Exploring the Regulatory Landscape of Dementia: Insights from Non-Coding RNAs
Abstract
1. Introduction
2. Dementia-Related Diseases and miRNAs
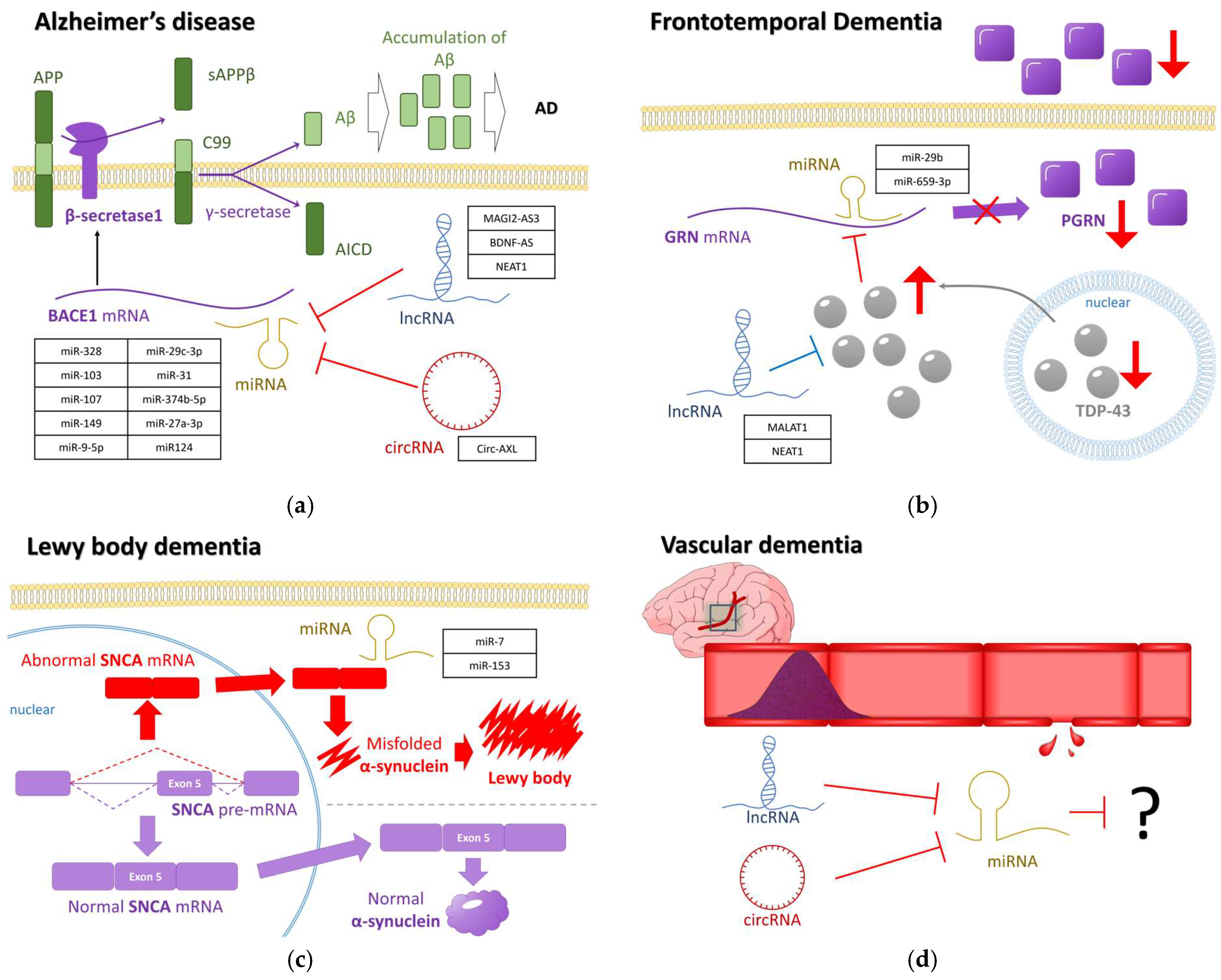
2.1. Alzheimer’s Disease and miRNA
2.1.1. Differentially Expressed miRNAs in AD
2.1.2. Exosomal miRNA and G-Quadruplex Structure in AD
2.2. Other Dementia-Related Diseases and miRNA
2.2.1. Differentially Expressed miRNAs in FTD
2.2.2. Differentially Expressed miRNAs in LBD
2.2.3. Differentially Expressed miRNAs in VD
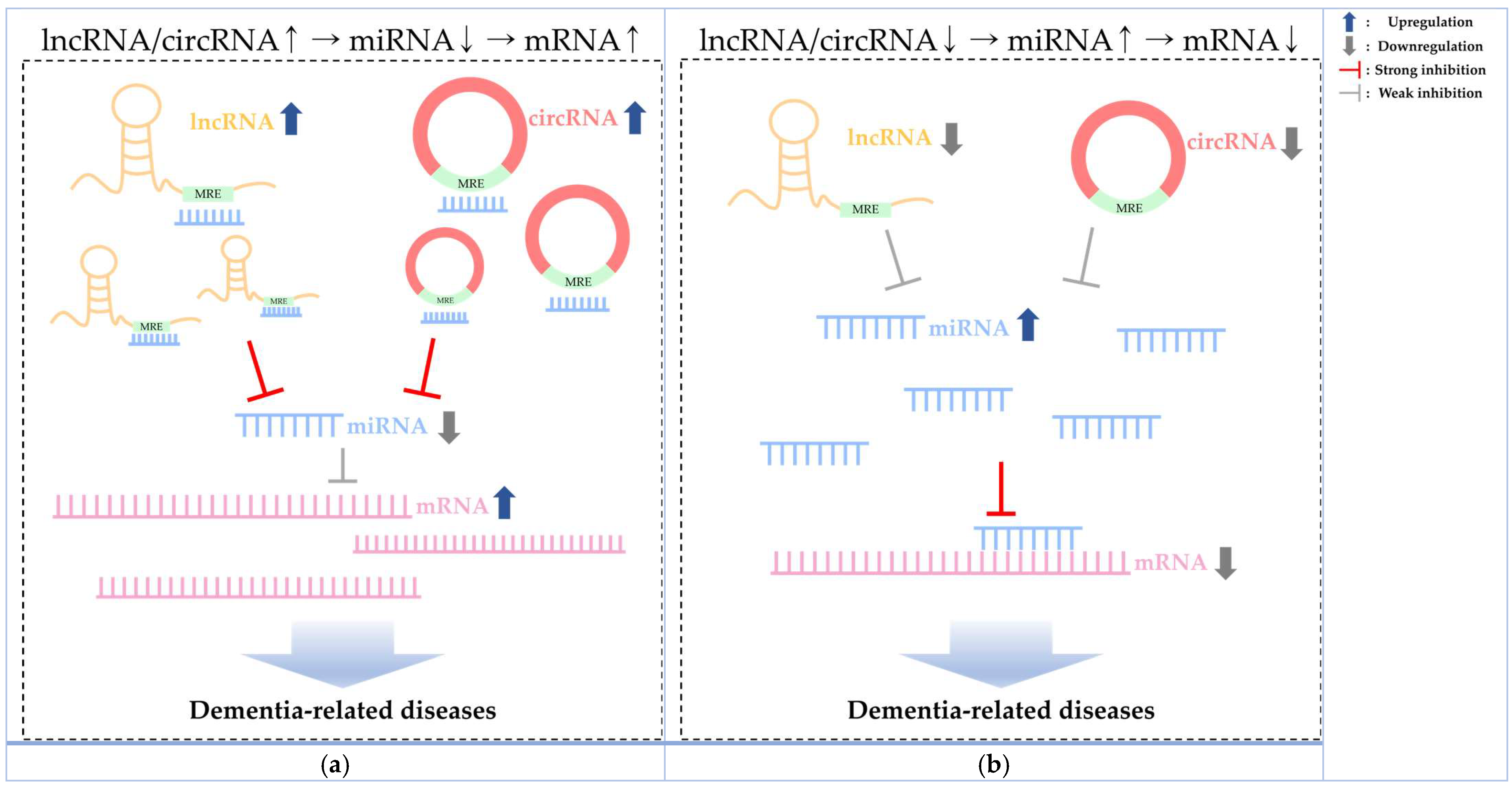
3. Dementia-Related Diseases and Other Non-Coding RNAs
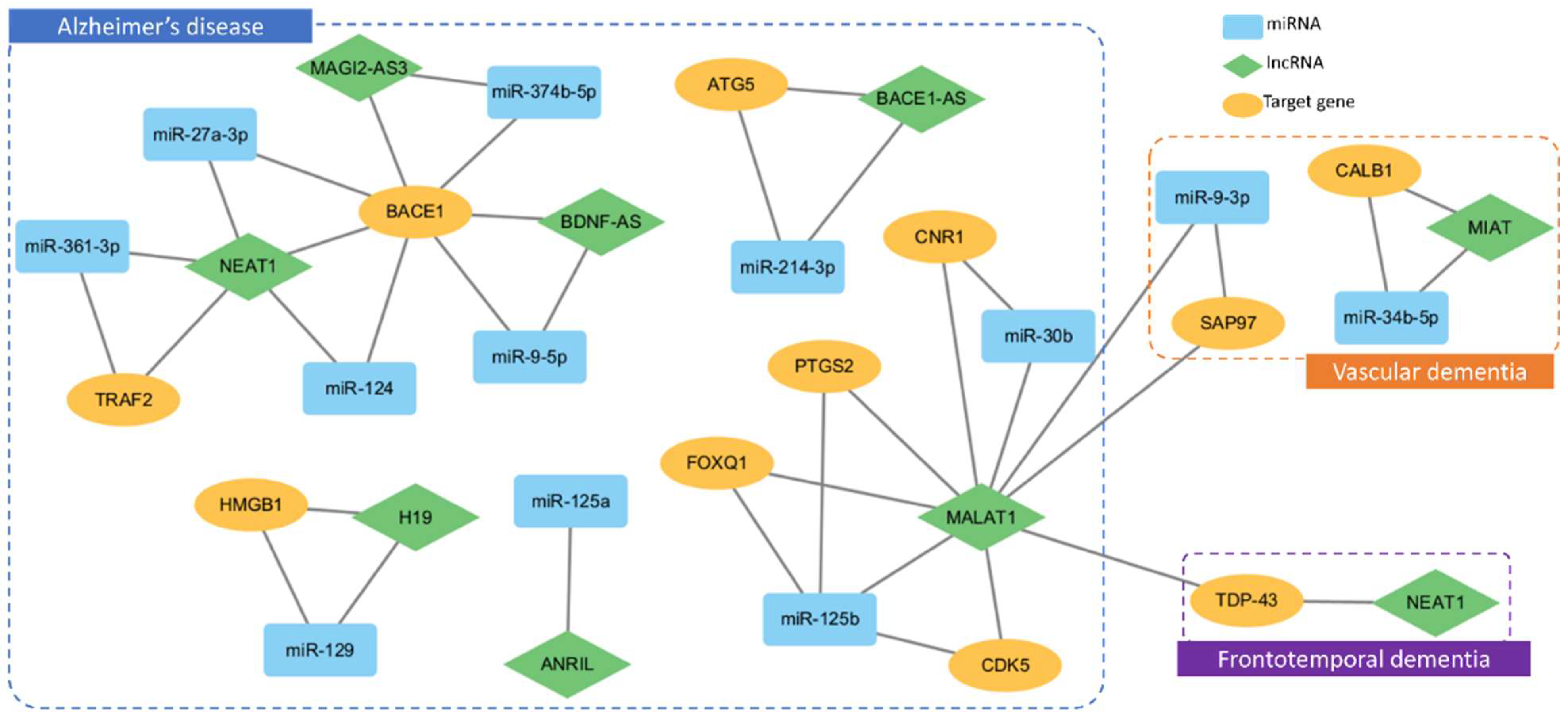
3.1. LncRNA
3.2. CircRNA
4. Dementia Pathogenesis with Genetic Factors and ncRNA Insights
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, B.; Kupeli, N.; Moore, K.J.; Sampson, E.L.; Davies, N. Defining end of life in dementia: A systematic review. J. Palliat. Med. 2021, 35, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Ellajosyula, R. Capacity issues and decision-making in dementia. Ann. Indian Acad. Neurol. 2016, 19 (Suppl. S1), S34–S39. [Google Scholar] [PubMed]
- Liang, C.S.; Li, D.J.; Yang, F.C.; Tseng, P.T.; Carvalho, A.F.; Stubbs, B.; Thompson, T.; Mueller, C.; Shin, J.I.; Radua, J.; et al. Mortality rates in Alzheimer’s disease and non-Alzheimer’s dementias: A systematic review and meta-analysis. Lancet Healthy Longev. 2021, 2, e479–e488. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Carter, J. Prevalence of all cause young onset dementia and time lived with dementia: Analysis of primary care health records. J. Dement. Care 2022, 30, 1–5. [Google Scholar]
- Chaudhry, A.; Houlden, H.; Rizig, M. Novel fluid biomarkers to differentiate frontotemporal dementia and dementia with Lewy bodies from Alzheimer’s disease: A systematic review. J. Neurol. Sci. 2020, 415, 116886. [Google Scholar] [CrossRef] [PubMed]
- Tayler, H.; Miners, J.S.; Güzel, Ö.; MacLachlan, R.; Love, S. Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer’s disease, vascular dementia and mixed dementia. Brain Pathol. 2021, 31, e12935. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Ayton, S.; Bush, A.I. The essential elements of Alzheimer’s disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, R.; Reddy, P.H. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Raho, E.M.; Lopriore, P.; Pace, A.P.; Latino, R.R.; Assogna, M.; Mancuso, M.; Gragnaniello, D.; Granieri, E.; Pugliatti, M.; et al. Frontotemporal Dementia, Where Do We Stand? A Narrative Review. Int. J. Mol. Sci. 2023, 24, 11732. [Google Scholar] [CrossRef] [PubMed]
- Pressman, P.S.; Miller, B.L. Diagnosis and management of behavioral variant frontotemporal dementia. Biol. Psychiatry 2014, 75, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Musa, G.; Slachevsky, A.; Muñoz-Neira, C.; Méndez-Orellana, C.; Villagra, R.; González-Billault, C.; Ibáñez, A.; Hornberger, M.; Lillo, P. Alzheimer’s Disease or Behavioral Variant Frontotemporal Dementia? Review of Key Points Toward an Accurate Clinical and Neuropsychological Diagnosis. J. Alzheimer’s Dis. 2020, 73, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, K.; Fukatsu, R.; Yamada, R.; Takamaru, Y.; Hara, Y.; Yasumura, S. Characteristics of initial symptoms and symptoms at diagnosis in probable dementia with Lewy body disease: Incidence of symptoms and gender differences. Psychogeriatrics 2020, 20, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Foguem, C.; Manckoundia, P. Lewy body disease: Clinical and pathological “overlap syndrome” between synucleinopathies (Parkinson disease) and tauopathies (Alzheimer disease). Curr. Neurol. Neurosci. Rep. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Surendranathan, A.; Kane, J.P.M.; Bentley, A.; Barker, S.A.H.; Taylor, J.; Thomas, A.J.; Allan, L.M.; McNally, R.J.; James, P.W.; McKeith, I.G.; et al. Clinical diagnosis of Lewy body dementia. BJPsych Open 2020, 6, e61. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.S.; Teodorczuk, A.; Watson, R. Dementia with Lewy bodies: Challenges in the diagnosis and management. Aust. N. Z. J. Psychiatry 2019, 53, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging Concepts in Vascular Dementia: A Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef]
- Hu, X.; De Silva, T.M.; Chen, J.; Faraci, F.M. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ. Res. 2017, 120, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lan, T.; Wang, Y.; Ren, L. Individual Insomnia Symptom and Increased Hazard Risk of Cardiocerebral Vascular Diseases: A Meta-Analysis. Front. Psychiatry 2021, 12, 654719. [Google Scholar] [CrossRef]
- Maclin, J.M.A.; Wang, T.; Xiao, S. Biomarkers for the diagnosis of Alzheimer’s disease, dementia Lewy body, frontotemporal dementia and vascular dementia. Gen. Psychiatry 2019, 32, e100054. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; Verberk, I.M.W.; Kindermans, J.; Abramian, A.; Vanbrabant, J.; Ball, A.J.; Pijnenburg, Y.; Lemstra, A.W.; Flier, W.M.; Stoops, E.; et al. Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia. Alzheimer’s Dement. 2022, 14, e12285. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.; Xiang, J.; Yang, D.; Zhang, Y.; Xiang, Q.; Li, J. lncRNA and circRNA expression profiles in the hippocampus of Abeta(25-35)-induced AD mice treated with Tripterygium glycoside. Exp. Ther. Med. 2023, 26, 426. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Muddashetty, R.S. Emerging Role of microRNAs in Dementia. J. Mol. Biol. 2019, 431, 743–1762. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Park, E.G.; Lee, D.H.; Lee, Y.J.; Bae, W.H.; Kim, H.S. The Tumorigenic Role of Circular RNA-MicroRNA Axis in Cancer. Int. J. Mol. Sci. 2023, 24, 3050. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.Y.; Chang, D.C.; Lin, S.L. The microRNA (miRNA): Overview of the RNA genes that modulate gene function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.L.; Enguita, F.J. A Structural View of miRNA Biogenesis and Function. Non-Coding RNA 2022, 8, 10. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Lund, E.; Dahlberg, J.E. Substrate selectivity of exportin 5 and Dicer in the biogenesis of microRNAs. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2006; Volume 71, pp. 59–66. [Google Scholar]
- Pratt, A.J.; MacRae, I.J. The RNA-induced silencing complex: A versatile gene-silencing machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef]
- Tan, P.H.; Yang, L.C.; Ji, R.R. Therapeutic potential of RNA interference in pain medicine. Open Pain J. 2009, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Afonso-Grunz, F.; Müller, S. Principles of miRNA–mRNA interactions: Beyond sequence complementarity. Cell. Mol. Life Sci. 2015, 72, 3127–3141. [Google Scholar] [CrossRef] [PubMed]
- Grammatikakis, I.; Lal, A. Significance of lncRNA abundance to function. Mamm. Genome 2022, 33, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Huaying, C.; Xing, J.; Luya, J.; Linhui, N.; Di, S.; Xianjun, D. A Signature of Five Long Non-Coding RNAs for Predicting the Prognosis of Alzheimer’s Disease Based on Competing Endogenous RNA Networks. Front. Aging Neurosci. 2020, 12, 598606. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L. Towards higher-resolution and in vivo understanding of lncRNA biogenesis and function. Nat. Methods 2022, 19, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Baird, A.M.; Brady, L.; Lim, M.; Gray, S.G.; McDermott, R.; Finn, S.P. Circular RNAs: Biogenesis, function and role in human diseases. Front. Mol. Biosci. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, M.; Calin, G.A. Circular RNAs in cancer–lessons learned from microRNAs. Front. Oncol. 2018, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, F. hsa_circ_0006916 Exerts Effect on Amyloid Beta-Induced Neuron Injury by Targeting miR-217/HOMER1. Ann. Clin. Lab. Sci. 2023, 53, 181–191. [Google Scholar] [PubMed]
- Moreno-García, L.; López-Royo, T.; Calvo, A.C.; Toivonen, J.M.; de la Torre, M.; Moreno-Martínez, L.; Molina, N.; Aparicio, P.; Zaragoza, P.; Manzano, R.; et al. Competing Endogenous RNA Networks as Biomarkers in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9582. [Google Scholar] [CrossRef] [PubMed]
- Ala, U. Competing Endogenous RNAs, Non-Coding RNAs and Diseases: An Intertwined Story. Cells 2020, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Jia, X.; He, R.; Zhang, X.; Zhang, W. Changing Expression Profiles of Messenger RNA, MicroRNA, Long Non-coding RNA, and Circular RNA Reveal the Key Regulators and Interaction Networks of Competing Endogenous RNA in Pulmonary Fibrosis. Front. Genet. 2020, 11, 558095. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, W.R.; Park, E.G.; Lee, D.H.; Kim, J.M.; Shin, H.J.; Jeong, H.S.; Roh, H.Y.; Kim, H.S. Exploring the Key Signaling Pathways and ncRNAs in Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 4548. [Google Scholar] [CrossRef]
- Fiannaca, A.; Paglia, L.L.; Rosa, M.L.; Rizzo, R.; Urso, A. miRTissue ce: Extending miRTissue web service with the analysis of ceRNA-ceRNA interactions. BMC Bioinform. 2020, 21, 199. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.R.; Abed, S.; Kouchakali, G.; Fattahi, F.; Sabaie, H.; Moslehian, M.S.; Sharifi-Bonab, M.; Hussen, B.M.; Taheri, M.; Ghafouri-Fard, S.; et al. Competing endogenous RNA (ceRNA) networks in Parkinson’s disease: A systematic review. Front. Cell. Neurosci. 2023, 17, 1044634. [Google Scholar] [CrossRef]
- Asanomi, Y.; Shigemizu, D.; Akiyama, S.; Sakurai, T.; Ozaki, K.; Ochiya, T.; Niida, S. Dementia subtype prediction models constructed by penalized regression methods for multiclass classification using serum microRNA expression data. Sci. Rep. 2021, 11, 20947. [Google Scholar] [CrossRef] [PubMed]
- Koriath, C.; Kenny, J.; Adamson, G.; Druyeh, R.; Taylor, W.; Beck, J.; Quinn, L.; Mok, T.H.; Dimitriadis, A.; Norsworthy, P.; et al. Predictors for a dementia gene mutation based on gene-panel next-generation sequencing of a large dementia referral series. Mol. Psychiatry 2020, 25, 3399–3412. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Zheng, Q.; Hao, S.; Yang, L.; Xia, Q.; Qi, C.; Ge, J. MicroRNAs in Alzheimer’s disease: Potential diagnostic markers and therapeutic targets. Biomed. Pharmacother. 2022, 148, 112681. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shui, X.; Diao, Y.; Chen, D.; Zhou, Y.; Lee, T.H. Potential Implications of miRNAs in the Pathogenesis, Diagnosis, and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 16259. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, J.; Duan, F.; Cong, L.; Qi, X. The role of the microRNA regulatory network in Alzheimer’s disease: A bioinformatics analysis. Arch. Med. Sci. 2022, 18, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chopra, N.; Nho, K.; Maloney, B.; Obukhov, A.G.; Nelson, P.T.; Counts, E.; Lahiri, D.K. Human microRNA (miR-20b-5p) modulates Alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences Alzheimer’s biomarkers. Mol. Psychiatry 2022, 27, 1256–1273. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Mu, Y.; Tian, H.; Wang, D.; Zhang, S.; Wang, H.; Liu, Y.; Di, C. MicroRNA-140 silencing represses the incidence of Alzheimer’s disease. Neurosci. Lett. 2021, 758, 135674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wu, Y.; Li, L.; Liu, C. MicroRNA-425-5p promotes tau phosphorylation and cell apoptosis in Alzheimer’s disease by targeting heat shock protein B8. J. Neural Transm. 2020, 127, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Li, Y.; He, R.; Li, X. Inhibition of microRNA-10b-5p up-regulates HOXD10 to attenuate Alzheimer’s disease in rats via the Rho/ROCK signalling pathway. J. Drug Target. 2021, 29, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Chen, Q.; Zhang, X. MicroRNA-455-5p/CPEB1 pathway mediates Abeta-related learning and memory deficits in a mouse model of Alzheimer’s disease. Brain Res. Bull. 2021, 177, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Li, Z.H.; Li, X.; Zheng, T.; Zhang, D.K. microRNA-592 blockade inhibits oxidative stress injury in Alzheimer’s disease astrocytes via the KIAA0319-mediated Keap1/Nrf2/ARE signaling pathway. Exp. Neurol. 2020, 324, 113128. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, K.Y.; Park, Y.; McLean, C.; Park, J.; Lee, J.H.; Kim, B.C.; Huh, Y.H.; Lee, K.H.; Song, W.K. Enhanced Expression of microRNA-1273g-3p Contributes to Alzheimer’s Disease Pathogenesis by Regulating the Expression of Mitochondrial Genes. Cells 2021, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Lee, S.; Lee, H.J.; Min, J.W.; Iwatsubo, T.; Teunissen, C.E.; Cho, H.J.; Ryu, J.H. Targeting MicroRNA-485-3p Blocks Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2022, 23, 3566. [Google Scholar] [CrossRef]
- Baby, N.; Alagappan, N.; Dheen, S.T.; Sajikumar, S. MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Aβ(1–42)-induced model of Alzheimer’s disease. Aging Cell 2020, 19, e13046. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, S.; Di, W.; Xia, M.; Dong, L.; Zhao, Y.; Ling, S.; He, J.; Xue, X.; Chen, X.; et al. Amyloid-beta protein and MicroRNA-384 in NCAM-Labeled exosomes from peripheral blood are potential diagnostic markers for Alzheimer’s disease. CNS Neurosci. Ther. 2022, 28, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, J.A.; Then, M.L.; McDougal, K.B.; Mihailescu, M.R. Characterization of a G-Quadruplex Structure in Pre-miRNA-1229 and in Its Alzheimer’s Disease-Associated Variant rs2291418: Implications for miRNA-1229 Maturation. Int. J. Mol. Sci. 2020, 21, 767. [Google Scholar] [CrossRef] [PubMed]
- Pena-Bautista, C.; Tarazona-Sanchez, A.; Braza-Boils, A.; Balaguer, A.; Ferre-Gonzalez, L.; Canada-Martinez, A.J.; Baquero, M.; Chafer-Pericas, C. Plasma microRNAs as potential biomarkers in early Alzheimer disease expression. Sci. Rep. 2022, 12, 15589. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.S.; Nygaard, A.B.; Christensen, T. miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia–an exploratory study. Transl. Neurodegener. 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Cong, Y.; Feng, N.; Liang, W.; Wu, Y. Up-regulated microRNA-132 reduces the cognition-damaging effect of sevoflurane on Alzheimer’s disease rats by inhibiting FOXA1. Genomics 2021, 113, 3644–3652. [Google Scholar] [CrossRef]
- He, B.; Chen, W.; Zeng, J.; Tong, W.; Zheng, P. MicroRNA-326 decreases tau phosphorylation and neuron apoptosis through inhibition of the JNK signaling pathway by targeting VAV1 in Alzheimer’s disease. J. Cell. Physiol. 2020, 235, 14. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zou, T.; Zhang, M.; Fan, W.; Zhang, T.; Jiang, Y.; Cai, Y.; Chen, F.; Chen, X.; Sun, Y.; et al. MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer’s disease. Theranostics 2021, 11, 4103–4121. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, M.; Guo, L.; Zhu, L.; Hou, J.; Zhang, L.; Pero, A.; Ng, S.; Gaamouch, F.E.; Elder, G.; et al. MicroRNA-195 rescues ApoE4-induced cognitive deficits and lysosomal defects in Alzheimer’s disease pathogenesis. Mol. Psychiatry 2021, 26, 4687–4701. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Zhang, Y. An investigation of microRNA-103 and microRNA-107 as potential blood-based biomarkers for disease risk and progression of Alzheimer’s disease. J. Clin. Lab. Anal. 2020, 34, e23006. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Morton, H.; Sawant, N.; Orlov, E.; Bunquin, L.E.; Pradeepkiran, J.A.; Alvir, R.; Reddy, P.H. MicroRNA-455-3p improves synaptic, cognitive functions and extends lifespan: Relevance to Alzheimer’s disease. Redox Biol. 2021, 48, 102182. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Lei, C.; Dong, Y. MicroRNA-149 is downregulated in Alzheimer’s disease and inhibits β-amyloid accumulation and ameliorates neuronal viability through targeting BACE1. Genet. Mol. Biol. 2021, 44, e20200064. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Morais, G.S., Jr.; Henriques, A.D.; Machado-Silva, W.; Perez, D.I.V.; Brito, C.J.; Camargos, E.F.; Moraes, C.F.; Nóbrega, O.T. Whole-Blood Levels of MicroRNA-9 Are Decreased in Patients With Late-Onset Alzheimer Disease. Am. J. Alzheimer’s Dis. Other Demen. 2020, 35, 1533317520911573. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, L.; Hu, Y.; Jiang, L.; Liang, N.; Chen, J.; Qin, H.; Tang, N. MicroRNA-107 Ameliorates Damage in a Cell Model of Alzheimer’s Disease by Mediating the FGF7/FGFR2/PI3K/Akt Pathway. J. Mol. Neurosci. 2020, 70, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Chen, S.; Wang, Y.; Yao, W.; Gao, X. MicroRNA-23b attenuates tau pathology and inhibits oxidative stress by targeting GnT-III in Alzheimer’s disease. Neuropharmacology 2021, 196, 108671. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Shen, X.; Cao, Y.; Qu, L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/beta-catenin pathway. Aging 2021, 13, 15285–15306. [Google Scholar] [CrossRef] [PubMed]
- Barros-Viegas, A.T.; Carmona, V.; Ferreiro, E.; Guedes, J.; Cardoso, A.M.; Cunha, P.; Almeida, L.P.; Oliveira, C.R.; Magalhães, J.P.; Peça, J.; et al. miRNA-31 Improves Cognition and Abolishes Amyloid-beta Pathology by Targeting APP and BACE1 in an Animal Model of Alzheimer’s Disease. Mol. Ther. Nucleic Acids 2020, 19, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Q. MicroRNA miR-212 regulates PDCD4 to attenuate Aβ25–35-induced neurotoxicity via PI3KAKT signaling pathway in Alzheimer’s disease. Biotechnol. Lett. 2020, 42, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chen, J.; Liu, Y.; Cui, X.; Wang, C.; Zong, S.; Wang, L.; Lu, Z. MicroRNA-22-3p ameliorates Alzheimer’s disease by targeting SOX9 through the NF-κB signaling pathway in the hippocampus. J. Neuroinflamm. 2022, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Qu, Y.; Zhang, X.; Xu, X.F. miRNA-126a-3p participates in hippocampal memory via alzheimer’s disease-related proteins. Cereb. Cortex 2022, 32, 4763–4781. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Shen, H.; Sheng, Y.; Guan, Q. ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer’s disease. J. Cell. Mol. Med. 2021, 25, 7513–7523. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, P.; Grasso, M.; Manzini, V.; Zeni, A.; Castelluzzo, M.; Fontana, F.; Talarico, G.; Castellano, A.E.; Rivabene, R.; Crestini, A.; et al. Identification of miRNAs regulating MAPT expression and their analysis in plasma of patients with dementia. Front. Mol. Neurosci. 2023, 16, 1127163. [Google Scholar] [CrossRef]
- Smith, P.Y.; Hernandez-Rapp, J.; Jolivette, F.; Lecours, C.; Bisht, K.; Goupil, C.; Dorval, V.; Parsi, S.; Morin, F.; Planel, E.; et al. miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 2015, 24, 6721–6735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, M.; Teng, Z.; Tang, Y.P.; Chen, C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. J. Neurosci. 2014, 34, 14919–14933. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Bossers, K.; Janky, R.S.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.R.; Thathiah, A.; Greenberg, D.; et al. Alteration of the micro RNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef]
- Mezache, L.; Mikhail, M.; Garofalo, M.; Nuovo, G.J. Reduced miR-512 and the elevated expression of its targets cFLIP and MCL1 localize to neurons with hyperphosphorylated tau protein in Alzheimer disease. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; Vos, A.D.; et al. The beta-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Zhu, J.; Wang, Y.C.; Wu, J.; Liu, J.R.; Guo, H.D. Effects of exosomal miRNAs in the diagnosis and treatment of Alzheimer’s disease. Mech. Ageing Dev. 2021, 200, 111593. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2020, 8, 616161. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014, 47, 531. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Shuang, M.E.N.G.; Ying, L.I.; Yao, L.U.; Yue, Z.H.A.O.; Wang, P.C. MicroRNA-135a in ABCA1-labeled Exosome is a Serum Biomarker Candidate for Alzheimer’s Disease. Biomed. Environ. Sci. 2021, 34, 19–28. [Google Scholar] [PubMed]
- Manna, I.; De Benedittis, S.; Quattrone, A.; Maisano, D.; Iaccino, E.; Quattrone, A. Exosomal miRNAs as potential diagnostic biomarkers in Alzheimer’s disease. Pharmaceuticals 2020, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zhao, B.; Zhao, J.; Li, S. Potential roles of exosomal microRNAs as diagnostic biomarkers and therapeutic application in Alzheimer’s disease. Neural Plast. 2017, 2017, 7027380. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Barlow, S.; Hoe, J.; Aitken, L. Persistent barriers and facilitators to seeking help for a dementia diagnosis: A systematic review of 30 years of the perspectives of carers and people with dementia. Int. Psychogeriatr. 2020, 32, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, H.G.; Zaki, L.A.; Goodkin, O.; Das, R.K.; Steketee, R.M.; Barkhof, F.; Vernooij, M.W. Correction to: Technical and clinical validation of commercial automated volumetric MRI tools for dementia diagnosis-a systematic review. Neuroradiology 2021, 63, 1955. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Benzinger, T.L. The Value of Neuroimaging in Dementia Diagnosis. Continuum 2022, 28, 800–821. [Google Scholar] [CrossRef] [PubMed]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-quadruplexes: A promising target for cancer therapy. Mol. Cancer 2021, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bidzinska, J.; Cimino-Reale, G.; Zaffaroni, N.; Folini, M. G-quadruplex structures in the human genome as novel therapeutic targets. Molecules 2013, 18, 12368–12395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, C.; Chen, H.; Li, X.; Li, Y.; Fan, X. A simple G-quadruplex molecular beacon-based biosensor for highly selective detection of microRNA. Biosens. Bioelectron. 2017, 87, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Karantzoulis, S.; Galvin, J.E. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev. Neurother. 2011, 11, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.A.; Lochner, K.A.; Thambisetty, M.; Wingo, T.S.; Posner, S.F.; Ling, S.M. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimer’s Dement. 2017, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Iacono, D.; Raiciulescu, S.; Olsen, C.; Perl, D.P. Traumatic Brain Injury Exposure Lowers Age of Cognitive Decline in AD and Non-AD Conditions. Front. Neurol. 2021, 12, 573401. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Herl, L.D.; Farese, R.V., Jr.; Gao, F.B. MicroRNA-29b regulates the expression level of human progranulin, a secreted glycoprotein implicated in frontotemporal dementia. PLoS ONE 2010, 5, e10551. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, P.; Grasso, M.; Fontana, F.; Crestini, A.; Puopolo, M.; Del Vescovo, V.; Venerosi, A.; Calamandrei, G.; Vencken, S.F.; Greene, C.M.; et al. Reduced miR-659-3p Levels Correlate with Progranulin Increase in Hypoxic Conditions: Implications for Frontotemporal Dementia. Front. Mol. Neurosci. 2016, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; McKeever, P.; Kim, T.; Graff, C.; van Swieten, J.C.; Karydas, A.; Boxer, A.; Rosen, H.; Miller, B.L.; Laforce, R., Jr.; et al. Downregulation of exosomal miR-204-5p and miR-632 as a biomarker for FTD: A GENFI study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Chen-Plotkin, A.S.; Unger, T.L.; Gallagher, M.D.; Bill, E.; Kwong, L.K.; Volpicelli-Daley, L.; Busch, J.I.; Akle, S.; Grossman, M.; Deerlin, V.V.; et al. TMEM106B, the Risk Gene for Frontotemporal Dementia, Is Regulated by the microRNA-132/212 Cluster and Affects Progranulin Pathways. J. Neurosci. 2012, 32, 11213–11227. [Google Scholar] [CrossRef] [PubMed]
- Gascon, E.; Lynch, K.; Ruan, H.; Almeida, S.; Verheyden, J.M.; Seeley, W.W.; Dickson, D.W.; Petrucelli, L.; Sun, D.; Jiao, J.; et al. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat. Med. 2014, 20, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, P.; Grasso, M.; Puopolo, M.; D’Acunto, E.; Talarico, G.; Crestini, A.; Gasparini, M.; Campopiano, R.; Gambardella, S.; Castellano, A.E.; et al. Circulating miR-127-3p as a Potential Biomarker for Differential Diagnosis in Frontotemporal Dementia. J. Alzheimer’s Dis. 2018, 65, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Tagliafierro, L.; Glenn, O.C.; Zamora, M.E.; Beach, T.G.; Woltjer, R.L.; Lutz, M.W.; Chiba-Falek, O. Genetic analysis of SNCA 3’UTR and its corresponding miRNAs in relation to Parkinson’s compared to Dementia with Lewy Bodies. Alzheimer’s. Dement. 2017, 13, 1237. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yu, J.; Wu, Z.; Si, W.; Li, X.; Liu, Y.; Zhou, J.; Deng, R.; Chen, D. MicroRNA-210-5p Contributes to Cognitive Impairment in Early Vascular Dementia Rat Model Through Targeting Snap25. Front. Mol. Neurosci. 2018, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; Wu, Z.; Si, W.; Ren, Z.; Zhang, S.; Zhou, J.; Chen, D. miR-134-5p/Foxp2/Syn1 is involved in cognitive impairment in an early vascular dementia rat model. Int. J. Mol. Med. 2019, 44, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.W.; Lin, L.T.; Huang, J.; Wang, X.R.; Su, X.T.; Cao, Y.; Fisher, M.; Liu, C.Z. Acupuncture Attenuates Inflammation in Microglia of Vascular Dementia Rats by Inhibiting miR-93-Mediated TLR4/MyD88/NF-kappaB Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8253904. [Google Scholar] [PubMed]
- Wei, C.; Xu, X.; Zhu, H.; Zhang, X.; Gao, Z. Promotive role of microRNA-150 in hippocampal neurons apoptosis in vascular dementia model rats. Mol. Med. Rep. 2021, 23, 257. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wang, H.; Li, Y.; Luo, Y.; Liu, Y.; Wang, F. Study on the Function of miR-134 on Cognitive Function of Vascular Dementia (VD) Rats and Mechanism about Oxidative Stress and Autophagy and Cofilin 2 Level. J. Biomater. Tiss. Eng. 2022, 12, 1994–2000. [Google Scholar] [CrossRef]
- Han, X.; Zhou, L.; Tu, Y.; Wei, J.; Zhang, J.; Jiang, G.; Shi, Q.; Ying, H. Circulating exo-miR-154-5p regulates vascular dementia through endothelial progenitor cell-mediated angiogenesis. Front. Cell. Neurosci. 2022, 16, 881175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Wang, S.; Bai, X.; Lv, P. miR-181a regulates mitophagy and improves cognitive function in vascular dementia via targeting PINK1/Parkin pathway. Mater. Express 2023, 13, 1234–1240. [Google Scholar] [CrossRef]
- Qian, X.; Xu, Q.; Li, G.; Bu, Y.; Sun, F.; Zhang, J. Therapeutic Effect of Idebenone on Rats with Vascular Dementia via the MicroRNA-216a/RSK2/NF-kappaB Axis. Neuropsychiatr. Dis. Treat. 2021, 17, 533–543. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Shi, Y.; Li, S.; Liu, J.; Li, X.; Zhong, W.; Pan, Q. Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res. Ther. 2022, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, J.; Zhou, B.; Chang, H. MiR-322-5p Alleviates Cell Injury and Impairment of Cognitive Function in Vascular Dementia by Targeting TSPAN5. Yonsei Med. J. 2022, 63, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kirola, L.; Mukherjee, A.; Mutsuddi, M. Recent Updates on the Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Mol. Neurobiol. 2022, 59, 5673–5694. [Google Scholar] [CrossRef] [PubMed]
- Matar, E.; Ehgoetz Martens, K.A.; Halliday, G.M.; Lewis, S.J. Clinical features of Lewy body dementia: Insights into diagnosis and pathophysiology. J. Neurol. 2020, 267, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Weis, L.; Schifano, R.; Pistonesi, F.; Fiorenzato, E.; Antonini, A.; Biundo, R. Differences in cognitive profiles between Lewy body and Parkinson’s disease dementia. J. Neural. Transm. 2020, 127, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wightman, D.P.; Savage, J.E.; Tissink, E.; Romero, C.; Jansen, I.E.; Posthuma, D. The genetic overlap between Alzheimer’s disease, amyotrophic lateral sclerosis, Lewy body dementia, and Parkinson’s disease. Neurobiol. Aging 2023, 127, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Reed, X.; Krohn, L.; Heilbron, K.; Bandres-Ciga, S.; Tan, M.; Gibbs, J.R.; Hernandez, D.G.; Kumaran, R.; Langston, R.; et al. Genetic modifiers of risk and age at onset in GBA associated Parkinson’s disease and Lewy body dementia. Brain 2020, 143, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, M.; Bilgic, B.; Tinaz, S.; Emre, M. Parkinson’s Disease Dementia and Lewy Body Disease. Semin. Neurol. 2019, 39, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Marsal-García, L.; Urbizu, A.; Arnaldo, L.; Campdelacreu, J.; Vilas, D.; Ispierto, L.; Gascón-Bayarri, J.; Reñé, R.; Álvarez, R.; Beyer, K. Expression Levels of an Alpha-Synuclein Transcript in Blood May Distinguish between Early Dementia with Lewy Bodies and Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 725. [Google Scholar] [CrossRef] [PubMed]
- Beishon, L.C.; Hosford, P.; Gurung, D.; Brassard, P.; Minhas, J.S.; Robinson, T.G.; Haunton, V.; Panerai, R.B. The role of the autonomic nervous system in cerebral blood flow regulation in dementia: A review. Auton. Neurosci. 2022, 240, 102985. [Google Scholar] [CrossRef] [PubMed]
- Prajjwal, P.; Marsool, M.D.M.; Inban, P.; Sharma, B.; Asharaf, S.; Aleti, S.; Gadam, S.; Sakini, A.S.A.; Hadi, D.D. Vascular dementia subtypes, pathophysiology, genetics, neuroimaging, biomarkers, and treatment updates along with its association with Alzheimer’s dementia and diabetes mellitus. Dis. Mon. 2023, 69, 101557. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Rakugi, H.; Morishita, R. Roles of vascular risk factors in the pathogenesis of dementia. Hypertens. Res. 2020, 43, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Y.; Ding, X.; Li, W. Identification of lncRNA/circRNA-miRNA-mRNA ceRNA Network as Biomarkers for Hepatocellular Carcinoma. Front. Genet. 2022, 13, 838869. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Holsinger, R.D. Roles of non-coding RNA in alzheimer’s disease pathophysiology. Int. J. Mol. Sci. 2023, 24, 12498. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Luo, T.; Zou, S.; Zhou, X.; Shen, W.; Ji, X.; Li, Q.; Wu, A. Differentially expressed lncRNAs and miRNAs with associated ceRNA networks in aged mice with postoperative cognitive dysfunction. Oncotarget 2017, 8, 55901. [Google Scholar] [CrossRef] [PubMed]
- Boniolo, F.; Hoffmann, M.; Roggendorf, N.; Tercan, B.; Baumbach, J.; Castro, M.A.; Robertson, A.G.; Saur, D.; List, M. spongEffects: ceRNA modules offer patient-specific insights into the miRNA regulatory landscape. Bioinformatics 2023, 39, btad276. [Google Scholar] [CrossRef] [PubMed]
- Guil, S.; Esteller, M. RNA–RNA interactions in gene regulation: The coding and noncoding players. Trends Biochem. Sci. 2015, 40, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Tay, Y. Noncoding RNA: RNA regulatory networks in cancer. Int. J. Mol. Sci. 2018, 19, 1310. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X.; Zhang, Y.; Zhang, Q.; Li, L. Comprehensive Analysis of ceRNA Regulation Network Involved in the Development of Coronary Artery Disease. Biomed Res. Int. 2021, 2021, 665815. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, H.; Tang, Y.; Wei, Y.; Wei, W.; Zhang, L.; Chen, J. The development and controversy of competitive endogenous RNA hypothesis in non-coding genes. Mol. Cell. Biochem. 2021, 476, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.C.R.; Mamede, I.; Luizon, M.R.; Gomes, K.B. Role of long non-coding RNAs in the pathophysiology of Alzheimer’s disease and other dementias. Mol. Biol. Rep. 2024, 51, 270. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Wasson, M.C.D.; Brown, J.M.; Fernando, W.; Marcato, P. LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 2021, 509, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, M.D.; McDonald, R.A.; Baker, A.H. lncRNA/MicroRNA interactions in the vasculature. Clin. Pharm. Therap. 2016, 99, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Taheriazam, A.; Orouei, S.; Fallah, S.; Sanaei, A.; Hejazi, E.S.; Kakavand, A.; Rezaei, S.; Heidari, H.; Behroozaghdam, M.; et al. LncRNA-miRNA axis in tumor progression and therapy response: An emphasis on molecular interactions and therapeutic interventions. Biomed. Pharmacother. 2022, 154, 113609. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.S.; Ebrahimi, R.; Al-e-Ahmad, A.; Panahi, G.; Meshkani, R.; Younesi, S.; Saadat, P.; Parsian, H. Competing Endogenous RNAs (CeRNAs): Novel Network in Neurological Disorders. Curr. Med. Chem. 2021, 28, 5983–6010. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The role of lncRNAs in gene expression regulation through mRNA stabilization. Non-Coding RNA 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Sabaie, H.; Amirinejad, N.; Asadi, M.R.; Jalaiei, A.; Daneshmandpour, Y.; Rezaei, O.; Taheri, M.; Rezazadeh, M. Molecular Insight Into the Therapeutic Potential of Long Non-coding RNA-Associated Competing Endogenous RNA Axes in Alzheimer’s Disease: A Systematic Scoping Review. Front. Aging Neurosci. 2021, 13, 742242. [Google Scholar] [CrossRef]
- Arun, G.; Aggarwal, D.; Spector, D.L. MALAT1 Long Non-Coding RNA: Functional Implications. Non-Coding RNA 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Z. lncRNA NEAT1: Key player in neurodegenerative diseases. Ageing Res. Rev. 2023, 86, 101878. [Google Scholar] [CrossRef] [PubMed]
- Maniati, M.S.; Maniati, M.; Yousefi, T.; Ahmadi-Ahangar, A.; Tehrani, S.S. New insights into the role of microRNAs and long noncoding RNAs in most common neurodegenerative diseases. J. Cell. Biochem. 2019, 120, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.R.; Hassani, M.; Kiani, S.; Sabaie, H.; Moslehian, M.S.; Kazemi, M.; Ghafouri-Fard, S.; Taheri, M.; Rezazadeh, M. The Perspective of Dysregulated LncRNAs in Alzheimer’s Disease: A Systematic Scoping Review. Front. Aging Neurosci. 2021, 13, 709568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, R. Deregulated lncRNA MAGI2-AS3 in Alzheimer’s disease attenuates amyloid-beta induced neurotoxicity and neuroinflammation by sponging miR-374b-5p. Exp. Gerontol. 2021, 144, 111180. [Google Scholar] [CrossRef]
- Ding, Y.; Luan, W.; Wang, Z.; Cao, Y. LncRNA BDNF-AS as ceRNA regulates the miR-9-5p/BACE1 pathway affecting neurotoxicity in Alzheimer’s disease. Arch. Gerontol. Geriatr. 2022, 99, 104614. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, Z.; Wang, J.; Feng, H. Expression relationship and significance of NEAT1 and miR-27a-3p in serum and cerebrospinal fluid of patients with Alzheimer’s disease. BMC Neurol. 2022, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Wang, G.Q.; Wang, N.N.; Yu, Q.Y.; Liu, R.L.; Shi, W.Q. The long-non-coding RNA NEAT1 is a novel target for Alzheimer’s disease progression via miR-124/BACE1 axis. Neurol. Res. 2019, 41, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Riku, Y.; Seilhean, D.; Duyckaerts, C.; Boluda, S.; Iguchi, Y.; Ishigaki, S.; Iwasaki, Y.; Yoshida, M.; Sobue, G.; Katsuno, M. Pathway from TDP-43-Related Pathology to Neuronal Dysfunction in Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration. Int. J. Mol. Sci. 2021, 22, 3843. [Google Scholar] [CrossRef] [PubMed]
- Gumina, V.; Onesto, E.; Colombrita, C.; Maraschi, A.; Silani, V.; Ratti, A. Inter-Species Differences in Regulation of the Progranulin-Sortilin Axis in TDP-43 Cell Models of Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 5866. [Google Scholar] [CrossRef] [PubMed]
- Tollervey, J.R.; Curk, T.; Rogelj, B.; Briese, M.; Cereda, M.; Kayikci, M.; König, J.; Hortobágyi, T.; Nishimura, A.L.; Župunski, V.; et al. Characterising the RNA targets and position-dependent splicing regulation by TDP-43; implications for neurodegenerative diseases. Nat. Neurosci. 2011, 14, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xiao, B.; Zhang, M. Potential Intersections between lncRNA, Vascular Cognitive Impairment, and Immunization Strategies: Insights and Future Directions. Vaccines 2024, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Hou, X.; Jin, C.; Chen, X.; Pan, C.; Fu, H.; Song, L.; Xue, J. HNSC exosome-derived MIAT improves cognitive disorders in rats with vascular dementia via the miR-34b-5p/CALB1 axis. Am. J. Transl. Res. 2021, 13, 10075–10093. [Google Scholar] [PubMed]
- Wang, P.; Mao, S.; Yi, T.; Wang, L. LncRNA MALAT1 Targets miR-9-3p to Upregulate SAP97 in the Hippocampus of Mice with Vascular Dementia. Biochem. Genet. 2023, 61, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Reddy, P.H. Non-Coding RNAs Based Molecular Links in Type 2 Diabetes, Ischemic Stroke, and Vascular Dementia. J. Alzheimer’s Dis. 2020, 75, 353–383. [Google Scholar] [CrossRef] [PubMed]
- Firat, H.; Nazi, S.A.A.; Bier, J.C.; Blanc, F.; Boutillier, S.; Danilin, S.; David, R.; Démonet, J.F.; Dubois, B.; Frisoni, G.B.; et al. ADDIA Consortium; ADKIT Consortium. lncRNAs as a novel source of diagnostic applications for early Alzheimer’s disease and other dementia types. Alzheimer’s Dement. 2020, 16, e039788. [Google Scholar] [CrossRef]
- Chen, L.; Shan, G. CircRNA in cancer: Fundamental mechanism and clinical potential. Cancer Lett. 2021, 505, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, J.; Zhang, K.Q. Structure, regulation, and function of linear and circular long non-coding RNAs. Front. Genet. 2020, 11, 150. [Google Scholar] [CrossRef]
- Lu, M. Circular RNA: Functions, applications and prospects. ExRNA 2020, 2, 1. [Google Scholar] [CrossRef]
- Puri, S.; Hu, J.; Sun, Z.; Lin, M.; Stein, T.D.; Farrer, L.A.; Wolozin, B.; Zhang, X. Identification of circRNAs linked to Alzheimer’s disease and related dementias. Alzheimer’s Dement. 2023, 19, 3389–3405. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chao, J.; Yao, H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol. Therapeut. 2018, 187, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, F.; Bao, S.; Sun, J. Systematic Characterization of Circular RNA-Associated CeRNA Network Identified Novel circRNA Biomarkers in Alzheimer’s Disease. Front. Bioeng. Biotechnol. 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Shang, H.; Chen, X.; Yang, S.; Qu, Y.; Ding, J.; Li, X. Circular RNA circ_0000950 promotes neuron apoptosis, suppresses neurite outgrowth and elevates inflammatory cytokines levels via directly sponging miR-103 in Alzheimer’s disease. Cell Cycle 2019, 18, 2197–2214. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tan, L.; Wang, X. Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci. Bull. 2019, 35, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Alexandrov, P.N.; Jaber, V.; Lukiw, W.J. Deficiency in the Ubiquitin Conjugating Enzyme UBE2A in Alzheimer’s Disease (AD) is Linked to Deficits in a Natural Circular miRNA-7 Sponge. Genes 2016, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, D.; Xu, B.; Yang, C.; Wang, L. Circular RNA circ_0005835 promotes promoted neural stem cells proliferation and differentiate to neuron and inhibits inflammatory cytokines levels through miR-576-3p in Alzheimer’s disease. Environ. Sci. Pollut. R. 2022, 29, 35934–35943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Yang, P.; Wu, J.; Pang, X.; Pang, C. Bioinformatics-based study reveals that AP2M1 is regulated by the circRNA-miRNA-mRNA interaction network and affects Alzheimer’s disease. Front. Genet. 2022, 13, 1049786. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.; Chen, Y.H.; Zhang, K. Identification of Circular RNA hsa_Circ_0003391 in Peripheral Blood Is Potentially Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 601965. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, X.; Fan, H.; Sun, J.; Ni, M.; Zhang, L.; Fang, F.; Zhang, W.; Ma, P. Circular RNA AXL increases neuron injury and inflammation through targeting microRNA-328 mediated BACE1 in Alzheimer’s disease. Neurosci. Lett. 2022, 776, 136531. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, X.; Luo, J.; Liu, H.; Zhong, S.; Chen, J. Expression of circular RNAs in the vascular dementia rats. Neurosci. Lett. 2020, 735, 135087. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Zhao, M.; Gao, Z. Neuro-protective roles of long non-coding RNA MALAT1 in Alzheimer’s disease with the involvement of the microRNA-30b/CNR1 network and the following PI3K/AKT activation. Exp. Mol. Pathol. 2020, 117, 104545. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Cai, P.; Chen, Z.; Yang, Q.; Chen, X.; Wang, X.; Zhuang, X. Long noncoding RNA MALAT1 and its target microRNA-125b are potential biomarkers for Alzheimer’s disease management via interactions with FOXQ1, PTGS2 and CDK5. Am. J. Transl. Res. 2020, 12, 5940–5954. [Google Scholar] [PubMed]
- Zhou, B.; Li, L.; Qiu, X.I.N.; Wu, J.; Xu, L.E.I.; Shao, W. Long non-coding RNA ANRIL knockdown suppresses apoptosis and pro-inflammatory cytokines while enhancing neurite outgrowth via binding microRNA-125a in a cellular model of Alzheimer’s disease. Mol. Med. Rep. 2020, 22, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Bao, H.L.; Dong, L.X.; Liu, Y.; Zhang, G.W.; An, F.M. Silenced lncRNA H19 and up-regulated microRNA-129 accelerates viability and restrains apoptosis of PC12 cells induced by Aβ25-35 in a cellular model of Alzheimer’s disease. Cell Cycle 2021, 20, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ge, Y.; Liu, Q.; Li, Y.X.; Chao, X.; Guan, J.J.; Diwu, Y.C.; Zhang, Q. LncRNA BACE1-AS Promotes Autophagy-Mediated Neuronal Damage Through The miR-214-3p/ATG5 Signalling Axis In Alzheimer’s Disease. Neuroscience 2021, 455, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, X.; Li, H.; Wang, J.; Lu, Z. Triptolide improves Alzheimer’s disease by regulating the NF-κB signaling pathway through the lncRNA NEAT1microRNA 361-3pTRAF2 axis. Exp. Ther. Med. 2023, 26, 440. [Google Scholar] [PubMed]
- Zhang, N.; Gao, Y.; Yu, S.; Sun, X.; Shen, K. Berberine attenuates Aβ42-induced neuronal damage through regulating circHDAC9/miR-142-5p axis in human neuronal cells. Life Sci. 2020, 252, 117637. [Google Scholar] [CrossRef] [PubMed]

| miRNA Expression in AD | miRNA | Target Gene | Clinical Value | Function | Study Model | Reference |
|---|---|---|---|---|---|---|
| UP | miR-20b-5p | APP | N/A | Reduce intracellular Ca2+ transients during neuronal membrane depolarization | Human tissues and in vitro | [57] |
| miR-140 | PINK1 | Biomarker and therapeutic target | Improve amyloid pathology and mitochondrial dysfunction while inhibiting cellular autophagy | In vivo | [58] | |
| miR-425-5p | HSPB8 | Therapeutic target | Reduce cell apoptosis and tau phosphorylation | Human tissues and in vitro | [59] | |
| miR-10b-5p | HOXD10 | Therapeutic target | Reduce nerve cell apoptosis, inflammatory response, and oxidative stress | In vivo | [60] | |
| miR-455-5p | CPEB1 | Therapeutic target | Synaptic plasticity and memory disorders | In vivo | [61] | |
| miR-592 | KIAA0319 | Therapeutic target | Promotion of oxidative stress injury in astrocytes | In vivo and in vitro | [62] | |
| miR-1273g-3p | TIMM13 | Biomarker and therapeutic target | Mitochondrial dysfunction | Human tissues and in vitro | [63] | |
| miR-485-3p | CD36 | Biomarker and therapeutic target | Inhibit microglial Aβ phagocytosis | In vivo and in vitro | [64] | |
| miR-134-5p | CREB-1 | Therapeutic target | Regulation of long-term plasticity and cellular correlation | In vivo and in vitro | [65] | |
| BDNF | ||||||
| miR-384 | - | Diagnostic biomarker | Involved in immune response | Human samples | [66] | |
| miR-1229-3p | SORL1 | Therapeutic target | Engaged in the processing and movement of Aβ | In vitro | [67] | |
| miR-29a-3p | C1QTNF6 | Diagnostic biomarker | Migration of neurons and evolution of the nervous system | Human samples | [68] | |
| ROBO1 | ||||||
| DAAM2 | ||||||
| let-7i-5p | - | Diagnostic biomarker | Regulating APP and BACE1, leading to AD pathology | Human samples | [69] | |
| miR-15a-5p | ||||||
| miR-34a | VAMP2 | Therapeutic target | Abnormalities in energy metabolism, resting state network activity, and synaptic plasticity | Human tissues and in vivo | [70] | |
| SYT1 | ||||||
| DOWN | miR-92a-3p | SYNJ1 | Diagnostic biomarker | Connected to protein and lipid pathways, transcription, structural function, and amyloid-beta clearance/cell signaling | Human samples | [68] |
| CBLN4 | ||||||
| BCL2L2 | ||||||
| NEFH | ||||||
| REST | ||||||
| miR-132 | FOXA1 | Therapeutic target | Improve cognitive impairment | In vivo | [71] | |
| miR-326 | VAV1 | Therapeutic target | Tau phosphorylation and neuronal apoptosis | In vivo | [72] | |
| miR-146a-5p | Nkd2 | Therapeutic target | Inhibit LPS/Aβ-induced neuroinflammation and regulate a microglial phenotype | In vivo and in vitro | [73] | |
| miR-195 | ApoE4 | Therapeutic target | Regulate tau hyperphosphorylation and secretion | Human tissues and in vivo | [74] | |
| miR-103 | BACE1 | Prognosis biomarker | Enhance neurite outgrowth and reduce neuronal apoptosis | Human tissues | [75] | |
| miR-107 | ||||||
| miR-455-3p | - | N/A | Improve neuronal activity and overall brain function | In vivo | [76] | |
| miR-146a | TRAF6 | N/A | Suppress astrocyte inflammation in AD | In vivo | [77] | |
| miR-149 | BACE1 | Diagnostic biomarker | Reduce accumulation of Aβ and improve the viability of neurons | Human serum and in vitro | [78] | |
| miR-9-5p | BACE1 | Diagnostic biomarker | Regulate differentiation of post-mitotic neurons from neural progenitor cells | Human serum | [79] | |
| SIRT1 | ||||||
| miR-107 | FGF7 | N/A | Ameliorate Aβ-induced inflammation and apoptosis | Human serum and in vitro | [80] | |
| miR-23b | GnT-III | Therapeutic target | Inhibit oxidative stress and activate the Akt/GSK-3β signaling pathway | In vivo and in vitro | [81] | |
| miR-29c-3p | BACE1 | Therapeutic target | Aβ-induced suppression of neuronal viability and increase in apoptosis | In vivo | [82] | |
| miR-31 | APP | Therapeutic target | Improve cognitive function and prevent the progression of the disease | In vivo and in vitro | [83] | |
| BACE1 | ||||||
| miR-212 | PDCD4 | N/A | Mitigate Aβ25-35 induced neurotoxicity through modulation of the PI3K/AKT pathway | Human serum and in vitro | [84] | |
| miR-22-3p | SOX9 | Therapeutic target | Enhance apoptosis attenuation and reduce Aβ accumulation | In vivo and in vitro | [85] | |
| miR-126a-3p | EFHD2 | Therapeutic target | Consolidate contextual fear memory | In vivo and in vitro | [86] | |
| miR-22 | GSDMD | Therapeutic target | Enhancing memory and motor abilities | In vivo | [87] | |
| miR-92a-3p | MAPT | Diagnostic biomarker | Control the expression of tau in a neuroblastoma | Human samples and in vitro | [88] | |
| miR-320a | ||||||
| miR-132 | MAPT | Therapeutic target | Partially recovered tau metabolism and memory | Human tissues, in vivo and in vitro | [89] | |
| miR-212 | ||||||
| miR-188-3p | BACE1 | Therapeutic target | Enhanced synaptic and cognitive function through decreased neuroinflammation and Aβ due to MAGL inhibition | In vivo and in vitro | [90] | |
| miR-132-3p | FOXO1a | N/A | Hyperphosphorylation of tau | Human tissues | [91] | |
| miR-512 | cFLIP | N/A | Apoptosis initiating factor, APAF-1 activity, activated caspase-3, elevated caspase-4 and caspase-8, and the TUNEL assay was negative in the regions where neurons displayed hyperphosphorylated tau | Human samples | [92] | |
| MCL1 |
| Name of Disease | miRNA Expression in Disease | miRNA | Target Gene | Clinical Value | Function | Study Model | Reference |
|---|---|---|---|---|---|---|---|
| FTD | UP | miR-29b | GRN | Therapeutic target | Induce progranulin deficiency and development of neurodegenerative diseases | In vitro | [110] |
| miR-659-3p | GRN | N/A | Neurotrophic, anti-inflammatory activity and act as neuroprotective against oxygen/glucose deprivation, oxidative injury, and hypoxia stress | In vivo and in vitro | [111] | ||
| DOWN | miR-632 | GRN | Diagnostic biomarker | Prevents apoptosis, averting degenerative changes in the frontal and temporal lobes | Human samples | [112] | |
| miR-132 | TMEM106B | Therapeutic target | Disrupt endosomal-lysosomal pathways, trapping PGRN in TMEM106B-positive compartments, elevating intracellular PGRN levels | Human tissues and in vitro | [113] | ||
| miR-212 | |||||||
| miR-124 | CHMP2B | Therapeutic target | Reduce AMPAR levels and partial rescue of behavioral deficits | Human tissues and in vivo | [114] | ||
| miR-127-3p | - | Diagnostic biomarker | Regulate neuronal differentiation | Human samples | [115] | ||
| LBD | UP | miR-7 | SNCA | N/A | The process of forming dopamine-producing neurons | Human tissues and in vitro | [116] |
| miR-153 | |||||||
| VD | UP | miR-210-5p | Snap25 | Therapeutic target | Synaptic loss impacting spatial learning and memory | In vivo | [117] |
| miR-134-5p | Foxp2 | Therapeutic target | Contribute to speech and language and associated neurodevelopmental disorders | In vivo and in vitro | [118] | ||
| miR-93 | TSPAN5 | Therapeutic target | Reduce inflammation and promote positive regulation of the TLR4 signaling pathway | In vivo | [119] | ||
| miR-150 | HOXA1 | Biomarker and therapeutic target | Increase cell apoptosis | In vivo and in vitro | [120] | ||
| miR-134 | Cofilin 2 | N/A | Regulate oxidative stress and autophagy | In vivo | [121] | ||
| miR-154-5p | PRKAA2 | Biomarker and therapeutic target | Impair EPC function and angiogenesis | Human samples and in vivo | [122] | ||
| miR-181a | PINK1 | N/A | Alleviate mitochondrial dysfunction and improve cognitive function | In vivo | [123] | ||
| Parkin | |||||||
| DOWN | miR-216a | RSK2 | Therapeutic target | Regulate oxidative stress and neuroinflammation | In vivo | [124] | |
| miR-132-3p | RASA1 | Therapeutic target | Improve neuronal and synaptic dysfunction by activating the Ras/Akt/GSK-3β pathway | In vivo | [125] | ||
| miR-322-5p | TSPAN5 | Therapeutic target | Improve cell apoptosis, inflammatory response, and cognitive function | In vivo and in vitro | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-m.; Kim, W.R.; Park, E.G.; Lee, D.H.; Lee, Y.J.; Shin, H.J.; Jeong, H.-s.; Roh, H.-Y.; Kim, H.-S. Exploring the Regulatory Landscape of Dementia: Insights from Non-Coding RNAs. Int. J. Mol. Sci. 2024, 25, 6190. https://doi.org/10.3390/ijms25116190
Kim J-m, Kim WR, Park EG, Lee DH, Lee YJ, Shin HJ, Jeong H-s, Roh H-Y, Kim H-S. Exploring the Regulatory Landscape of Dementia: Insights from Non-Coding RNAs. International Journal of Molecular Sciences. 2024; 25(11):6190. https://doi.org/10.3390/ijms25116190
Chicago/Turabian StyleKim, Jung-min, Woo Ryung Kim, Eun Gyung Park, Du Hyeong Lee, Yun Ju Lee, Hae Jin Shin, Hyeon-su Jeong, Hyun-Young Roh, and Heui-Soo Kim. 2024. "Exploring the Regulatory Landscape of Dementia: Insights from Non-Coding RNAs" International Journal of Molecular Sciences 25, no. 11: 6190. https://doi.org/10.3390/ijms25116190
APA StyleKim, J.-m., Kim, W. R., Park, E. G., Lee, D. H., Lee, Y. J., Shin, H. J., Jeong, H.-s., Roh, H.-Y., & Kim, H.-S. (2024). Exploring the Regulatory Landscape of Dementia: Insights from Non-Coding RNAs. International Journal of Molecular Sciences, 25(11), 6190. https://doi.org/10.3390/ijms25116190







