Anti-Inflammatory Activity of No-Ozone Cold Plasma in Porphyromonas gingivalis Lipopolysaccharide-Induced Periodontitis Rats
Abstract
:1. Introduction
2. Results
2.1. NCP Treatment for Up to 5 Min Has No Effects on Cell Viability
2.2. NCP Inhibited PG-LPS-Mediated Inflammatory Reactions of HGFs
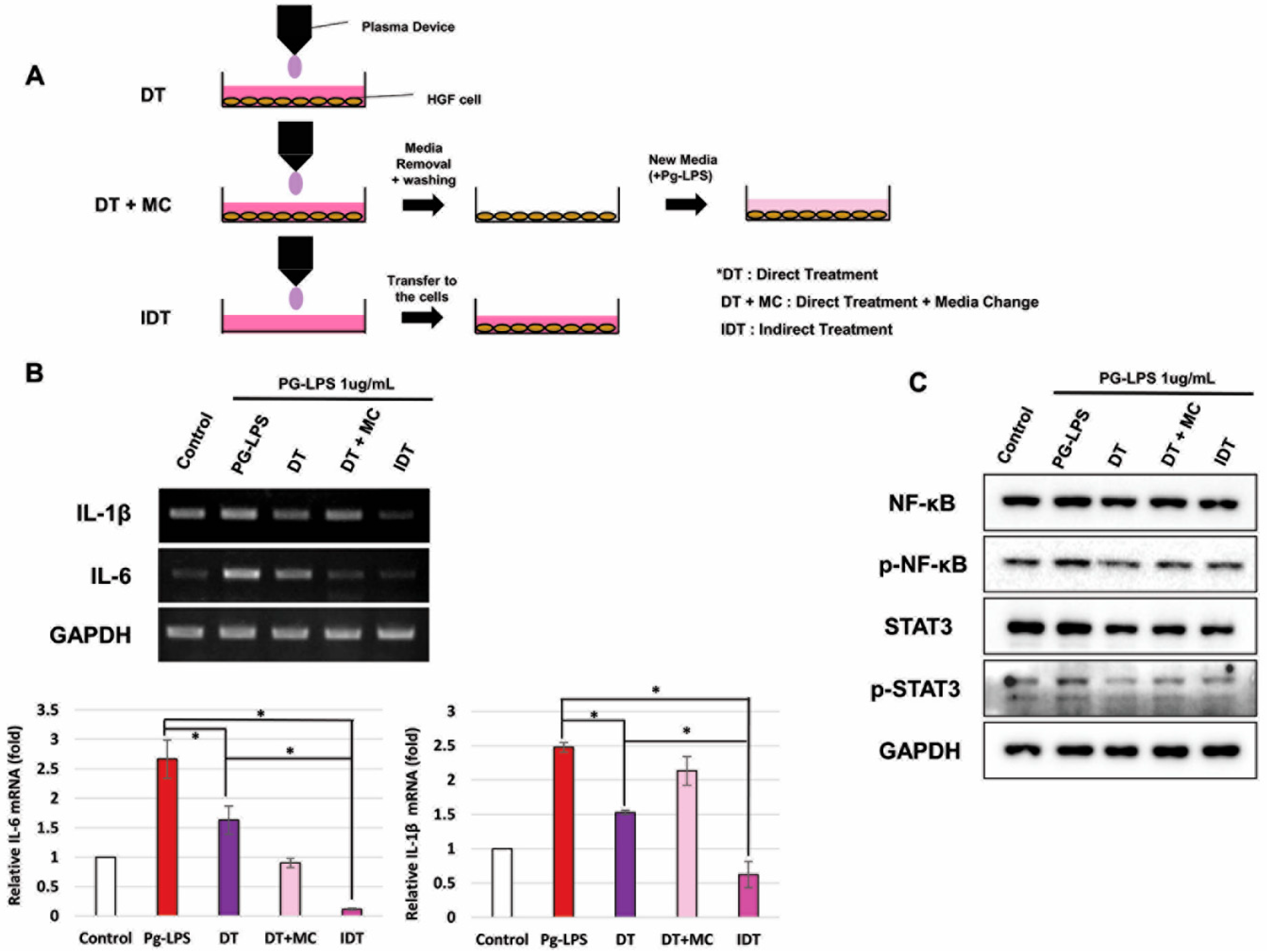
2.3. NCP-Mediated Changes in PG-LPS-Containing Media Are Essential for Anti-Inflammatory Activity
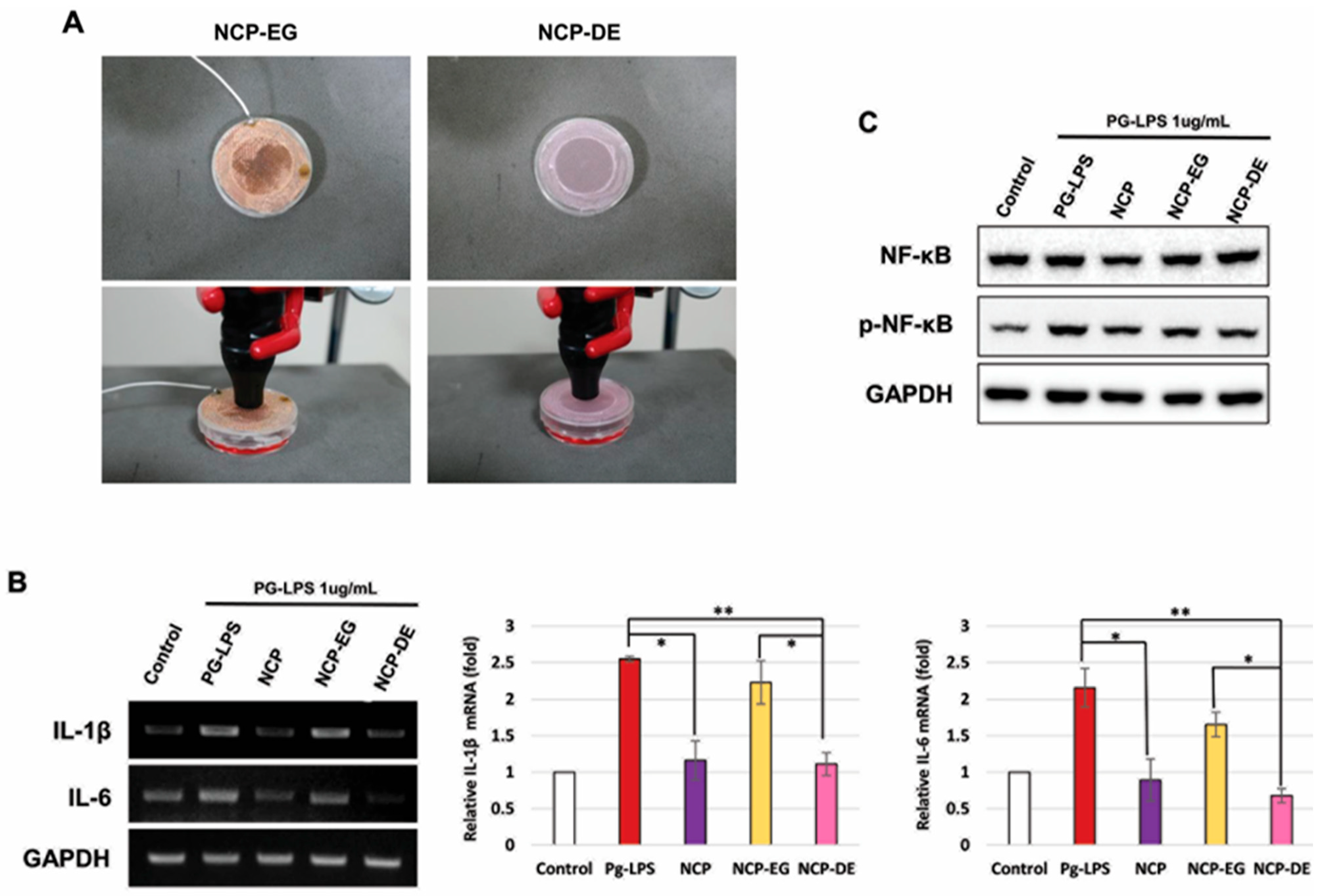
2.4. Charged NCP Particles Were Responsible for NCP-Mediated Anti-Inflammatory Activity in the PG-LPS-Treated HGFs
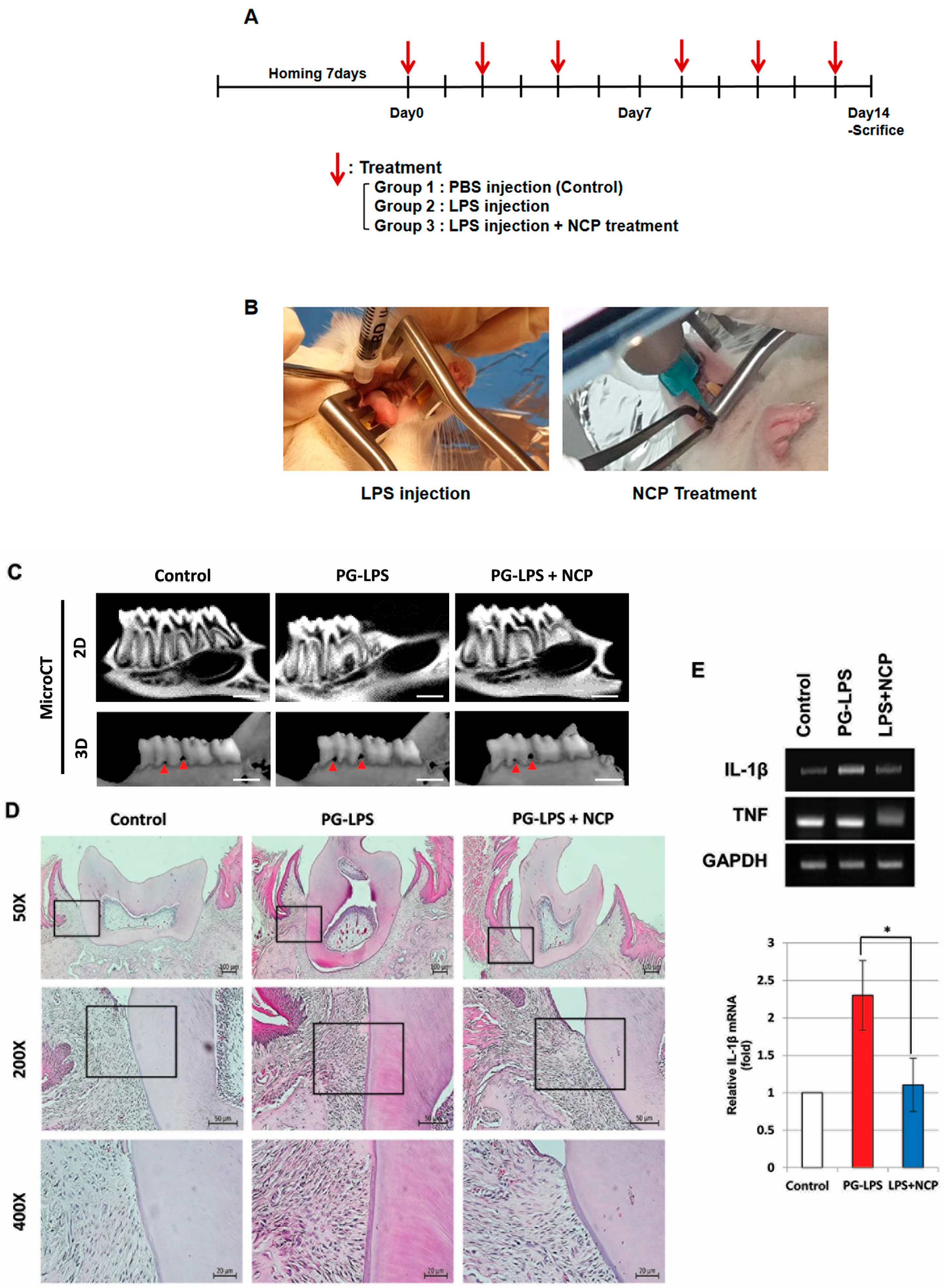
2.5. NCP Reduces the Inflammatory Phenotype of PG-LPS-Induced Periodontitis in Rats
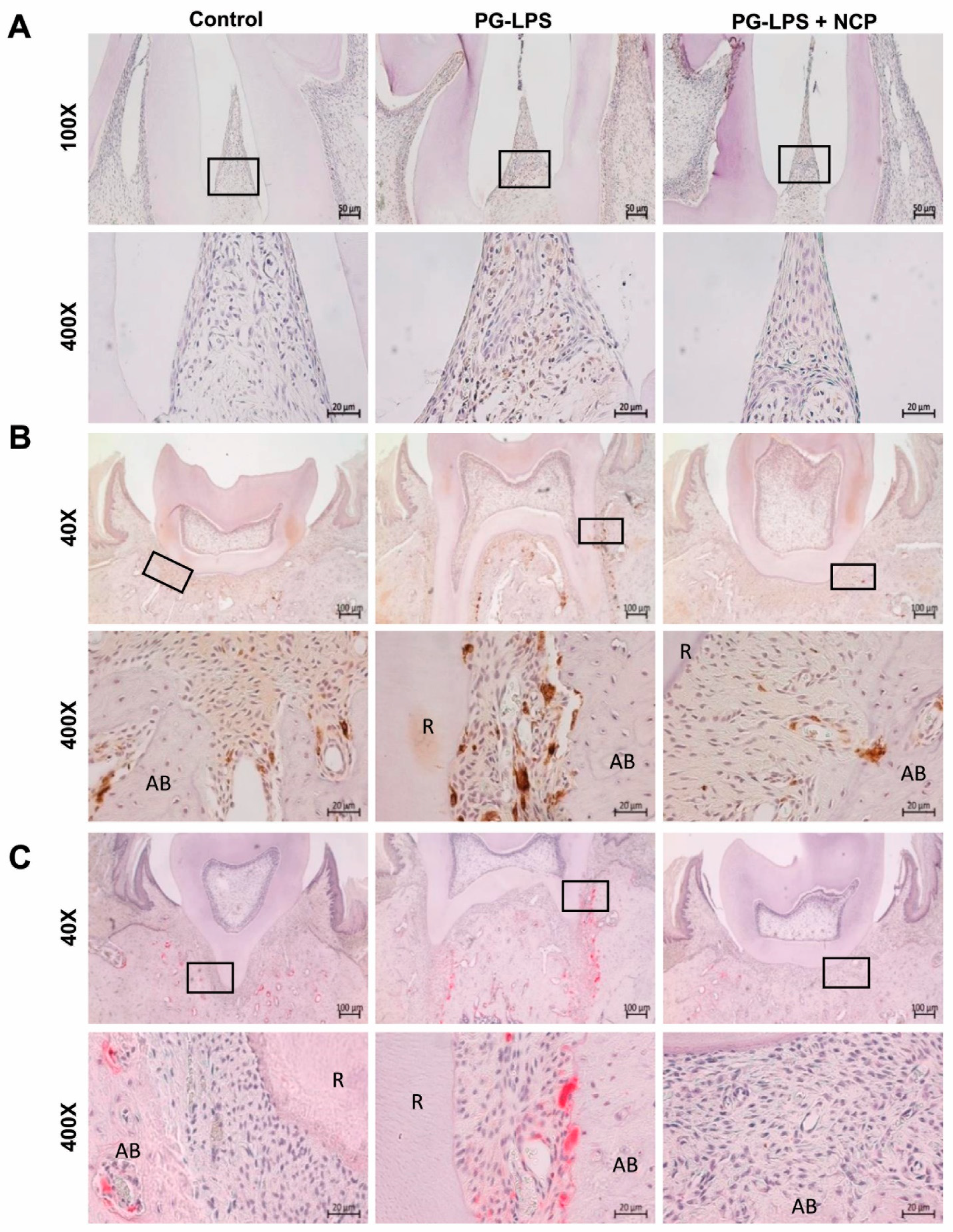
2.6. NCP Treatment Inhibited the Accumulation of T Cells and Macrophages and Blocked the Activation of Osteoclasts in the Lesion
3. Discussion
4. Methods and Materials
4.1. Plasma Device
4.2. In Vitro Study
4.2.1. Cells
4.2.2. Sulforhodamine B (SRB) Cell Growth Assay
4.2.3. Western Blot
4.2.4. RNA Extraction and RT-PCR
4.2.5. NCP Treatment Methods on Cells
4.2.6. NCP Treatment in the Presence of Two Types of Mesh
4.3. In Vivo Study
4.3.1. Periodontitis Animal Model
4.3.2. Micro-CT
4.3.3. RNA Extraction and RT-PCR
4.3.4. Hematoxylin and Eosin (H&E) Staining
4.3.5. Immunohistochemistry
4.3.6. Tartrate-Resistant Acid Phosphatase (TRAP) Assay
4.4. Ethical Statement
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.C.; Kostov, G.K.; Pessoa, R.S.; de Abreu, G.M.A.; Lima, G.M.G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Socrasky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent Jr, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Nakamura, K.; Ishiyama, K.; Yamada, Y.; Shirato, M.; Niwano, Y.; Kayaba, C.; Ikeda, K.; Takagi, A.; Yamaguchi, T.; et al. Adjunctive antimicrobial chemotherapy based on hydrogen peroxide photolysis for non-surgical treatment of moderate to severe periodontitis: A randomized controlled trial. Sci. Rep. 2017, 7, 12247. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, S.; Ari, G.; Rajendran, S.; Mahendra, J.; Namasvayam, A. A review on use of lasers in periodontitis. Ann. Rom. Soc. Cell Biol. 2020, 24, 1056–1061. [Google Scholar]
- Ma, J.; Yang, X.; Sun, Y.; Yang, J. Thermal damage in three-dimensional vivo bio-tissues induced by moving heat sources in laser therapy. Sci. Rep. 2019, 9, 10987. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Yadav, D.K.; Ki, S.H.; Choi, E.H.; Han, I. Inactivation of Infectious Bacteria Using Nonthermal Biocompatible Plasma Cabinet Sterilizer. Int. J. Mol. Sci. 2020, 21, 8321. [Google Scholar] [CrossRef] [PubMed]
- JungBauer, G.; Moser, D.; Muller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antibocrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, K.H.; Park, S.Y.; Yoon, S.Y.; Kim, G.H.; Lee, Y.M.; Rhyu, I.C.; Seol, Y.J. The bactericidal effect of an atmospheric-pressure plasma jet on Porphyromonas gingivalis biofilms on sandblasted and acid-etched titanium discs. J. Periodontal. Implant. Sci. 2019, 49, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, I.; Odagiri, H.; Park, G.; Sanghavi, R.; Oshita, T.; Togi, A.; Yoshikawa, K.; Mizutani, K.; Takeuchi, Y.; Kobayashi, H.; et al. Anti-inflammatory effects of cold atmospheric plasma irradiation on the THP-1 human acute monocytic leukemia cell line. PLoS ONE 2023, 18, 0292267. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.; Seo, I.H.; Chae, M.W.; Park, J.W.; Choi, E.H.; Uhm, H.S.; Baik, K.Y. Effects of Non-thermal Plasma Activated Water on the Anti-cancer Immune Activities of Macrophages. Clin. Plasam. Med. 2018, 9, 3. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective Killing of Melanoma Cells With Non-Thermal Atmospheric Pressure Plasma and p-FAK Antibody Conjugated Gold Nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, Y.S.; Song, K.; Lee, H.J.; Hong, J.W.; Kim, G.C. Skin renewal activity of non-thermal plasma through the activation of beta-catenin in keratinocytes. Sci. Rep. 2017, 7, 6416. [Google Scholar]
- Choi, J.H.; Song, Y.S.; Lee, H.J.; Hong, J.W.; Kim, G.C. Inhibition of inflammatory reactions in 2,4-Dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Sci. Rep. 2016, 6, 27376. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.B.R.; Choi, J.H.; Ji, J.; Song, K.W.; Lee, H.J.; Kim, G.C. Increment of growth factors in mouse skin treated with non-thermal plasma. Int. J. Med. Sci. 2018, 15, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xin, Y.; Hamblin, M.R.; Jiang, X. Applications of cold atmospheric plasma for transdermal drug delivery: A review. Drug Deliv. Transl. Res. 2021, 11, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Nam, S.H.; Song, Y.S.; Lee, H.W.; Lee, H.J.; Song, K.; Hong, J.W.; Kim, G.C. Treatment with low-temperature atmospheric pressure plasma enhances cutaneous delivery of epidermal growth factor by regulating E-cadherin-mediated cell junctions. Arch. Dermatol. Res. 2014, 306, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater Today Bio. 2023, 13, 100200. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, E.; Kim, W.J. Health Effects of Ozone on Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83, S6–S11. [Google Scholar] [CrossRef]
- Park, N.S.; Yun, S.E.; Lee, H.Y.; Lee, H.J.; Choi, J.H.; Kim, G.C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Sci. Rep. 2022, 12, 7597. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Martinez, C.; Martinez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Ambili, R.; Janam, P.; Babu, P.S.S.; Prasad, M.; Vinod, D.; Kumar, P.R.A.; Kumary, T.V.; Nair, S.A. Differential expression of transcription factors NF-κB and STAT3 in periodontal ligament fibroblasts and gingiva of healthy and diseased individuals. Arch. Oral Biol. 2017, 82, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ramboiu, S.; Nicolae, F.M.; Soanca, A.; Gheorghe, D.N.; Popescu, D.M.; Ungureanu, B.S.; Mărgăritescu, D.N.; Turcu-Știolica, A.; Boldeanu, M.V.; Șurlin, V.M.; et al. Periodontal status and inflammatory markers in gingival crevicular fluid of patients with periodontitis and colorectal cancer. J. Mind Med. Sci. 2023, 10, 347–355. [Google Scholar] [CrossRef]
- Cheng, Z.; Meade, J.; Mankia, K.; Emery, P.; Devine, D.A. Periodontal disease and periodontal bacteria as triggers for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078. [Google Scholar] [CrossRef] [PubMed]
- Rivas, C.A.; Suliman, S.; Almarhoumi, R.; Vega, M.E.; Rojas, C.; Monasterio, G.; Galindo, M.; Vernal, R.; Kantarci, A. Regulatory T cell phenotype and anti-osteoclastogenic function in experimental periodontitis. Sci. Rep. 2020, 10, 19018. [Google Scholar]
- Yin, L.; Li, X.; Hou, J. Macrophages in periodontitis: A dynamic shift between tissue destruction and repair. Jpn. Dent. Sci. Rev. 2022, 58, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Stefan, S.P.; Hienz, A.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar]
- Leira, Y.; Iglesias-Rey, R.; Comez-Lado, N.; Aguiar, P.; Sobrino, T.; D’Aiuto, F.; Castillo, J.; Blanco, J.; Campos, F. Periodontitis and vascular inflammatory biomarkers: An experimental in vivo study in rats. Odontology 2020, 108, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.A.; Walters, L.M. Tartrate-resistant Acid Phosphatase in Bone and Cartilage Following Decalcification and Cold-Embedding in Plastic. J. Histochem. Cytochem. 1987, 35, 203–206. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-H.; Jang, Y.-S.; Joo, J.-Y.; Kim, G.-C.; Choi, J.-H. Anti-Inflammatory Activity of No-Ozone Cold Plasma in Porphyromonas gingivalis Lipopolysaccharide-Induced Periodontitis Rats. Int. J. Mol. Sci. 2024, 25, 6161. https://doi.org/10.3390/ijms25116161
Park K-H, Jang Y-S, Joo J-Y, Kim G-C, Choi J-H. Anti-Inflammatory Activity of No-Ozone Cold Plasma in Porphyromonas gingivalis Lipopolysaccharide-Induced Periodontitis Rats. International Journal of Molecular Sciences. 2024; 25(11):6161. https://doi.org/10.3390/ijms25116161
Chicago/Turabian StylePark, Kwang-Ha, Yoon-Seo Jang, Ji-Young Joo, Gyoo-Cheon Kim, and Jeong-Hae Choi. 2024. "Anti-Inflammatory Activity of No-Ozone Cold Plasma in Porphyromonas gingivalis Lipopolysaccharide-Induced Periodontitis Rats" International Journal of Molecular Sciences 25, no. 11: 6161. https://doi.org/10.3390/ijms25116161





