Colonic Tuft Cells: The Less-Recognized Therapeutic Targets in Inflammatory Bowel Disease and Colorectal Cancer
Abstract
1. Introduction
2. The Structure of the Intestinal Mucosa
3. Morphological Characteristics and Origins of Tuft Cells
4. Differentiation of Tuft Cells
5. Identification of Human Intestinal Tuft Cells
6. Tuft Cell Receptors, Chemical Sensing, and Signal Transduction Mediators
7. The Role of Tuft Cells in IBD
8. Tuft-Cell-Based IBD Treatment Options and Their Controversies
9. The Role of Tuft Cells in Colorectal Cancer
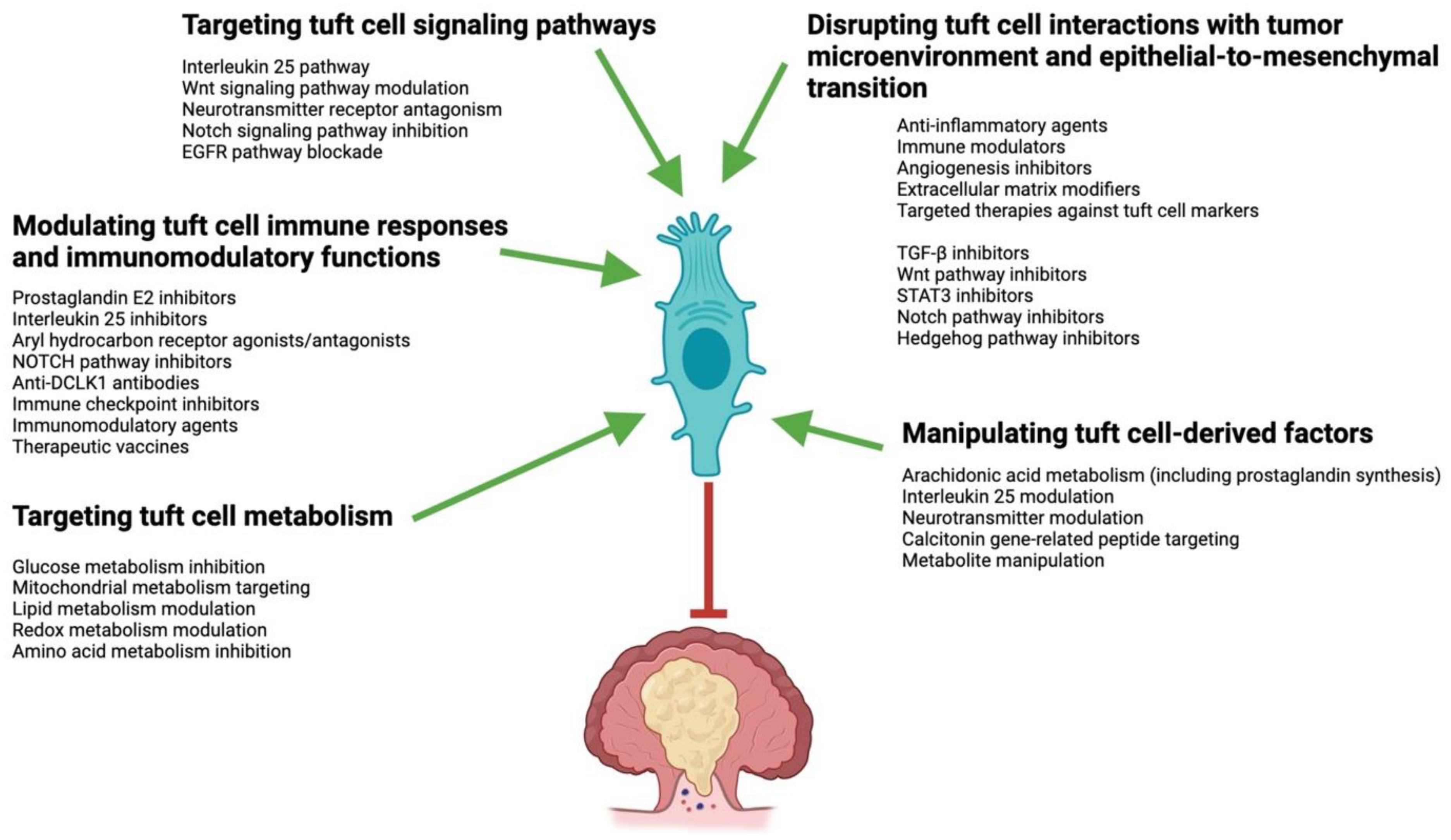
10. Tuft-Cell-Based CRC Therapies and Their Considerations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Ireland, H.; Houghton, C.; Howard, L.; Winton, D.J. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Dev. Dyn. 2005, 233, 1332–1336. [Google Scholar] [CrossRef]
- Sato, T.; Van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; Van De Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef]
- Parker, H.E.; Gribble, F.M.; Reimann, F. The role of gut endocrine cells in control of metabolism and appetite. Exp. Physiol. 2014, 99, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Frey, A.; Kraehenbuhl, J.P. Epithelial M cells: Gateways for mucosal infection and immunization. Cell 1996, 86, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.-F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 Requirements Define Tuft Cells as a New Secretory Cell Type in the Intestinal Epithelium. J. Cell Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Saqui-Salces, M.; Keeley, T.M.; Grosse, A.S.; Qiao, X.T.; El-Zaatari, M.; Gumucio, D.L.; Samuelson, L.C.; Merchant, J.L. Gastric Tuft Cells Express DCLK1 and Are Expanded in Hyperplasia. Histochem. Cell Biol. 2011, 136, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Jay, P. Intestinal tuft cells: Epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol. 2016, 9, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.; Baena, V.; Kaelberer, M.M.; Getaneh, F.; Chinchilla, S.; Bohórquez, D.V. The Intestinal Tuft Cell Nanostructure in 3D. Sci. Rep. 2017, 7, 1652. [Google Scholar] [CrossRef] [PubMed]
- Luciano, L.; Reale, E. A New Morphological Aspect of the Brush Cells of the Mouse Gallbladder Epithelium. Cell Tissue Res. 1979, 201, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Luciano, L.; Reale, E. Brush Cells of the Mouse Gallbladder—A Correlative Light- and Electron-Microscopical Study. Cell Tissue Res. 1990, 262, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Luciano, L.; Groos, S.; Reale, E. Brush Cells of Rodent Gallbladder and Stomach Epithelia Express Neurofilaments. J. Histochem. Cytochem. 2003, 51, 187–198. [Google Scholar] [CrossRef]
- Cheng, X.; Voss, U.; Ekblad, E. Tuft Cells: Distribution and Connections With Nerves and Endocrine Cells in Mouse Intestine. Exp. Cell Res. 2018, 369, 105–111. [Google Scholar] [CrossRef]
- Cheng, X.; Voss, U.; Ekblad, E. A Novel Serotonin-Containing Tuft Cell Subpopulation in Mouse Intestine. Cell Tissue Res. 2019, 376, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Middelhoff, M.; Westphalen, C.B.; Hayakawa, Y.; Yan, K.S.; Gershon, M.D.; Wang, T.C.; Quante, M. Dclk1-Expressing Tuft Cells: Critical Modulators of the Intestinal Niche? Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G285–G299. [Google Scholar] [CrossRef]
- Haber, A.L.; Biton, M.; Rogel, N.; Herbst, R.H.; Shekhar, K.; Smillie, C.; Burgin, G.; Delorey, T.M.; Howitt, M.R.; Katz, Y.; et al. A Single-Cell Survey of the Small Intestinal Epithelium. Nature 2017, 551, 333–339. [Google Scholar] [CrossRef]
- Kjærgaard, S.; Jensen, T.S.; Feddersen, U.R.; Bindslev, N.; Grunddal, K.V.; Poulsen, S.S.; Rasmussen, H.B.; Budtz-Jørgensen, E.; Berner-Hansen, M. Decreased Number of Colonic Tuft Cells in Quiescent Ulcerative Colitis Patients. Eur. J. Gastroenterol. Hepatol. 2021, 33, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Van den Born, M.; Van Es, J.H.; Clevers, H. Enteroendocrine Cells Switch Hormone Expression Along the Crypt-to-Villus BMP Signalling Gradient. Nat. Cell Biol. 2018, 20, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Grunddal, K.V.; Tonack, S.; Egerod, K.L.; Thompson, J.J.; Petersen, N.; Engelstoft, M.S.; Vagne, C.; Keime, C.; Gradwohl, G.; Offermanns, S.; et al. Adhesion Receptor Adgrg2/Gpr64 Is in the GI-Tract Selectively Expressed in Mature Intestinal Tuft Cells. Mol. Metab. 2021, 101231. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Asfaha, S.; Hayakawa, Y.; Takemoto, Y.; Lukin, D.J.; Nuber, A.H.; Brandtner, A.; Setlik, W.; Remotti, H.; Muley, A.; et al. Long-Lived Intestinal Tuft Cells Serve as Colon Cancer-Initiating Cells. J. Clin. Investig. 2014, 124, 1283–1295. [Google Scholar] [CrossRef]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, C.; Nevo, S.; Giladi, A.; Kadouri, N.; Pouzolles, M.; Gerbe, F.; David, E.; Machado, A.; Chuprin, A.; Tóth, B.; et al. Single-Cell Mapping of the Thymic Stroma Identifies IL-25-Producing Tuft Epithelial Cells. Nature 2018, 559, 622–626. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A Revised Airway Epithelial Hierarchy Includes CFTR-Expressing Ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-Cell-Derived IL-25 Regulates an Intestinal ILC2-Epithelial Response Circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.A.; Banerjee, A.; McKinley, E.T.; Simmons, A.J.; Ping, J.; Roland, J.T.; Franklin, J.L.; Liu, Q.; Gerdes, M.J.; Coffey, R.J.; et al. Unsupervised Trajectory Analysis of Single-Cell RNA-Seq and Imaging Data Reveals Alternative Tuft Cell Origins in the Gut. Cell Syst. 2018, 6, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.S.; Aslam, U.; Ashcroft, D.M.; Rostami-Hodjegan, A. Meta-Analysis of the Turnover of Intestinal Epithelia in Preclinical Animal Species and Humans. Drug Metab. Dispos. 2014, 42, 2016–2022. [Google Scholar] [CrossRef]
- Kuga, D.; Ushida, K.; Mii, S.; Enomoto, A.; Asai, N.; Nagino, M.; Takahashi, M.; Asai, M. Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut. J. Histochem. Cytochem. 2017, 65, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Seno, H.; Fukuoka, A.; Ueo, T.; Yamaga, Y.; Maruno, T.; Nakanishi, N.; Kanda, K.; Komekado, H.; Kawada, M.; et al. Dclk1 Distinguishes Between Tumor and Normal Stem Cells in the Intestine. Nat. Genet. 2013, 45, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, M.; Khandanpour, C.; Möröy, T.; Fujiyama, T.; Hoshino, M.; Klisch, T.J.; Ding, Q.; Gan, L.; Wang, J.; Martín, M.G.; et al. Origin of the Brush Cell Lineage in the Mouse Intestinal Epithelium. Dev. Biol. 2012, 362, 194–218. [Google Scholar] [CrossRef]
- Yamashita, J.; Ohmoto, M.; Yamaguchi, T.; Matsumoto, I.; Hirota, J. Skn-1a/Pou2f3 Functions as a Master Regulator to Generate Trpm5-Expressing Chemosensory Cells in Mice. PLoS ONE. 2017, 12, e0189340. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal Epithelial Tuft Cells Initiate Type 2 Mucosal Immunity to Helminth Parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef]
- O’Leary, C.E.; Schneider, C.; Locksley, R.M. Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu. Rev. Immunol. 2019, 37, 47–72. [Google Scholar] [CrossRef]
- Plasschaert, L.W.; Žilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A Single-Cell Atlas of the Airway Epithelium Reveals the CFTR-Rich Pulmonary Ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Banerjee, A.; Herring, C.A.; Chen, B.; Kim, H.; Simmons, A.J.; Southard-Smith, A.N.; Allaman, M.M.; White, J.R.; Macedonia, M.C.; Mckinley, E.T.; et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology 2020, 159, 2101–2115. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Hsieh, J.J.; Liu, C.Y.; Appel, K.L.; Waddell, A.; Almohazey, D.; Katada, K.; Bernard, J.K.; Bucar, E.B.; Gadeock, S.; et al. Sprouty2 Limits Intestinal Tuft and Goblet Cell Numbers Through GSK3β-Mediated Restriction of Epithelial IL-33. Nat. Commun. 2021, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Lee, D.; Jeong, J.W.; Lee, S.-H.; Ryu, J.; Oh, S.K.; Yang, H.; Fang, S.; Kim, S. Gut Epithelial Inositol Polyphosphate Multikinase Alleviates Experimental Colitis via Governing Tuft Cell Homeostasis. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 1235–1256. [Google Scholar] [CrossRef] [PubMed]
- Aigbologa, J.; Connolly, M.; Buckley, J.M.; O’Malley, D. Mucosal Tuft Cell Density Is Increased in Diarrhea-Predominant Irritable Bowel Syndrome Colonic Biopsies. Front. Psychiatry 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, J.; Helminen, O.; Huhta, H.; Kauppila, J.H.; Miinalainen, I.; Ronkainen, V.P.; Saarnio, J.; Lehenkari, P.P.; Karttunen, T.J. Doublecortin-Like Kinase 1-Positive Enterocyte—A New Cell Type in Human Intestine. APMIS 2016, 124, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Schütz, B.; Ruppert, A.L.; Strobel, O.; Lazarus, M.; Urade, Y.; Büchler, M.W.; Weihe, E. Distribution Pattern and Molecular Signature of Cholinergic Tuft Cells in Human Gastro-Intestinal and Pancreatic-Biliary Tract. Sci. Rep. 2019, 9, 17466. [Google Scholar] [CrossRef] [PubMed]
- McKinley, E.T.; Sui, Y.; Al-Kofahi, Y.; Millis, B.A.; Tyska, M.J.; Roland, J.T.; Santamaria-Pang, A.; Ohland, C.L.; Jobin, C.; Franklin, J.L.; et al. Optimized Multiplex Immunofluorescence Single-Cell Analysis Reveals Tuft Cell Heterogeneity. JCI Insight 2017, 2, e93487. [Google Scholar] [CrossRef] [PubMed]
- Billipp, T.E.; Nadjsombati, M.S.; von Moltke, J. Tuning Tuft Cells: New Ligands and Effector Functions Reveal Tissue-Specific Function. Curr. Opin. Immunol. 2021, 68, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.J.; Roland, J.T.; Asai, M.; Kaji, I. Distribution of Duodenal Tuft Cells Is Altered in Pediatric Patients With Acute and Chronic Enteropathy. BioMed Res. 2020, 41, 113–118. [Google Scholar] [CrossRef]
- Höfer, D.; Drenckhahn, D. Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry 1992, 98, 237–242. [Google Scholar] [CrossRef]
- Simmons, A.; Banerjee, A.; McKinley, E.; Scurrah, C.; Franklin, J.; Gerdes, M.; Irish, J.; Coffey, R.; Lau, K. Cytometry-based single-cell analysis of intact epithelial signaling reveals MAPK activation divergent from TNF-α-induced apoptosis in vivo. Mol. Syst. Biol. 2015, 11, 835. [Google Scholar] [CrossRef]
- Bezençon, C.; le Coutre, J.; Damak, S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 2007, 32, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Ren, W.; Ohmoto, M.; Urban, J.F.; Matsumoto, I.; Margolskee, R.F.; Jiang, P. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. USA 2018, 115, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Young, R.L.; Cooper, N.J.; Horowitz, M.; Blackshaw, L.A. Phenotypic characterization of taste cells of the mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1420–G1428. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Brulin, B.; Makrini, L.; Legraverend, C.; Jay, P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 2009, 137, 2179–2180. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Parkhurst, C.N.; Zhang, W.; Zhou, L.; Yano, H.; Arifuzzaman, M.; Artis, D. The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity. Sci. Immunol. 2021, 6, eabe3218. [Google Scholar] [CrossRef] [PubMed]
- Bezençon, C.; Fürholz, A.; Raymond, F.; Mansourian, R.; Métairon, S.; Le Coutre, J.; Damak, S. Murine Intestinal Cells Expressing Trpm5 Are Mostly Brush Cells and Express Markers of Neuronal and Inflammatory Cells. J. Comp. Neurol. 2008, 509, 514–525. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhu, X.; Geng, J.; Xu, Y.; Wu, R.; Li, C.; Fan, D.; Qin, X.; Du, Y.; Tian, Y.; et al. Intestinal Tuft-2 cells exert antimicrobial immunity via sensing bacterial metabolite N-undecanoylglycine. Immunity 2022, 55, 686–700.e7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef] [PubMed]
- Howitt, M.R.; Cao, Y.G.; Gologorsky, M.B.; Li, J.A.; Haber, A.L.; Biton, M.; Lang, J.; Michaud, M.; Regev, A.; Garrett, W.S. The Taste Receptor TAS1R3 Regulates Small Intestinal Tuft Cell Homeostasis. ImmunoHorizons 2020, 4, 23–32. [Google Scholar] [CrossRef]
- Hass, N.; Schwarzenbacher, K.; Breer, H. T1R3 Is Expressed in Brush Cells and Ghrelin-Producing Cells of Murine Stomach. Cell Tissue Res. 2010, 339, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Gowda, G.N.; Fontana, M.F.; et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49, 33–41. [Google Scholar] [CrossRef]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.-E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 2018, 174, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Elmentaite, R.; Kumasaka, N.; Roberts, K.; Fleming, A.; Dann, E.; King, H.W.; Kleshchevnikov, V.; Dabrowska, M.; Pritchard, S.; Bolt, L.; et al. Cells of the Human Intestinal Tract Mapped Across Space and Time. Nature 2021, 597, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Ahuja, V.; Paul, J. Attenuated Gabaergic Signaling in Intestinal Epithelium Contributes to Pathogenesis of Ulcerative Colitis. Dig. Dis. Sci. 2017, 62, 2768–2779. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, Q.; Sun, X.; Chen, D.; Wei, C.; Yu, X.; Liu, C.; Li, Y.; Li, J. Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis. Front. Immunol. 2018, 9, 987. [Google Scholar] [CrossRef] [PubMed]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft Cells, Taste-Chemosensory Cells, Orchestrate Parasite Type 2 Immunity in the Gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.B.; Schnoeller, C.; Berkachy, R.; Darby, M.; Pillaye, J.; Oudhoff, M.J.; Parmar, N.; Mackowiak, C.; Sedda, D.; Quesniaux, V.; et al. Acetylcholine Production by Group 2 Innate Lymphoid Cells Promotes Mucosal Immunity to Helminths. Sci. Immunol. 2021, 6, eabd0359. [Google Scholar] [CrossRef] [PubMed]
- Terashima, A.; Watarai, H.; Inoue, S.; Sekine, E.; Nakagawa, R.; Hase, K.; Iwamura, C.; Nakajima, H.; Nakayama, T.; Taniguchi, M. A Novel Subset of Mouse NKT Cells Bearing the IL-17 Receptor B Responds to IL-25 and Contributes to Airway Hyperreactivity. J. Exp. Med. 2008, 205, 2727–2733. [Google Scholar] [CrossRef]
- Neill, D.R.; Mckenzie, A.N.J. Nuocytes and Beyond: New Insights Into Helminth Expulsion. Trends Parasitol. 2019, 27, 214–221. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and Cancer. Nat. Rev. Cancer. 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Oyesola, O.O.; Shanahan, M.T.; Kanke, M.; Mooney, B.M.; Webb, L.M.; Smita, S.; Matheson, M.K.; Campioli, P.; Pham, D.; Früh, S.P.; et al. PGD2 and CRTH2 Counteract Type 2 Cytokine–Elicited Intestinal Epithelial Responses During Helminth Infection. J. Exp. Med. 2021, 218, e20202178. [Google Scholar] [CrossRef]
- Dai, L.; King, D.W.; Perera, D.S.; Lubowski, D.Z.; Burcher, E.; Liu, L. Inverse Expression of Prostaglandin E2-Related Enzymes Highlights Differences Between Diverticulitis and Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Hendel, J.; Nielsen, O.H. Expression of Cyclooxygenase-2 mRNA in Active Inflammatory Bowel Disease. Am. J. Gastroenterol. 1997, 92, 1170–1173. [Google Scholar] [PubMed]
- Montrose, D.C.; Nakanishi, M.; Murphy, R.C.; Zarini, S.; McAleer, J.P.; Vella, A.T.; Rosenberg, D.W. The Role of PGE2 in Intestinal Inflammation and Tumorigenesis. Prostaglandins Other Lipid Mediat. 2015, 116–117, 26–36. [Google Scholar] [CrossRef]
- McGinty, J.W.; Ting, H.-A.; Billipp, T.E.; Nadjsombati, M.S.; Khan, D.M.; Barrett, N.A.; Liang, H.-E.; Matsumoto, I.; von Moltke, J. Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-Helminth Immunity in the Small Intestine But Are Dispensable for Anti-Protist Immunity. Immunity 2020, 52, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Howitt, M.R. A Tuft Act to Follow: Leukotrienes Take the Stage in Anti-Worm Immunity. Immunity 2020, 52, 426–428. [Google Scholar] [CrossRef]
- O’Leary, C.E.; Feng, X.; Cortez, V.S.; Locksley, R.M.; Schneider, C. Interrogating the Small Intestine Tuft Cell–ILC2 Circuit Using In Vivo Manipulations. Curr. Protoc. 2021, 1, e77. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Jurastow, I.; Nandigama, R.; Appenzeller, S.; Li, L.; Vogel, J.; Wiederhold, S.; Althaus, M.; Empting, M.; Altmüller, J.; et al. Tracheal Brush Cells Release Acetylcholine in Response to Bitter Tastants for Paracrine and Autocrine Signaling. FASEB J. 2020, 34, 316–332. [Google Scholar] [CrossRef]
- Perniss, A.; Liu, S.; Boonen, B.; Keshavarz, M.; Ruppert, A.-L.; Timm, T.; Pfeil, U.; Soultanova, A.; Kusumakshi, S.; Delventhal, L.; et al. Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides. Immunity 2020, 52, 683–699. [Google Scholar] [CrossRef]
- Schütz, B.; Jurastow, I.; Bader, S.; Ringer, C.; von Engelhardt, J.; Chubanov, V.; Gudermann, T.; Diener, M.; Kummer, W.; Krasteva-Christ, G.; et al. Chemical Coding and Chemosensory Properties of Cholinergic Brush Cells in the Mouse Gastrointestinal and Biliary Tract. Front. Physiol. 2015, 6, 87. [Google Scholar] [PubMed]
- Jönsson, M.; Norrgård, Ö.; Forsgren, S. Presence of a Marked Nonneuronal Cholinergic System in Human Colon: Study of Normal Colon and Colon in Ulcerative Colitis. Inflamm. Bowel Dis. 2007, 13, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Tahaghoghi-Hajghorbani, S.; Ajami, A.; Ghorbanalipoor, S.; Hosseini-Khah, Z.; Taghiloo, S.; Khaje-Enayati, P.; Hosseini, V. Protective effect of TSLP and IL-33 cytokines in ulcerative colitis. Auto. Immun. Highlights 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Bas, J.; Jay, P.; Gerbe, F. Intestinal tuft cells: Sentinels, what else? Semin. Cell Dev. Biol. 2023, 150–151, 35–42. [Google Scholar]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, T.; Ji, X.Y.; Liu, C.; Yadav, P.K.; Wu, R.; Yang, P.; Liu, Z. IL-25 Downregulates Th1/Th17 Immune Response in an IL-10—Dependent Manner in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 720–728. [Google Scholar] [CrossRef] [PubMed]
- de Silva, P.; Korzenik, J. The changing epidemiology of inflammatory bowel disease: Identifying new high-risk populations. Clin. Gastroenterol. Hepatol. 2015, 13, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Schölmerich, J.; Fellermann, K.; Seibold, F.W.; Rogler, G.; Langhorst, J.; Howaldt, S.; Novacek, G.; Petersen, A.M.; Bachmann, O.; Matthes, H.; et al. A Randomised, Double-blind, Placebo-controlled Trial of Trichuris suis ova in Active Crohn’s Disease. J. Crohns Colitis. 2017, 11, 390–399. [Google Scholar] [PubMed]
- Huang, X.; Zeng, L.R.; Chen, F.S.; Zhu, J.P.; Zhu, M.H. Trichuris suis ova therapy in inflammatory bowel disease: A meta-analysis. Medicine 2018, 97, e12087. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.W.; Elliott, D.E.; Qadir, K.; Urban, J.F., Jr.; Thompson, R.; Weinstock, J.V. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 2003, 98, 2034–2041. [Google Scholar] [CrossRef]
- Yi, J.; Bergstrom, K.; Fu, J.; Shan, X.; McDaniel, J.M.; McGee, S.; Qu, D.; Houchen, C.W.; Liu, X.; Xia, L. Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis. Cell Death Differ. 2019, 26, 1656–1669. [Google Scholar] [CrossRef]
- O’donnell, S.; Borowski, K.; Espin-Garcia, O.; Milgrom, R.; Kabakchiev, B.; Stempak, J.; Panikkath, D.; Eksteen, B.; Xu, W.; Steinhart, A.H.; et al. The Unsolved Link of Genetic Markers and Crohn’s Disease Progression: A North American Cohort Experience. Inflamm. Bowel Dis. 2019, 25, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- McLarren, K.W.; Cole, A.E.; Weisser, S.B.; Voglmaier, N.S.; Conlin, V.S.; Jacobson, K.; Popescu, O.; Boucher, J.-L.; Sly, L.M. SHIP-deficient mice develop spontaneous intestinal inflammation and arginase-dependent fibrosis. Am. J. Pathol. 2011, 179, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, E.N.; Weisser, S.B.; Lo, Y.; Kozicky, L.K.; Jen, R.; Brugger, H.K.; Menzies, S.C.; McLarren, K.W.; Nackiewicz, D.; van Rooijen, N.; et al. Activity of SHIP, Which Prevents Expression of Interleukin 1β, Is Reduced in Patients With Crohn’s Disease. Gastroenterology 2016, 150, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, J.P. COX-Expressing Tuft Cells Initiate Crohn’s Disease-like Intestinal Inflammation in SHIP-/- Mice. Graduate Thesis, University of British Columbia, Vancouver, BC, Canada, 2019. Available online: https://open.library.ubc.ca/collections/ubctheses/24/items/1.0387210 (accessed on 5 March 2024).
- Iqbal, S.; Rezaul Karim, M.; Yang, D.C.; Mathiyalagan, R.; Chan Kang, S. Tuft cells–the immunological interface and role in disease regulation. Int. Immunopharmacol. 2023, 118, 110018. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Sun, S.; Xie, Y.; Pan, C.; Li, M.; Li, C.; Liu, Y.; Xu, Z.; Liu, W.; et al. Indolepropionic acid reduces obesity-induced metabolic dysfunction through colonic barrier restoration mediated via tuft cell-derived IL-25. FEBS J. 2022, 289, 5985–6004. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeong, D.Y.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. Insight into the role of TSLP in inflammatory bowel diseases. Autoimmun. Rev. 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.B.; Cyr, S.L.; Arima, K.; McDonald, R.A.; Levit, N.A.; Nestle, F.O. Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis. Dermatol. Ther. 2022, 12, 1501–1533. [Google Scholar] [CrossRef] [PubMed]
- Bjerkan, L.; Sonesson, A.; Schenck, K. Multiple Functions of the New Cytokine-Based Antimicrobial Peptide Thymic Stromal Lymphopoietin (TSLP). Pharmaceuticals 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Jou, E.; Rodriguez-Rodriguez, N.; McKenzie, A.N.J. Emerging roles for IL-25 and IL-33 in colorectal cancer tumorigenesis. Front. Immunol. 2022, 13, 981479. [Google Scholar] [CrossRef]
- Chang, C.; Chen, G.; Wu, W.; Chen, D.; Chen, S.; Gao, J.; Feng, Y.; Zhen, G. Exogenous IL-25 ameliorates airway neutrophilia via suppressing macrophage M1 polarization and the expression of IL-12 and IL-23 in asthma. Respir. Res. 2023, 24, 260. [Google Scholar] [CrossRef]
- Canè, L.; Poto, R.; Palestra, F.; Pirozzi, M.; Parashuraman, S.; Iacobucci, I.; Ferrara, A.L.; La Rocca, A.; Mercadante, E.; Pucci, P.; et al. TSLP is localized in and released from human lung macrophages activated by T2-high and T2-low stimuli: Relevance in asthma and COPD. Eur. J. Intern. Med. 2024, 124, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Liu, X.; Jiang, T.; Huang, G.; Cai, H.; Xing, D.; Mao, Y.; Zheng, X. Integration analysis using bioinformatics and experimental validation on the clinical and biological significance of TSLP in cancers. Cell Signal. 2023, 111, 110874. [Google Scholar] [CrossRef] [PubMed]
- Obata-Ninomiya, K.; de Jesus Carrion, S.; Hu, A.; Ziegler, S.F. Emerging role for thymic stromal lymphopoietin-responsive regulatory T cells in colorectal cancer progression in humans and mice. Sci. Transl. Med. 2022, 14, eabl6960. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.Y.X.; Viswanath, D.I.; Huston, D.P.; Grattoni, A. Engineering platforms for localized long-acting immune modulation. J. Allergy Clin. Immunol. 2024, 153, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.P.; Melchor, S.J.; Petri, W.A., Jr. Tuft Cells: New Players in Colitis. Trends Mol. Med. 2016, 22, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Hoving, J.C. Targeting IL-13 as a Host-Directed Therapy Against Ulcerative Colitis. Front. Cell Infect. Microbiol. 2018, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, C.; Trivedi, S.; Wijesundara, D.K.; Jackson, R.J. IL-4 and IL-13 receptors: Roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor. Rev. 2014, 25, 437–442. [Google Scholar] [CrossRef]
- Braddock, M.; Hanania, N.A.; Sharafkhaneh, A.; Colice, G.; Carlsson, M. Potential Risks Related to Modulating Interleukin-13 and Interleukin-4 Signalling: A Systematic Review. Drug Saf. 2018, 41, 489–509. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S. Role of interleukin-13 in fibrosis, particularly systemic sclerosis. Biofactors. 2013, 39, 593–596. [Google Scholar] [CrossRef]
- Kotas, M.E.; O’Leary, C.E.; Locksley, R.M. Tuft Cells: Context- and Tissue-Specific Programming for a Conserved Cell Lineage. Annu. Rev. Pathol. 2023, 18, 311–335. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1602–1616.e6. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, H.; He, C.; Xin, S.; Wang, B.; Zhang, S.; Gong, F.; Yu, X.; Pan, L.; Sun, F.; et al. An update on the biological characteristics and functions of tuft cells in the gut. Front. Cell Dev. Biol. 2023, 10, 1102978. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010, 328, 228–231. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Honda, K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012, 12, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Asano, N.; Meng, G.; Yamashita, K.; Arai, Y.; Sakurai, T.; Kudo, M.; Fuss, I.J.; Kitani, A.; Shimosegawa, T.; et al. NOD2 downregulates colonic inflammation by IRF4-mediated inhibition of K63-linked polyubiquitination of RICK and TRAF6. Mucosal Immunol. 2014, 7, 1312–1325. [Google Scholar] [CrossRef]
- Yang, Z.; Fuss, I.J.; Watanabe, T.; Asano, N.; Davey, M.P.; Rosenbaum, J.T.; Strober, W.; Kitani, A. NOD2 transgenic mice exhibit enhanced MDP-mediated down-regulation of TLR2 responses and resistance to colitis induction. Gastroenterology 2007, 133, 1510–1521. [Google Scholar] [CrossRef]
- Lee, J.; Gonzales-Navajas, J.M.; Raz, E. The polarizing-tolerizing” mechanism of intestinal epithelium: Its relevance to colonic homeostasis. Semin. Immunopathol. 2008, 30, 3–9. [Google Scholar] [CrossRef]
- Karikó, K.; Weissman, D.; Welsh, F.A. Inhibition of toll-like receptor and cytokine signaling—A unifying theme in ischemic tolerance. J. Cereb. Blood Flow. Metab. 2004, 24, 1288–1304. [Google Scholar] [CrossRef]
- BeLow, M.; Osipo, C. Notch Signaling in Breast Cancer: A Role in Drug Resistance. Cells 2020, 9, 2204. [Google Scholar] [CrossRef]
- Takebe, N.; Nguyen, D.; Yang, S.X. Targeting notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol. Ther. 2014, 141, 140–149. [Google Scholar] [CrossRef]
- Radojcic, V.; Maillard, I. Notch Signaling and Alloreactivity. Transplantation 2016, 100, 2593–2600. [Google Scholar] [CrossRef]
- Han, W.; Yu, Y.; Liu, X.Y. Local signals in stem cell-based bone marrow regeneration. Cell Res. 2006, 16, 189–195. [Google Scholar] [CrossRef]
- Jaiswal, A.; Singh, R. Homeostases of epidermis and hair follicle, and development of basal cell carcinoma. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188795. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.L.; Wu, H. Notch signaling, brain development, and human disease. Pediatr. Res. 2005, 57, 104R–109R. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.H.; Joshi, S.; Yang, Y.; Tune, J.D.; Zhao, M.T.; Yang, H. Bioengineering Systems for Modulating Notch Signaling in Cardiovascular Development, Disease, and Regeneration. J. Cardiovasc. Dev. Dis. 2021, 8, 125. [Google Scholar] [CrossRef]
- Khoramjoo, S.M.; Kazemifard, N.; Baradaran Ghavami, S.; Farmani, M.; Shahrokh, S.; Asadzadeh Aghdaei, H.; Sherkat, G.; Zali, M.R. Overview of Three Proliferation Pathways (Wnt, Notch, and Hippo) in Intestine and Immune System and Their Role in Inflammatory Bowel Diseases (IBDs). Front. Med. 2022, 9, 865131. [Google Scholar] [CrossRef]
- D’Assoro, A.B.; Leon-Ferre, R.; Braune, E.B.; Lendahl, U. Roles of Notch Signaling in the Tumor Microenvironment. Int. J. Mol. Sci. 2022, 23, 6241. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, B.; Asahara, T.; Alev, C. Overview of Basic Mechanisms of Notch Signaling in Development and Disease. Adv. Exp. Med. Biol. 2020, 1227, 9–27. [Google Scholar]
- Arora, P.; Andersen, D.; Moll, J.M.; Danneskiold-Samsøe, N.B.; Xu, L.; Zhou, B.; Kladis, G.; Rausch, P.; Workman, C.T.; Kristiansen, K.; et al. Small Intestinal Tuft Cell Activity Associates With Energy Metabolism in Diet-Induced Obesity. Front. Immunol. 2021, 12, 629391. [Google Scholar] [CrossRef]
- Eshleman, E.M.; Rice, T.; Potter, C.; Waddell, A.; Hashimoto-Hill, S.; Woo, V.; Field, S.; Engleman, L.; Lim, H.-W.; Schumacher, M.A.; et al. Microbiota-derived butyrate restricts tuft cell differentiation via histone deacetylase 3 to modulate intestinal type 2 immunity. Immunity 2024, 57, 319–332.e6. [Google Scholar] [CrossRef] [PubMed]
- Repossi, G.; Das, U.N.; Eynard, A.R. Molecular Basis of the Beneficial Actions of Resveratrol. Arch. Med. Res. 2020, 51, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Fujinami, N.; Arita, M. Polyunsaturated Fatty Acid-Derived Lipid Mediators That Regulate Epithelial Homeostasis. Biol. Pharm. Bull. 2022, 45, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Goulart, R.A.; Carvalho, A.C.A.; Barbalho, S.M. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci. 2019, 20, 4851. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Wang, H.Y.; Chen, T.X. Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed. Res. Int. 2019, 2019, 6764919. [Google Scholar] [CrossRef] [PubMed]
- Poveda, M.C.; Britton, C.; Devaney, E.; McNeilly, T.N.; Gerbe, F.; Jay, P.; Maizels, R.M. Tuft Cells: Detectors, Amplifiers, Effectors and Targets in Parasite Infection. Cells 2023, 12, 2477. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Moore, C.M.; Gurrola, J.G.; Goldberg, A.N.; Alvarez, R.; Yamato, S.; Bratcher, P.E.; Shaughnessy, C.A.; Zeitlin, P.L.; Zhang, I.H.; et al. IL-13-programmed airway tuft cells produce PGE2, which promotes CFTR-dependent mucociliary function. JCI Insight 2022, 7, e159832. [Google Scholar] [CrossRef]
- Yu, S.; Sun, Y.; Shao, X.; Zhou, Y.; Yu, Y.; Kuai, X.; Zhou, C. Leaky Gut in IBD: Intestinal Barrier-Gut Microbiota Interaction. J. Microbiol. Biotechnol. 2022, 32, 825–834. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Monaco, H.; Sambataro, E.; Clemente, J.C. Novel technologies to characterize and engineer the microbiome in inflammatory bowel disease. Gut Microbes 2022, 14, 2107866. [Google Scholar] [CrossRef]
- Achufusi, T.G.O.; Sharma, A.; Zamora, E.A.; Manocha, D. Small Intestinal Bacterial Overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods. Cureus 2020, 12, e8860. [Google Scholar] [CrossRef]
- Chlebicz-Wójcik, A.; Śliżewska, K. Probiotics, Prebiotics, and Synbiotics in the Irritable Bowel Syndrome Treatment: A Review. Biomolecules 2021, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Jelinsky, S.A.; Derksen, M.; Bauman, E.; Verissimo, C.S.; van Dooremalen, W.T.M.; Roos, J.L.; Barón, C.H.; Caballero-Franco, C.; Johnson, B.G.; Rooks, M.G.; et al. Molecular and Functional Characterization of Human Intestinal Organoids and Monolayers for Modeling Epithelial Barrier. Inflamm. Bowel Dis. 2023, 29, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Hadas, E.; Bozek, A.; Cudak, A.; Ciuk, A.; Jarząb, J. Examples of adverse effects after biological therapy. Postep. Dermatol. Alergol. 2020, 37, 712–718. [Google Scholar] [CrossRef]
- Campar, A.; Isenberg, D.A. Life-Threatening Complications of Biological Therapies. In Autoimmune Diseases; Springer: London, UK, 2011; pp. 375–403. [Google Scholar]
- Hansen, S.L.; Larsen, H.L.; Pikkupeura, L.M.; Maciag, G.; Guiu, J.; Müller, I.; Clement, D.L.; Mueller, C.; Johansen, J.V.; Helin, K.; et al. An organoid-based CRISPR-Cas9 screen for regulators of intestinal epithelial maturation and cell fate. Sci. Adv. 2023, 9, eadg4055. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.; Ho, G.T. Therapeutic Potential of Human Intestinal Organoids in Tissue Repair Approaches in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2023, 29, 1488–1498. [Google Scholar] [CrossRef]
- Harris, N. Immunology. The enigmatic tuft cell in immunity. Science 2016, 351, 1264–1265. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yan, W.; Fu, Y. New sights of immunometabolism and agent progress in colitis associated colorectal cancer. Front. Pharmacol. 2024, 14, 1303913. [Google Scholar] [CrossRef]
- Vega, K.J.; May, R.; Sureban, S.M.; A Lightfoot, S.; Qu, D.; Reed, A.; Weygant, N.; Ramanujam, R.; Souza, R.; Madhoun, M.; et al. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett’s esophagus and esophageal adenocarcinoma. J. Gastroenterol. Hepatol. 2012, 27, 773–780. [Google Scholar] [CrossRef]
- Quante, M.; Bhagat, G.; Abrams, J.A.; Marache, F.; Good, P.; Lee, M.D.; Lee, Y.; Friedman, R.; Asfaha, S.; Dubeykovskaya, Z.; et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012, 21, 36–51. [Google Scholar] [CrossRef]
- Roulis, M.; Kaklamanos, A.; Schernthanner, M.; Bielecki, P.; Zhao, J.; Kaffe, E.; Frommelt, L.-S.; Qu, R.; Knapp, M.S.; Henriques, A.; et al. Paracrine Orchestration of Intestinal Tumorigenesis by a Mesenchymal Niche. Nature 2020, 580, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Fibroblasts Fuel Intestinal Tumorigenesis. Cell Res. 2020, 30, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Lightfoot, S.A.; Hoskins, A.B.; Lerner, M.; Brackett, D.J.; Postier, R.G.; Ramanujam, R.; Mohammed, A.; Rao, C.V.; et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011, 71, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Ramalingam, S.; Subramaniam, D.; Natarajan, G.; Wyche, J.H.; Anant, S.; Houchen, C.W. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology 2009, 137, 649–659.e6592. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Liu, Z.; Liu, D.; Chen, H.; Wang, Y.; Sun, B. LncRNA CCAT1 participates in pancreatic ductal adenocarcinoma progression by forming a positive feedback loop with c-Myc. Carcinogenesis 2023, 45, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dai, X.; Zhu, Z.; Fan, D.; Jiang, S.; Dong, Y.; Chen, B.; Xie, Q.; Yao, Z.; Li, Q.; et al. Reciprocal regulation of lncRNA MEF and c-Myc drives colorectal cancer tumorigenesis. Neoplasia 2024, 49, 100971. [Google Scholar] [CrossRef] [PubMed]
- Burman, A.; Kaji, I. Luminal Chemosensory Cells in the Small Intestine. Nutrients 2021, 13, 3712. [Google Scholar] [CrossRef]
- Lei, H.; Yu, D.; Xue, Y.-B.; Li, Y.-H.; Gong, S.-M.; Peng, Y.-Y.; Liu, K.-F.; Buratto, D.; Yang, Y.; Zhang, S.-S.; et al. Tuft cells utilize taste signaling molecules to respond to the pathobiont microbe Ruminococcus gnavus in the proximal colon. Front. Immunol. 2023, 14, 1259521. [Google Scholar] [CrossRef]
- Ting, H.A.; von Moltke, J. The Immune Function of Tuft Cells at Gut Mucosal Surfaces and Beyond. J. Immunol. 2019, 202, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Mu, L.; Huang, K.; Hu, Y.; Yan, C.; Zhao, H.; Ma, C.; Li, X.; Tao, D.; Qin, J. Hypoxic colorectal cancer cells promote metastasis of normoxic cancer cells depending on IL-8/p65 signaling pathway. Cell Death Dis. 2020, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.T.; Rajasekaran, V.; Blackmur, J.P.; O’callaghan, A.; Donnelly, K.; Timofeeva, M.; Vaughan-Shaw, P.G.; Din, F.V.N.; Dunlop, M.G.; Farrington, S.M. Transcriptional dynamics of colorectal cancer risk associated variation at 11q23.1 correlate with tuft cell abundance and marker expression in silico. Sci. Rep. 2022, 12, 13609. [Google Scholar] [CrossRef] [PubMed]
- Sugita, R.; Kuwabara, H.; Sugimoto, K.; Kubota, K.; Imamura, Y.; Kiho, T.; Tengeiji, A.; Kawakami, K.; Shimada, K. A Novel Selective Prostaglandin E2 Synthesis Inhibitor Relieves Pyrexia and Chronic Inflammation in Rats. Inflammation 2016, 39, 907–915. [Google Scholar] [CrossRef] [PubMed]
- O’keefe, R.N.; Carli, A.L.E.; Baloyan, D.; Chisanga, D.; Shi, W.; Afshar-Sterle, S.; Eissmann, M.F.; Poh, A.R.; Pal, B.; Seillet, C.; et al. A tuft cell—ILC2 signaling circuit provides therapeutic targets to inhibit gastric metaplasia and tumor development. Nat. Commun. 2023, 14, 6872. [Google Scholar] [CrossRef] [PubMed]
- Ualiyeva, S.; Lemire, E.; Aviles, E.C.; Wong, C.; Boyd, A.A.; Lai, J.; Liu, T.; Matsumoto, I.; Barrett, N.A.; Boyce, J.A.; et al. Tuft cell-produced cysteinyl leukotrienes and IL-25 synergistically initiate lung type 2 inflammation. Sci. Immunol. 2021, 6, eabj0474. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, N.; Xiao, F.; Shi, X.; Zhu, B.; Rui, K.; Tian, J.; Lu, L. New insights into the function of Interleukin-25 in disease pathogenesis. Biomark. Res. 2023, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Wang, T.C. The Tuft Cell-ILC2 Circuit Integrates Intestinal Defense and Homeostasis. Cell 2018, 174, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Jayaraman, A.; Safe, S.; Chapkin, R.S. Targeting the aryl hydrocarbon receptor in stem cells to improve the use of food as medicine. Curr. Stem Cell Rep. 2020, 6, 109–118. [Google Scholar] [CrossRef]
- Ding, L.; Weygant, N.; Ding, C.; Lai, Y.; Li, H. DCLK1 and tuft cells: Immune-related functions and implications for cancer immunotherapy. Crit. Rev. Oncol. Hematol. 2023, 191, 104118. [Google Scholar] [CrossRef]
- Li, L.; Ma, M.; Duan, T.; Sui, X. The critical roles and therapeutic implications of tuft cells in cancer. Front. Pharmacol. 2022, 13, 1047188. [Google Scholar] [CrossRef] [PubMed]
- Gargaun, S.; Gargaun, L. A novel method aimed at counteracting the side effects caused by prostaglandin e2 deficiency during non-steroidal anti-inflammatory drug therapy. Antiinflamm. Antiallergy Agents Med. Chem. 2014, 13, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Dai, Y. Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 2020, 126, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Gazzaniga, F.S.; Kasper, D.L.; Sharpe, A.H. Microbiota-dependent regulation of costimulatory and coinhibitory pathways via innate immune sensors and implications for immunotherapy. Exp. Mol. Med. 2023, 55, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Weygant, N.; Qu, D.; Berry, W.L.; May, R.; Chandrakesan, P.; Owen, D.B.; Sureban, S.M.; Ali, N.; Janknecht, R.; Houchen, C.W. Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent activity against colorectal and pancreatic cancer through inhibition of doublecortin-like kinase 1. Mol. Cancer 2014, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Weygant, N.; Qu, D.; Chandrakesan, P.; Bannerman-Menson, E.; Ali, N.; Pantazis, P.; Westphalen, C.B.; Wang, T.C.; et al. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014, 351, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.M.; Nabet, B.; Raghavan, S.; Liu, Y.; Leggett, A.L.; Kuljanin, M.; Kalekar, R.L.; Yang, A.; He, S.; Wang, J.; et al. Discovery of a selective inhibitor of doublecortin like kinase 1. Nat. Chem. Biol. 2020, 16, 635–643. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, S.; Chen, L.; Dong, X.; Zhang, L.; Wu, C.; Ye, K.; Shao, F.; Zhu, Z.; Thorne, R.F. Research Progress of DCLK1 Inhibitors as Cancer Therapeutics. Curr. Med. Chem. 2022, 29, 2261–2273. [Google Scholar] [CrossRef]
- Sureban, S.M.; May, R.; Mondalek, F.G.; Qu, D.; Ponnurangam, S.; Pantazis, P.; Anant, S.; Ramanujam, R.P.; Houchen, C.W. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J. Nanobiotechnology 2011, 9, 40. [Google Scholar] [CrossRef]
- Ge, Y.; Weygant, N.; Qu, D.; May, R.; Berry, W.L.; Yao, J.; Chandrakesan, P.; Zheng, W.; Zhao, L.; Zhao, K.L.; et al. Alternative splice variants of DCLK1 mark cancer stem cells, promote self-renewal and drug-resistance, and can be targeted to inhibit tumorigenesis in kidney cancer. Int. J. Cancer 2018, 143, 1162–1175. [Google Scholar] [CrossRef]
- Qiao, S.; Zhao, Y.; Geng, S.; Li, Y.; Hou, X.; Liu, Y.; Lin, F.-H.; Yao, L.; Tian, W. A novel double-targeted nondrug delivery system for targeting cancer stem cells. Int. J. Nanomed. 2016, 11, 6667–6678. [Google Scholar] [CrossRef]
- Sureban, S.M.; Berahovich, R.; Zhou, H.; Xu, S.; Wu, L.; Ding, K.; May, R.; Qu, D.; Bannerman-Menson, E.; Golubovskaya, V.; et al. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers 2019, 12, 54. [Google Scholar] [CrossRef]
- Liu, H.; Wen, T.; Zhou, Y.; Fan, X.; Du, T.; Gao, T.; Li, L.; Liu, J.; Yang, L.; Yao, J.; et al. DCLK1 Plays a Metastatic-Promoting Role in Human Breast Cancer Cells. Biomed. Res. Int. 2019, 2019, 1061979. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.Y.; Jeon, S.E.; Choi, J.H.; Lee, C.J.; Jang, T.Y.; Yun, H.J.; Lee, Y.; Kim, P.; Cho, S.H.; et al. DCLK1 promotes colorectal cancer stemness and aggressiveness via the XRCC5/COX2 axis. Theranostics 2022, 12, 5258–5271. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, L.; Lin, Z.; Yu, D.; Jin, M.; Zhou, P.; Ren, J.; Cheng, J.; Yang, K.; Wu, G.; et al. Targeting DCLK1 overcomes 5-fluorouracil resistance in colorectal cancer through inhibiting CCAR1/β-catenin pathway-mediated cancer stemness. Clin. Transl. Med. 2022, 12, e743. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ye, J.; Wang, H.; Sun, M.; Zhang, Y.; Sang, X.; Zhuang, Z. Application of toll-like receptors (TLRs) and their agonists in cancer vaccines and immunotherapy. Front. Immunol. 2023, 14, 1227833. [Google Scholar] [CrossRef]
- Gang, W.; Wang, J.; Guan, R.; Yan, S.; Shi, F.; Zhang, J.; Li, Z.; Gao, J.; Fu, X. Strategy to targeting the immune resistance and novel therapy in colorectal cancer. Cancer Med. 2018, 7, 1578–1603. [Google Scholar] [CrossRef]
- Cammareri, P.; Vincent, D.F.; Hodder, M.C.; Ridgway, R.A.; Murgia, C.; Nobis, M.; Campbell, A.D.; Varga, J.; Huels, D.J.; Subramani, C.; et al. TGFβ pathway limits dedifferentiation following WNT and MAPK pathway activation to suppress intestinal tumourigenesis. Cell Death Differ. 2017, 24, 1681–1693. [Google Scholar] [CrossRef]
- Lindholm, H.T.; Parmar, N.; Drurey, C.; Poveda, M.C.; Vornewald, P.M.; Ostrop, J.; Díez-Sanchez, A.; Maizels, R.M.; Oudhoff, M.J. BMP signaling in the intestinal epithelium drives a critical feedback loop to restrain IL-13-driven tuft cell hyperplasia. Sci. Immunol. 2022, 7, eabl6543. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, C.J.; Choi, J.H.; Kim, J.H.; Kim, J.W.; Kim, J.Y.; Nam, J.S. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J. Exp. Clin. Cancer Res. 2019, 38, 399. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, R.; Xiao, Z.; Huang, X.; Yao, J.; Liu, J.; An, G.; Ge, Y. Targeting DCLK1 attenuates tumor stemness and evokes antitumor immunity in triple-negative breast cancer by inhibiting IL-6/STAT3 signaling. Breast Cancer Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Shi, N.; Wang, Z.; Zhu, H.; Liu, W.; Zhao, M.; Jiang, X.; Zhao, J.; Ren, C.; Zhang, Y.; Luo, L. Research progress on drugs targeting the TGF-β signaling pathway in fibrotic diseases. Immunol. Res. 2022, 70, 276–288. [Google Scholar] [CrossRef]
- Yang, X.; Xu, L.; Yang, L.; Xu, S. Research progress of STAT3-based dual inhibitors for cancer therapy. Bioorg Med. Chem. 2023, 91, 117382. [Google Scholar] [CrossRef]
- Javed, Z.; Iqbal, M.J.; Rasheed, A.; Sadia, H.; Raza, S.; Irshad, A.; Koch, W.; Kukula-Koch, W.; Głowniak-Lipa, A.; Cho, W.C.; et al. Regulation of Hedgehog Signaling by miRNAs and Nanoformulations: A Possible Therapeutic Solution for Colorectal Cancer. Front. Oncol. 2021, 10, 607607. [Google Scholar] [CrossRef]
- Vijai, M.; Baba, M.; Ramalingam, S.; Thiyagaraj, A. DCLK1 and its interaction partners: An effective therapeutic target for colorectal cancer. Oncol. Lett. 2021, 22, 850. [Google Scholar] [CrossRef]
- Wang, Y.C.; Cao, Y.; Pan, C.; Zhou, Z.; Yang, L.; Lusis, A.J. Intestinal cell type-specific communication networks underlie homeostasis and response to Western diet. J. Exp. Med. 2023, 220, e20221437. [Google Scholar] [CrossRef]
- Rezakhani, S.; Gjorevski, N.; Lutolf, M.P. Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials 2021, 276, 121020. [Google Scholar] [CrossRef]
- Cao, Z.; Weygant, N.; Chandrakesan, P.; Houchen, C.W.; Peng, J.; Qu, D. Tuft and Cancer Stem Cell Marker DCLK1: A New Target to Enhance Anti-Tumor Immunity in the Tumor Microenvironment. Cancers 2020, 12, 3801. [Google Scholar] [CrossRef]


| Markers’ Main Functions | Marker | Immunoreactivity on Other Intestinal Cells (TC-Specificity) |
|---|---|---|
| Structural | ||
| Villin-1 | also appears in other intestinal epithelial cells, but the basal staining in this case is tuft-cell-specific [50] | |
| CK18 (Cytokeratin-18) | also appears on some secretory cells [51] | |
| Chemical sensing | ||
| TRPM5 (Transient Receptor Potential Cation Channel Subfamily M Member 5) | also appears on enteroendocrine cells [52] | |
| PLCβ2 (Phospholipase C β2) | also appears on enteroendocrine cells [52] | |
| SUCNR1 (Succinate Receptor 1) | not known [53] | |
| GNAT3 (G Protein Subunit Alpha Transducin 3) | also appears on some enteroendocrine cells [54] | |
| Neuronal | ||
| DCLK1 (Doublecortin Like Kinase 1) | also appears on some enteroendocrine cells [36,55] | |
| ChAT (Choline acetyltransferase) | not known [56] | |
| Immunological | ||
| COX-1/2 (Cyclooxygenase-1/2) | not known [13,57] | |
| HPGDS (Hematopoietic Prostaglandin D Synthase) | not known [13] | |
| ALOX5 (Arachidonate 5-lipoxygenase) | not known [57] | |
| IL25 (Interleukin 25) | not known [31,38] | |
| SiglecF (Sialic Acid Binding Ig-like Lectin F) | not known [38] | |
| PTPRC (Protein Tyrosine Phosphatase Receptor Type C) | not known [23,58] | |
| SH2D6 (SH2 Domain Containing 6) | not known [23,58] | |
| Transcription factor | ||
| Pou2f3 (POU class 2 homeobox 3) | not known [38] | |
| Gfi1B (Growth Factor Independent 1B Transcriptional Repressor) | not known [36,38] | |
| Spib (SBIP transcription factor) | not known [58] | |
| SOX-9 (SRY-box transcription factor 9) | it also appears on Paneth and intestinal stem cells [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipos, F.; Műzes, G. Colonic Tuft Cells: The Less-Recognized Therapeutic Targets in Inflammatory Bowel Disease and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 6209. https://doi.org/10.3390/ijms25116209
Sipos F, Műzes G. Colonic Tuft Cells: The Less-Recognized Therapeutic Targets in Inflammatory Bowel Disease and Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(11):6209. https://doi.org/10.3390/ijms25116209
Chicago/Turabian StyleSipos, Ferenc, and Györgyi Műzes. 2024. "Colonic Tuft Cells: The Less-Recognized Therapeutic Targets in Inflammatory Bowel Disease and Colorectal Cancer" International Journal of Molecular Sciences 25, no. 11: 6209. https://doi.org/10.3390/ijms25116209
APA StyleSipos, F., & Műzes, G. (2024). Colonic Tuft Cells: The Less-Recognized Therapeutic Targets in Inflammatory Bowel Disease and Colorectal Cancer. International Journal of Molecular Sciences, 25(11), 6209. https://doi.org/10.3390/ijms25116209







