Proton Radiation Therapy: A Systematic Review of Treatment-Related Side Effects and Toxicities
Abstract
1. Introduction
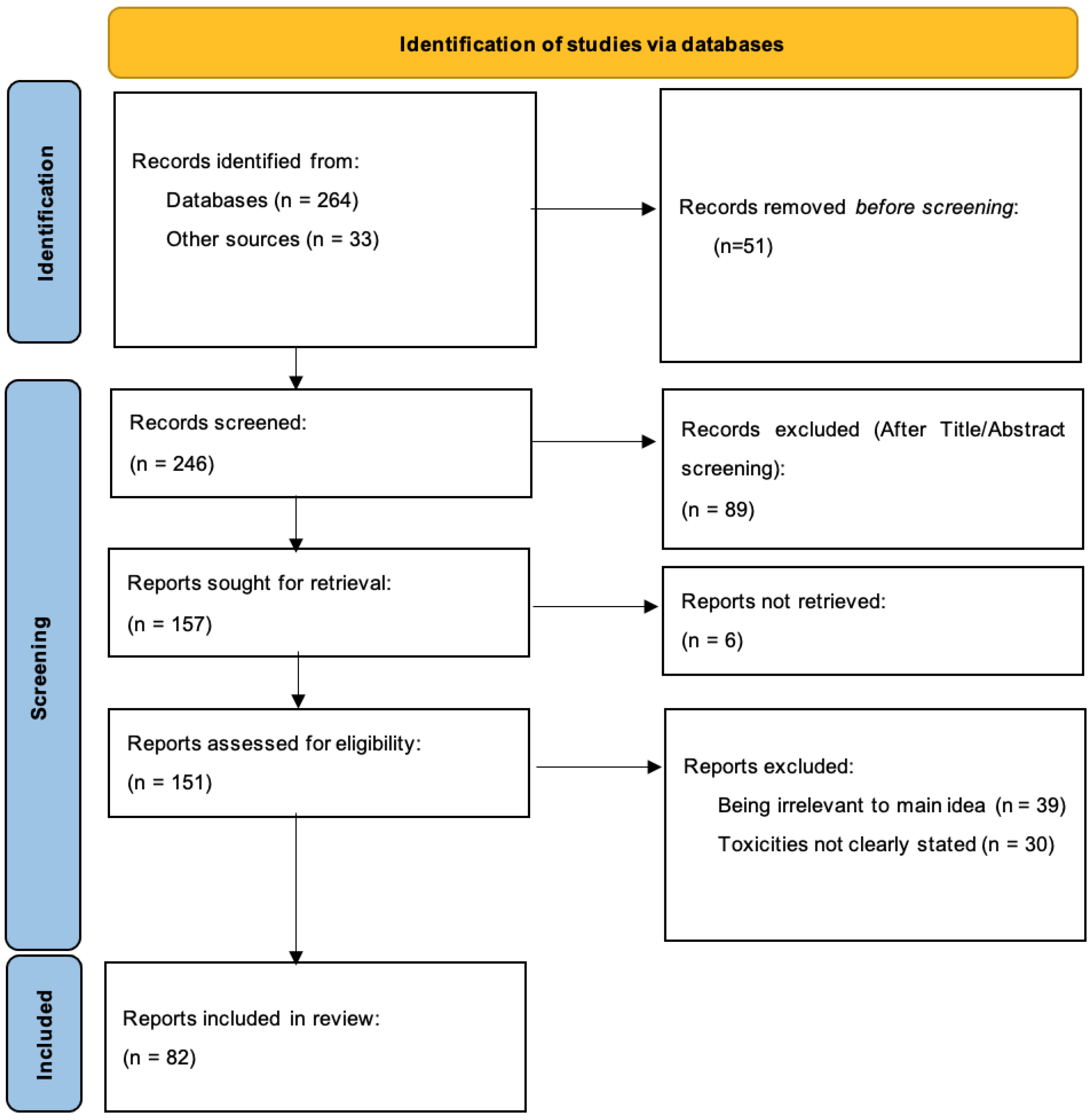
2. Materials and Methods
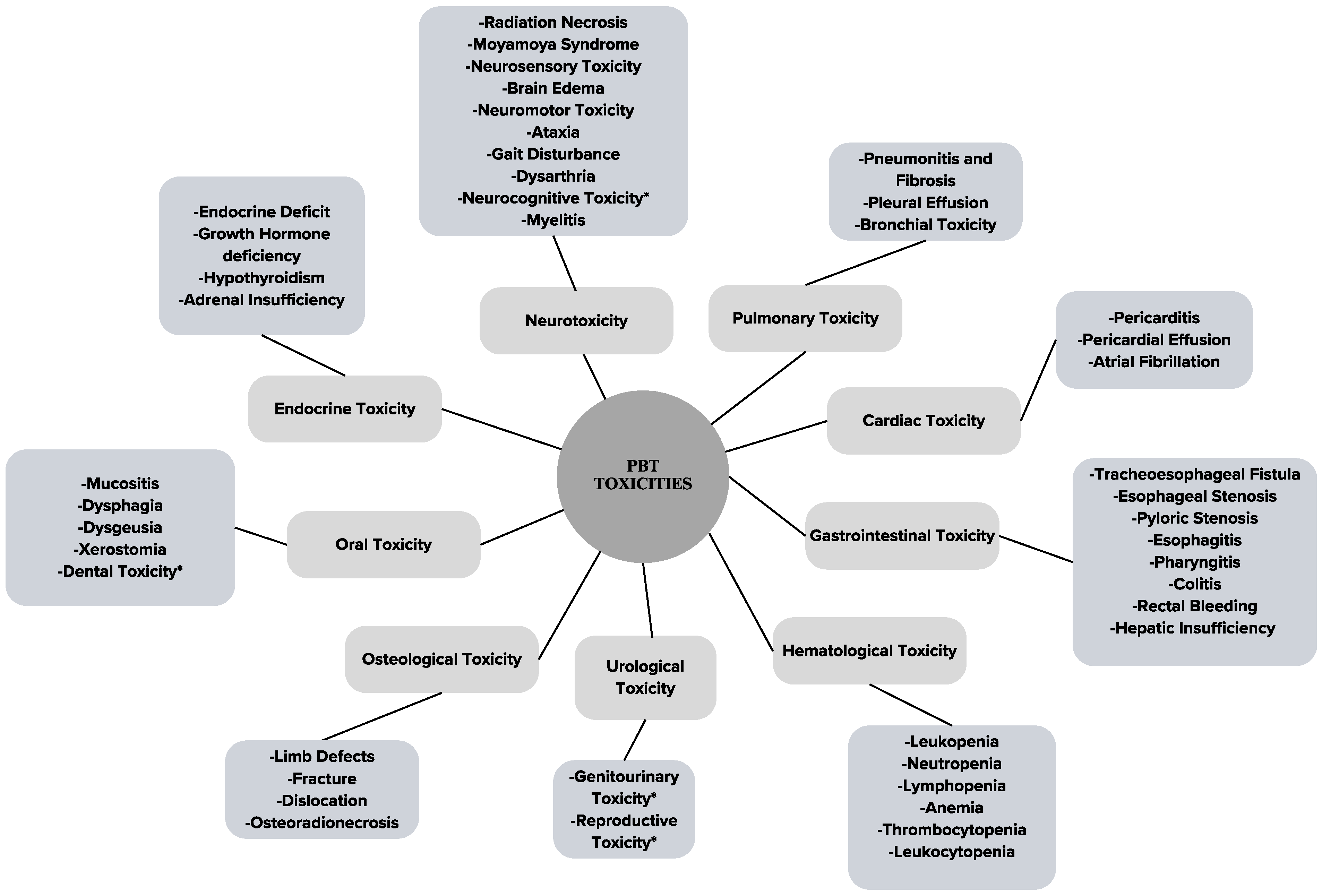
3. Results
3.1. Proton Radiation Therapy (PBT)
3.2. Neurotoxicities
3.2.1. Radiation Necrosis
3.2.2. Moyamoya Syndrome
3.2.3. Neurosensory Toxicities
3.2.4. Brain Edema
3.2.5. Neuromotor/Neuromuscular
3.2.6. Ataxia
3.2.7. Gait Disturbance
3.2.8. Dysarthria
3.2.9. Neurocognitive
Memory Impairment
Dysphasia
Somnolence Syndrome
3.2.10. Myelitis
3.3. Pulmonary Toxicities
3.3.1. Pneumonitis and Fibrosis
3.3.2. Pleural Effusion
3.3.3. Bronchial Toxicities
3.4. Cardiac Toxicities
3.4.1. Pericarditis
3.4.2. Pericardial Effusion
3.4.3. Atrial Fibrillation
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Fractionated Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Pericarditis | |||||
| Y. L. Lin [111] | PBS (single-field breast) † | Acute | 64.40 GyRBE (total) 56 GyRBE in 28 fr (upper mediastinal, retrosternal lymphatics and entire sternum) | Spinal cord: 0.06 Gy (mean) Right brachial plexus: 43.70 Gy (mean) Left lung: 7.38 Gy (mean) Right lung: 14.79 Gy (mean) Esophagus: 22.89 Gy (mean) Heart: 13.06 Gy (mean) Heart volume receiving ≥30 Gy: 20% | Spinal cord: 50 Gy (max) Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) |
| Laughlin et al. [124] | IMPT | Unspecified (chronic restrictive pericarditis) | 64.8 GyRBE (total) | NA | Spinal cord: 50 Gy (max) Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) |
| Pericardial effusion | |||||
| Lin et al. [100] | PSPT or PBS * | Asymptomatic | 50.4 Gy/28 fr | Lung: 4.8 Gy (mean) Heart: 11.3 Gy (mean) Liver: 2.4 Gy (mean) Spinal cord: 38.3 Gy (mean) | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy (mean) Spinal cord: 50 Gy (max) |
| Ning et al. [126] | PSPT or IMRT * | Grades 2, 3 | 74 Gy (median) in 2 Gy/fr | Heart: 12.2 Gy (median mean) | Heart: <26 Gy (mean) |
| McCusker MG et al. [127] | PBS | Unspecified | 54 Gy (total dose) | Pleura: 45.0 Gy in 1.8 Gy fractions Right hemithorax: 4.0 Gy sequential boost in 2.0 Gy fractions | Right lung V40 ≤ 67% Left lung mean ≤ 2 Gy Left lung V20 ≤ 5%, Total lungs minus GTV ≤ 20 Gy |
| Atrial fibrillation | |||||
| Lin et al. [100] | PSPT or PBS * | NA | 50.4 Gy/28 fr | Lung: 4.8 Gy (mean) Heart: 11.3 Gy (mean) Liver: 2.4 Gy (mean) Spinal cord: 38.3 Gy (mean) | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy (mean) Spinal cord: 50 Gy (max) |
| Chang et al. [94] | Dose-escalated hypofractionated PSPT | Grade 2: 2 patients | 87.5 GyRBE in 35 fr (2.5 GyRBE/fr) | Dose limit: Bronchial tree and large blood vessels: 87.5 GyRBE < 10 cm3 Heart: 70 Gy < 10% | Heart: <26 Gy (mean) |
3.5. Gastrointestinal Toxicities
3.6. Hematological Toxicities
3.7. Urological Toxicities
3.7.1. Genitourinary Toxicities
3.7.2. Reproductive-Related Toxicities
3.8. Osteological Toxicities
3.9. Oral-Health-Related Toxicities
3.10. Endocrine Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. Estimated Number of New Cases in 2020, World, Both Sexes, All Ages (excl. NMSC). Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 6 March 2023).
- World Cancer Research Fund International. Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 6 March 2023).
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Van Luijk, P. Biological considerations when comparing proton therapy with photon therapy. In Seminars in Radiation Oncology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 77–87. [Google Scholar]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chang, J.Y. Proton therapy in clinical practice. Chin. J. Cancer 2011, 30, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Eley, J.G.; Onyeuku, N.E.; Rice, S.R.; Wright, C.C.; McGovern, N.E.; Sank, M.; Zhu, M.; Vujaskovic, Z.; Simone, C.B., 2nd; et al. Proton Therapy Delivery and Its Clinical Application in Select Solid Tumor Malignancies. J. Vis. Exp. 2019, 144, e58372. [Google Scholar] [CrossRef]
- Mohan, R.; Das, I.J.; Ling, C.C. Empowering Intensity Modulated Proton Therapy through Physics and Technology: An Overview. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 304–316. [Google Scholar] [CrossRef]
- Brodin, N.P.; Kabarriti, R.; Schechter, C.B.; Pankuch, M.; Gondi, V.; Kalnicki, S.; Garg, M.K.; Tomé, W.A. Individualized quality of life benefit and cost-effectiveness estimates of proton therapy for patients with oropharyngeal cancer. Radiat. Oncol. 2021, 16, 19. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Fu, J. Treatment of Radiation-Induced Brain Necrosis. Oxid. Med. Cell. Longev. 2021, 2021, 4793517. [Google Scholar] [CrossRef]
- Miyatake, S.; Nonoguchi, N.; Furuse, M.; Yoritsune, E.; Miyata, T.; Kawabata, S.; Kuroiwa, T. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol. Med. Chir. 2015, 55, 50–59. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Zeng, Q.S.; Li, C.F.; Zhang, K.; Liu, H.; Kang, X.S.; Zhen, J.H. Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J. Neurooncol. 2007, 84, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ruben, J.D.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.A.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.O.; McDonald, M.W.; Simoneaux, V.; Buchsbaum, J.C. Reirradiation with Proton Therapy for Recurrent Gliomas. Int. J. Part. Ther. 2015, 2, 11–18. [Google Scholar] [CrossRef]
- Kalapurakal, J.A.; Goldman, S.; Stellpflug, W.; Curran, J.; Sathiaseelan, V.; Marymont, M.H.; Tomita, T. Phase I study of intraoperative radiotherapy with photon radiosurgery system in children with recurrent brain tumors: Preliminary report of first dose level (10 Gy). Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Gaito, S.; Hwang, E.J.; France, A.; Aznar, M.C.; Burnet, N.; Crellin, A.; Holtzman, A.L.; Indelicato, D.J.; Timmerman, B.; Whitfield, G.A.; et al. Outcomes of Patients Treated in the UK Proton Overseas Programme: Central Nervous System Group. Clin. Oncol. 2023, 35, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.; Gulidov, I.; Gogolin, D.; Lepilina, O.; Golovanova, O.; Semenov, A.; Dujenko, S.; Medvedeva, K.; Koryakin, S.; Ivanov, S.; et al. A Clinical Case of 5 Times Irradiated Recurrent Orbital Hemangiopericytoma. Case Rep. Oncol. 2021, 14, 78–84. [Google Scholar] [CrossRef]
- Bronk, J.K.; Amer, A.; Khose, S.; Flint, D.; Adair, A.; Yepes, P.; Grosshans, D.; Johnson, J.; Chung, C. Brain Radiation Necrosis Outside the Target Volume After Proton Radiation Therapy: Analyses of Multiparametric Imaging and Proton Biologic Effectiveness. Adv. Radiat. Oncol. 2022, 7, 101044. [Google Scholar] [CrossRef]
- Gunther, J.R.; Rahman, A.R.; Dong, W.; Yehia, Z.A.; Kebriaei, P.; Rondon, G.; Pinnix, C.C.; Milgrom, S.A.; Allen, P.K.; Dabaja, B.S.; et al. Craniospinal irradiation prior to stem cell transplant for hematologic malignancies with CNS involvement: Effectiveness and toxicity after photon or proton treatment. Pract. Radiat. Oncol. 2017, 7, e401–e408. [Google Scholar] [CrossRef]
- Merchant, T.E.; Chitti, R.M.; Li, C.; Xiong, X.; Sanford, R.A.; Khan, R.B. Factors associated with neurological recovery of brainstem function following postoperative conformal radiation therapy for infratentorial ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 496–503. [Google Scholar] [CrossRef]
- Gentile, M.S.; Yeap, B.Y.; Paganetti, H.; Goebel, C.P.; Gaudet, D.E.; Gallotto, S.L.; Weyman, E.A.; Morgan, M.L.; MacDonald, S.M.; Giantsoudi, D.; et al. Brainstem Injury in Pediatric Patients with Posterior Fossa Tumors Treated with Proton Beam Therapy and Associated Dosimetric Factors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 719–729. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Flampouri, S.; Rotondo, R.L.; Bradley, J.A.; Morris, C.G.; Aldana, P.R.; Sandler, E.; Mendenhall, N.P. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014, 53, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.L.; Okcu, M.F.; Munsell, M.F.; Kumbalasseriyil, N.; Grosshans, D.R.; McAleer, M.F.; Chintagumpala, M.; Khatua, S.; Mahajan, A. Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, A.L.; Rutenberg, M.S.; De Leo, A.N.; Rao, D.; Patel, J.; Morris, C.G.; Indelicato, D.J.; Mendenhall, W.M. The incidence of brainstem toxicity following high-dose conformal proton therapy for adult skull-base malignancies. Acta Oncol. 2022, 61, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Grewal, A.; O’Reilly, S.; Lustig, R.; Kurtz, G.; Minturn, J.E.; Shah, A.C.; Waanders, A.J.; Belasco, J.B.; Cole, K.A.; et al. Risk of brainstem necrosis in pediatric patients with central nervous system malignancies after pencil beam scanning proton therapy. Acta Oncol. 2019, 58, 1752–1756. [Google Scholar] [CrossRef]
- Gordon, K.; Gulidov, I.; Koryakin, S.; Smyk, D.; Makeenkova, T.; Gogolin, D.; Lepilina, O.; Golovanova, O.; Semenov, A.; Dujenko, S.; et al. Proton therapy with a fixed beamline for skull-base chordomas and chondrosarcomas: Outcomes and toxicity. Radiat. Oncol. 2021, 16, 238. [Google Scholar] [CrossRef]
- Chhabra, A.M.; Rice, S.R.; Holtzman, A.; Choi, J.I.; Hasan, S.; Press, R.H.; Chang, J.; Halasz, L.; Tsai, H.K.; Wang, C.J.; et al. Clinical outcomes and toxicities of 100 patients treated with proton therapy for chordoma on the proton collaborative group prospective registry. Radiother. Oncol. 2023, 183, 109551. [Google Scholar] [CrossRef]
- Desai, S.S.; Paulino, A.C.; Mai, W.Y.; Teh, B.S. Radiation-induced moyamoya syndrome. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1222–1227. [Google Scholar] [CrossRef]
- Suzuki, J.; Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef]
- Murphy, E.S.; Xie, H.; Merchant, T.E.; Yu, J.S.; Chao, S.T.; Suh, J.H. Review of cranial radiotherapy-induced vasculopathy. J. Neurooncol. 2015, 122, 421–429. [Google Scholar] [CrossRef]
- Greenfield, B.J.; Jaramillo, S.; Abboud, M.; Mahajan, A.; Paulino, A.C.; McGovern, S.; McAleer, M.F.; Chintagumpala, M.; Okcu, M.F.; Khatua, S.; et al. Outcomes for pediatric patients with central nervous system germ cell tumors treated with proton therapy. Clin. Transl. Radiat. Oncol. 2016, 1, 9–14. [Google Scholar] [CrossRef]
- Scala, M.; Vennarini, S.; Garrè, M.L.; Tortora, D.; Cianchetti, M.; Fellin, F.; Lorentini, S.; Pavanello, M. Radiation-Induced Moyamoya Syndrome After Proton Therapy in Child with Clival Chordoma: Natural History and Surgical Treatment. World Neurosurg. 2019, 123, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Zwagerman, N.T.; Foster, K.; Jakacki, R.; Khan, F.H.; Yock, T.I.; Greene, S. The development of Moyamoya syndrome after proton beam therapy. Pediatr. Blood Cancer 2014, 61, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Haydon, D.H.; Caird, J.; Leonard, J.R. Radiation-Induced Moyamoya Syndrome after Proton Beam Therapy in the Pediatric Patient: A Case Series. Pediatr. Neurosurg. 2016, 51, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.C.; Constine, L.S.; Rubin, P.; Williams, J.P. Normal tissue development, homeostasis, senescence, and the sensitivity to radiation injury across the age spectrum. Semin. Radiat. Oncol. 2010, 20, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bavle, A.; Srinivasan, A.; Choudhry, F.; Anderson, M.; Confer, M.; Simpson, H.; Gavula, T.; Thompson, J.S.; Clifton, S.; Gross, N.L. Systematic review of the incidence and risk factors for cerebral vasculopathy and stroke after cranial proton and photon radiation for childhood brain tumors. Neuro-Oncol. Pract. 2021, 8, 31–39. [Google Scholar] [CrossRef]
- Elkatatny, A.; Ismail, M.; Ibrahim, K.M.M.; Aly, M.H.; Fouda, M.A. The incidence of radiation-induced moyamoya among pediatric brain tumor patients who received photon radiation versus those who received proton beam therapy: A systematic review. Neurosurg. Rev. 2023, 46, 146. [Google Scholar] [CrossRef]
- Read, R. Nongranulomatous inflammation: Uveitis, endophthalmitis, panophthalmitis, and sequelae. In Duane’s Foundation of Clinical Ophthalmology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Nakagawa, N.; Morimoto, T.; Miyamura, T.; Suzuki, S.; Shimojo, H.; Nishida, K. A case of retinoblastoma resulting in phthisis bulbi after proton beam radiation therapy. Am. J. Ophthalmol. Case Rep. 2022, 28, 101715. [Google Scholar] [CrossRef]
- Zehetmayer, M.; Menapace, R. Choroidal melanomas near the optic disk or macula. Long-term results after proton beam irradiation: A report of 3 cases. Ophthalmologica 1993, 206, 18–23. [Google Scholar] [CrossRef]
- Foti, P.V.; Inì, C.; Broggi, G.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; et al. Histopathologic and MR Imaging Appearance of Spontaneous and Radiation-Induced Necrosis in Uveal Melanomas: Initial Results. Cancers 2022, 14, 215. [Google Scholar] [CrossRef]
- Foti, P.V.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Raffaele, L.; Salamone, V.; Caltabiano, R.; Broggi, G.; Puzzo, L.; et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part I: MR imaging with pathologic correlation and technical considerations. Insights Imaging 2021, 12, 66. [Google Scholar] [CrossRef]
- De Leo, A.N.; Holtzman, A.L.; Ho, M.W.; Morris, C.G.; Rutenberg, M.S.; Rotondo, R.L.; Bates, J.E.; Indelicato, D.J.; Rao, D.; Asa Hasan, M.; et al. Vision loss following high-dose proton-based radiotherapy for skull-base chordoma and chondrosarcoma. Radiother. Oncol. 2021, 158, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Riechardt, A.I.; Pilger, D.; Cordini, D.; Seibel, I.; Gundlach, E.; Hager, A.; Joussen, A.M. Neovascular glaucoma after proton beam therapy of choroidal melanoma: Incidence and risk factors. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Cordini, D.; Hager, A.; Riechardt, A.I.; Rehak, M.; Böker, A.; Böhmer, D.; Heufelder, J.; Joussen, A.M. Cataract development in patients treated with proton beam therapy for uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Seibel, I.; Cordini, D.; Hager, A.; Tillner, J.; Riechardt, A.I.; Heufelder, J.; Davids, A.M.; Rehak, M.; Joussen, A.M. Predictive risk factors for radiation retinopathy and optic neuropathy after proton beam therapy for uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Leopold, D. Distortion of olfactory perception: Diagnosis and treatment. Chem. Senses 2002, 27, 611–615. [Google Scholar] [CrossRef]
- Rosenzweig, S.J.; Lazarev, S.; Hasan, S.; Fox, J.; Choi, J.I.; Simone, C.B., 2nd; Wolden, S.L. Phantosmia Among Pediatric, Adolescent, and Young Adult Patients Receiving Proton Beam Therapy. Adv. Radiat. Oncol. 2022, 7, 100881. [Google Scholar] [CrossRef]
- Raghavan, K.C.; Camfield, A.S.; Lucas, J.; Ismael, Y.; Rossi, M.G.; Anghelescu, D.L. Propofol Total Intravenous Anesthesia as an Intervention for Severe Radiation-Induced Phantosmia in an Adolescent with Ependymoma. J. Adolesc. Young Adult Oncol. 2020, 9, 299–302. [Google Scholar] [CrossRef]
- Landier, W. Ototoxicity and cancer therapy. Cancer 2016, 122, 1647–1658. [Google Scholar] [CrossRef]
- Koetsier, K.S.; Hensen, E.F.; Niemierko, A.; Dewyer, N.A.; Chapman, P.H.; Lamba, N.; Bussière, M.R.; van Vulpen, M.; McKenna, M.J.; Loeffler, J.S.; et al. Outcome and Toxicity of Proton Therapy for Vestibular Schwannoma: A Cohort Study. Otol. Neurotol. 2021, 42, 1560–1571. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Bradley, J.A.; Rotondo, R.L.; Nanda, R.H.; Logie, N.; Sandler, E.S.; Aldana, P.R.; Ranalli, N.J.; Beier, A.D.; Morris, C.G.; et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018, 57, 644–648. [Google Scholar] [CrossRef]
- Fuji, H.; Nakasu, Y.; Ishida, Y.; Horiguchi, S.; Mitsuya, K.; Kashiwagi, H.; Murayama, S. Feasibility of proton beam therapy for chordoma and chondrosarcoma of the skull base. Skull Base 2011, 21, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Rotondo, R.L.; Mailhot Vega, R.B.; Holtzman, A.L.; Looi, W.S.; Morris, C.G.; Sandler, E.S.; Aldana, P.R.; Bradley, J.A. Local Control After Proton Therapy for Pediatric Chordoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.C.; Mahajan, A.; Ye, R.; Grosshans, D.R.; Fatih Okcu, M.; Su, J.; McAleer, M.F.; McGovern, S.; Mangona, V.A.; Chintagumpala, M. Ototoxicity and cochlear sparing in children with medulloblastoma: Proton vs. photon radiotherapy. Radiother. Oncol. 2018, 128, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 2007, 22, E1. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, V.; Vanninen, R.; Rauramaa, T. Raised intracranial pressure and brain edema. Handb. Clin. Neurol. 2017, 145, 25–37. [Google Scholar] [CrossRef]
- Valk, P.E.; Dillon, W.P. Radiation injury of the brain. AJNR Am. J. Neuroradiol. 1991, 12, 45–62. [Google Scholar]
- Sanford, N.N.; Yeap, B.Y.; Larvie, M.; Daartz, J.; Munzenrider, J.E.; Liebsch, N.J.; Fullerton, B.; Pan, E.; Loeffler, J.S.; Shih, H.A. Prospective, Randomized Study of Radiation Dose Escalation with Combined Proton-Photon Therapy for Benign Meningiomas. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 787–796. [Google Scholar] [CrossRef]
- Smart, D. Radiation Toxicity in the Central Nervous System: Mechanisms and Strategies for Injury Reduction. Semin. Radiat. Oncol. 2017, 27, 332–339. [Google Scholar] [CrossRef]
- Ashizawa, T.; Xia, G. Ataxia. Continuum 2016, 22, 1208–1226. [Google Scholar] [CrossRef]
- Upadhyay, R.; Liao, K.; Grosshans, D.R.; McGovern, S.L.; Frances McAleer, M.; Zaky, W.; Chintagumpala, M.M.; Mahajan, A.; Nana Yeboa, D.; Paulino, A.C. Quantifying the risk and dosimetric variables of symptomatic brainstem injury after proton beam radiation in pediatric brain tumors. Neuro Oncol. 2022, 24, 1571–1581. [Google Scholar] [CrossRef]
- Saeed, A.M.; Khairnar, R.; Sharma, A.M.; Larson, G.L.; Tsai, H.K.; Wang, C.J.; Halasz, L.M.; Chinnaiyan, P.; Vargas, C.E.; Mishra, M.V. Clinical Outcomes in Patients with Recurrent Glioblastoma Treated with Proton Beam Therapy Reirradiation: Analysis of the Multi-Institutional Proton Collaborative Group Registry. Adv. Radiat. Oncol. 2020, 5, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Chung, C.; Liu, D.D.; McAvoy, S.; Grosshans, D.; Al Feghali, K.; Mahajan, A.; Li, J.; McGovern, S.L.; McAleer, M.F.; et al. A prospective phase II randomized trial of proton radiotherapy vs intensity-modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro Oncol. 2021, 23, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Ataullah, A.H.M.; De Jesus, O. Gait Disturbances; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yang, T.J.; Wijetunga, N.A.; Yamada, J.; Wolden, S.; Mehallow, M.; Goldman, D.A.; Zhang, Z.; Young, R.J.; Kris, M.G.; Yu, H.A.; et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol. 2021, 23, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.; Parker, N.K.; Kelly, H.; Miller, N. Speech therapy for children with dysarthria acquired before three years of age. Cochrane Database Syst. Rev. 2016, 7, Cd006937. [Google Scholar] [CrossRef]
- Bower, A.S.; Fisayo, A.; Baehring, J.M.; Roy, B. Clinical Reasoning: A 73-Year-Old Woman with Episodic Dysarthria and Horizontal Binocular Diplopia. Neurology 2022, 98, 767–772. [Google Scholar] [CrossRef]
- Shih, H.A.; Sherman, J.C.; Nachtigall, L.B.; Colvin, M.K.; Fullerton, B.C.; Daartz, J.; Winrich, B.K.; Batchelor, T.T.; Thornton, L.T.; Mancuso, S.M.; et al. Proton therapy for low-grade gliomas: Results from a prospective trial. Cancer 2015, 121, 1712–1719. [Google Scholar] [CrossRef]
- Jairam, V.; Park, H.S.; Yu, J.B.; Bindra, R.S.; Contessa, J.N.; Jethwa, K.R. Practice Patterns Related to Mitigation of Neurocognitive Decline in Patients Receiving Whole Brain Radiation Therapy. Adv Radiat. Oncol. 2022, 7, 100949. [Google Scholar] [CrossRef]
- Hopper, A.; Salans, M.; Karunamuni, R.; Hattangadi-Gluth, J.A. Neurocognitive considerations in the treatment of meningioma with radiation therapy: Applications for quantitative neuroimaging and precision radiation medicine. J. Neurooncol. 2023, 161, 277–286. [Google Scholar] [CrossRef]
- Sherman, J.C.; Colvin, M.K.; Mancuso, S.M.; Batchelor, T.T.; Oh, K.S.; Loeffler, J.S.; Yeap, B.Y.; Shih, H.A. Neurocognitive effects of proton radiation therapy in adults with low-grade glioma. J. Neurooncol. 2016, 126, 157–164. [Google Scholar] [CrossRef]
- Ladra, M.M.; Szymonifka, J.D.; Mahajan, A.; Friedmann, A.M.; Yong Yeap, B.; Goebel, C.P.; MacDonald, S.M.; Grosshans, D.R.; Rodriguez-Galindo, C.; Marcus, K.J.; et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J. Clin. Oncol. 2014, 32, 3762–3770. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.R.; Ramaswamy, V.; Paulino, A.C.; Hodgson, D.; Mahajan, A.; et al. Superior Intellectual Outcomes After Proton Radiotherapy Compared with Photon Radiotherapy for Pediatric Medulloblastoma. J. Clin. Oncol. 2020, 38, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Monje, M. Cranial radiation therapy and damage to hippocampal neurogenesis. Dev. Disabil. Res. Rev. 2008, 14, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Alterio, D.; Gerardi, M.; Cella, L.; Spoto, R.; Zurlo, V.; Sabbatini, A.; Fodor, C.; D’avino, V.; Conson, M.; Valoriani, F. Radiation-induced acute dysphagia. Strahlenther. Onkol. 2017, 193, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C. Encyclopedia of Sleep; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Harjani, R.R.; Gururajachar, J.M.; Krishnaswamy, U. Comprehensive assessment of Somnolence Syndrome in patients undergoing radiation to the brain. Rep. Pract. Oncol. Radiother. 2016, 21, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.G.; Wong, C.S. Radiation-induced apoptosis of oligodendrocytes and its association with increased ceramide and down-regulated protein kinase B/Akt activity. Int. J. Radiat. Biol. 2004, 80, 39–51. [Google Scholar] [CrossRef]
- Khan, M.; Ambady, P.; Kimbrough, D.; Shoemaker, T.; Terezakis, S.; Blakeley, J.; Newsome, S.D.; Izbudak, I. Radiation-Induced Myelitis: Initial and Follow-Up MRI and Clinical Features in Patients at a Single Tertiary Care Institution during 20 Years. AJNR Am. J. Neuroradiol. 2018, 39, 1576–1581. [Google Scholar] [CrossRef]
- Arroyo-Hernández, M.; Maldonado, F.; Lozano-Ruiz, F.; Muñoz-Montaño, W.; Nuñez-Baez, M.; Arrieta, O. Radiation-induced lung injury: Current evidence. BMC Pulm. Med. 2021, 21, 9. [Google Scholar] [CrossRef]
- Verma, V.; Simone, C.B., 2nd; Werner-Wasik, M. Acute and Late Toxicities of Concurrent Chemoradiotherapy for Locally-Advanced Non-Small Cell Lung Cancer. Cancers 2017, 9, 120. [Google Scholar] [CrossRef]
- Xia, C.; Shi, W.; Zhang, Y.; Ding, L.; Gao, L.; Wang, Q.; Shao, L.; Dong, L.; Gao, Y. Prevention and treatment of radiation-induced lung injury. Future Med. Chem. 2020, 12, 2161–2173. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd. Thoracic Radiation Normal Tissue Injury. Semin. Radiat. Oncol. 2017, 27, 370–377. [Google Scholar] [CrossRef]
- Gomez, D.R.; Gillin, M.; Liao, Z.; Wei, C.; Lin, S.H.; Swanick, C.; Alvarado, T.; Komaki, R.; Cox, J.D.; Chang, J.Y. Phase 1 study of dose escalation in hypofractionated proton beam therapy for non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.S.; Nichols, R.C.; Flampouri, S.; Li, Z.; Morris, C.G.; Pham, D.C.; Mohindra, P.; Hartsell, W.; Mohammed, N.; Chon, B.H.; et al. Hypofractionated Proton Therapy with Concurrent Chemotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial from the University of Florida and Proton Collaborative Group. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Jeter, M.D.; Gomez, D.; Nguyen, Q.N.; Komaki, R.; Zhang, X.; Zhu, X.; O’Reilly, M.; Fossella, F.V.; Xu, T.; Wei, X.; et al. Simultaneous Integrated Boost for Radiation Dose Escalation to the Gross Tumor Volume with Intensity Modulated (Photon) Radiation Therapy or Intensity Modulated Proton Therapy and Concurrent Chemotherapy for Stage II to III Non-Small Cell Lung Cancer: A Phase 1 Study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Lee, J.J.; Komaki, R.; Gomez, D.R.; O’Reilly, M.S.; Fossella, F.V.; Blumenschein, G.R., Jr.; Heymach, J.V.; Vaporciyan, A.A.; Swisher, S.G.; et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Oguri, M.; Iwata, H.; Hattori, Y.; Hashimoto, S.; Nomura, K.; Hayashi, K.; Toshito, T.; Akita, K.; Baba, F.; et al. Long-term survival outcomes and quality of life of image-guided proton therapy for operable stage I non-small cell lung cancer: A phase 2 study. Radiother. Oncol. 2024, 196, 110276. [Google Scholar] [CrossRef]
- Yegya-Raman, N.; Berman, A.T.; Ciunci, C.A.; Friedes, C.; Berlin, E.; Iocolano, M.; Wang, X.; Lai, C.; Levin, W.P.; Cengel, K.A.; et al. Phase 2 Trial of Consolidation Pembrolizumab After Proton Reirradiation for Thoracic Recurrences of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 56–65. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Ly, N.B.; Komaki, R.; Levy, L.B.; Gomez, D.R.; Chang, J.Y.; Allen, P.K.; Mehran, R.J.; Lu, C.; Gillin, M.; et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother. Oncol. 2015, 115, 367–372. [Google Scholar] [CrossRef]
- Chang, J.Y.; Zhang, W.; Komaki, R.; Choi, N.C.; Chan, S.; Gomez, D.; O’Reilly, M.; Jeter, M.; Gillin, M.; Zhu, X.; et al. Long-term outcome of phase I/II prospective study of dose-escalated proton therapy for early-stage non-small cell lung cancer. Radiother. Oncol. 2017, 122, 274–280. [Google Scholar] [CrossRef]
- Chi, A.; Chen, H.; Wen, S.; Yan, H.; Liao, Z. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother. Oncol. 2017, 123, 346–354. [Google Scholar] [CrossRef]
- Chang, J.Y.; Verma, V.; Li, M.; Zhang, W.; Komaki, R.; Lu, C.; Allen, P.K.; Liao, Z.; Welsh, J.; Lin, S.H.; et al. Proton Beam Radiotherapy and Concurrent Chemotherapy for Unresectable Stage III Non-Small Cell Lung Cancer: Final Results of a Phase 2 Study. JAMA Oncol. 2017, 3, e172032. [Google Scholar] [CrossRef]
- Harada, H.; Fuji, H.; Ono, A.; Kenmotsu, H.; Naito, T.; Yamashita, H.; Asakura, H.; Nishimura, T.; Takahashi, T.; Murayama, S. Dose escalation study of proton beam therapy with concurrent chemotherapy for stage III non-small cell lung cancer. Cancer Sci. 2016, 107, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.S.; Nichols, R.C.; Flampouri, S.; Pankuch, M.; Morris, C.G.; Pham, D.C.; Mohindra, P.; Hartsell, W.F.; Mohammed, N.; Chon, B.H.; et al. Chemoradiation with Hypofractionated Proton Therapy in Stage II-III Non-Small Cell Lung Cancer: A Proton Collaborative Group Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.B.; Hickey, S.; DePauw, N.; Yeap, B.Y.; Batin, E.; Gadd, M.A.; Specht, M.; Isakoff, S.J.; Smith, B.L.; Liao, E.C.; et al. Phase II Study of Proton Beam Radiation Therapy for Patients with Breast Cancer Requiring Regional Nodal Irradiation. J. Clin. Oncol. 2019, 37, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Hobbs, B.P.; Verma, V.; Tidwell, R.S.; Smith, G.L.; Lei, X.; Corsini, E.M.; Mok, I.; Wei, X.; Yao, L.; et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 1569–1579. [Google Scholar] [CrossRef]
- Nakajima, K.; Iwata, H.; Ogino, H.; Hattori, Y.; Hashimoto, S.; Toshito, T.; Hayashi, K.; Akita, K.; Baba, F.; Nakamae, K.; et al. Clinical outcomes of image-guided proton therapy for histologically confirmed stage I non-small cell lung cancer. Radiat. Oncol. 2018, 13, 199. [Google Scholar] [CrossRef]
- Oshiro, Y.; Okumura, T.; Kurishima, K.; Homma, S.; Mizumoto, M.; Ishikawa, H.; Onizuka, M.; Sakai, M.; Goto, Y.; Hizawa, N.; et al. High-dose concurrent chemo-proton therapy for Stage III NSCLC: Preliminary results of a Phase II study. J. Radiat. Res. 2014, 55, 959–965. [Google Scholar] [CrossRef]
- Tseng, Y.D.; Hoppe, B.S.; Dedeckova, K.; Patel, C.G.; Hill-Kayser, C.E.; Miller, D.M.; Maity, A.; Mendenhall, N.P.; Mailhot Vega, R.B.; Yock, T.I.; et al. Risk of Pneumonitis and Outcomes After Mediastinal Proton Therapy for Relapsed/Refractory Lymphoma: A PTCOG and PCG Collaboration. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 220–230. [Google Scholar] [CrossRef]
- Nagano, T.; Kotani, Y.; Fujii, O.; Demizu, Y.; Niwa, Y.; Ohno, Y.; Nishio, W.; Itoh, T.; Murakami, M.; Nishimura, Y. A case of acute exacerbation of idiopathic pulmonary fibrosis after proton beam therapy for non-small cell lung cancer. Jpn. J. Clin. Oncol. 2012, 42, 965–969. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, J.; Xiong, Y.; Tan, K.; Zhang, X.; Yu, Y.; Dong, H.; Lu, X.; Zhu, G.; Cui, H. Radiation induced lung injury (RILI) after postoperative intensity modulated proton therapy (IMPT) in a patient with stage III locally advanced lung adenocarcinoma: A case report. Transl. Cancer Res. 2022, 11, 3400–3408. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, 877. [Google Scholar] [CrossRef]
- Saguil, A.; Wyrick, K.; Hallgren, J. Diagnostic approach to pleural effusion. Am. Fam. Physician 2014, 90, 99–104. [Google Scholar] [PubMed]
- Wojno, K.J.; Olson, J.L.; Sherman, M.E. Cytopathology of pleural effusions after radiotherapy. Acta Cytol. 1994, 38, 1–8. [Google Scholar] [PubMed]
- Siva, S.; MacManus, M.; Kron, T.; Best, N.; Smith, J.; Lobachevsky, P.; Ball, D.; Martin, O. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS ONE 2014, 9, e109560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Day, R.M.; Jin, J.Y.; Quint, L.; Williams, H.; Ferguson, C.; Yan, L.; King, M.; Albsheer, A.; Matuszak, M.; et al. Thoracic radiation-induced pleural effusion and risk factors in patients with lung cancer. Oncotarget 2017, 8, 97623–97632. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L. Reirradiation of recurrent breast cancer with proton beam therapy: A case report and literature review. World J. Clin. Oncol. 2019, 10, 256–268. [Google Scholar] [CrossRef]
- Lindberg, K.; Onjukka, E. Medical consequences of radiation exposure of the bronchi-what can we learn from high-dose precision radiation therapy? J. Radiol. Prot. 2021, 41, S355. [Google Scholar] [CrossRef]
- Cho, Y.C.; Kim, J.H.; Park, J.H.; Shin, J.H.; Ko, H.K.; Song, H.Y. Fluoroscopically guided balloon dilation for benign bronchial stricture occurring after radiotherapy in patients with lung cancer. Cardiovasc. Intervent. Radiol. 2014, 37, 750–755. [Google Scholar] [CrossRef]
- Salik, I.; Vashisht, R.; Sharma, S.; Abramowicz, A. Bronchopleural Fistula; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef]
- Quintero-Martinez, J.A.; Cordova-Madera, S.N.; Villarraga, H.R. Radiation-Induced Heart Disease. J. Clin. Med. 2021, 11, 146. [Google Scholar] [CrossRef]
- Gjyshi, O.; Xu, T.; Elhammali, A.; Boyce-Fappiano, D.; Chun, S.G.; Gandhi, S.; Lee, P.; Chen, A.B.; Lin, S.H.; Chang, J.Y.; et al. Toxicity and Survival After Intensity-Modulated Proton Therapy versus Passive Scattering Proton Therapy for NSCLC. J. Thorac. Oncol. 2021, 16, 269–277. [Google Scholar] [CrossRef]
- Janopaul-Naylor, J.; Kanter, K.R.; Flampouri, S.; Nguyen, V.; Olson, T.A.; Eaton, B.R. Adjuvant chemoradiation for high-grade cardiac leiomyosarcoma in a child: Case report and review of literature. Pediatr. Blood Cancer 2021, 68, e29241. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Madera, S.; Garcia-Bello, L.; Bruno, G.; Di Stefano, C.; Quintero-Martinez, J.; Laack, N.; Corbin, K.; Whitaker, T.; Pellikka, P.; Herrmann, J. Comprehensive strain analysis in oncological patients undergoing thoracic radiotherapy: 1-year follow-up of a prospective study comparing proton vs. photon beam therapy. Eur. Heart J. 2021, 42, ehab724.024. [Google Scholar] [CrossRef]
- Frankart, A.J.; Nagarajan, R.; Pater, L. The impact of proton therapy on cardiotoxicity following radiation treatment. J. Thromb. Thrombolysis 2021, 51, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, E.; Le Guevelou, J.; Chaikh, A.; Danhier, S.; Geffrelot, J.; Levy, C.; Saloux, E.; Habrand, J.-L.; Thariat, J. Proton therapy for locally advanced breast cancer: A systematic review of the literature. Cancer Treat. Rev. 2018, 63, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.F.; Viscusi, M.M.; Van Tassell, B.W.; Abbate, A. Interleukin-1 blockade for the treatment of pericarditis. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 46–53. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef]
- Laughlin, B.S.; Stoker, J.; Vern-Gross, T. Proton Beam Therapy for Unresectable Mediastinal and Pericardial Spindle Cell Sarcoma: A Case Report. Int. J. Part. Ther. 2023, 10, 43–50. [Google Scholar] [CrossRef]
- Vakamudi, S.; Ho, N.; Cremer, P.C. Pericardial Effusions: Causes, Diagnosis, and Management. Prog. Cardiovasc. Dis. 2017, 59, 380–388. [Google Scholar] [CrossRef]
- Ning, M.S.; Tang, L.; Gomez, D.R.; Xu, T.; Luo, Y.; Huo, J.; Mouhayar, E.; Liao, Z. Incidence and predictors of pericardial effusion after chemoradiation therapy for locally advanced non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 70–79. [Google Scholar] [CrossRef]
- McCusker, M.G.; Scilla, K.A.; Simone, C.B., 2nd; Sachdeva, A.; Miller, K.D.; Burke, A.P.; Rolfo, C. Proton Beam Therapy and Immune Checkpoint Inhibitors in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2019, 14, e185–e187. [Google Scholar] [CrossRef]
- Bradley, D.J.; Shen, W.K. Overview of management of atrial fibrillation in symptomatic elderly patients: Pharmacologic therapy versus AV node ablation. Clin. Pharmacol. Ther. 2007, 81, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef] [PubMed]
- Abugroun, A.; Ahmed, F.; Singh, N.; Nadiri, M. Late Onset Chemo/Radiation Induced Tracheoesophageal Fistula in Squamous Cell Cancer of the Lung. World J. Oncol. 2017, 8, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Rutenberg, M.S.; Hoppe, B.S.; Starr, J.S.; Awad, Z.; Thomas, M.; Morris, C.G.; Johnson, P.; Henderson, R.H.; Jones, J.C.; Gharia, B.; et al. Proton Therapy with Concurrent Chemotherapy for Thoracic Esophageal Cancer: Toxicity, Disease Control, and Survival Outcomes. Int. J. Part. Ther. 2023, 9, 18–29. [Google Scholar] [CrossRef]
- Fernandes, A.; Berman, A.T.; Mick, R.; Both, S.; Lelionis, K.; Lukens, J.N.; Ben-Josef, E.; Metz, J.M.; Plastaras, J.P. A Prospective Study of Proton Beam Reirradiation for Esophageal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 483–487. [Google Scholar] [CrossRef]
- Takada, A.; Nakamura, T.; Takayama, K.; Makita, C.; Suzuki, M.; Azami, Y.; Kato, T.; Tsukiyama, I.; Hareyama, M.; Kikuchi, Y.; et al. Preliminary treatment results of proton beam therapy with chemoradiotherapy for stage I-III esophageal cancer. Cancer Med. 2016, 5, 506–515. [Google Scholar] [CrossRef]
- DeCesaris, C.M.; McCarroll, R.; Mishra, M.V.; Glass, E.; Greenwald, B.D.; Carr, S.; Burrows, W.; Mehra, R.; Regine, W.F.; Simone, C.B., 2nd; et al. Assessing Outcomes of Patients Treated with Re-Irradiation Utilizing Proton Pencil-Beam Scanning for Primary or Recurrent Malignancies of the Esophagus and Gastroesophageal Junction. J. Thorac. Oncol. 2020, 15, 1054–1064. [Google Scholar] [CrossRef]
- Antunes, C.; Sharma, A. Esophagitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stewart, W.D. Pharyngitis. Ind. Med. Gaz. 1889, 24, 356–359. [Google Scholar]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Sawada, T.; Mizumoto, M.; Oshiro, Y.; Numajiri, H.; Shimizu, S.; Hiroshima, Y.; Nakamura, M.; Iizumi, T.; Okumura, T.; Sakurai, H. Long-term follow up of a patient with a recurrent desmoid tumor that was successfully treated with proton beam therapy: A case report and literature review. Clin. Transl. Radiat. Oncol. 2021, 27, 32–35. [Google Scholar] [CrossRef]
- Hong, T.S.; Ryan, D.P.; Borger, D.R.; Blaszkowsky, L.S.; Yeap, B.Y.; Ancukiewicz, M.; Deshpande, V.; Shinagare, S.; Wo, J.Y.; Boucher, Y.; et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Hoehn, M.E.; Khan, R.B.; Sabin, N.D.; Klimo, P.; Boop, F.A.; Wu, S.; Li, Y.; Burghen, E.A.; Jurbergs, N.; et al. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): A single-arm, phase 2 study. Lancet Oncol. 2023, 24, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Colaco, R.J.; Hoppe, B.S.; Flampouri, S.; McKibben, B.T.; Henderson, R.H.; Bryant, C.; Nichols, R.C.; Mendenhall, W.M.; Li, Z.; Su, Z.; et al. Rectal toxicity after proton therapy for prostate cancer: An analysis of outcomes of prospective studies conducted at the university of Florida Proton Therapy Institute. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Smith, T.L.; Henderson, R.H.; Hoppe, B.S.; Mendenhall, W.M.; Nichols, R.C.; Morris, C.G.; Williams, C.R.; Su, Z.; Li, Z.; et al. Five-Year Biochemical Results, Toxicity, and Patient-Reported Quality of Life After Delivery of Dose-Escalated Image Guided Proton Therapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.J.; Choi, S.; Nogueras-Gonzalaez, G.M.; Nguyen, Q.N.; Mahmood, U.; Frank, S.J.; Mathai, B.; Zhu, X.R.; Sahoo, N.; Gillin, M.; et al. Proton Beam Therapy for Localized Prostate Cancer: Results from a Prospective Quality-of-Life Trial. Int. J. Part. Ther. 2016, 3, 27–36. [Google Scholar] [CrossRef]
- Chilukuri, S.; Sundar, S.; Patro, K.; Sawant, M.; Sivaraman, R.; Arjunan, M.; Panda, P.K.; Sharma, D.; Jalali, R. Comparison of Estimated Late Toxicities between IMPT and IMRT Based on Multivariable NTCP Models for High-Risk Prostate Cancers Treated with Pelvic Nodal Radiation. Int. J. Part. Ther. 2022, 9, 42–53. [Google Scholar] [CrossRef]
- Sumiya, T.; Mizumoto, M.; Oshiro, Y.; Baba, K.; Murakami, M.; Shimizu, S.; Nakamura, M.; Hiroshima, Y.; Ishida, T.; Iizumi, T.; et al. Transitions of Liver and Biliary Enzymes during Proton Beam Therapy for Hepatocellular Carcinoma. Cancers 2020, 12, 1840. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Liu, C.M.; Wang, Y.M.; Hsu, H.C.; Huang, E.Y.; Huang, T.T.; Lee, C.H.; Hung, S.P.; Huang, B.S. Proton versus photon radiotherapy for primary hepatocellular carcinoma: A propensity-matched analysis. Radiat. Oncol. 2020, 15, 159. [Google Scholar] [CrossRef]
- Kawashima, M.; Furuse, J.; Nishio, T.; Konishi, M.; Ishii, H.; Kinoshita, T.; Nagase, M.; Nihei, K.; Ogino, T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J. Clin. Oncol. 2005, 23, 1839–1846. [Google Scholar] [CrossRef]
- Bush, D.A.; Volk, M.; Smith, J.C.; Reeves, M.E.; Sanghvi, S.; Slater, J.D.; deVera, M. Proton beam radiotherapy versus transarterial chemoembolization for hepatocellular carcinoma: Results of a randomized clinical trial. Cancer 2023, 129, 3554–3563. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tokuuye, K.; Fukumitsu, N.; Igaki, H.; Hata, M.; Kagei, K.; Sugahara, S.; Ohara, K.; Matsuzaki, Y.; Akine, Y. Repeated proton beam therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.B.; DeStephano, D.M.; Jeffers, B.; Horowitz, D.P.; Soulos, P.R.; Gross, C.P.; Cheng, S.K. Updated Analysis of Comparative Toxicity of Proton and Photon Radiation for Prostate Cancer. J. Clin. Oncol. 2024, 42, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Vennarini, S.; Del Baldo, G.; Lorentini, S.; Pertile, R.; Fabozzi, F.; Merli, P.; Megaro, G.; Scartoni, D.; Carai, A.; Tornesello, A.; et al. Acute Hematological Toxicity during Cranio-Spinal Proton Therapy in Pediatric Brain Embryonal Tumors. Cancers 2022, 14, 1653. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, B.A.; Wang, H.; Noel, M.S.; He, A.R.; Marshall, J.L.; Weiner, L.M.; Fishbein, T.M.; Hodgins, N.E.; Winslow, E.R.; Jackson, P.G.; et al. Phase 1 Study of Hypofractionated Proton Beam Radiation Therapy in Adjuvant Pancreatic Cancer (PROTON-PANC). Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Routman, D.M.; Garant, A.; Lester, S.C.; Day, C.N.; Harmsen, W.S.; Sanheuza, C.T.; Yoon, H.H.; Neben-Wittich, M.A.; Martenson, J.A.; Haddock, M.G.; et al. A Comparison of Grade 4 Lymphopenia with Proton versus Photon Radiation Therapy for Esophageal Cancer. Adv. Radiat. Oncol. 2019, 4, 63–69. [Google Scholar] [CrossRef]
- Barney, C.L.; Brown, A.P.; Grosshans, D.R.; McAleer, M.F.; de Groot, J.F.; Puduvalli, V.; Tucker, S.L.; Crawford, C.N.; Gilbert, M.R.; Brown, P.D.; et al. Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro Oncol. 2014, 16, 303–309. [Google Scholar] [CrossRef]
- Liu, K.X.; Ioakeim-Ioannidou, M.; Susko, M.S.; Rao, A.D.; Yeap, B.Y.; Snijders, A.M.; Ladra, M.M.; Vogel, J.; Zaslowe-Dude, C.; Marcus, K.J.; et al. A Multi-institutional Comparative Analysis of Proton and Photon Therapy-Induced Hematologic Toxicity in Patients with Medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 726–735. [Google Scholar] [CrossRef]
- Takaoka, T.; Yanagi, T.; Tanaka, A.; Kiriyama, Y.; Tanaka, Y.; Kondo, T.; Takano, S.; Takahashi, S.; Shibamoto, Y.; Tomita, N.; et al. Acute genitourinary toxicity of pencil beam scanning proton therapy for localized prostate cancer: Utility of the transition zone index and average urinary flow rate in predicting acute urinary retention. Jpn. J. Clin. Oncol. 2023, 53, 419–428. [Google Scholar] [CrossRef]
- Makishima, H.; Ishikawa, H.; Tanaka, K.; Mori, Y.; Mizumoto, M.; Ohnishi, K.; Aihara, T.; Fukumitsu, N.; Okumura, T.; Sakurai, H. A retrospective study of late adverse events in proton beam therapy for prostate cancer. Mol. Clin. Oncol. 2017, 7, 547–552. [Google Scholar] [CrossRef]
- Uezono, H.; Indelicato, D.J.; Rotondo, R.L.; Mailhot Vega, R.B.; Bradfield, S.M.; Morris, C.G.; Bradley, J.A. Treatment Outcomes After Proton Therapy for Ewing Sarcoma of the Pelvis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 974–981. [Google Scholar] [CrossRef]
- Chiang, J.S.; Yu, N.Y.; Sheedy, J.T.; Hayden, R.E.; Lemish, P.R.; Karlin, N.J.; Mishra, N.; Sio, T.T. Radiotherapeutic Management of Synchronous Prostate and Rectal Cancers Using Proton Beam Therapy. Int. J. Part. Ther. 2021, 8, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.S.; Nichols, R.C.; Henderson, R.H.; Morris, C.G.; Williams, C.R.; Costa, J.; Marcus, R.B., Jr.; Mendenhall, W.M.; Li, Z.; Mendenhall, N.P. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer 2012, 118, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Meixner, E.; Wark, A.; Forster, T.; Weykamp, F.; Lang, K.; König, L.; Lindel, K.; Oelmann-Avendano, J.T.; Krisam, J.; Schneeweiss, A.; et al. Health-related quality of life and patient-reported symptoms after postoperative proton beam radiotherapy of cervical and endometrial cancer: 2-year results of the prospective phase II APROVE-trial. Radiat. Oncol. 2023, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Kirk, M.; Lin, L. Proton Therapy for Vaginal Reirradiation. Int. J. Part. Ther. 2016, 3, 320–326. [Google Scholar] [CrossRef]
- Barcellini, A.; Ditto, A.; Mirandola, A.; Roccio, M.; Imparato, S.; Raspagliesi, F.; Orlandi, E. Is a tailored strategy using proton beam radiotherapy for reirradiation advantageous for elderly women? A case report. Tumori 2021, 107, Np67–Np72. [Google Scholar] [CrossRef]
- Raggio, B.S.; Winters, R. Modern management of osteoradionecrosis. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 254–259. [Google Scholar] [CrossRef]
- Singh, A.; Kitpanit, S.; Neal, B.; Yorke, E.; White, C.; Yom, S.K.; Randazzo, J.D.; Wong, R.J.; Huryn, J.M.; Tsai, C.J.; et al. Osteoradionecrosis of the Jaw Following Proton Radiation Therapy for Patients with Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 151–159. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Yang, P.; Blanchard, P.; Garden, A.S.; Gunn, B.; Fuller, C.D.; Chambers, M.; Hutcheson, K.A.; Ye, R.; et al. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother. Oncol. 2017, 123, 401–405. [Google Scholar] [CrossRef]
- Bush, D.A.; Cheek, G.; Zaheer, S.; Wallen, J.; Mirshahidi, H.; Katerelos, A.; Grove, R.; Slater, J.D. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: Results of a 12-year experience at Loma Linda University Medical Center. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 964–968. [Google Scholar] [CrossRef]
- Foster-Thomas, E.; Aznar, M.; Indelicato, D.; Pan, S.; Hwang, E.; Sitch, P.; Horner, K.; Smith, E.; Gaito, S. Late Dental Toxicities After Proton Chemoradiation for Rhabdomyosarcoma: A Pediatric Case Report. Int. J. Part. Ther. 2023, 9, 50–57. [Google Scholar] [CrossRef]
- Hopcraft, M.S.; Tan, C. Xerostomia: An update for clinicians. Aust. Dent. J. 2010, 55, 238–244; quiz 353. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Ye, R.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Phan, J.; Sturgis, E.M.; Ferrarotto, R.; et al. Xerostomia-related quality of life for patients with oropharyngeal carcinoma treated with proton therapy. Radiother. Oncol. 2020, 142, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, X.; Jiang, B.; Chen, J.; Wang, X.; Wang, L.; Sahoo, N.; Zhu, X.R.; Ye, R.; Blanchard, P.; et al. Intensity-modulated proton therapy for oropharyngeal cancer reduces rates of late xerostomia. Radiother. Oncol. 2021, 160, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.; Bryant, J.; Hartsell, W.; Larson, G.; Badiyan, S.; Laramore, G.E.; Katz, S.; Tsai, H.; Vargas, C. Minimal acute toxicity from proton beam therapy for major salivary gland cancer. Acta Oncol. 2020, 59, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.E. Endocrine late effects of cancer treatment. Endocrinol. Metab. Clin. N. Am. 2005, 34, 769–789, xi. [Google Scholar] [CrossRef]
- Fangusaro, J.R.; Littlejohn, E.E. Endocrine late effects: Manifestations and treatments. Cancer Treat Res. 2009, 150, 155–182. [Google Scholar] [CrossRef]
- Bishop, A.J.; Greenfield, B.; Mahajan, A.; Paulino, A.C.; Okcu, M.F.; Allen, P.K.; Chintagumpala, M.; Kahalley, L.S.; McAleer, M.F.; McGovern, S.L. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: Multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 354–361. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Patterson, B.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.J.; Ebb, D.; MacDonald, S.M.; et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016, 18, 881–887. [Google Scholar] [CrossRef]
- Littley, M.; Shalet, S.; Beardwell, C.; Ahmed, S.; Applegate, G.; Sutton, M. Hypopituitarism following external radiotherapy for pituitary tumours in adults. QJM Int. J. Med. 1989, 70, 145–160. [Google Scholar]
- Aldrich, K.D.; Horne, V.E.; Bielamowicz, K.; Sonabend, R.Y.; Scheurer, M.E.; Paulino, A.C.; Mahajan, A.; Chintagumpala, M.; Okcu, M.F.; Brown, A.L. Comparison of hypothyroidism, growth hormone deficiency, and adrenal insufficiency following proton and photon radiotherapy in children with medulloblastoma. J. Neurooncol. 2021, 155, 93–100. [Google Scholar] [CrossRef]
- Bielamowicz, K.; Okcu, M.F.; Sonabend, R.; Paulino, A.C.; Hilsenbeck, S.G.; Dreyer, Z.; Suzawa, H.; Bryant, R.; Adesina, A.; Dauser, R. Hypothyroidism after craniospinal irradiation with proton or photon therapy in patients with medulloblastoma. Pediatr. Hematol. Oncol. 2018, 35, 257–267. [Google Scholar] [CrossRef]
- Uemura, S.; Demizu, Y.; Hasegawa, D.; Fujikawa, T.; Inoue, S.; Nishimura, A.; Tojyo, R.; Nakamura, S.; Kozaki, A.; Saito, A.; et al. The comparison of acute toxicities associated with craniospinal irradiation between photon beam therapy and proton beam therapy in children with brain tumors. Cancer Med. 2022, 11, 1502–1510. [Google Scholar] [CrossRef]
- Patients Treated with Protons and C-Ions Worldwide 2007–2012. Available online: https://www.ptcog.site/index.php/patient-statistics-2 (accessed on 2 May 2023).
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Fractionated Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Radiation necrosis | |||||
| Galle et al. [16] | PSPT † | Grade 1: 1 patient Unspecified: 1 patient (required surgical intervention) | 89.4 Gy (cumulative dose) 118 Gy (cumulative dose) | NA | |
| Gaito et al. [18] | USPT or PSPT | Grade 5: 2 patients | 54 GyRBE (median) | NA | |
| Gordon et al. [19] | PBS † | Unspecified | 60 Gy with 2.0 Gy/fr | D95 = 56.3 GyRBE Left temporal lobe: 56.5 GyRBE (max dose) Brainstem: 16.1 GyRBE (max dose) Chiasma: 29.8 GyRBE (max dose) Right optical nerve: 7.8 GyRBE (max dose) Hypophysis: 3.0 GyRBE (mean) Midbrain: 1.0 GyRBE (mean) | Brain: <60 Gy (max) Brainstem: <54 Gy (max) Optic chiasm: <55 Gy |
| Bronk et al. [20] | PSPT | NA | 60 GyRBE/30 fr | D95: <60 Gy RBE (in 6 of 10 lesions) | |
| Gunther et al. [21] | PSPT (CSI) | Grade 4: 1 patient | 30 Gy (patient treatment) 21.8 Gy (median) | NA | |
| Brainstem toxicity | |||||
| Indelicato et al. [24] | PSPT | Grade 2: 7 patients Grade 3: 1 patient Grade 4: 2 patients Grade 5: 1 patient | 54 CGE (median) | Brainstem: >50.4 CGE Brainstem V54 CGE: 26% | Brainstem: <54 Gy (max) |
| Holtzman et al. [26] | PSPT or PBS | Grade 2: 1 patient Grade 3: 1 patient | 73.8 GyRBE (median) | Brainstem: 59 GyRBE (Dmax) | Brainstem: <54 Gy (max) |
| Vogel et al. [27] | PBS *,† | NA | 54 GyRBE (median) | CSI: 36 GyRBE (median) Brainstem: 55.4 GyRBE (median max dose) Brainstem: 98 GyRBE (cumulative median max dose) | Brain: <60 Gy (max) Brainstem: <54 Gy (max) |
| Gordon et al. [28] | PBS | Grade 5 | 70.0 GyRBE (median) in 2 GyRBE/fr | Brainstem: 64.0 GyRBE (Dmax) | Brainstem: <54 Gy (max) |
| Moyamoya syndrome | |||||
| Greenfield et al. [33] | PSPT or PBS * | NA | 50.4 GyRBE, Initial: 36.0 GyRBE Boost: 14.4 GyRBE | NA | |
| Scala et al. [34] | PBS | NA | 72.0 GyRBE in 40 fr of 1.8 GyRBE | Tumor bed: 50.4 GyRBE, boost to tumor nodules: 21.6 GyRBE | |
| Zwagerman et al. [35] | PSPT * | NA | Tumor bed: 52.2 CGE | Hypothalamus: 49.8 CGE (mean), pituitary: 47.8 CGE (mean), Circle of Willis estimated dose: 37–45 CGE | |
| Reynolds et al. [36] | PSPT *, PSPT (2 patients) | NA | 50.4 CGE/28 fr of 1.8 CGE 59.6 CGE/30 fr of 1.9 CGE | NA | |
| Phthisis bulbi | |||||
| Nakagawa et al. [41] | PBS * | NA | 45 Gy/25 fr | NA | |
| Zehetmayer et al. [42] | PSPT | NA | 60 CGE | NA | |
| Phantosomia | |||||
| Rosenzweig et al. [50] | PBS-IMPT | Mild to moderate (12 patients) | Median dose: 23.4 Gy/1.8 Gy per fr | NA | |
| Raghavan et al. [51] | PBS | Severe | NA | Craniospinal axis: 36 CGE Boost thecal sac: 54.0 CGE Boost focal thoracic spine areas: 50.4 CGE | Brain: <60 Gy (max) Spinal cord: 50 Gy (max) |
| Hearing loss | |||||
| Koetsier et al. [53] | PSPT (stereotactic single-dose proton radiosurgery or fractionated stereotactic PBT) | NA | 12 GyRBE or 54 GyRBE or 50.4 | Cochlea mean dose: 44.2 GyRBE (fractionated arm) 8.9 GyRBE (single dose arm) | Cochlea: ≤45 Gy (mean) |
| Indelicato et al. [54] | PSPT | NA | 59.4 GyRBE (patients >3 years old) 54 GyRBE (patients ≤3 years old) | NA | |
| Otitis | |||||
| Fuji et al. [55] | PSPT | Grade 2 | 63 GyRBE (median) | Dose constraint: 60 GyRBE (optic nerves and chiasma) <67 GyRBE (D90 max brainstem) <60 GyRBE (max dose to center of brainstem) | Optic nerve/chiasm: <55 Gy Brainstem: <54 Gy (max) |
| Indelicato et al. [56] | PSPT | 2 cases of unspecified grade | 73.8 GyRBE (median) | NA | |
| Brain edema | |||||
| Greenfield et al. [33] | PSPT or PBS | 4 cases requiring medical intervention | 52.2 GyRBE (median) | NA | |
| Sanford et al. [61] | PSPT or PBS | Grade 4: 1 patient | 55.8 GyRBE | NA | |
| Neuromotor deficits | |||||
| Sanford et al. [61] | PSPT or PBS | Grade 4: 1 patient | 55.8 GyRBE | NA | |
| Ataxia | |||||
| Upadhyay et al. [64] | PSPT or PBS | Grades 2, 3, 4, 5 | 54 CGyE (median) | Mean Dmax brainstem: 55.9 Gy | Brainstem: <54 Gy (max) |
| Saeed et al. [65] | PSPT or PBS *,† | Grade 3 | 46.2 Gy (median)/2.2 Gy per fr (median) | NA | |
| Brown et al. [66] | PSPT or PBS * | Grade 1 | 50 Gy and 60 Gy in 30 fr | Whole brain: 65.19 Gy (max) Right lens: 162.81 cGy (max) Left lens: 213.99 cGy (max) Right cochlea: 11.52 Gy (max) Left cochlea: 9.28 Gy (max) Pituitary: 21.35 Gy (max) Right hippocampus: 34.58 Gy (max) Left hippocampus: 38.15 Gy (max) | Brain: <60 Gy (max) Cochlea: ≤45 Gy (mean) |
| Sanford et al. [61] | PSPT or PBS | Grade 1: 1 patient (55.8 Gy dose) Grade 2: 1 patient (55.8 Gy dose) Grade 1: 1 patient (63.0 Gy dose) Grade 2: 2 patients (63.0 Gy dose) | 55.8 GyRBE or 63.0 GyRBE | NA | |
| Gait disturbance | |||||
| Yang et al. [68] | PBS (hypofractionated proton CSI) | Grade 2 | 30 GyRBE in 10 fr | NA | |
| Dysarthria | |||||
| Shih et al. [71] | PSPT | Grade 1 | 54 GyRBE/30 fr | NA | |
| Sanford et al. [61] | PSPT or PBS | Grade 1: 1 patient | 55.8 GyRBE | NA | |
| Cognitive disturbance | |||||
| Saeed et al. [65] | PBS or PSPT *,† | Grade 3 | 46.2 Gy (median)/2.2 Gy per fr (median) | NA | |
| Gaito et al. [18] | USPT or PSPT | Grade 3: 9 patients | 54 GyRBE (median) | NA | |
| Memory impairment | |||||
| Yang et al. [68] | PBS (hypofractionated proton CSI) | Grade 1 | 30 GyRBE in 10 fr | NA | |
| Gaito et al. [18] | USPT or PSPT | Grade 3 | 54 GyRBE (median) | NA | |
| Brown et al. [66] | PBS or PSPT * | Grade 1: 3 patients | Mean whole brain: 19.38 GyRBE, Max: 65.19 GyRBE | Whole brain: 65.19 Gy (max) Right lens: 162.81 cGy (max) Left lens: 213.99 cGy (max) Right cochlea: 11.52 Gy (max) Left cochlea: 9.28 Gy (max) Pituitary: 21.35 Gy (max) Right hippocampus: 34.58 Gy (max) Left hippocampus: 38.15 Gy (max) | Brain: <60 Gy (max) Cochlea: ≤45 Gy (mean) |
| Dysphasia | |||||
| Brown et al. [66] | PSPT or PBS * | Grade 1 | Mean whole brain: 19.38 GyRBE, Max: 65.19 GyRBE | Whole brain: 65.19 Gy (max) Right lens: 162.81 cGy (max) Left lens: 213.99 cGy (max) Right cochlea: 11.52 Gy (max) Left cochlea: 9.28 Gy (max) Pituitary: 21.35 Gy (max) Right hippocampus: 34.58 Gy (max) Left hippocampus: 38.15 Gy (max) | Brain: <60 Gy (max) Cochlea: ≤45 Gy (mean) |
| Somnolence syndrome | |||||
| Greenfield et al. [33] | PSPT or PBS | Required medical intervention | 52.2 GyRBE (median) | NA | |
| Myelitis | |||||
| Gordon et al. [19] | PBS | Grade 3 | 70.0 GyRBE (median) in 2 GyRBE/fr | Brainstem: 52.85 GyRBE (Dmax) Chiasma: 45.9 GyRBE (Dmax) Right optical nerve: 43.2 GyRBE (Dmax) Left optical nerve: 44.8 GyRBE (Dmax) Spinal cord: 20.4 GyRBE (Dmax) Right temporal lobe: 18 GyRBE (mean) Left temporal lobe: 16.5 GyRBE (mean) Hypophysis: 52.1 GyRBE (mean) | Brainstem: <54 Gy (max) Optic nerve/chiasm: <55 Gy Spinal cord: 50 Gy (max) |
| Gaito et al. [18] | USPT or PSPT | Grade 4 | 54 GyRBE (median) | NA | |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Fractionated Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Pneumonitis | |||||
| Gomez et al. [87] | Hypofractionated PSPT | Grade 3 | 60 GyRBE in 15 fr of 4 GyRBE | Lung: 13.0 GyRBE (mean) | Lung: 7 Gy (mean) |
| Hoppe et al. [88] | Hypofractionated PSPT, USPT, or PBS * | Grade 4 | 60.01 GyRBE in 3.53 GyRBE/fr | Lung: 10 GyRBE (mean) | Lung: 7 Gy (mean) |
| Jeter et al. [89] | PBS IMPT * | Grades 3 and 5 | 78 Gy | Lung: 15 Gy (mean) | Lung: 7 Gy (mean) |
| Liao et al. [90] | PSPT * | Grade 3 | 74 or 66 GyRBE | Lung: 16.1 GyRBE (mean) Esophagus: 23.6 GyRBE (mean) Heart: 5.9 GyRBE (mean) | Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) |
| Nguyen et al. [93] | PSPT * | Grade 3 | 74 GyRBE | Spinal cord: Dmax <45 Gy Lung: ≤20 Gy (mean) Esophagus: Dmax ≤ 80 Gy Heart: <26 Gy (mean) Kidney: 20 Gy to <32% of bilateral kidney Liver: <30 Gy (mean) | Spinal cord: 50 Gy (max) Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) Kidney: <15–18 Gy (mean) Liver: <30–32 Gy (mean) |
| Chang et al. [94] | Dose-escalated hypofractionated PSPT | Grade 2: 4 patients, Grade 3: 1 patient | 87.5 GyRBE in 35 fr (2.5 GyRBE/fr) | Dose limit: Bronchial tree and large blood vessels: 87.5 GyRBE < 10 cm3 Heart: 70 Gy < 10% | Heart: <26 Gy (mean) |
| Nakajima et al. [91] | Grade 2: 4 patients, Grade 3: 1 patient | 66 GyRBE in 10 fractions (peripheral lesions), 72.6 GyRBE in 22 fractions (central lesions) | Lung: 4.6 GyRBE (mean) Heart: 0 GyRBE (mean) | Lung: 7 Gy (mean) Heart: <26 Gy (mean) | |
| Yegya-Raman et al. [92] | PBS or PSPT | Grade ≥ 2: 4, Grade 3: 1 | 60–70 Gy in fractions | Lung: 6.9 Gy (mean) Heart: 2.5 Gy (mean) Esophagus: 9.7 Gy (mean) | Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) |
| Fibrosis | |||||
| Nagano et al. [104] | PSPT | Exacerbation of previous fibrosis | 66 GyRBE/10 fr | NA | |
| Hu et al. [105] | PBS IMPT * | NA | 50 GyE/25 fr | Lung: 56.06 GyE (max) Heart: 55 GyE (max) Esophagus: 51.97 GyE (max) Spinal cord: 11.91 GyE (max) | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Esophagus: <34 Gy (mean) Spinal cord: 50 Gy (max) |
| Pleural Effusion | |||||
| Y. L. Lin [111] | PBS (single-field breast) † | NA | 64.40 GyRBE (total) 56 GyRBE in 28 fr (upper mediastinal, retrosternal lymphatics and entire sternum) | Spinal cord: 0.06 Gy (mean) Right brachial plexus: 43.70 Gy (mean) Left lung: 7.38 Gy (mean) Right lung: 14.79 Gy (mean) Esophagus: 22.89 Gy (mean) Heart: 13.06 Gy (mean) Heart volume receiving ≥30 Gy: 20% | Spinal cord: 50 Gy (max) Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) |
| Lin et al. [100] | PSPT or PBS * | Asymptomatic: 13 patients Surgical intervention: 1 patient medical intervention: 1 patient | 50.4 Gy/28 fr | Lung: 4.8 Gy (mean) Heart: 11.3 Gy (mean) Liver: 2.4 Gy (mean) Spinal cord: 38.3 Gy (mean) | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy (mean) Spinal cord: 50 Gy (max) |
| Hoppe et al. [98] | Hypofractionated PSPT, USPT, or PBS * | Grade 2 | 60 GyRBE in 2.5 to 3.53 GyRBE/fr | NA | |
| Chang et al. [96] | PSPT * | Grade 3: 2 patients | 74 GyRBE | NA | |
| Bronchial stricture | |||||
| Chang et al. [96] | PSPT * | Grade 2: 2 Patients | 74 GyRBE | NA | |
| Bronchial fistula | |||||
| Chang et al. [96] | PSPT * | Grade 4 | 74 GyRBE | NA | |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Fractionated Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Tracheoesophageal fistula | |||||
| Gomez et al. [87] | Hypofractionated PSPT † | Grade 5 | 52.5 Gy (15 fr of 3.5 Gy) | Esophagus: 21.0 Gy (mean), 55.6 Gy (max) | Esophagus: <34 Gy (mean) |
| Rutenberg et al. [131] | PSPT * | Grade 5 | 64.8 GyRBE | 45 Gy GyRBE (initial dose to gross disease and elective lymph nodes) Boost range: 5.4–19.8 GyRBE Dose constraints: Lung: 6.5 GyRBE (mean) Heart: 7.8 GyRBE (mean) | Lung: 7 Gy (mean) (mean) Heart: <26 Gy (mean) |
| DeCesaris CM et al. [134] | PBS IMPT ‡ | Grade 4 | 53.4 Gy (median) | Spinal cord: 13.10 Gy (median) Total lung mean: 3.70 Gy Liver mean: 0.30 Gy Heart mean: 0.70 Gy | Lung: 7 Gy (mean) (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy |
| Esophageal stenosis | |||||
| Rutenberg et al. [131] | PSPT * | Grade 3 | 54 GyRBE | 45 Gy GyRBE (initial dose to gross disease and elective lymph nodes) Boost range: 5.4–19.8 GyRBE Dose constraints: Lung: 6.5 GyRBE (mean) Heart: 7.8 GyRBE (mean) | Lung: 7 Gy (mean) (mean) Heart: <26 Gy (mean) |
| Fernandes et al. [132] | PSPT or PBS *,‡ | Grade 3 | 54.0 Gy (median reirradiation dose) 109.8 Gy (median cumulative dose) | NA | |
| Takada et al. [133] | PSPT * | Grade 3 | 33–39.6 Gy in 15–18 fr | Dose constraints: Lung: <20 Gy (mean) Lung V20: <35% Heart V40: <40% Liver V30: < 30% Spinal cord: <45 Gy | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy (mean) Spinal cord: 50 Gy (max) |
| Nguyen et al. [93] | PSPT * | Grade 4 | 74 Gy | Spinal cord: Dmax <45 Gy Lung: ≤20 Gy (mean) Esophagus: Dmax ≤ 80 Gy Heart: <26 Gy (mean) Kidney: 20 Gy to <32% of bilateral kidney Liver: <30 Gy (mean) | Spinal cord: 50 Gy (max) Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) Kidney: <15–18 Gy (mean) Liver: <30–32 Gy (mean) |
| Pyloric stenosis | |||||
| Rutenberg et al. [131] | PSPT * | Grade 3 | 50.4 Gy (median) | 45 GyRBE (initial dose to gross disease and elective lymph nodes) Boost range: 5.4–19.8 GyRBE Dose constraints: Lung: 6.5 GyRBE (mean) Heart: 7.8 GyRBE (mean) | Lung: 7 Gy (mean) (mean) Heart: <26 Gy (mean) |
| Esophageal fistula | |||||
| Takada et al. [133] | PSPT * | Grade 3 | 33–39.6 Gy in 15–18 fr | Esophagus lesion: 75.6 Gy Dose constraints: Lung: <20 Gy (mean) Lung V20: <35% Heart V40: <40% Liver V30: < 30% Spinal cord: <45 Gy | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy (mean) Spinal cord: 50 Gy (max) |
| Aorto-esophageal fistula | |||||
| Yegya-Raman et al. [92] | PBS or PSPT | Grade 5 | 60–70 Gy in fractions | Lung: 6.9 Gy (mean) Heart: 2.5 Gy (mean) Esophagus: 9.7 Gy (mean) | Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) |
| Esophagitis | |||||
| Chang et al. [96] | PSPT * | Grades 3–4 | 74 Gy | NA | |
| Nguyen et al. [93] | PSPT * | Grade 3 | 60–74 Gy | Spinal cord: Dmax <45 Gy Lung: ≤20 Gy (mean) Esophagus: Dmax ≤ 80 Gy Heart: <26 Gy (mean) Kidney: 20 Gy to <32% of bilateral kidney Liver: <30 Gy (mean) | Spinal cord: 50 Gy (max) Lung: 7 Gy (mean) Esophagus: <34 Gy (mean) Heart: <26 Gy (mean) Kidney: <15–18 Gy (mean) Liver: <30–32 Gy (mean) |
| Takada et al. [133] | PSPT * | Grade 3 | 33–39.6 Gy in 15–18 fr | Dose constraints: Lung: <20 Gy (mean) Lung V20: <35% Heart V40: <40% Liver V30: < 30% Spinal cord: <45 Gy | Lung: 7 Gy (mean) Heart: <26 Gy (mean) Liver: <30–32 Gy (mean) Spinal cord: 50 Gy (max) |
| Pharyngitis | |||||
| Sawada et al. [138] | PSPT | Grade 2 | 60 GyRBE in 30 fr | NA | |
| Colitis | |||||
| Hong et al. [139] | PSPT * | Grade 3 | 5 daily doses of 5 GyE in 1 week | NA | |
| Gastritis | |||||
| Merchant et al. [140] | PSPT | Grade 3 | 54 GyRBE | NA | NA |
| Rectal bleeding | |||||
| Colaco et al. [141] | PSPT (single field prostate) | Grade 3 | 78 GyRBE (median) | Prostate (high- and low-risk patients): 78 GyRBE Prostate and proximal seminal vesicles (intermediate-risk patients): dose escalation 78 to 82 GyRBE Dose constraints: Rectal wall: V70 < 30%, V50 < 50% Bladder wall: V82 < 7 cm3, V80 < 8 cm3,V30 < 35 cm3 | Rectum: V70 < 20% V50 < 50% Bladder: V80 < 15% |
| Bryant et al. [142] | PSPT | Grade 3 | 74–82 Gy at 2 Gy/fr | NA | |
| Pugh et al. [143] | PSPT or PBS IMPT | Grade 3 | 75.6–78 Gy | NA | |
| Elevated biliary enzymes/bilirubin | |||||
| Sumiya et al. [145] | PSPT | Grade 2–3 | 66 Gy/10 fr 72.6 Gy/22 fr 74 Gy/37 fr | NA | |
| Kawashima et al. [147] | PSPT | Grade 3 | 72 GyE/16 fr (median) | Percent of liver noncancerous regions receiving ≥30 GyE exceeded 25% | Liver: <30–32 Gy (mean) |
| Bush et al. [148] | PSPT | Grade 3: 2 cases, Grade 4: 1 case | 70.2 Gy/15 fr | No more than 33% liver received 30 Gy | Liver: <30–32 Gy (mean) |
| Radiation-induced liver disease | |||||
| Cheng et al. [146] | PSPT | NA | 96.56 GyRBE (median) | NA | |
| Hepatic insufficiency | |||||
| Kawashima et al. [147] | PSPT | Grade 5 | 72 GyE/16 fr (median) | Percent of liver noncancerous regions receiving ≥30 GyE exceeded 25% | Liver: <30–32 Gy (mean) |
| Hepatic failure | |||||
| Hashimoto et al. [149] | PSPT | Grade 4 | 1st course: 72 Gy/16 fr (median) following courses: 66 Gy/16 fr (median) | NA | |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs |
|---|---|---|---|---|
| Leukopenia | ||||
| Vennarini et al. [151] | PBS (CSI) * | Grade 3–4 | 54 Gy (median) | 36 Gy (median CSI dose) |
| Barney et al. [154] | PSPT (CSI) * | Grade 3 | 54 Gy (median) | 30.6 Gy (median CSI dose) |
| Yang et al. [68] | PBS (hypofractionated CSI) | Grade 3 | 30 GyRBE in 10 fr | NA |
| Liu et al. [155] | PSPT (CSI) | Grade 4 | 54 Gy (median) | 23.4 Gy (median CSI dose) |
| Neutropenia | ||||
| Vennarini et al. [151] | PBS (CSI) | Grade 3–4 | 54 Gy (median) | 36 Gy (median CSI dose) |
| Oshiro et al. [102] | PSPT * | Grade 3–4 | 74 Gy in 37 fr (primary site) 66 Gy in 33 fr (lymph nodes) | NA |
| Yegya-Raman et al. [92] | PBS or PSPT | Grade 3: 2, Grade 4: 1 | 60–70 Gy in fractions | Lung: 6.9 Gy (mean) Heart: 2.5 Gy (mean) Esophagus: 9.7 Gy (mean) |
| Weinberg et al. [152] | PBS (hypofractionated) * | Grade 3 | 5 Gy × 5 fractions | NA |
| Lymphopenia | ||||
| Routman et al. [153] | PBS * | Grade 4 | 50 Gy (median) | NA |
| Yang et al. [68] | PBS (hypofractionated CSI) | Grade 4 | 30 GyRBE in 10 fr | NA |
| Liu et al. [155] | PSPT (Proton CSI) | Grade 4 | 54 Gy (median) | 23.4 Gy (median CSI dose) |
| Yegya-Raman et al. [92] | PBS or PSPT | Grade 3: 2, Grade 4: 1 | 60–70 Gy in fractions | Lung: 6.9 Gy (mean) Heart: 2.5 Gy (mean) Esophagus: 9.7 Gy (mean) |
| Anemia | ||||
| Barney et al. [154] | PSPT (CSI) * | Grade 2 | 54 Gy (median) | 30.6 Gy (median CSI dose) |
| Yegya-Raman et al. [92] | PBS or PSPT | Grade 1: 3 | 60–70 Gy in fractions | Lung: 6.9 Gy (mean) Heart: 2.5 Gy (mean) Esophagus: 9.7 Gy (mean) |
| Yang et al. [68] | PBS (hypofractionated CSI) | Grade 3 | 30 GyRBE in 10 fr | NA |
| Liu et al. [155] | PSPT (CSI) | Grade 3 | 54 Gy (median) | 23.4 Gy (median CSI dose) |
| Thrombocytopenia | ||||
| Barney et al. [154] | PSPT (CSI) * | Grade 3–4 | 54 Gy (median) | 30.6 Gy (median CSI dose) |
| Oshiro et al. [102] | PSPT * | Grade 3 | 74 Gy in 37 fr (primary site) 66 Gy in 33 fr (lymph nodes) | NA |
| Yang et al. [68] | PBS (hypofractionated CSI) | Grade 4 | 30 GyRBE in 10 fr | NA |
| Leukocytopenia | ||||
| Oshiro et al. [102] | PBT * | Grade 3 | 74 Gy in 37 fr (primary site) 66 Gy in 33 fr (lymph nodes) | NA |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Urinary retention | |||||
| Takaoka et al. [156] | PBS (NFPT or MHPT) | Grade 1–2 | 76–78 Gy in 38–39 fr or 60–63 Gy in 20–21 fr | 12.3 Gy or 11.1 Gy (median bladder dose) | Bladder: <65 Gy (max) |
| Hematuria | |||||
| Takaoka et al. [156] | PBS (NFPT or MHPT) | Grade 1–2 | 76–78 Gy in 38–39 fr or 60–63 Gy in 20–21 fr | 12.3 Gy or 11.1 Gy (median bladder dose) | Bladder: <65 Gy (max) |
| Makishima et al. [157] | PSPT | Grade 3 | Low risk: 74 Gy/37 fr Intermediate or high risk: 78 Gy/39 fr | Median rectal V30: 32.5%, V80: 12.5% of prescribed dose | Rectum: V50 < 50% V75 < 15% |
| Bryant et al. [142] | PSPT | Grade 3–4 | 74–82 Gy at 2 Gy/fr | NA | |
| Urinary frequency | |||||
| Takaoka et al. [156] | PBS (NFPT or MHPT) | Grade 1–2 | 76–78 Gy in 38–39 fr or 60–63 Gy in 20–21 fr | 12.3 Gy or 11.1 Gy (median bladder dose) | Bladder: <65 Gy (max) |
| Makishima et al. [157] | PSPT | Grade 2 | Low risk: 74 Gy/37 fr Intermediate or high risk: 78 Gy/39 fr | Median rectal V30: 32.5%, V80: 12.5% of prescribed dose | Rectum: V50 < 50% V75 < 15% |
| Meixner et al. [161] | PBS * | <Grade 3 | 50.4 Gy (median) in 25–28 fr | NA | |
| Urinary urgency | |||||
| Takaoka et al. [156] | PBS (NFPT or MHPT) | Grade 1–2 | 76–78 Gy in 38–39 fr or 60–63 Gy in 20–21 fr | 12.3 Gy or 11.1 Gy (median bladder dose) | Bladder: <65 Gy (max) |
| Urinary incontinence | |||||
| Takaoka et al. [156] | PBS (NFPT or MHPT) | Grade 1–2 | 76–78 Gy in 38–39 fr or 60–63 Gy in 20–21 fr | 12.3 Gy or 11.1 Gy (median bladder dose) | Bladder: <65 Gy (max) |
| Hoppe et al. [160] | PSPT | 76–82 CGE or 70–72.5 CGE | ≥40 CGE (mean penile bulb dose) | Penile bulb: D90 < 50 Gy | |
| Meixner et al. [161] | PBS * | <Grade 3 | 50.4 Gy (median) in 25–28 fr | NA | |
| Urinary tract pain | |||||
| Takaoka et al. [156] | PBS (NFPT or MHPT) | Grade 1–2 | 76–78 Gy in 38–39 fr or 60–63 Gy in 20–21 fr | 12.3 Gy or 11.1 Gy (median bladder dose) | Bladder: <65 Gy (max) |
| Non-infectious cystitis | |||||
| Makishima et al. [157] | PSPT | Grade 3 | Low risk: 74 Gy/37 fr Intermediate or high risk: 78 Gy/39 fr | Median rectal V30: 32.5%, V80: 12.5% of prescribed dose | Rectum: V50 < 50% V75 < 15% |
| Bladder irritation | |||||
| Bryant et al. [142] | PSPT | Grade 3 | 74–82 Gy at 2 Gy/fr | NA | |
| Urinary obstruction | |||||
| Bryant et al. [142] | PSPT | Grade 3 | 74–82 Gy at 2 Gy/fr | NA | |
| Bowel perforation | |||||
| Uezono et al. [158] | PSPT (preoperative PBT) * | Grade 3 | 50.4 Gy | NA | |
| Erectile Dysfunction | |||||
| Chiang et al. [159] | PBS * | 50 GyE/25 fr Boost: 78 Gy/39 fr | 50.9 Gy (max dose to small bowel) | Small bowel: Individual loops: V15 < 120 cc Peritoneal cavity: V45 < 195 cc | |
| Hoppe et al. [160] | PSPT | 76–82 CGE or 70–72.5 CGE | ≥40 CGE (mean penile bulb dose) | Penile bulb: D90 < 50 Gy | |
| Nocturia | |||||
| Meixner et al. [161] | PBS * | <Grade 3 | 50.4 Gy (median) in 25–28 fr | NA | |
| Vaginal bleeding | |||||
| Meixner et al. [161] | PBS * | <Grade 3 | 50.4 Gy (median) in 25–28 fr | NA | |
| Vaginal mucosal toxicity | |||||
| Li et al. [162] | PSPT *,† | Grade 1–2 | NA | Rectum: 63.8 Gy Bladder: 58 Gy | Rectum: V50 < 50% V75 < 15% Bladder: <65 Gy (max) |
| Vaginal dryness | |||||
| Barcellini et al. [163] | PBS † | Grade 1 | 39 Gy/13 fr | NA | |
| Premature ovarian deficiency | |||||
| Uezono et al. [158] | PSPT (preoperative PBT) * | Grade 2 | 50.4 Gy | NA | |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Osteoradionecrosis | |||||
| Singh et al. [165] | USPT or PBS * | Grade 2–3 | 66 Gy (median) | ORN site Average Dmean: 71.5 Gy Average Dmax: 75.1 Gy | Mandible: ≤70 Gy (max) Femoral heads: V50 < 5% |
| Zhang et al. [166] | PBS IMPT * | Grade 1 | 25.6 Gy (mean) | Mandible: >50 Gy | Mandible: ≤70 Gy (max) Femoral heads: V50 < 5% |
| Unequal limb length | |||||
| Uezono et al. [158] | PSPT (postoperative PBT) * | Grade 2 | 54 Gy | NA | |
| Hip dislocation | |||||
| Uezono et al. [158] | PSPT (postoperative PBT) * | Grade 3 | 50.4 Gy | NA | |
| Rib fracture | |||||
| Chang et al. [94] | PSPT (dose-escalated hypofractionated PBT) | Grade 2 | 87.5 GyRBE in 35 fr (2.5 GyRBE/fr) | Dose limit to bronchial tree and large blood vessels: 87.5 Gy < 10 cm3 Dose limit to heart: 70 Gy < 10% | Heart: V25 < 10% Mandible: ≤70 Gy (max) Femoral heads: V50 < 5% |
| Bush et al. [167] | Hypofractionated PSPT | Grade 2 | Dose escalation: 51 to 60 to 70 Gy in 10 fr | NA | |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs | QUANTEC Photon 3DCRT Dose Constraints |
|---|---|---|---|---|---|
| Microdontia | |||||
| Foster-Thomas et al. [168] | PSPT *,† | NA | 50.4 Gy in 28 fr | Maxilla: 30 Gy (mean) Mandible: 25.9 Gy (mean) | Mandible ≤ 70 Gy (max) |
| Hypodontia | |||||
| Foster-Thomas et al. [168] | PSPT *,† | NA | 50.4 Gy in 28 fr | Maxilla: 30 Gy (mean) Mandible: 25.9 Gy (mean) | Mandible ≤ 70 Gy (max) |
| Hypermineralization | |||||
| Foster-Thomas et al. [168] | PSPT *,† | NA | 50.4 Gy in 28 fr | Maxilla: 30 Gy (mean) Mandible: 25.9 Gy (mean) | Mandible ≤ 70 Gy (max) |
| Abrupt root development | |||||
| Foster-Thomas et al. [168] | PSPT *,† | NA | 50.4 Gy in 28 fr | Maxilla: 30 Gy (mean) Mandible: 25.9 Gy (mean) | Mandible ≤ 70 Gy (max) |
| Partial/lack of eruption of teeth | |||||
| Foster-Thomas et al. [168] | PSPT *,† | NA | 50.4 Gy in 28 fr | Maxilla: 30 Gy (mean) Mandible: 25.9 Gy (mean) | Mandible ≤ 70 Gy (max) |
| Xerostomia | |||||
| Bagley et al. [170] | PBS IMPT * | NA | 69.3 Gy (median) | Mean doses: Parotid: 27.8 ± 5.7 Gy Submandibular: 39.2 ± 21.9 Gy Contralateral parotid: 17.5 ± 9.0 Gy Contralateral submandibular: 39.2 ± 21.9 Gy Oral cavity: 22.4 ± 10.6 Gy | Mandible ≤ 70 Gy (max) |
| Cao et al. [171] | PBS IMPT * | NA | With chemotherapy: CTV1: 70 Gy CTV2: 63 Gy CTV3: 57 Gy Without chemotherapy: CTV1: 66 Gy CTV2: 60 Gy CTV3: 54 Gy | Dose constraints: Brain: <60 Gy (max) Brainstem and optical apparatus: <54 Gy (max) Spinal cord: <45 Gy (max) Cornea, cochlea: <35 Gy (max) Larynx: <35 Gy (mean) Oral cavity: <30 Gy (mean) Salivary glands: <26 Gy (mean) | Parotid (bilateral) ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) |
| Chuong et al. [172] | PBS or USPT * | Grade 2 | 66.5 GyE (median) in 33 fr | Dose constraints: Optic nerve/chiasm: 54 Gy (max) Spinal cord and brainstem: <45 Gy (max) Larynx: <45 Gy (mean) Oral cavity: <30 Gy (mean) Contralateral submandibular: <20 Gy (mean) Contralateral parotid: <10 Gy (mean) | Parotid bilateral ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) |
| Dysgeusia | |||||
| Chuong et al. [172] | PBS or USPT * | Grade 2 | 66.5 GyE (median) in 33 fr | Dose constraints: Optic nerve/chiasm: 54 Gy (max) Spinal cord and brainstem: <45 Gy (max) Larynx: <45 Gy (mean) Oral cavity: <30 Gy (mean) Contralateral submandibular: <20 Gy (mean) Contralateral parotid: <10 Gy (mean) | Parotid bilateral ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) |
| Dysphagia | |||||
| Chuong et al. [172] | PBS or USPT * | Grade 2 | 66.5 GyE (median) in 33 fr | Dose constraints: Optic nerve/chiasm: 54 Gy (max) Spinal cord and brainstem: <45 Gy (max) Larynx: <45 Gy (mean) Oral cavity: <30 Gy (mean) Contralateral submandibular: <20 Gy (mean) Contralateral parotid: <10 Gy (mean) | Parotid bilateral ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) Esophagus < 34 Gy (mean) |
| Chuong et al. [172] | PBS or USPT * | Grade 3 | 66.5 GyE (median) in 33 fr | Dose constraints: Optic nerve/chiasm: 54 Gy (max) Spinal cord and brainstem: <45 Gy (max) Larynx: <45 Gy (mean) Oral cavity: <30 Gy (mean) Contralateral submandibular: <20 Gy (mean) Contralateral parotid: <10 Gy (mean) | Parotid bilateral ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) Esophagus < 34 Gy (mean) |
| Oral mucositis | |||||
| Chuong et al. [172] | PBS or USPT * | Grade 2 | 66.5 GyE (median) in 33 fr | Dose constraints: Optic nerve/chiasm: 54 Gy (max) Spinal cord and brainstem: <45 Gy (max) Larynx: <45 Gy (mean) Oral cavity: <30 Gy (mean) Contralateral submandibular: <20 Gy (mean) Contralateral parotid: <10 Gy (mean) | Parotid bilateral ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) |
| Chuong et al. [172] | PBS or USPT * | Grade 3 | 66.5 GyE (median) in 33 fr | Dose constraints: Optic nerve/chiasm: 54 Gy (max) Spinal cord and brainstem: <45 Gy (max) Larynx: <45 Gy (mean) Oral cavity: <30 Gy (mean) Contralateral submandibular: <20 Gy (mean) Contralateral parotid: <10 Gy (mean) | Parotid bilateral ≤ 25 Gy (mean) Mandible ≤ 70 Gy (max) |
| Paper | Type of PBT | Toxicity Grade | Prescribed Dose | Dose to Structure/OARs |
|---|---|---|---|---|
| Endocrine deficits | ||||
| Sanford et al. [61] | PSPT or PBS | Grade 2: 6 patients (55.8 Gy dose) Grade 4: 1 patient (55.8 Gy dose) Grade 2: 10 patients (63.0 Gy dose) | 55.8 GyRBE or 63.0 GyRBE | NA |
| Indelicato et al. [54] | PSPT | Grade 2+: 13 patients | 59.4 GyRBE (patients > 3 years old) 54 GyRBE (patients ≤ 3 years old) | NA |
| Growth hormone deficiency | ||||
| Greenfield et al. [33] | PSPT or PBS * | 1 patient unspecified grade with hypothyroidism, 7 patients with unspecified grade | 52.2 GyRBE (median) | 38 Gy (hypothalamic–pituitary mean dose) |
| Ladra et al. [75] | PSPT * | Grade 2: 3 patients | 50.4 GyRBE (median) | NA |
| Eaton et al. [176] | PSPT | 21 patients unspecified grade | 54–55.8 Gy as 1.8 Gy/fr | 23.4 Gy (median CSI dose) |
| Panhypopituitarism | ||||
| Bishop et al. [175] | PSPT | NA | 50.4 GyRBE (median) | NA |
| Adrenal insufficiency | ||||
| Eaton et al. [176] | PSPT | 2 patients unspecified grade | 54–55.8 Gy as 1.8 Gy/fr | 23.4 Gy (median CSI dose) |
| Shih et al. [71] | PSPT | 4 patients unspecified grade | 54 GyRBE/30 fr | NA |
| Sex hormone deficiency | ||||
| Eaton et al. [176] | PSPT | 1 patient unspecified grade | 54–55.8 Gy as 1.8 Gy/fr | 23.4 Gy (median CSI dose) |
| Hypothyroidism | ||||
| Eaton et al. [176] | PSPT | 9 patients unspecified grade | 54–55.8 Gy as 1.8 Gy/fr | 23.4 (median CSI dose) |
| Shih et al. [71] | PSPT | 3 patients unspecified grade | 54 GyRBE/30 fr | NA |
| Greenfield et al. [33] | PSPT or PBS * | 1 patient unspecified grade with growth hormone deficiency | 52.2 GyRBE (median) | 38 Gy (hypothalamic–pituitary mean dose) |
| Hypogonadism | ||||
| Shih et al. [71] | PSPT | 2 patients unspecified grade | 54 GyRBE/30 fr | NA |
| Greenfield et al. [33] | PSPT or PBS * | 1 patient unspecified grade | 52.2 GyRBE (median) | 48 Gy (hypothalamic–pituitary mean dose) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, P.P.; Chami, P.; Daou, R.; Hajj, J.; Lin, H.; Chhabra, A.M.; Simone, C.B., II; Lee, N.Y.; Hajj, C. Proton Radiation Therapy: A Systematic Review of Treatment-Related Side Effects and Toxicities. Int. J. Mol. Sci. 2024, 25, 10969. https://doi.org/10.3390/ijms252010969
Salem PP, Chami P, Daou R, Hajj J, Lin H, Chhabra AM, Simone CB II, Lee NY, Hajj C. Proton Radiation Therapy: A Systematic Review of Treatment-Related Side Effects and Toxicities. International Journal of Molecular Sciences. 2024; 25(20):10969. https://doi.org/10.3390/ijms252010969
Chicago/Turabian StyleSalem, Peter P., Perla Chami, Remy Daou, Joseph Hajj, Haibo Lin, Arpit M. Chhabra, Charles B. Simone, II, Nancy Y. Lee, and Carla Hajj. 2024. "Proton Radiation Therapy: A Systematic Review of Treatment-Related Side Effects and Toxicities" International Journal of Molecular Sciences 25, no. 20: 10969. https://doi.org/10.3390/ijms252010969
APA StyleSalem, P. P., Chami, P., Daou, R., Hajj, J., Lin, H., Chhabra, A. M., Simone, C. B., II, Lee, N. Y., & Hajj, C. (2024). Proton Radiation Therapy: A Systematic Review of Treatment-Related Side Effects and Toxicities. International Journal of Molecular Sciences, 25(20), 10969. https://doi.org/10.3390/ijms252010969






