DNA-PKcs Inhibition Sensitizes Human Chondrosarcoma Cells to Carbon Ion Irradiation via Cell Cycle Arrest and Telomere Capping Disruption
Abstract
:1. Introduction
2. Results
2.1. The Processes of Cellular Growth Regulation
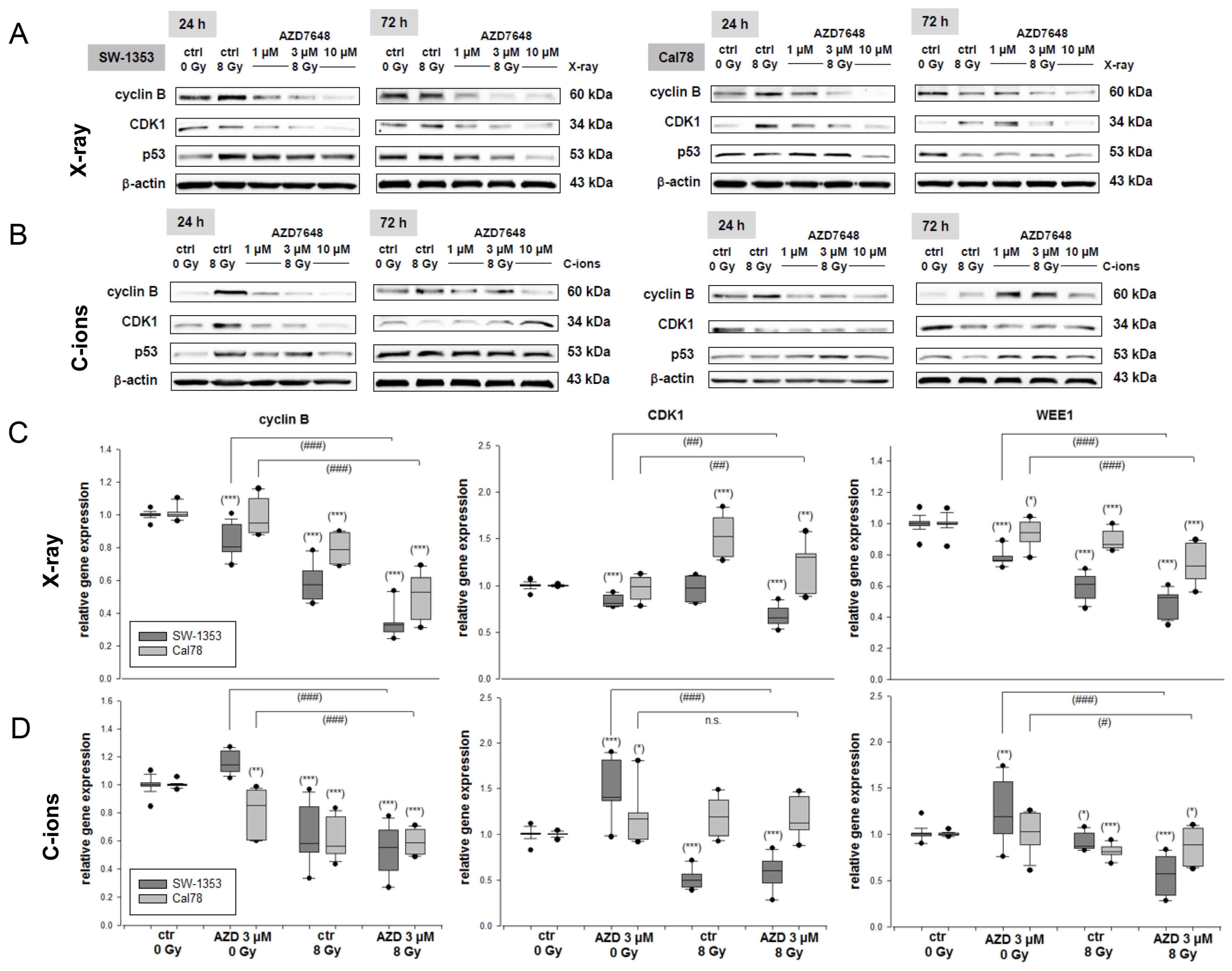
2.2. The Additive Effect of Combined Treatment with the AZD7648 on Cell Cycle Distribution
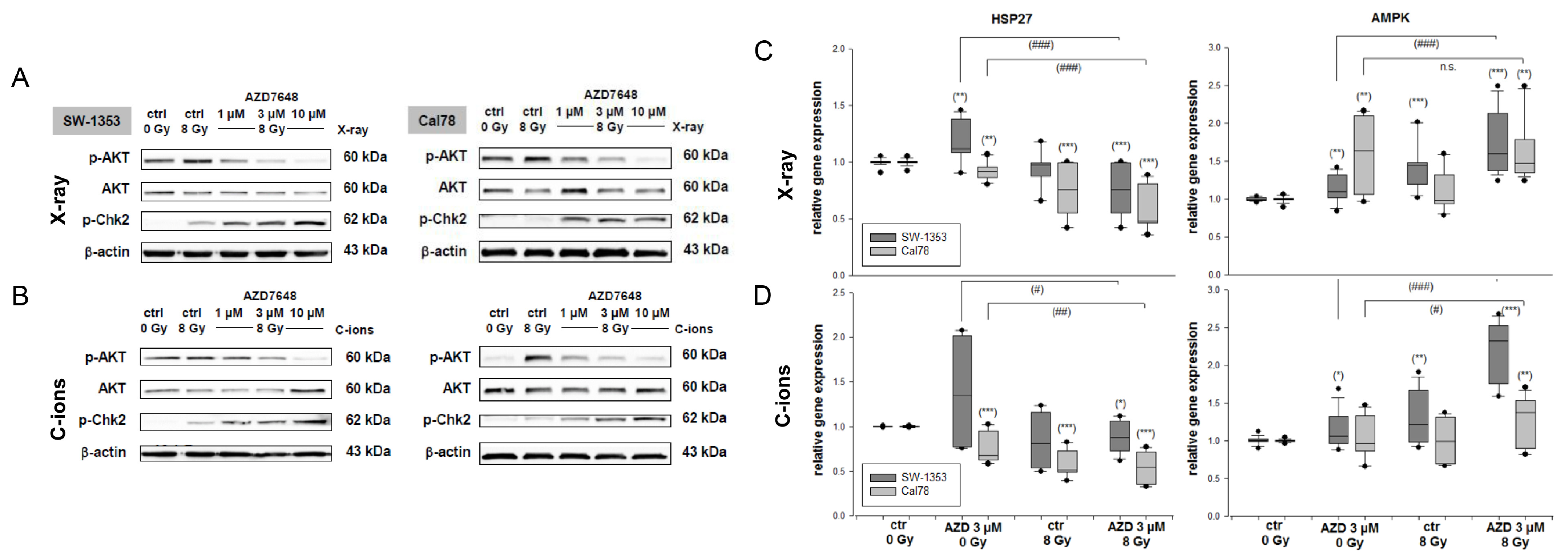
2.3. Combined Treatment with DNA-PKcs Inhibition and Particle IR Affected AKT and Chk2 Phosphorylation and the Expression of Protein Stability Marker and Energy Sensor
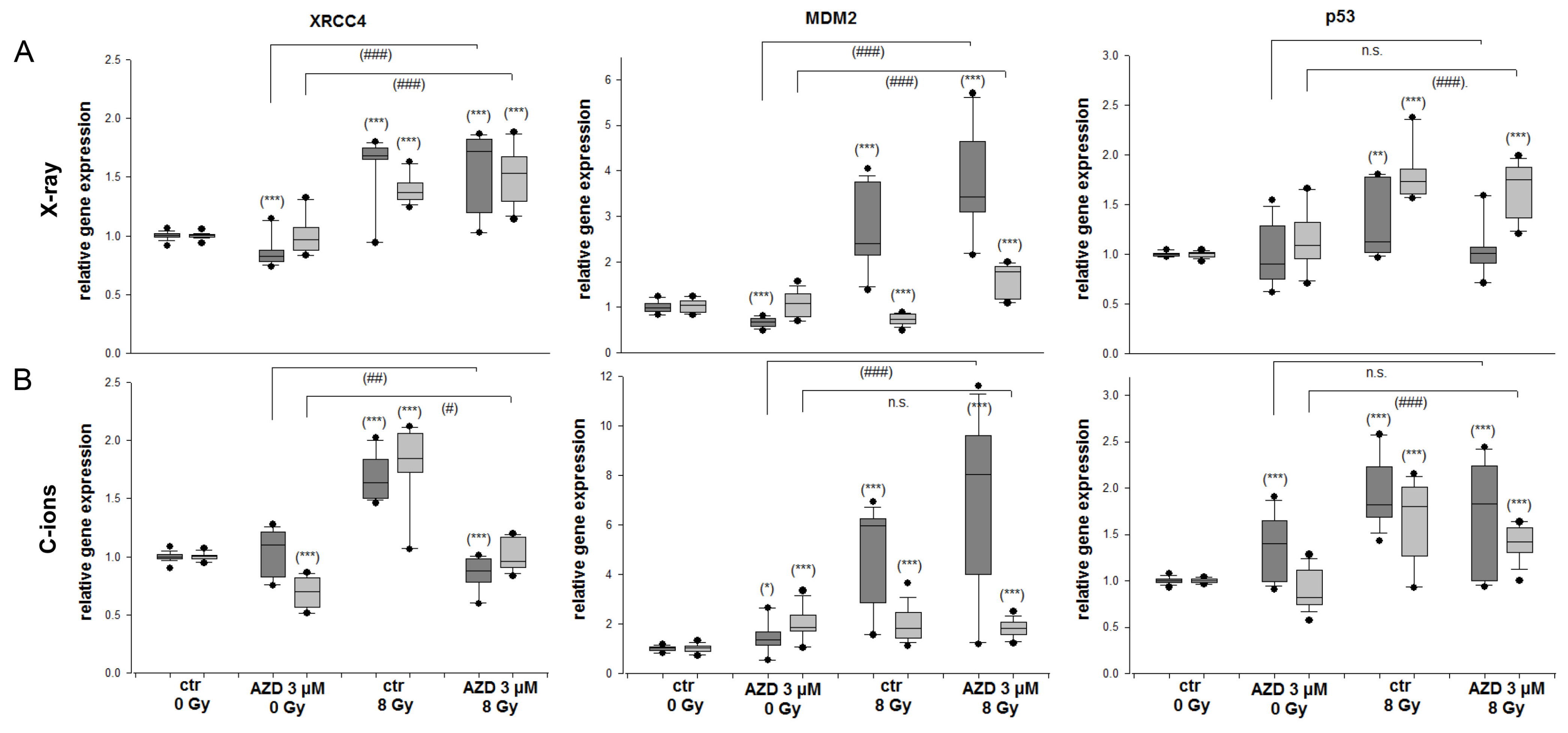
2.4. DNA Repair and MDM2-p53 in Response to Radiation and DNA-PKcs Inhibition
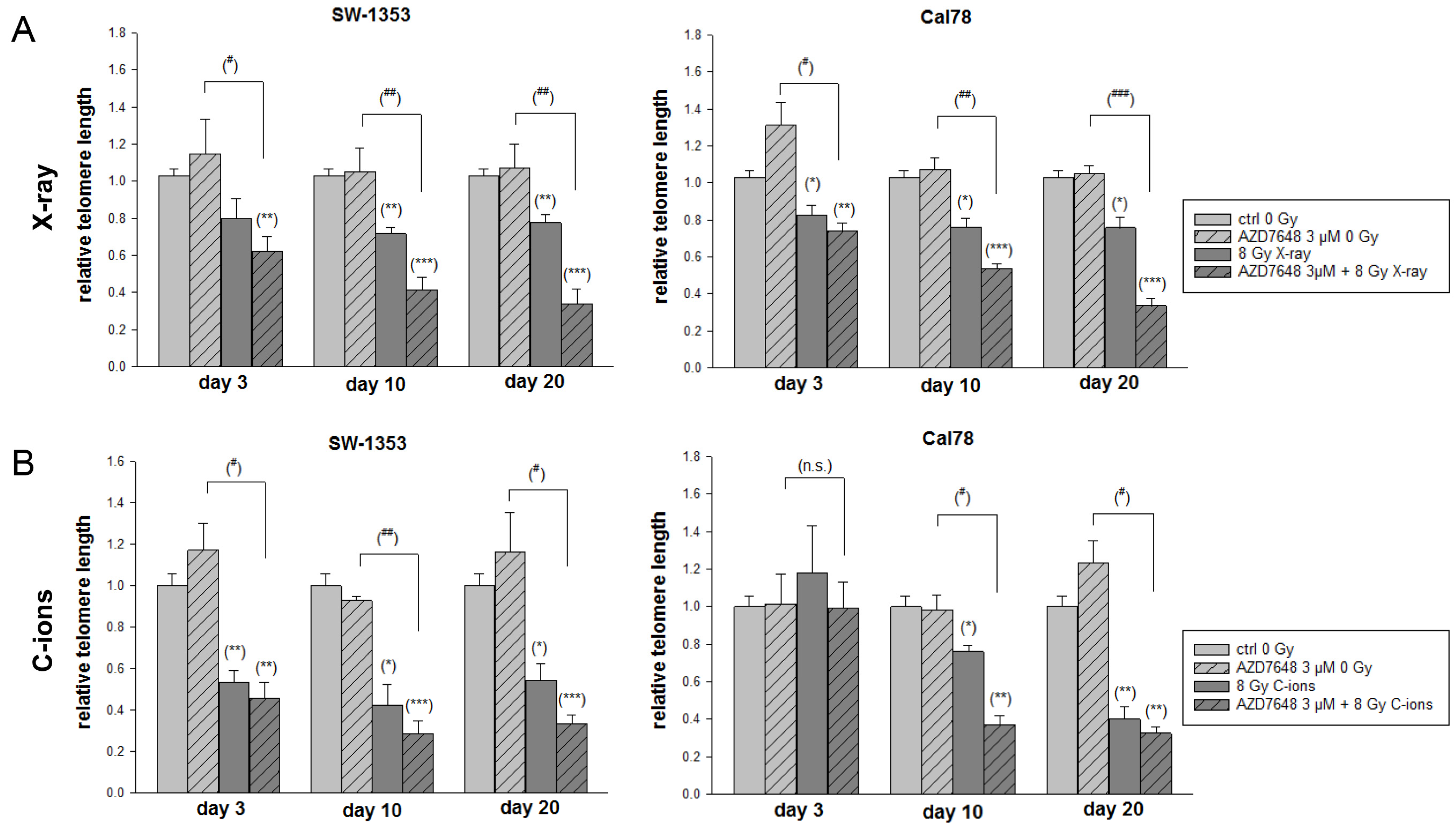
2.5. DNA-PKcs Inhibition Reduced the NHEJ DNA Repair and Resulted in Abbreviated Telomere Lengths after IR
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Experimental Irradiation Conditions
4.3. Viability and Proliferation Analysis
4.4. Flow Cytometry Cell Cycle Analysis
4.5. Protein Expression Analysis
4.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Telomere Length Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laitinen, M.K.; Parry, M.C.; Le Nail, L.R.; Wigley, C.H.; Stevenson, J.D.; Jeys, L.M. Locally recurrent chondrosarcoma of the pelvis and limbs can only be controlled by wide local excision. Bone Joint J. 2019, 101-B, 266–271. [Google Scholar] [CrossRef]
- Zajac, A.E.; Kopeć, S.; Szostakowski, B.; Spałek, M.J.; Fiedorowicz, M.; Bylina, E.; Filipowicz, P.; Szumera-Ciećkiewicz, A.; Tysarowski, A.; Czarnecka, A.M.; et al. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers 2021, 13, 2390. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M.; Hogendoorn, P.C.W.; Mertens, F. Chondrosarcoma. In World Health Organization Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC Press: Lyon, France, 2013; Volume 5, pp. 264–274. [Google Scholar]
- Guan, X.; Gao, J.; Hu, J.; Hu, W.; Yang, J.; Qiu, X.; Hu, C.; Kong, L.; Lu, J.J. The preliminary results of proton and carbon ion therapy for chordoma and chondrosarcoma of the skull base and cervical spine. Radiat. Oncol. 2019, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Kuess, P.; Böhlen, T.T.; Lechner, W.; Elia, A.; Georg, D.; Palmans, H. Lateral response heterogeneity of Bragg peak ionization chambers for narrow-beam photon and proton dosimetry. Phys. Med. Biol. 2017, 62, 9189–9206. [Google Scholar] [CrossRef] [PubMed]
- Mizoe, J.E. Review of carbon ion radiotherapy for skull base tumors (especially chordomas). Rep. Pract. Oncol. Radiother. 2016, 21, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Fiore, M.R.; Barcellini, A.; Iannalfi, A.; Vischioni, B.; Ronchi, S.; Bonora, M.; Riva, G.; Vai, A.; Facoetti, A.; et al. Outcome and Toxicity of Carbon Ion Radiotherapy for Axial Bone and Soft Tissue Sarcomas. Anticancer. Res. 2020, 40, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Cavallo, I.; Gandini, S.; Ingargiola, R.; Pecorilla, M.; Imparato, S.; Rossi, E.; Mirandola, A.; Ciocca, M.; Orlandi, E.; et al. Particle Radiotherapy for Skull Base Chondrosarcoma: A Clinical Series from Italian National Center for Oncological Hadrontherapy. Cancers 2021, 13, 4423. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Glänzer, D.; Eck, N.; Kerschbaum-Gruber, S.; Mara, E.; Deycmar, S.; Madl, T.; Kashofer, K.; Georg, P.; Leithner, A.; et al. Activation of efficient DNA repair mechanisms after photon and proton irradiation of human chondrosarcoma cells. Sci. Rep. 2021, 11, 24116. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Glänzer, D.; Eck, N.; Stasny, K.; Falkner, A.; Leithner, A.; Georg, D. The ATR Inhibitor VE-821 Enhances the Radiosensitivity and Suppresses DNA Repair Mechanisms of Human Chondrosarcoma Cells. Int. J. Mol. Sci. 2023, 4, 2315. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell. 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Davis, A.J.; Chen, B.P.; Chen, D.J. DNA-PK: A dynamic enzyme in a versatile DSB repair pathway. DNA Repair 2014, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jette, N.; Lees-Miller, S.P. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog. Biophys. Mol. Biol. 2015, 117, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Stiff, T.; O’Driscoll, M.; Rief, N.; Iwabuchi, K.; Löbrich, M.; Jeggo, P.A. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004, 64, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.F.; Knudsen, K.E. Beyond DNA repair: DNA-PK function in cancer. Cancer Discov. 2014, 4, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W. Telomere length regulation. Annu. Rev. Biochem. 1996, 65, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Hande, M.P. DNA repair factors and telomere-chromosome integrity in mammalian cells. Cytogenet. Genome Res. 2004, 104, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.M.; O’Connor, K.P.; Mahajan, A.; Carlson, M.L.; Van Gompel, J.J. Carbon ion radiotherapy for skull base chordomas and chondrosarcomas: A systematic review and meta-analysis of local control, survival, and toxicity outcomes. J. Neurooncol. 2020, 147, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, F.; Scifoni, E.; Durante, M. New Ions for Therapy. Int. J. Part. Ther. 2015, 2, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Fok, J.H.L.; Ramos-Montoya, A.; Vazquez-Chantada, M.; Wijnhoven, P.W.G.; Follia, V.; James, N.; Farrington, P.M.; Karmokar, A.; Willis, S.E.; Cairns, J.; et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat. Commun. 2019, 10, 5065. [Google Scholar] [CrossRef]
- Anastasia, A.; Dellavedova, G.; Ramos-Montoya, A.; James, N.; Chiorino, G.; Russo, M.; Baakza, H.; Wilson, J.; Ghilardi, C.; Cadogan, E.B.; et al. The DNA-PK Inhibitor AZD7648 Sensitizes Patient-Derived Ovarian Cancer Xenografts to Pegylated Liposomal Doxorubicin and Olaparib Preventing Abdominal Metastases. Mol. Cancer Ther. 2022, 21, 555–567. [Google Scholar] [CrossRef]
- Wade, M.A.; Sunter, N.J.; Fordham, S.E.; Long, A.; Masic, D.; Russell, L.J.; Harrison, C.J.; Rand, V.; Elstob, C.; Bown, N.; et al. c-MYC is a radiosensitive locus in human breast cells. Oncogene 2015, 34, 4985–4994. [Google Scholar] [CrossRef] [PubMed]
- Vafa, O.; Wade, M.; Kern, S.; Beeche, M.; Pandita, T.K.; Hampton, G.M.; Wahl, G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol. Cell. 2002, 9, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- de Jong, Y.; van Oosterwijk, J.G.; Kruisselbrink, A.B.; Briaire-de Bruijn, I.H.; Agrogiannis, G.; Baranski, Z.; Cleven, A.H.; Cleton-Jansen, A.M.; van de Water, B.; Danen, E.H.; et al. Targeting survivin as a potential new treatment for chondrosarcoma of bone. Oncogenesis 2016, 5, e222. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [PubMed]
- Xu, N.; Lao, Y.; Zhang, Y.; Gillespie, D.A. Akt: A double-edged sword in cell proliferation and genome stability. J. Oncol. 2012, 2012, 951724. [Google Scholar] [CrossRef] [PubMed]
- Viniegra, J.G.; Martínez, N.; Modirassari, P.; Hernández Losa, J.; Parada Cobo, C.; Sánchez-Arévalo Lobo, V.J.; Aceves Luquero, C.I.; Alvarez-Vallina, L.; Ramón y Cajal, S.; Rojas, J.M.; et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is med iated through ATM. J. Biol. Chem. 2005, 280, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.J.; Coxon, P.Y.; Powell, D.W.; Webster, R.; Klein, J.B.; Pierce, W.; Ping, P.; McLeish, K.R. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J. Biol. Chem. 2001, 276, 3517–3523. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Dylgjeri, E.; Knudsen, K.E. DNA-PKcs: A Targetable Protumorigenic Protein Kinase. Cancer Res. 2022, 82, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.E. Mdm2 in the response to radiation. Mol. Cancer Res. 2004, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- van Oosterwijk, J.G.; van Ruler, M.A.; Briaire-de Bruijn, I.H.; Herpers, B.; Gelderblom, H.; van de Water, B.; Bovée, J.V. Src kinases in chondrosarcoma chemoresistance and migration: Dasatinib sensitises to doxorubicin in TP53 mutant cells. Br. J. Cancer 2013, 109, 1214–1222. [Google Scholar] [CrossRef]
- Yue, X.; Bai, C.; Xie, D.; Ma, T.; Zhou, P.K. DNA-PKcs: A Multi-Faceted Player in DNA Damage Response. Front. Genet. 2020, 11, 607428. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Zhang, S.; Chen, B.P.C. DNA-dependent protein kinase in telomere maintenance and protection. Cell. Mol. Biol. Lett. 2020, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Xie, Y.; Tanaka, K.; Wang, B.; Zhang, H. DNA-PKcs inhibition sensitizes cancer cells to carbon-ion irradiation via telomere capping disruption. PLoS ONE 2013, 8, e72641. [Google Scholar] [CrossRef]
- Kohno, R.; Koto, M.; Ikawa, H.; Lee, S.H.; Sato, K.; Hashimoto, M.; Inaniwa, T.; Shirai, T. High-Linear Energy Transfer Irradiation in Clinical Carbon-Ion Beam With the Linear Energy Transfer Painting Technique for Patients With Head and Neck Cancer. Adv. Radiat. Oncol. 2023, 9, 101317. [Google Scholar] [CrossRef]



| SW-1353 | Cal78 | |||||
|---|---|---|---|---|---|---|
| G1/G0 | S | G2/M | G1/G0 | S | G2/M | |
| ctrl 0 Gy | 69.2 ± 1.9 | 15.6 ± 2.5 | 15.2 ± 4.5 | 67.6 ± 0.4 | 18.3 ± 1.6 | 14.1 ± 2.0 |
| 3 µM AZD 0 Gy | 63.2 ± 3.4 n.s. | 25.9 ± 1.9 n.s. | 10.8 ± 3.0 n.s. | 76.6 ± 4.5 * | 18.5 ± 2.5 n.s. | 4.9 ± 2.5 * |
| X-ray 8 Gy | 61.6 ± 0.8 * | 7.8 ± 1.8 * | 30.7 ± 2.5 * | 65.8 ± 8.0 n.s. | 8.9 ± 0.9 ** | 25.3 ± 8.8 n.s. |
| AZD 3 µM + X-ray 8 Gy | 46.6 ± 2.1 ***, ## | 1.9 ± 0.0 *, # | 51.4 ± 1.6 ***, ### | 9.1 ± 1.8 ***, ## | 10.3 ± 0.4 *, # | 80.5 ± 0.4 ***, ### |
| C-ions 8 Gy | 37.8 ± 0.3 *** | 2.8 ± 0.8 *** | 59.4 ± 0.9 *** | 33.8 ± 1.7 *** | 5.4 ± 2.4 *** | 60.9 ± 3.8 *** |
| AZD 3 µM + C-ions 8 Gy | 15.1 ± 2.7 ***, ## | 0.5 ± 0.2 *** | 84.6 ± 2.3 ***, ### | 3.8 ± 1.8 ***, ### | 6.4 ± 1.6 *** | 90.1 ± 1.2 ***, ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohberger, B.; Barna, S.; Glänzer, D.; Eck, N.; Leithner, A.; Georg, D. DNA-PKcs Inhibition Sensitizes Human Chondrosarcoma Cells to Carbon Ion Irradiation via Cell Cycle Arrest and Telomere Capping Disruption. Int. J. Mol. Sci. 2024, 25, 6179. https://doi.org/10.3390/ijms25116179
Lohberger B, Barna S, Glänzer D, Eck N, Leithner A, Georg D. DNA-PKcs Inhibition Sensitizes Human Chondrosarcoma Cells to Carbon Ion Irradiation via Cell Cycle Arrest and Telomere Capping Disruption. International Journal of Molecular Sciences. 2024; 25(11):6179. https://doi.org/10.3390/ijms25116179
Chicago/Turabian StyleLohberger, Birgit, Sandra Barna, Dietmar Glänzer, Nicole Eck, Andreas Leithner, and Dietmar Georg. 2024. "DNA-PKcs Inhibition Sensitizes Human Chondrosarcoma Cells to Carbon Ion Irradiation via Cell Cycle Arrest and Telomere Capping Disruption" International Journal of Molecular Sciences 25, no. 11: 6179. https://doi.org/10.3390/ijms25116179






