Synthesis and Study of Building Blocks with Dibenzo[b,f]oxepine: Potential Microtubule Inhibitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Computational Aspects
2.2. Molecular Docking
2.3. Synthesis, Spectroscopic Data, and Photochemical Characteristics
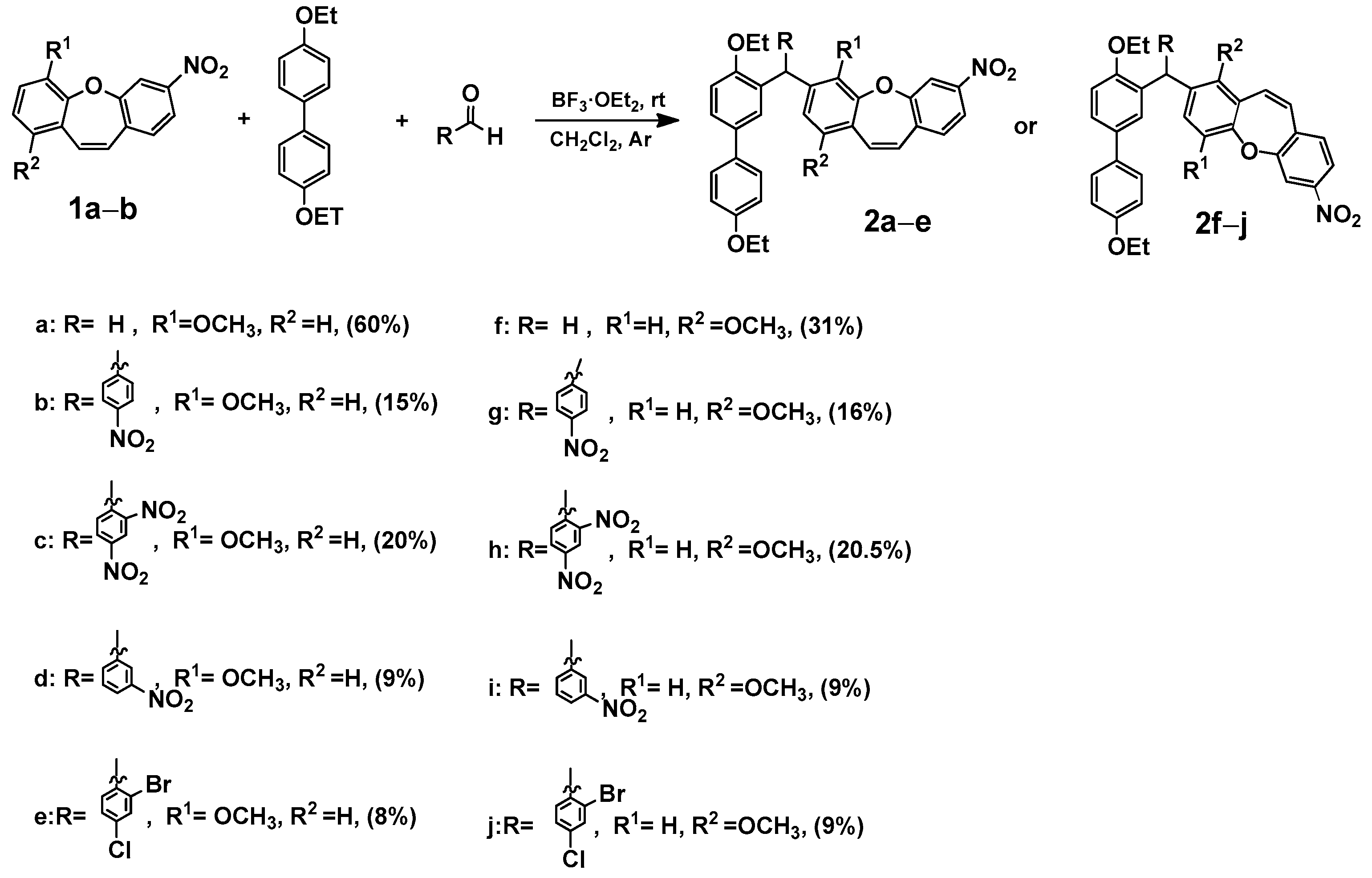
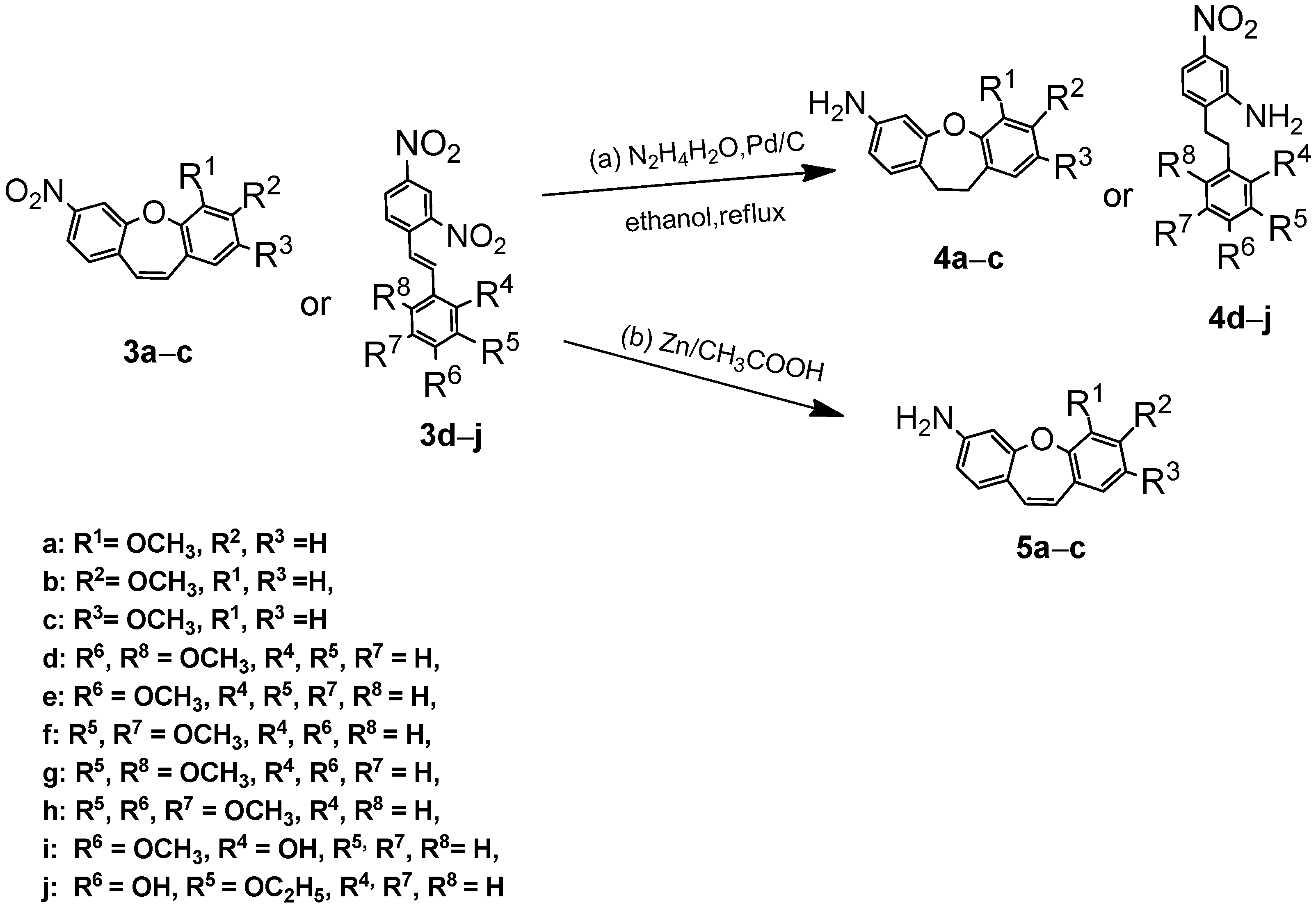
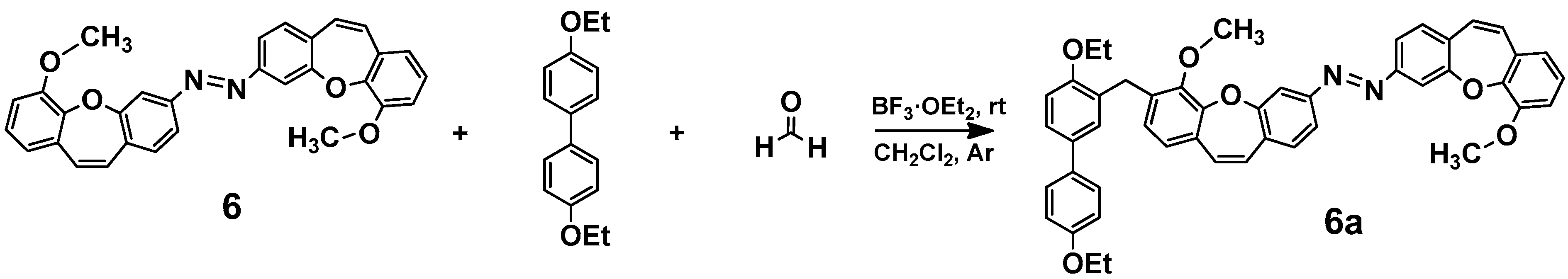
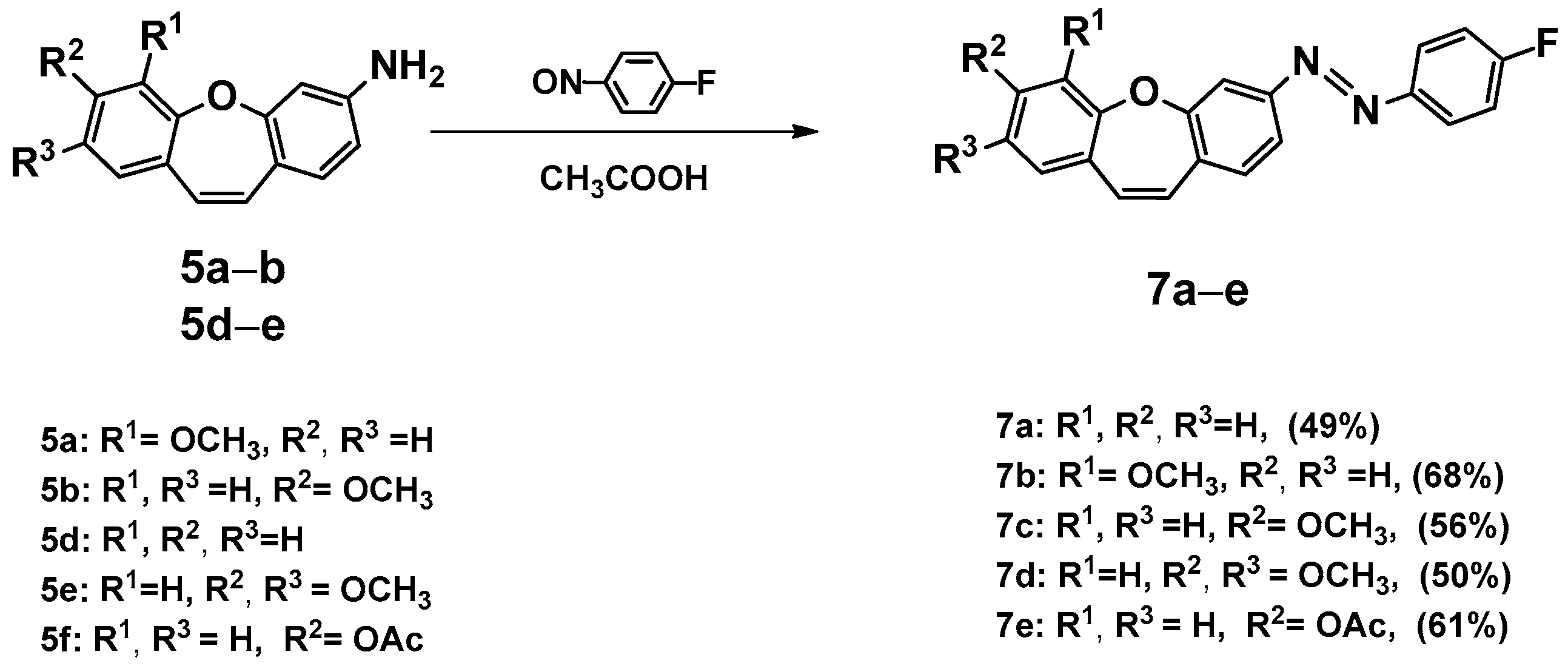

3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fojo, T. The Role of Microtubules in Cell Biology, Neurobiology, and Oncology; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- Brouhard, G.J.; Rice, L.M. Microtubule Dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 6, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Lerch, M.; Hansen, M.; van Dam, G.; Szymański, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. 2016, 55, 10978–10999. [Google Scholar] [CrossRef]
- Barrett, C.J.; Mamiya, J.I.; Yagerc, K.G.; Ikeda, T. Photo-mechanical effects in azobenzene-containing soft materials. Soft Matter. 2007, 3, 1249–1261. [Google Scholar] [PubMed]
- Tobiasz, P.; Borys, F.; Borecka, M.; Krawczyk, H. Synthesis and investigations of building blocks with dibenzo[b,f ]oxepine for use in photopharmacology. Int. J. Mol. Sci. 2021, 22, 11033. [Google Scholar] [CrossRef] [PubMed]
- Garbicz, D.; Mielecki, D.; Wrzesiński, M.; Pilżys, T.; Marcinkowski, M.; Piwowarski, J.; Dębski, J.; Palak, E.; Szczeciński, P.; Krawczyk, H.; et al. Evaluation of anti-cancer activity of stilbene and methoxydibenzo[b,f]oxepin derivatives. Curr. Cancer Drug Targets 2018, 18, 706–717. [Google Scholar] [CrossRef]
- Krawczyk, H. Dibenzo[b,f]oxepine Molecules Used in Biological Systems and Medicine. Int. J. Mol. Sci. 2023, 24, 12066. [Google Scholar] [CrossRef]
- Harris, T.W.; Smith, H.E.; Mobley, P.L.; Manier, D.H.; Sulser, F. Affinity of 10-(4-methylpiperazino)dibenz[b,f]oxepins for clozapine and spiroperidol binding sites in rat brain. J. Med. Chem. 1982, 25, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Bharath, Y.; Thirupathi, B.; Ranjit, G.; Mohapatra, D.K. An Efficient Synthesis of Dibenzo[b,f]oxepins by Ring-Closing Metathesis. Asian J. Org. Chem. 2013, 2, 848–851. [Google Scholar] [CrossRef]
- Moreno, D.R.R.; Giorgi, G.; Salas, C.O.; Tapia, R.A. New Short Strategy for the Synthesis of the Dibenz[b,f]oxepin Scaffold. Molecules 2013, 18, 14797–14806. [Google Scholar] [CrossRef]
- Storz, T.; Vangrevelinghe, E.; Dittmar, P. Synthesis and Wagner-Meerwein Rearrangement of 9-(α-Hydroxyalkyl) xanthenes to 10-Substituted Dibenz [b,f] oxepins: Scope, Limitations and ab initio Calculations. Synthesis 2005, 15, 2562–2570. [Google Scholar] [CrossRef]
- Heesgaard, J.T.; Mogens, L.; Morten, J.; Broendsted, N.M. Three-Step Synthesis of (Thio)xanthene and Dibenzothiepine/Dibenzoxepine by an Intramolecular Mizoroki-Heck Reaction of Diaryl (Thio)Ethers. Synlett 2012, 23, 418–422. [Google Scholar]
- Choi, Y.L.; Lim, H.S.; Lim, H.J.; Heo, J.-N. One-Pot Transition-Metal-Free Synthesis of Dibenzo[b,f]oxepins from 2-Halobenzaldehydes. Org. Lett. 2012, 14, 5102–5105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; He, Q.; Xie, Y.; Yang, C. Copper-Assisted/Copper-Free Synthesis of Functionalized Dibenzo[b,f]oxepins and Their Analogs via a One-Pot Tandem Reaction. Helv. Chim. Acta 2013, 96, 296–308. [Google Scholar] [CrossRef]
- Koichi, N.; Ken, N.; Yui, A.; Tadashi, K. Total Synthesis of Bauhinoxepin J: A Biologically Active Dibenzo[b,f]oxepin Isolated from Bauhinia purpurea. Eur. J. Org. Chem. 2011, 4985–4988. [Google Scholar]
- Krawczyk, H.; Wrzesiński, M.; Mielecki, D.; Szczeciński, P.; Grzesiuk, E. Synthesis of derivatives of methoydibenzo[b,f]oxepine in the presence of sodium azide. Tetrahedron 2016, 72, 3877–3884. [Google Scholar] [CrossRef]
- Tobiasz, P.; Poterała, M.; Jaskowska, E. Synthesis and investigation of new cyclic molecules using the stilbene scaffold. RSC Adv. 2018, 8, 30678–30682. [Google Scholar] [CrossRef]
- Zhou, J.; Giannakakou, P. Targeting Microtubules for Cancer Chemotherapy. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, P.R.; Mondhe, D.M. Potential anticancer role of colchicine-based derivatives: An overview. Anti-Cancer Drugs 2017, 28, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Covell, D.G.; Pei, X.F.; Ewell, J.B.; Nguyen, N.Y.; Brossi, A.; Hamel, E. Mapping the binding site of colchicinoids on beta -tubulin. 2-Chloroacetyl-2-demethylthiocolchicine covalently reacts predominantly with cysteine 239 and secondarily with cysteine 354. J. Biol. Chem. 2000, 275, 40443–40452. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- RCSB Protein Data Bank—RCSB PDB. Available online: http://www.rcsb.org/pdb/home/home.do (accessed on 23 February 2004).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision E.01, Gaussian Inc., Wallingford. Available online: https://gaussian.com/citation/ (accessed on 27 May 2024).
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. Available online: https://vina.scripps.edu/ (accessed on 27 May 2024). [CrossRef] [PubMed]
- Luo, Y.; Qiu, K.M.; Lu, X.; Liu, K.; Fu, J.; Zhu, H.L. Synthesis, biological evaluation, and molecular modeling of cinnamic acyl sulfonamide derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2011, 19, 4730–4738. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J. Substituent Effects on Nuclear Shielding. Ann. Rep. NMR Spectrosc. 1984, 15, 1–104. [Google Scholar]
- Lin, C.-K.; Yang, J.-S. Fluorescence response of TICT-active aminostilbenes to copper(II) ions: Redox reaction vs ion recognition. Res. Chem. Intermed. 2013, 39, 19–32. [Google Scholar] [CrossRef]
- Borys, F.; Tobiasz, P.; Joachimiak, E.; Fabczak, H.; Krawczyk, H. Systematic studies on anti-cancer evaluation of stilbene and dibenzo[b,f]oxepine derivatives. Molecules 2023, 28, 3558. [Google Scholar] [CrossRef]
- Bleger, D.; Hecht, S. Visible-Light-Activated Molecular Switches. Angew. Chem. Int. Ed. 2015, 54, 11338–11349. [Google Scholar] [CrossRef] [PubMed]
- Bleger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. o-Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef]
- Agnetta, L.; Bermudez, M.; Riefolo, F.; Matera, C.; Claro, E.; Messerer, R.; Littmann, T.; Wolber, G.; Holzgrabe, U.; Decker, M. Fluorination of Photoswitchable Muscarinic Agonists Tunes Receptor Pharmacology and Photochromic Properties. J. Med. Chem. 2019, 62, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Kara, Y.S. Substituent effect study on experimental 13C NMR chemical shifts of (3-(substitutedphenyl)-cis-4,5-dihydroisoxazole-4,5-diyl)bis(methylene)diacetate derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Holik, M. Second-order regression of 13C substituent chemical shifts with Taft’s sigma constants. Magn. Reson. Chem. 1992, 30, 189–199. [Google Scholar] [CrossRef]
- Ewing, D.F. 13C substituent effects in monosubstituted benzenes. Magn. Reson. Chem. 1979, 12, 499–524. [Google Scholar] [CrossRef]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Photocontrol of Antibacterial Activity: Shifting from 531 UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef] [PubMed]
- Wutz, D.-F.; Gluhacevic, D.; Chakrabarti, A.; Schmidtkunz, K.; Robaa, D.; Erdmann, F.; Romier, C.; Sippl, W.; Jung, M.; König, B. Photochromic Histone Deacetylase Inhibitors Based on Dithienylethenes and Fulgimides. Org. Biomol. Chem. 2017, 15, 4882–4896. [Google Scholar] [CrossRef] [PubMed]
- Kalka, K.; Merk, H.; Mukhtar, H.J. Photodynamic therapy in dermatology. J. Am. Acad. Dermatol. 2000, 42, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Wäldchen, S.; Lehmann, J.; Klein, T.; van de Linde, S.; Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 2015, 5, 15348. [Google Scholar] [CrossRef] [PubMed]
- Alachouzos, G.; Schulte, A.M.; Mondal, A.; Szymanski, W.; Feringa, B.L. Computational Design, Synthesis, and Photochemistry of Cy7-PPG, an Efficient NIR-Activated Photolabile Protecting Group for Therapeutic Applications. Angew. Chem. Int. Ed. 2022, 134, e202201308. [Google Scholar] [CrossRef]
- Borys, F.; Tobiasz, P.; Sobel, J.; Krawczyk, H. Synthesis and Study of Dibenzo[b,f]oxepine Combined with Fluoroazobenzenes—New Photoswitches for Application in Biological Systems. Molecules 2022, 27, 5836. [Google Scholar] [CrossRef]
- Kirchner, S.; Pianowski, Z. Photopharmacology of antimitotic agents. Int. J. Mol. Sci. 2022, 23, 5657. [Google Scholar] [CrossRef] [PubMed]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
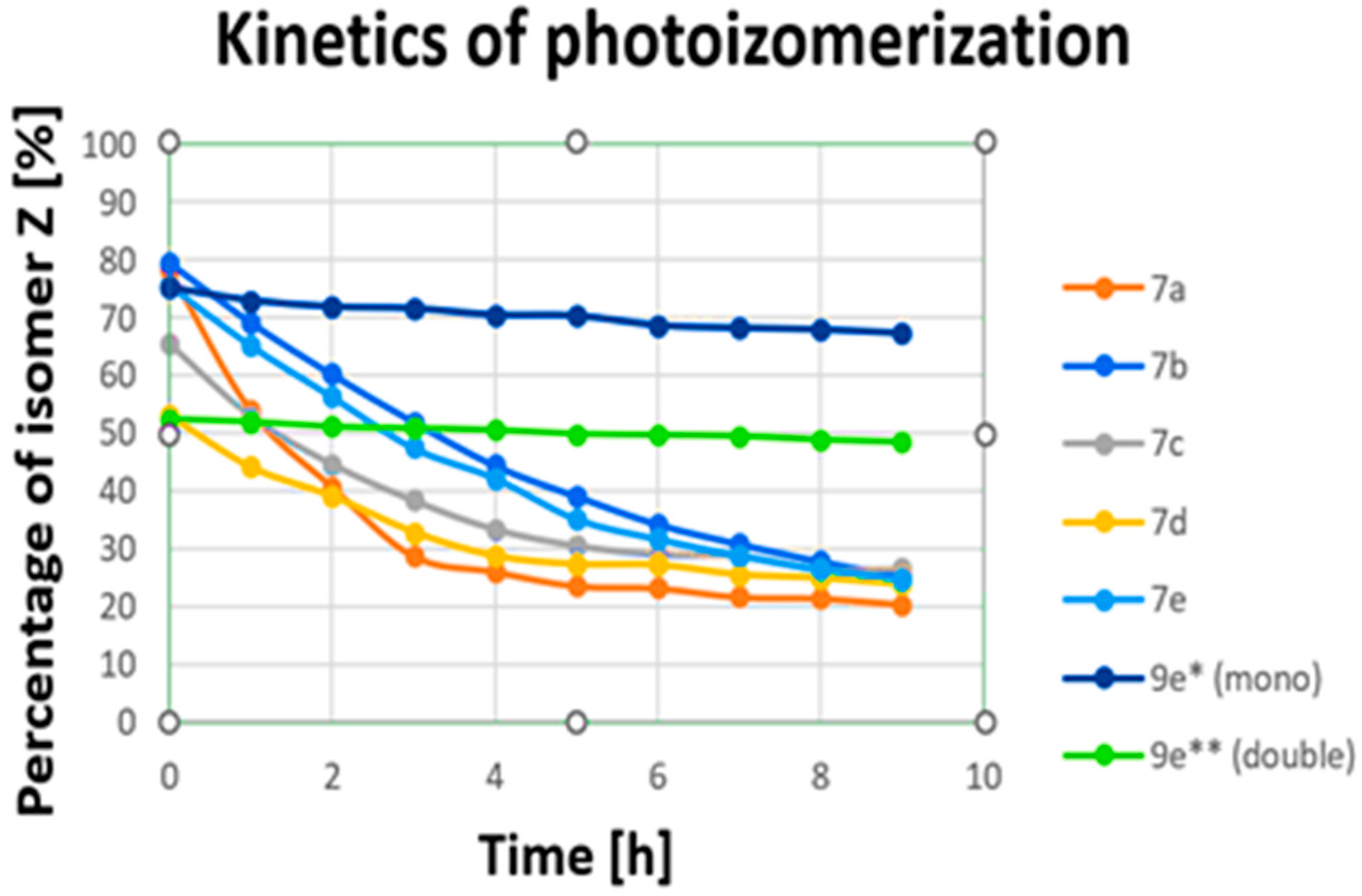
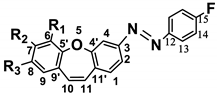 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C4′ | C5′ | C6 | C7 | C8 | C9 | C9′ | C10 | C11 | C11′ | C12 | C13 | C14 | C15 | |
| (7a) R1–R3 = H, | 129.68 | 120.85 | 153.67 | 114.56 | 157.87 | 156.98 | 121.52 | 130.28 | 125.14 | 129.48 | 130.35 | 131.46 | 129.33 | 133.28 | 149.13 | 124.98 | 116.09 | 164.49 |
| (7b) R2,R3 = H, R1 = OCH3 | −0.16 | −0.05 | −0.04 | 1.47 | −0.11 | −11.91 | 30.38 | −17.44 | −5.32 | −4.49 | 1.27 | −0.13 | 0.03 | 0.24 | 0.04 | 0.00 | −0.05 | −0.05 |
| (7c) R1,R3 = H, R2 = OCH3 | −0.11 | −0.01 | −0.02 | 0.13 | −0.41 | 1.25 | −14.31 | 31.78 | −13.89 | 0.79 | −7.10 | −0.19 | −2.26 | 0.44 | 0.23 | −0.02 | −0.02 | 0.06 |
| (7d) R1 = H, R2,R3 = OCH3 | −0.12 | 0.16 | −0.14 | −0.36 | −0.23 | −6.32 | −18.19 | 20.45 | 21.02 | −18.22 | −8.38 | −0.24 | −1.51 | 0.28 | 0.01 | −0.04 | 0.00 | −0.03 |
| (7e) R1,R3 = H, R2 = OCOCH3 | 0.01 | 0.44 | −0.02 | −0.18 | −0.58 | 0.46 | −6.30 | 21.87 | −6.74 | 0.27 | −2.19 | −0.89 | −0.19 | −0.21 | −0.03 | 0.01 | −0.01 | 0.00 |
| (a) | |||||||||
| Percentage of Isomer Z [%] | |||||||||
| Wavelength [nm] | Compound | ||||||||
| 7a | 7b | 7c | 7d | 7e | 9e ** (double) | 9e * (mono) | 9f ** (double) | 9f * (mono) | |
| 390 nm | 77.66 | 79.24 | 67.23 | 52.63 | 73.48 | 63.18 | 29.9 | 73.49 | 44.71 |
| 400 nm | 77.03 | 74.08 | 65.97 | 53.8 | 68.01 | 61.99 | 26.01 | 70.62 | 30.61 |
| 430 nm | 36.39 | 16.16 | 42.67 | 29.92 | 8.28 | 16.98 | 8.62 | 22.47 | 8.69 |
| 470 nm | 27.03 | 25.54 | 43.73 | 39.31 | 26.46 | 38.15 | 51.85 | 23.96 | 31.05 |
| 505 nm | 22.61 | 22.12 | 30.39 | 31.47 | 21.92 | 50.31 | 70.49 | 20.98 | 32.39 |
| 515 nm | 22.48 | 22.02 | 32.22 | 31.8 | 22.07 | 51.17 | 69.3 | 21.53 | 32.26 |
| 525 nm | 21.18 | 17.35 | 28.43 | 26.77 | 19.24 | 53.88 | 69.33 | 17.14 | 20.15 |
| 535 nm | 23.28 | 19.88 | 31.57 | 28.76 | 21.21 | 52.49 | 75.17 | 19.33 | 28.68 |
| 590 nm | 0.96 | 2.32 | 4.17 | 3.38 | 3.06 | 28.15 | 14.51 | 1.88 | 0.83 |
| 610 nm | 0 | 0 | 0 | 0 | 0 | 5.2 | 1.92 | 0 | 0 |
| (b) | |||||||||
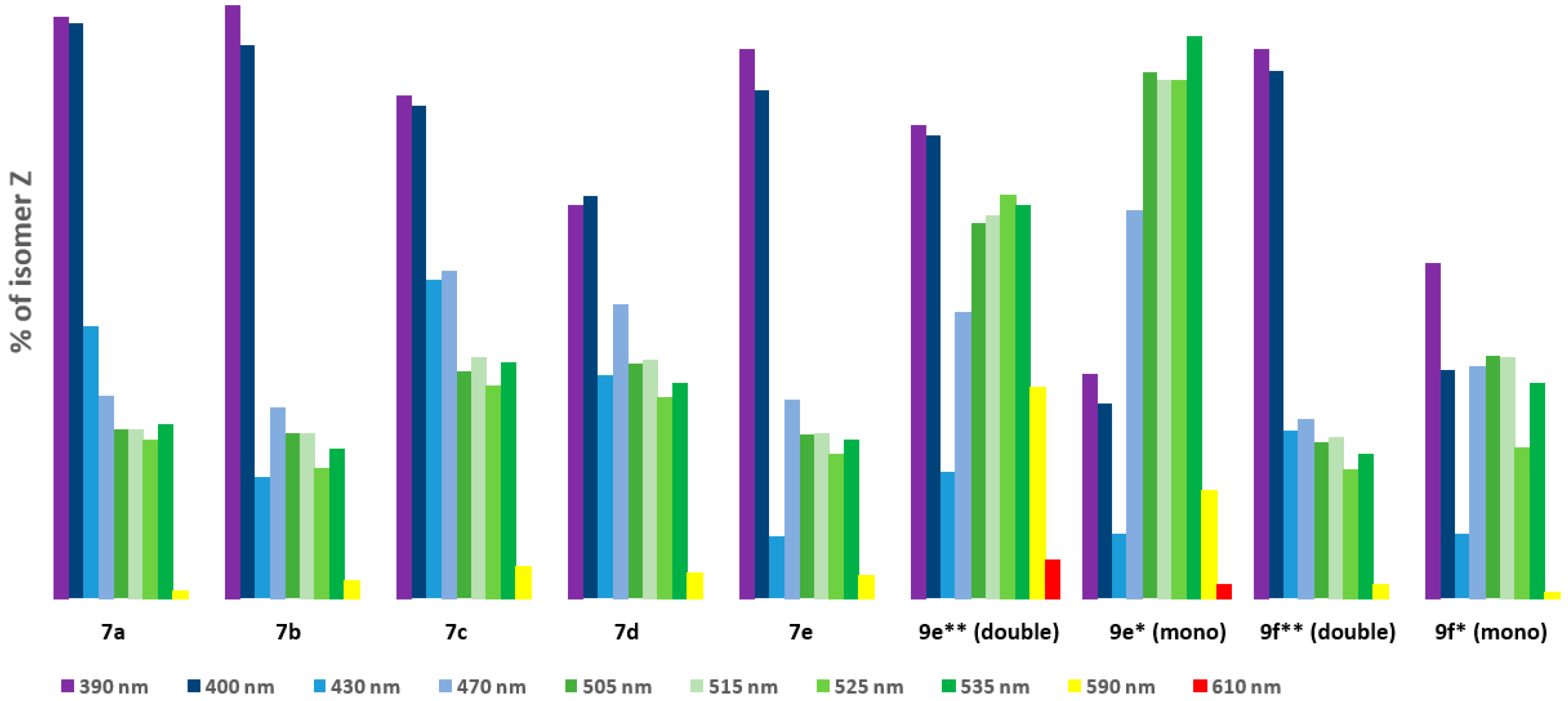 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobiasz, P.; Borys, F.; Kucharska, M.; Poterała, M.; Krawczyk, H. Synthesis and Study of Building Blocks with Dibenzo[b,f]oxepine: Potential Microtubule Inhibitors. Int. J. Mol. Sci. 2024, 25, 6155. https://doi.org/10.3390/ijms25116155
Tobiasz P, Borys F, Kucharska M, Poterała M, Krawczyk H. Synthesis and Study of Building Blocks with Dibenzo[b,f]oxepine: Potential Microtubule Inhibitors. International Journal of Molecular Sciences. 2024; 25(11):6155. https://doi.org/10.3390/ijms25116155
Chicago/Turabian StyleTobiasz, Piotr, Filip Borys, Marta Kucharska, Marcin Poterała, and Hanna Krawczyk. 2024. "Synthesis and Study of Building Blocks with Dibenzo[b,f]oxepine: Potential Microtubule Inhibitors" International Journal of Molecular Sciences 25, no. 11: 6155. https://doi.org/10.3390/ijms25116155
APA StyleTobiasz, P., Borys, F., Kucharska, M., Poterała, M., & Krawczyk, H. (2024). Synthesis and Study of Building Blocks with Dibenzo[b,f]oxepine: Potential Microtubule Inhibitors. International Journal of Molecular Sciences, 25(11), 6155. https://doi.org/10.3390/ijms25116155







