19, 2600. [Google Scholar] [CrossRef] [PubMed]
- Aperia, A.; Brismar, H.; Uhlén, P. Mending Fences: Na,K-ATPase Signaling via Ca2+ in the Maintenance of Epithelium Integrity. Cell Calcium 2020, 88, 102210. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.; Pierre, S.V.; Askari, A. Association of PI3K-Akt Signaling Pathway with Digitalis-Induced Hypertrophy of Cardiac Myocytes. Am. J. Physiol. Cell Physiol. 2007, 293, C1489–C1497. [Google Scholar] [CrossRef]
- Wu, J.; Akkuratov, E.E.; Bai, Y.; Gaskill, C.M.; Askari, A.; Liu, L. Cell Signaling Associated with Na(+)/K(+)-ATPase: Activation of Phosphatidylinositide 3-Kinase IA/Akt by Ouabain Is Independent of Src. Biochemistry 2013, 52, 9059–9067. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Askari, A.; Xie, Z. Involvement of Src and Epidermal Growth Factor Receptor in the Signal-Transducing Function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, T.; Tian, J.; Xie, J.X.; Zhao, X.; Liu, L.; Shapiro, J.I.; Xie, Z. NaKtide, a Na/K-ATPase-Derived Peptide Src Inhibitor, Antagonizes Ouabain-Activated Signal Transduction in Cultured Cells. J. Biol. Chem. 2009, 284, 21066–21076. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Maxwell, K.; Yan, Y.; Liu, J.; Chaudhry, M.A.; Xie, Z.; Shapiro, J.I. pNaKtide Inhibits Na/K-ATPase Signaling and Attenuates Obesity. J. Clin. Med. Sci. 2023, 7, 1000238. [Google Scholar] [PubMed]
- Aizman, O.; Uhlén, P.; Lal, M.; Brismar, H.; Aperia, A. Ouabain, a Steroid Hormone That Signals with Slow Calcium Oscillations. Proc. Natl. Acad. Sci. USA 2001, 98, 13420–13424. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa-Naito, A.; Uhlén, P.; Lal, M.; Aizman, O.; Mikoshiba, K.; Brismar, H.; Zelenin, S.; Aperia, A. Cell Signaling Microdomain with Na,K-ATPase and Inositol 1,4,5-Trisphosphate Receptor Generates Calcium Oscillations. J. Biol. Chem. 2003, 278, 50355–50361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Malmersjö, S.; Li, J.; Ando, H.; Aizman, O.; Uhlén, P.; Mikoshiba, K.; Aperia, A. Distinct Role of the N-Terminal Tail of the Na,K-ATPase Catalytic Subunit as a Signal Transducer. J. Biol. Chem. 2006, 281, 21954–21962. [Google Scholar] [CrossRef] [PubMed]
- Panizza, E.; Zhang, L.; Fontana, J.M.; Hamada, K.; Svensson, D.; Akkuratov, E.E.; Scott, L.; Mikoshiba, K.; Brismar, H.; Lehtiö, J.; et al. Ouabain-Regulated Phosphoproteome Reveals Molecular Mechanisms for Na+, K+–ATPase Control of Cell Adhesion, Proliferation, and Survival. FASEB J. 2019, 33, 10193. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Wang, Y.; Askari, A. (Na+ + K+)-ATPase: Inactivation and Degradation Induced by Oxygen Radicals. Int. J. Biochem. 1992, 24, 621–626. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Nie, Y.; Shapiro, J.I. Na/K-ATPase Signaling and Salt Sensitivity: The Role of Oxidative Stress. Antioxidants 2017, 6, 18. [Google Scholar] [CrossRef]
- Liu, J.; Nie, Y.; Chaudhry, M.; Bai, F.; Chuang, J.; Sodhi, K.; Shapiro, J.I. The Redox-Sensitive Na/K-ATPase Signaling in Uremic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 1256. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.E.; Miller, R.B.; Thiesfeldt, S.; Lakhani, H.V.; Shapiro, J.I.; Sodhi, K. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Aging: Implications in Obesity and Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 2139. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Shapiro, J.I.; Sodhi, K. The Role of Na/K-ATPase Signaling in Oxidative Stress Related to Obesity and Cardiovascular Disease. Molecules 2016, 21, 1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lee, W.; Bian, J.-S. Recent Advances in the Study of Na+/K+-ATPase in Neurodegenerative Diseases. Cells 2022, 11, 4075. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.H.; Balandeh, E.; Hasani, J.; Karimian, M.; Arabshahi, V.; Pourfarzam, M.; Bahmani, F.; Namazi, G. The Oxidative Status and Na+/K+-ATPase Activity in Obsessive-Compulsive Disorder: A Case Control Study. BioMed Res. Int. 2024, 2024, 9979582. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, S.S.; Peper-Nascimento, J.; Aguiar-Geraldo, J.M.; Hilsendeger, A.; Daminelli, T.; Juruena, M.F.; El-Mallakh, R.S.; Quevedo, J. Biological Rhythms Are Correlated with Na+, K+-ATPase and Oxidative Stress Biomarkers: A Translational Study on Bipolar Disorder. J. Affect. Disord. 2023, 340, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shapiro, A.P.; Mopidevi, B.R.; Chaudhry, M.A.; Maxwell, K.; Haller, S.T.; Drummond, C.A.; Kennedy, D.J.; Tian, J.; Malhotra, D.; et al. Protein Carbonylation of an Amino Acid Residue of the Na/K-ATPase A1 Subunit Determines Na/K-ATPase Signaling and Sodium Transport in Renal Proximal Tubular Cells. J. Am. Heart Assoc. 2016, 5, e003675. [Google Scholar] [CrossRef] [PubMed]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V.A.; Kamanina, Y.V.; Ziganshin, R.H.; Meng, X.; Anashkina, A.A.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-Glutathionylation of the Na,K-ATPase Catalytic α Subunit Is a Determinant of the Enzyme Redox Sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef]
- Kutz, L.C.; Cui, X.; Xie, J.X.; Mukherji, S.T.; Terrell, K.C.; Huang, M.; Wang, X.; Wang, J.; Martin, A.J.; Pessoa, M.T.; et al. The Na/K-ATPase A1/Src Interaction Regulates Metabolic Reserve and Western Diet Intolerance. Acta Physiol. Oxf. Engl. 2021, 232, e13652. [Google Scholar] [CrossRef]
- Orlov, S.N.; Klimanova, E.A.; Tverskoi, A.M.; Vladychenskaya, E.A.; Smolyaninova, L.V.; Lopina, O.D. Na+i,K+i-Dependent and -Independent Signaling Triggered by Cardiotonic Steroids: Facts and Artifacts. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 635. [Google Scholar] [CrossRef]
- Lopina, O.D.; Fedorov, D.A.; Sidorenko, S.V.; Bukach, O.V.; Klimanova, E.A. Sodium Ions as Regulators of Transcription in Mammalian Cells. Biochem. Mosc. 2022, 87, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Klimanova, E.A.; Tverskoi, A.M.; Koltsova, S.V.; Sidorenko, S.V.; Lopina, O.D.; Tremblay, J.; Hamet, P.; Kapilevich, L.V.; Orlov, S.N. Time- and Dose Dependent Actions of Cardiotonic Steroids on Transcriptome and Intracellular Content of Na+ and K+: A Comparative Analysis. Sci. Rep. 2017, 7, 45403. [Google Scholar] [CrossRef] [PubMed]
- Tverskoi, A.M.; Sidorenko, S.V.; Klimanova, E.A.; Akimova, O.A.; Smolyaninova, L.V.; Lopina, O.D.; Orlov, S.N. Effects of Ouabain on Proliferation of Human Endothelial Cells Correlate with Na+,K+-ATPase Activity and Intracellular Ratio of Na+ and K. Biochem. Biokhimiia 2016, 81, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Marco-Haviv, Y.; Baran, N.; Manor, H. DNA Molecules Can Drive the Assembly of Other DNA Molecules into Specific Four-Stranded Structures. J. Mol. Biol. 1999, 286, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.J. Ion Pumps in Epithelial Cells: Sorting, Stabilization, and Polarity. Am. J. Physiol. 1997, 272, G1304–G1313. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Robbins, E.S.; Dolan, W.J.; Rotunno, C.A.; Sabatini, D.D. Polarized Monolayers Formed by Epithelial Cells on a Permeable and Translucent Support. J. Cell Biol. 1978, 77, 853–880. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Ehrenfeld, J.; Meza, I.; Martínez-Palomo, A. Structural and Functional Membrane Polarity in Cultured Monolayers of MDCK Cells. J. Membr. Biol. 1980, 52, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.G.; Flores-Maldonado, C.; Lázaro, A.; Shoshani, L.; Flores-Benitez, D.; Larré, I.; Cereijido, M. Ouabain Binding to Na+,K+-ATPase Relaxes Cell Attachment and Sends a Specific Signal (NACos) to the Nucleus. J. Membr. Biol. 2004, 198, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. Epithelial Cell Adhesion and the Regulation of Gene Expression. Trends Cell Biol. 2003, 13, 310–318. [Google Scholar] [CrossRef]
- Ponce, A.; Larre, I.; Castillo, A.; García-Villegas, R.; Romero, A.; Flores-Maldonado, C.; Martinez-Rendón, J.; Contreras, R.G.; Cereijido, M. Ouabain Increases Gap Junctional Communication in Epithelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 34, 2081–2090. [Google Scholar] [CrossRef]
- Larre, I.; Lazaro, A.; Contreras, R.G.; Balda, M.S.; Matter, K.; Flores-Maldonado, C.; Ponce, A.; Flores-Benitez, D.; Rincon-Heredia, R.; Padilla-Benavides, T.; et al. Ouabain Modulates Epithelial Cell Tight Junction. Proc. Natl. Acad. Sci. USA 2010, 107, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Ortuño-Pineda, C.; Flores-Maldonado, C.; Larre, I.; Martínez Rendón, J.; Hinojosa, L.; Ponce, A.; Ogazón, A.; Serrano, M.; Valdes, J.; et al. Ouabain Modulates the Adherens Junction in Renal Epithelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 52, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Larre, I.; Castillo, A.; Flores-Maldonado, C.; Verdejo-Torres, O.; Contreras, R.G.; Cereijido, M. Ouabain Modulates the Distribution of Connexin 43 in Epithelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 39, 1329–1338. [Google Scholar] [CrossRef]
- Cereijido, M.; Jimenez, L.; Hinojosa, L.; Castillo, A.; Martínez-Rendon, J.; Ponce, A. Ouabain-Induced Changes in the Expression of Voltage-Gated Potassium Channels in Epithelial Cells Depend on Cell-Cell Contacts. Int. J. Mol. Sci. 2022, 23, 13257. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Larre, I.; Jimenez, L.; Roldán, M.L.; Shoshani, L.; Cereijido, M. Ouabain’s Influence on TRPV4 Channels of Epithelial Cells: An Exploration of TRPV4 Activity, Expression, and Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 16687. [Google Scholar] [CrossRef] [PubMed]
- Larre, I.; Castillo, A.; Flores-Maldonado, C.; Contreras, R.G.; Galvan, I.; Muñoz-Estrada, J.; Cereijido, M. Ouabain Modulates Ciliogenesis in Epithelial Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 20591–20596. [Google Scholar] [CrossRef] [PubMed]
- Gurler, B.; Gencay, G.; Baloglu, E. Hypoxia and HIF-1α Regulate the Activity and Expression of Na,K-ATPase Subunits in H9c2 Cardiomyoblasts. Curr. Issues Mol. Biol. 2023, 45, 8277–8288. [Google Scholar] [CrossRef] [PubMed]
- Busanello, E.N.B.; Viegas, C.M.; Moura, A.P.; Tonin, A.M.; Grings, M.; Vargas, C.R.; Wajner, M. In Vitro Evidence That Phytanic Acid Compromises Na(+),K(+)-ATPase Activity and the Electron Flow through the Respiratory Chain in Brain Cortex from Young Rats. Brain Res. 2010, 1352, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Torequl Islam, M.; Shimul Bhuia, M.; Paulo Martins de Lima, J.; Paulo Araujo Maia, F.; Beatriz Herminia Ducati, A.; Douglas Melo Coutinho, H. Phytanic Acid, an Inconclusive Phytol Metabolite: A Review. Curr. Res. Toxicol. 2023, 5, 100120. [Google Scholar] [CrossRef]
- Thazhathuputhenpurayil, S.M.; Natarajan, M. Effect of Propolis on Membrane Bound Enzymes Linked with Breast Cancer. Bioinformation 2022, 18, 1192–1195. [Google Scholar] [CrossRef]
- Williams, D.; Mehrabian, M.; Arshad, H.; Eid, S.; Sackmann, C.; Zhao, W.; Wang, X.; Ghodrati, F.; Verkuyl, C.E.; Watts, J.C.; et al. The Cellular Prion Protein Interacts with and Promotes the Activity of Na,K-ATPases. PLoS ONE 2021, 16, e0258682. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-R.; Yang, Q.; Xiang, S.-Y.; Zhang, P.-H.; Ye, Y.; Chen, Y.; Xu, K.-W.; Ren, X.-Y.; Mei, H.-X.; Shen, C.-X.; et al. Rosuvastatin Enhances Alveolar Fluid Clearance in Lipopolysaccharide-Induced Acute Lung Injury by Activating the Expression of Sodium Channel and Na,K-ATPase via the PI3K/AKT/Nedd4-2 Pathway. J. Inflamm. Res. 2021, 14, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Y.; Kapur, A.; Felder, M.; Barroilhet, L.; Pattnaik, B.R.; Patankar, M.S. Oxidative Stress Induced by the Anti-Cancer Agents, Plumbagin, and Atovaquone, Inhibits Ion Transport through Na+/K+-ATPase. Sci. Rep. 2020, 10, 19585. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.S.; Paltian, J.J.; Domingues, W.B.; Costa, G.P.; Alves, D.; Giongo, J.L.; Campos, V.F.; Luchese, C.; Wilhelm, E.A. Pharmacological Modulation of Na+, K+-ATPase as a Potential Target for OXA-Induced Neurotoxicity: Correlation between Anxiety and Cognitive Decline and Beneficial Effects of 7-Chloro-4-(Phenylselanyl) Quinoline. Brain Res. Bull. 2020, 162, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-D.; Zhang, Y.-W.; Wang, B.-Y.; Bai, L.; Lu, S.-F.; Zhu, L.-L.; Bai, M.; Li, Y.-C.; Xu, E.-P. Effects of Resveratrol on the Levels of ATP, 5-HT and GAP-43 in the Hippocampus of Mice Exposed to Chronic Unpredictable Mild Stress. Neurosci. Lett. 2020, 735, 135232. [Google Scholar] [CrossRef] [PubMed]
- Hodeify, R.; Chakkour, M.; Rida, R.; Kreydiyyeh, S. PGE2 Upregulates the Na+/K+ ATPase in HepG2 Cells via EP4 Receptors and Intracellular Calcium. PLoS ONE 2021, 16, e0245400. [Google Scholar] [CrossRef] [PubMed]
- Nepal, N.; Arthur, S.; Haynes, J.; Palaniappan, B.; Sundaram, U. Mechanism of Na-K-ATPase Inhibition by PGE2 in Intestinal Epithelial Cells. Cells 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Hou, Y.; Yue, N.; Zhou, X.; Wang, Y.; Wang, L.; Li, A.; Zhang, J. The Effects of Aqueous Extract of Maca on Energy Metabolism and Immunoregulation. Eur. J. Med. Res. 2020, 25, 24. [Google Scholar] [CrossRef]
- Soratto Heitich Ferrazza, M.H.; Delwing-Dal Magro, D.; Salamaia, E.; Guareschi, T.E.; Fernandes Erzinger, L.F.; Maia, T.P.; Siebert, C.; Dos Santos, T.M.; de Souza Wyse, A.T.; Borgmann, G.; et al. Sub-chronic Administration of Lead Alters Markers of Oxidative Stress, Acetylcholinesterase and Na+K+-ATPase Activities in Rat Brain. Acta Neurobiol. Exp. 2023, 83, 216–225. [Google Scholar] [CrossRef]
- Yoo, M.H.; Lee, S.-J.; Kim, W.; Kim, Y.; Kim, Y.-B.; Moon, K.-S.; Lee, B.-S. Bisphenol A Impairs Renal Function by Reducing Na+/K+-ATPase and F-Actin Expression, Kidney Tubule Formation in Vitro and in Vivo. Ecotoxicol. Environ. Saf. 2022, 246, 114141. [Google Scholar] [CrossRef]
- Barbosa, D.J.; Capela, J.P.; Ferreira, L.M.; Branco, P.S.; Fernandes, E.; de Lourdes Bastos, M.; Carvalho, F. Ecstasy Metabolites and Monoamine Neurotransmitters Upshift the Na+/K+ ATPase Activity in Mouse Brain Synaptosomes. Arch. Toxicol. 2022, 96, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.C.; Gao, Z.; Riley, A.M.; Furkert, D.; Wittwer, C.; Dutta, A.; Rojas, T.; Semenza, E.R.; Felder, R.A.; Pluznick, J.L.; et al. The Inositol Pyrophosphate 5-InsP7 Drives Sodium-Potassium Pump Degradation by Relieving an Autoinhibitory Domain of PI3K P85α. Sci. Adv. 2020, 6, eabb8542. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.K.; Ahmad, A.; Mummalaneni, S.; Daneva, Z.; Dempsey, S.K.; Li, N.; Li, P.-L.; Lyall, V. Mechanism of Diuresis and Natriuresis by Cannabinoids: Evidence for Inhibition of Na+-K+-ATPase in Mouse Kidney Thick Ascending Limb Tubules. J. Pharmacol. Exp. Ther. 2021, 376, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Yu, H.; Larre, I.; Dube, P.R.; Kennedy, D.J.; Tang, W.H.W.; Westfall, K.; Pierre, S.V.; Xie, Z.; et al. Regulation of Na/K-ATPase Expression by Cholesterol: Isoform Specificity and the Molecular Mechanism. Am. J. Physiol. Cell Physiol. 2020, 319, C1107–C1119. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Tokhtaeva, E.; Jimenez, J.L.; Wen, Y.; Naini, B.V.; Heard, A.N.; Kim, S.; Capri, J.; Cohn, W.; Whitelegge, J.P.; et al. Helicobacter Pylori Infection Impairs Chaperone-Assisted Maturation of Na-K-ATPase in Gastric Epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G931–G945. [Google Scholar] [CrossRef] [PubMed]
- Mázala-de-Oliveira, T.; de Figueiredo, C.S.; de Rezende Corrêa, G.; da Silva, M.S.; Miranda, R.L.; de Azevedo, M.A.; Cossenza, M.; Dos Santos, A.A.; Giestal-de-Araujo, E. Ouabain-Na+/K+-ATPase Signaling Regulates Retinal Neuroinflammation and ROS Production Preventing Neuronal Death by an Autophagy-Dependent Mechanism Following Optic Nerve Axotomy In Vitro. Neurochem. Res. 2022, 47, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Takei, G.L.; Hayashi, K. Na+/K+-ATPase A4 Regulates Sperm Hyperactivation While Na+/K+-ATPase A1 Regulates Basal Motility in Hamster Spermatozoa. Theriogenology 2020, 157, 48–60. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.I.; Gonçalves-de-Albuquerque, C.F.; de Moraes, B.P.T.; Garcia, D.G.; Burth, P. Na/K-ATPase: Their Role in Cell Adhesion and Migration in Cancer. Biochimie 2021, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Rubi, M.; Jimenez, L.; Martinez-Rendon, J.; Cereijido, M.; Ponce, A. Ouabain Promotes Gap Junctional Intercellular Communication in Cancer Cells. Int. J. Mol. Sci. 2020, 22, 358. [Google Scholar] [CrossRef]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Rimpelová, S. Na+/K+-ATPase Revisited: On Its Mechanism of Action, Role in Cancer, and Activity Modulation. Molecules 2021, 26, 1905. [Google Scholar] [CrossRef]
- Staehr, C.; Aalkjaer, C.; Matchkov, V.V. The Vascular Na,K-ATPase: Clinical Implications in Stroke, Migraine, and Hypertension. Clin. Sci. Lond. Engl. 1979 2023, 137, 1595–1618. [Google Scholar] [CrossRef]
- Obradovic, M.; Stanimirovic, J.; Panic, A.; Bogdanovic, N.; Sudar-Milovanovic, E.; Cenic-Milosevic, D.; Isenovic, E.R. Regulation of Na+/K+-ATPase by Estradiol and IGF-1 in Cardio-Metabolic Diseases. Curr. Pharm. Des. 2017, 23, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-N.; Sun, Y.-J.; Pan, S.; Li, J.-X.; Qu, Y.-E.; Li, Y.; Wang, Y.-L.; Gao, Z.-B. Na+-K+-ATPase, a Potent Neuroprotective Modulator against Alzheimer Disease. Fundam. Clin. Pharmacol. 2013, 27, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Blair, J.E.A.; Filippatos, G.S.; Macarie, C.; Ruzyllo, W.; Korewicki, J.; Bubenek-Turconi, S.I.; Ceracchi, M.; Bianchetti, M.; Carminati, P.; et al. Effects of Istaroxime on Diastolic Stiffness in Acute Heart Failure Syndromes: Results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: A Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) Trial. Am. Heart J. 2009, 157, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P. Rostafuroxin: An Ouabain-Inhibitor Counteracting Specific Forms of Hypertension. Biochim. Biophys. Acta 2010, 1802, 1254–1258. [Google Scholar] [CrossRef]
- Askari, A. The Sodium Pump and Digitalis Drugs: Dogmas and Fallacies. Pharmacol. Res. Perspect. 2019, 7, e00505. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, R.G.; Torres-Carrillo, A.; Flores-Maldonado, C.; Shoshani, L.; Ponce, A. Na+/K+-ATPase: More than an Electrogenic Pump. Int. J. Mol. Sci. 2024, 25, 6122. https://doi.org/10.3390/ijms25116122
Contreras RG, Torres-Carrillo A, Flores-Maldonado C, Shoshani L, Ponce A. Na+/K+-ATPase: More than an Electrogenic Pump. International Journal of Molecular Sciences. 2024; 25(11):6122. https://doi.org/10.3390/ijms25116122
Chicago/Turabian StyleContreras, Ruben G., Antonio Torres-Carrillo, Catalina Flores-Maldonado, Liora Shoshani, and Arturo Ponce. 2024. "Na+/K+-ATPase: More than an Electrogenic Pump" International Journal of Molecular Sciences 25, no. 11: 6122. https://doi.org/10.3390/ijms25116122
APA StyleContreras, R. G., Torres-Carrillo, A., Flores-Maldonado, C., Shoshani, L., & Ponce, A. (2024). Na+/K+-ATPase: More than an Electrogenic Pump. International Journal of Molecular Sciences, 25(11), 6122. https://doi.org/10.3390/ijms25116122





