Lead Decreases Bone Morphogenetic Protein-7 (BMP-7) Expression and Increases Renal Cell Carcinoma Growth in a Sex-Divergent Manner
Abstract
:1. Introduction
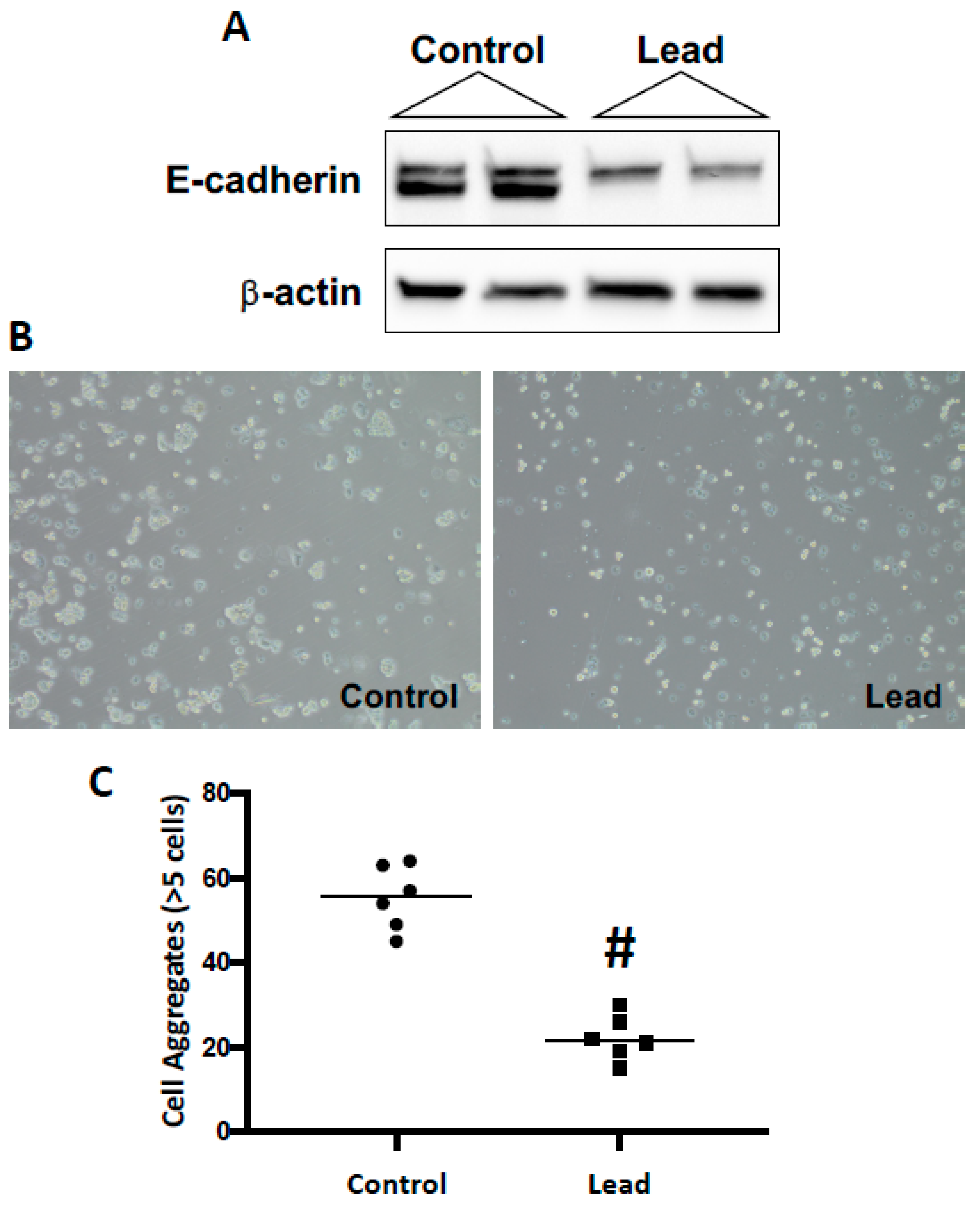
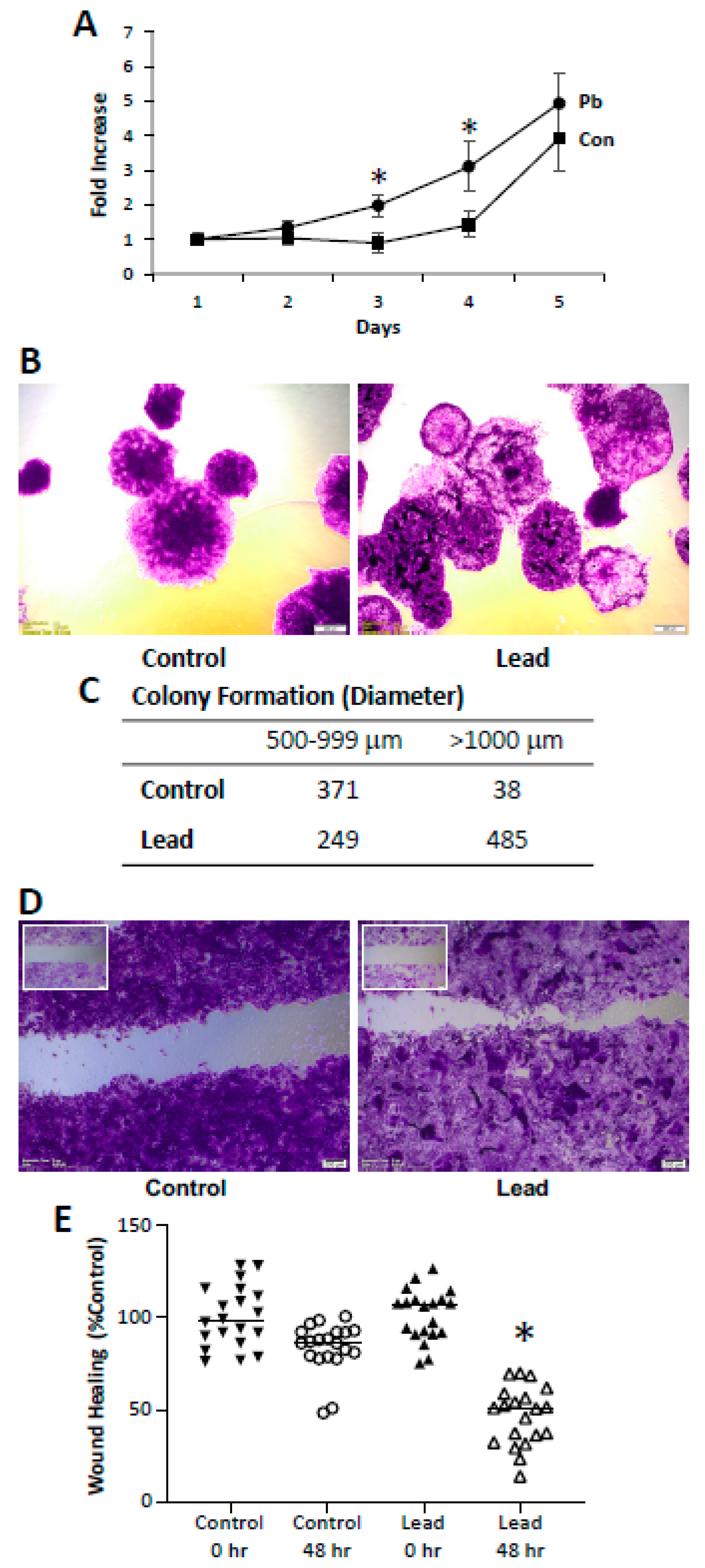
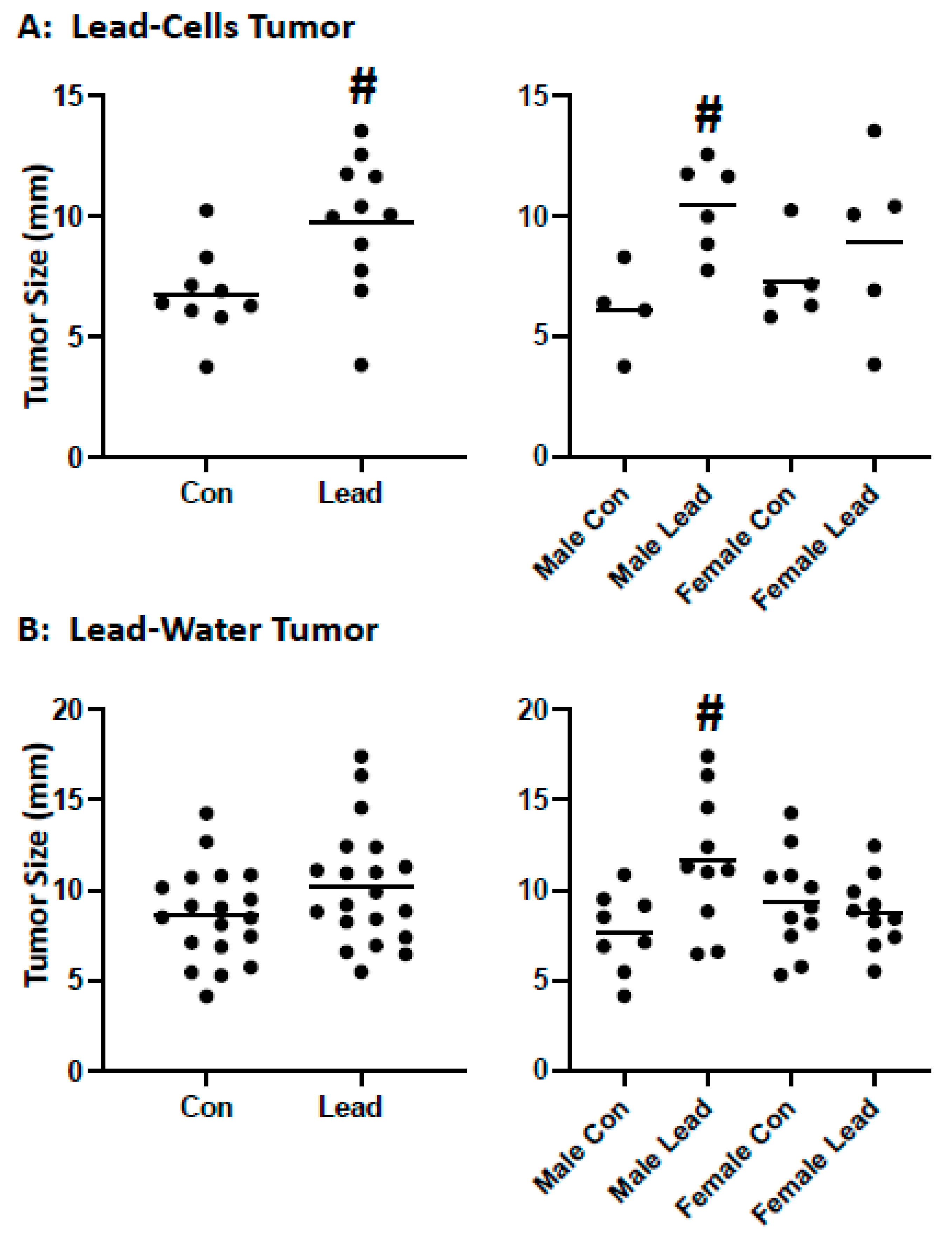
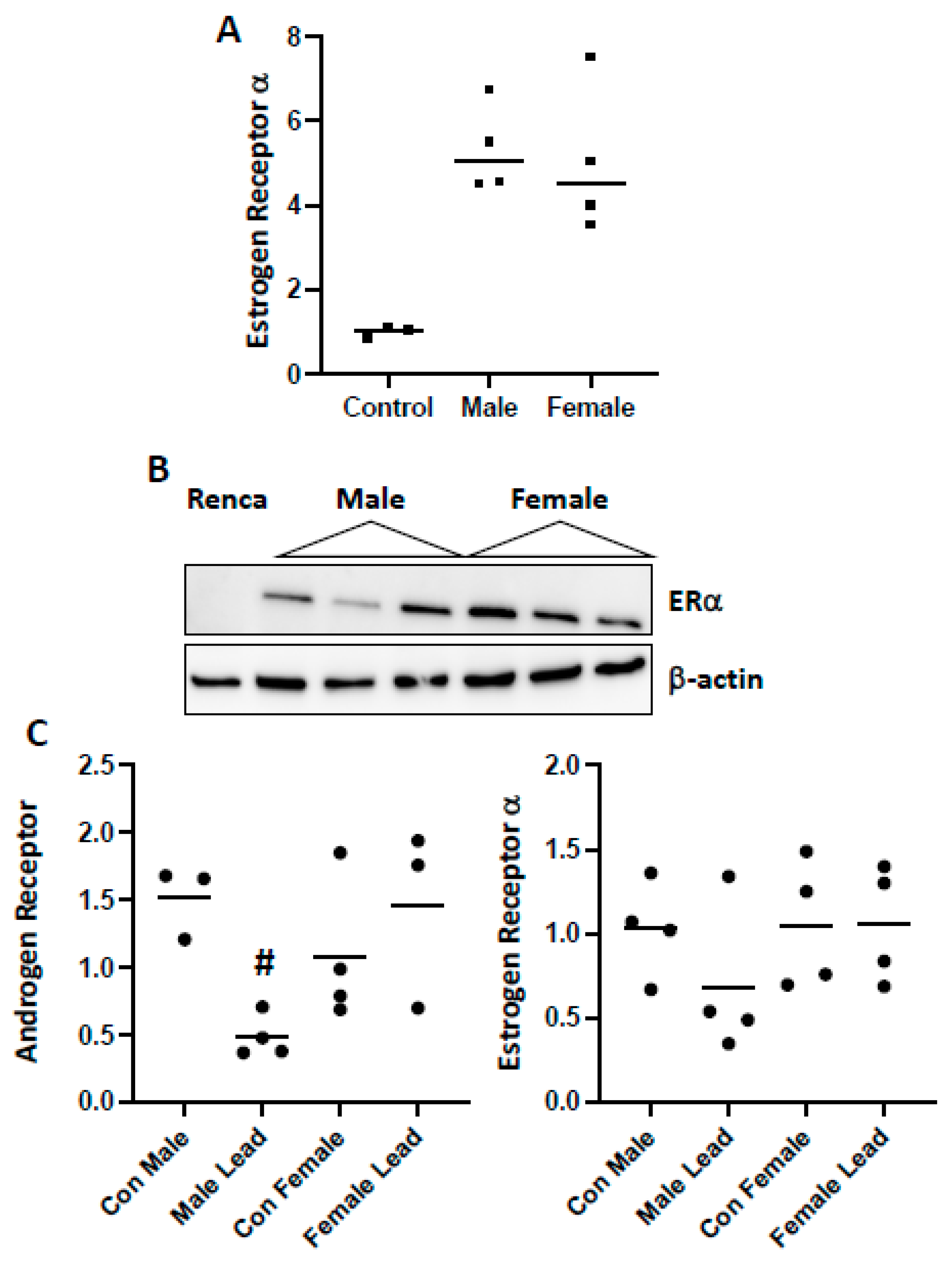
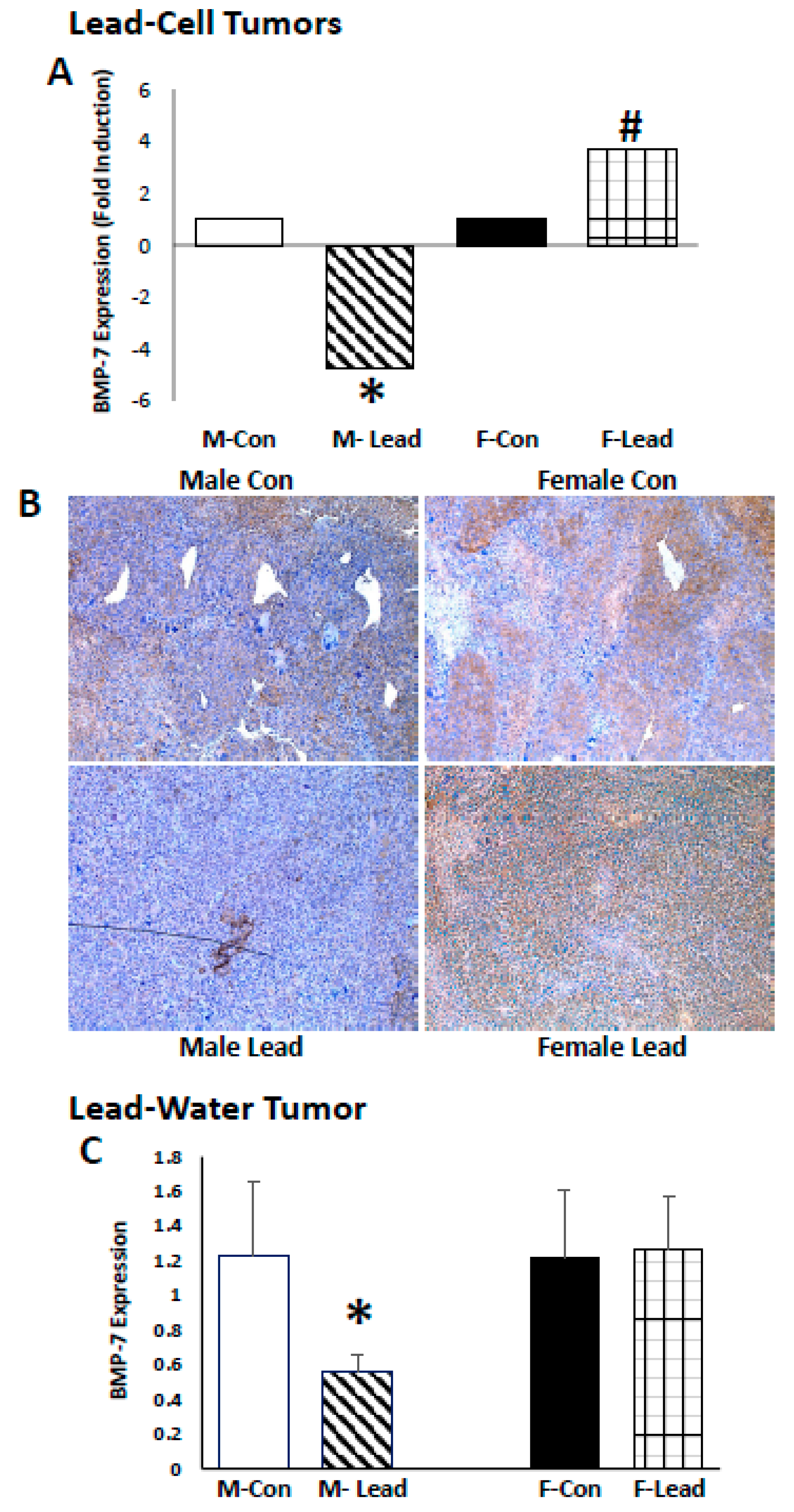
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Western Blot
4.3. Cell Aggregation
4.4. Proliferation
4.5. Colony Formation
4.6. Wound Healing
4.7. Heterotopic Tumors
4.8. Plate-Based Array/qPCR
4.9. Immunohistochemistry
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance Epidemiology and End Results. SEER Stat Fact Sheets. National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html (accessed on 27 May 2024).
- McLaughlin, J.K.; Lipworth, L.; Tarone, R.E.; Blot, W.J. Cancer Epidemiology and Prevention; Kidney cancer; Oxford University Press: Oxford, UK, 2006; pp. 1087–1100. [Google Scholar]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Thompson, R.H.; Ordonez, M.A.; Iasonos, A.; Secin, F.P.; Guillonneau, B.; Russo, P.; Touijer, K. Renal cell carcinoma in young and old patients—Is there a difference? J. Urol. 2008, 180, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.; Spalding, S.; Walsh, B.; Wainer, J.; Pickens, S.; Royster, M.; Villanacci, J.; Little, B.B. Chronic environmental and occupational lead exposure and kidney function among African Americans: Dallas Lead Project II. Int. J. Environ. Res. Public Health 2018, 15, 2875. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Cadmium and kidney function: Concentrations, variabilities, and associations across various stages of glomerular function. J. Trace Elem. Med. Biol. 2019, 256, 113361. [Google Scholar] [CrossRef]
- Ekong, E.; Jaar, B.; Weaver, V. Lead-related nephrotoxicity: A review of epidemiologic evidence. Kidney Int. 2006, 70, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; He, J.; Vupputuri, S.; Coresh, J.; Batuman, V. Blood lead and chronic kidney disease in the general United States population: Results from NHANES III. Kidney Int. 2003, 63, 1044–1050. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health concerns. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Apostolou, A.; Garcia-Esquinas, E.; Fadrowski, J.J.; McLain, P.; Weaver, V.M.; Navas-Acien, A. Secondhand tobacco smoke: A source of lead exposure in US children and adolescents. Am. J. Public. Health 2012, 102, 714–722. [Google Scholar] [CrossRef]
- Tsivian, M.; Moreira, D.M.; Caso, J.R.; Mouraviev, V.; Polascik, T.J. Cigarette smoking is associated with advanced renal cell carcinoma. J. Clin. Oncol. 2011, 29, 2027–2031. [Google Scholar] [CrossRef]
- Boffetta, P.; Fontana, L.; Stewart, P.; Zaridze, D.; Szeszenia-Dabrowska, N.; Janout, V.; Bencko, V.; Foretova, L.; Jinga, V.; Matveev, V.; et al. Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: A case-control study from Central and Eastern Europe. Occup. Environ. Med. 2011, 68, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Southard, E.B.; Roff, A.; Fortugno, T.; Richie Jr, J.P.; Kaag, M.; Chinchilli, V.M.; Virtamo, J.; Albanes, D.; Weinstein, S.; Wilson, R.T. Lead, calcium uptake, and related genetic variants in association with renal cell carcinoma in a cohort of male Finnish smokers. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 191–201. [Google Scholar] [CrossRef]
- Calvo, F.B.; Santos Junior, D.; Rodrigues, C.J.; Krug, F.J.; Marumo, J.T.; Schor, N.; Bellini, M.H. Variation in the distribution of trace elements in renal cell carcinoma. Biol. Trace Elem. Res. 2009, 130, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Pirincci, N.; Gecit, I.; Gunes, M.; Kaba, M.; Tanik, S.; Yuksel, M.B.; Arslan, H.; Demir, H. Levels of serum trace elements in renal cell carcinoma cases. Asian Pac. J. Cancer Prev. 2013, 14, 499–502. [Google Scholar] [CrossRef]
- Peired, A.J.; Campi, R.; Angelotti, M.L.; Antonelli, G.; Conte, C.; Lazzeri, E.; Becherucci, F.; Calistri, L.; Serni, S.; Romagnani, P. Sex and Gender differences in kidney cancer: Clinical and experimental evidence. Cancers 2021, 13, 4588. [Google Scholar] [CrossRef]
- Scelo, G.; Li, P.; Chanudet, E.; Muller, D.C. Variability of sex disparities in cancer incidence over 30 years: The striking case of kidney cancer. Eur. Urol. Focus. 2018, 4, 586–590. [Google Scholar] [CrossRef]
- Harlander, S.; Schönenberger, D.; Toussaint, N.C.; Prummer, M.; Catalano, A.; Brandt, L.; Moch, H.; Wild, P.J.; Frew, I.J. Combined Vhl, Trp53 and Rb1 mutation causes clear cell renal cell carci-noma in mice. Nat. Med. 2017, 23, 869–877. [Google Scholar] [CrossRef]
- Yuan, P.; Ge, Y.; Liu, X.; Wang, S.; Ye, Z.; Xu, H.; Chen, Z. The association of androgen receptor expression with renal cell carcinoma risk: A systemic review and meta-analysis. Pathol. Oncol. Res. 2020, 26, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liang, L.; Li, L.; Dang, Q.; Song, W.; Yeh, S.; He, D.; Chang, C. The expression and evaluation of androgen receptor in human renal cell carcinoma. Urology 2014, 83, 510.e19–510.e24. [Google Scholar] [CrossRef]
- Ha, Y.-S.; Lee, G.T.; Modi, P.; Kwon, Y.S.; Ahn, H.; Kim, W.-J.; Kim, I.Y. Increased expression of androgen receptor mRNA in human renal cell carcinoma cells is associated with poor prognosis in patients with localized renal cell carcinoma. J. Urol. 2015, 194, 1441–1448. [Google Scholar] [CrossRef]
- Noh, S.J.; Kang, M.J.; Kim, K.M.; Bae, J.S.; Park, H.S.; Moon, W.S.; Chung, M.J.; Lee, H.; Lee, D.G.; Jang, K.Y. Acetylation status of p53 and the expression of DB1, SIRT1, and androgen receptor are asso-ciated with survival in clear cell renal cell carcinoma patients. Pathology 2013, 45, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Mozaffari, H.R.; Payandeh, M.; Sadeghi, E. Renal cell carcinoma: A systemic review and meta-analysis on expression of androgen receptor. Biomed. Res. Ther. 2018, 5, 2820–2826. [Google Scholar] [CrossRef]
- Langdon, S.P. Estrogen receptor signaling in cancer. Cancers 2020, 12, 2744. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Lin, C.M.; Huang, C.J.; Chen, S.K.; Wu, S.T.; Chiang, H.S.; Ku, W.C. Dual roles of 17-b estradiol in estrogen receptor-dependent growth inhibition in renal cell carcinoma. Cancer Genom. Proteom. 2016, 13, 219–230. [Google Scholar]
- Yu, C.P.; Ho, J.Y.; Huang, Y.T.; Cha, T.L.; Sun, G.H.; Yu, D.S.; Chang, F.W.; Chen, S.P.; Hsu, R.J. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-b activation. PLoS ONE 2013, 8, e56667. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yeh, C.R.; Sun, Y.; Lin, C.; Chou, J.; Ou, Z.; Chang, C.; Qi, J.; Yeh, S. Estrogen receptor b promotes renal cell carcinoma progression via regulating LncRNA HO-TAIR-miR-128/200c/204/217 associated CeRNA network. Oncogene 2018, 37, 5037–5053. [Google Scholar] [CrossRef] [PubMed]
- El-Deek, H.E.M.; Ahmed, A.M.; Hassan, T.S.; Morsy, A.M. Expression and localization of estrogen receptors in human renal cell carcinoma and their clinical significance. Int. J. Clin. Exp. Pathol. 2018, 11, 3176–3185. [Google Scholar] [PubMed]
- Urist, M.R. Bone morphogenetic protein: The molecularization of skeletal system development. J. Bone Miner. Res. 1997, 12, 343–346. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGFb/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signaling skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Gomez-Puerto, M.C.; Iyengar, P.V.; De Vinuesa, A.G.; ten Dijke, P.; Sanchez-Duffhues, G. Bone morphogenetic protein receptor signal transduction in human disease. J. Pathol. 2019, 247, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.L.; Suzuki, R.; Huang, T.L.; Jing, E.; Schulz, T.J.; Lee, K.; Taniguchi, C.M.; Espinoza, D.O.; McDougall, L.E.; Zhang, H.; et al. Bone morphogentic proteiun 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012, 26, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Park, H.J.; Lee, S.K. The dual role of bone morphogenetic proteins in cancer. Mol. Ther. Oncolytics 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhao, Y.; Ye, Y.; Yu, J. Opposing roles and potential antagonistic mechanism between TGF-b and BMP pathways: Impli-cations for cancer progression. EBioMedicine 2019, 41, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.T.; Lyons, K.M.; Robertson, E.J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes. Dev. 1995, 9, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Ewart, L.M.; Masterson, E.; Tennant, S.; Grebnev, G.; Prunotto, M.; Pomposiello, S.; Conde-Knape, K.; Martin, F.M.; Godson, C. BMP7-induced-Pten inhibits Akt and prevents renal fibrosis. Biochim. Biophys. Acta 2017, 1863, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.; Hruska, K.; Guo, G.; Wang, S.; Chen, Q.; Klahr, S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates return of renal function. J. Am. Soc. Nephrol. 2002, 13 (Suppl. S1), S14–S21. [Google Scholar] [CrossRef]
- Naber, H.P.; Wiercinska, E.; Pardali, E.; van Laar, T.; Nirmala, E.; Sundqvist, A.; van Dam, H.; van der Horst, G.; van der Pluijm, G.; Heckmann, B.; et al. BMP-7 inhibits TGF-b-induced invasion of breast cancer cells through inhibition of integrin b(3) expression. Cell Oncol. 2012, 35, 19–28. [Google Scholar] [CrossRef]
- Ying, X.; Sun, Y.; He, P. Bone morphogenetic protein-7 inhibits EMT-associated genes in breast cancer. Cell. Physiol. Biochem. 2015, 37, 1271–1278. [Google Scholar] [CrossRef]
- Buijs, J.T.; Rentsch, C.A.; van der Horst, G.; van Overveld, P.G.; Wetterwald, A.; Schwaninger, R.; Henriquez, N.V.; Dijke, P.T.; Borovecki, F.; Markwalder, R.; et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone me-tastasis in vivo. Am. J. Pathol. 2007, 171, 1047–1057. [Google Scholar] [CrossRef]
- Buijs, J.T.; Henriquez, N.V.; van Overveld, P.G.; van der Horst, G.; Que, I.; Schwaninger, R.; Rentsch, C.; Dijke, P.T.; Cleton-Jansen, A.-M.; Driouch, K.; et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007, 67, 8742–8751. [Google Scholar] [CrossRef] [PubMed]
- Markić, D.; Ćelić, T.; Španjol, J.; Gršković, A.; Bobinac, D.; Fučkar, Ž. Expression of bone morphogenetic protein-7, its receptors and Smad1/5/8 in normal human kidney and renal cell cancer. Coll. Antropol. 2010, 34 (Suppl. S2), 149–153. [Google Scholar] [PubMed]
- Basic-Jukic, N.; Hudolin, T.; Radic-Antolic, M.; Coric, M.; Zadro, R.; Kastelan, Z.; Pasini, J.; Bandic-Pavlovic, D.; Kes, P. Bone morphogenetic protein-7 expression is down-regulated in human clear cell renal carcinoma. J. Nephrol. 2010, 24, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.-C.C. In vitro and in vivo studies on the effects of bone morphogenetic protein-7 on human kidney and lung tumor cells. Int. J. Biomed. Sci. 2010, 6, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.G.; Jin, D.F.; Sanders, M.M. Estrogen opposes the apoptotic effects of bone morphogenetic protein 7 on tissue remodeling. Mol. Cell. Biol. 2000, 20, 4626–4634. [Google Scholar] [CrossRef] [PubMed]
- Mikami, S.; Katsube, K.-I.; Oya, M.; Ishida, M.; Kosaka, T.; Mizuno, R.; Mukai, M.; Okada, Y. Expression of Snail and Slug in renal cell carcinoma: E-cadherin repressor Snail is associated with cancer invasion and prognosis. Lab. Investig. 2011, 91, 1443–1458. [Google Scholar] [CrossRef] [PubMed]
- Adibi, M.; Thomas, A.Z.; Borregales, L.D.; Merrill, M.M.; Slack, R.S.; Chen, H.-C.; Sircar, K.; Murugan, P.; Tamboli, P.; Jonasch, E.; et al. Percentage of sarcomatoid component as a prognostic indicator for survival in renal cell carcinoma with sarcomatoid dedifferentiation. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 427.e17–427.e23. [Google Scholar] [CrossRef] [PubMed]
- Alevizakos, M.; Gaitanidis, A.; Nasioudis, D.; Msaouel, P.; Appleman, L.J. Sarcomatoid renal cell carcinoma: Population-based study of 879 patients. Clin. Genitourin. Cancer 2019, 17, e447–e453. [Google Scholar] [CrossRef]
- Xu, H.; Xu, W.-H.; Ren, F.; Wang, J.; Wang, H.-K.; Cao, L.; Shi, G.-H.; Qu, Y.-Y.; Zhang, H.-L.; Ye, D.-W. Prognostic value of epithelial-mesenchymal transition markers in clear cell renal cell carcinoma. Aging 2020, 12, 866–883. [Google Scholar] [CrossRef]
- Kasten-Jolly, J.; Lawrence, D.A. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol. Appl. Pharmacol. 2017, 334, 142–157. [Google Scholar] [CrossRef]
- Polanska, K.; Hanke, W.; Pawlas, N.; Wesolowska, E.; Jankowska, A.; Jagodic, M.; Mazej, D.; Dominowska, J.; Grzesiak, M.; Mirabella, F.; et al. Sex-dependent impact of low-level lead exposure during prenatal period on child psy-chomotor functions. Int. J. Environ. Res. Public. Health 2018, 15, 2263. [Google Scholar] [CrossRef]
- Singh, G.; Singh, V.; Sobolewski, M.; Cory-Slechta, D.A.; Schneider, J.S. Sex-dependent effects of developmental lead exposure on brain. Front. Genet. 2018, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Shalaby, A.A.; Elaziz, R.T.A.; Bahr, H.I. Chlorella vulgaris or Spirulina platensis mitigate lead acetate-induced testicular oxidative stress and apoptosis with regard to androgen receptor expression in rats. Environ. Sci. Pollut. Res. 2021, 28, 39126–39138. [Google Scholar] [CrossRef]
- Besong, E.E.; Akhigbe, T.M.; Ashonibare, P.J.; Oladipo, A.A.; Obimma, J.N.; Hamed, M.A.; Adeyemi, D.H.; Akhigbe, R.E. Zinc improves sexual performance and erectile function by preventing penile oxidative injury and upregulating circulating testosterone in lead-exposed rats. Redox Rep. 2023, 28, 2225675. [Google Scholar] [CrossRef]
- Rodamilans, M.; Mtz-Osaba, M.J.; To-Figueras, J.; Rivera-Fillat, F.; Torra, M.; Pérez, P.; Corbella, J. Inhibition of intratesticular testosterone synthesis by inorganic lead. Toxicol. Lett. 1988, 42, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Reyes, M.A.; Antaño-Martínez, A.R.; Alcaraz-Contreras, Y.; Alegría-Torres, J.A.; Robles, J.; Yáñez-Barrientos, E.; Martinez-Alfaro, M. Melatonin therapy reverses lead exposure-induced tes-ticular damage in rats despite the lack of effect of serum testosterone levels. J. Toxicol. Sci. 2023, 48, 481–486. [Google Scholar] [CrossRef]
- Wahab, O.A.; Princely, A.C.; Oluwadamilare, A.A.; Oore-Oluwapo, D.O.; Blessing, A.O.; Alfred, E.F. Clomiphene citrate ameliorated lead acetate-induced reproductive toxicity in male Wistar rats. JBRA Assist. Reprod. 2019, 23, 336–343. [Google Scholar]
- Tchernitchin, N.N.; Clavero, A.; Mena, M.A.; Unda, C.; Villagra, R.; Cumsille, M.; Tchernitchin, A.N. Effect of chronic exposure to lead on estrogen action in the prepubertal rat uterus. Environ. Toxicol. 2003, 18, 268–277. [Google Scholar] [CrossRef]
- Tchernitchin, A.N.; Gaete, L.; Bustamante, R.; Báez, A. Effect of prenatal exposure to lead on estrogen action in the prepubertal rat uterus. ISRN Obstet. Gynecol. 2011, 2011, 329692. [Google Scholar] [CrossRef]
- Omrčen, H.; Cvek, S.Z.; Batičić, L.; Šućurović, S.; Kezele, T.G. Gender-related differences in BMP expression and adult hippocampal neurogenesis within joint-hippocampal axis in a rat model of rheumatoid arthritis. Int. J. Mol. Sci. 2021, 22, 12163. [Google Scholar] [CrossRef]
- Thomas, R.; Anderson, W.A.; Raman, V.; Reddi, A.H. Androgen-dependent gene expression of bone morphogenetic protein 7 in mouse prostate. Prostate 1998, 37, 236–245. [Google Scholar] [CrossRef]
- Schwalbe, M.; Sänger, J.; Eggers, R.; Naumann, A.; Schmidt, A.; Höffken, K.; Clement, J.H. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int. J. Oncol. 2003, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Sultana, H.; Paulius, K.; Zhang, G. Steroid regulation of proliferation and osteogenic differentiation of bone marrow stromal cells: A gender difference. J. Steroid Biochem. Mol. Biol. 2009, 114, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Fog-Poulsen, K.; Nørregaard, R.; Djurhuus, J.C.; Olsen, L.H. Gender-dependent bladder response to one-day bladder outlet obstruction. J. Pediatr. Urol. 2021, 17, 170.e1–170.e10. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Manson, S.R.; de Araujo, I.B.B.A.; Austin, P.F.; Djurhuus, J.C.; Olsen, L.H.; Nørregaard, R. The release of 24 h infravesical obstruction in mice: Changes in molecular, morpho-logical, and functional parameters for 14-day observation. Front. Med. 2022, 9, 892746. [Google Scholar] [CrossRef]
- Bakulski, K.M.; Dou, J.F.; Thompson, R.C.; Lee, C.; Middleton, L.Y.; Perera, B.P.U.; Ferris, S.P.; Jones, T.R.; Neier, K.; Zhou, X.; et al. Single-cell analysis of the gene expression effects of developmental lead (Pb) exposure on the mouse hippocampus. Toxicol. Sci. 2020, 176, 396–409. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunz, E.A.; Anderson, H.; Ernst, R.M.; Price, S.; Good, D.; Vieira-Potter, V.; Parrish, A.R. Lead Decreases Bone Morphogenetic Protein-7 (BMP-7) Expression and Increases Renal Cell Carcinoma Growth in a Sex-Divergent Manner. Int. J. Mol. Sci. 2024, 25, 6139. https://doi.org/10.3390/ijms25116139
Grunz EA, Anderson H, Ernst RM, Price S, Good D, Vieira-Potter V, Parrish AR. Lead Decreases Bone Morphogenetic Protein-7 (BMP-7) Expression and Increases Renal Cell Carcinoma Growth in a Sex-Divergent Manner. International Journal of Molecular Sciences. 2024; 25(11):6139. https://doi.org/10.3390/ijms25116139
Chicago/Turabian StyleGrunz, Elizabeth A., Haley Anderson, Rebecka M. Ernst, Spencer Price, D’Artanyan Good, Victoria Vieira-Potter, and Alan R. Parrish. 2024. "Lead Decreases Bone Morphogenetic Protein-7 (BMP-7) Expression and Increases Renal Cell Carcinoma Growth in a Sex-Divergent Manner" International Journal of Molecular Sciences 25, no. 11: 6139. https://doi.org/10.3390/ijms25116139





