Mitochondrial Oxidative Stress Regulates FOXP3+ T-Cell Activity and CD4-Mediated Inflammation in Older Adults with Frailty
Abstract
1. Introduction
2. Results
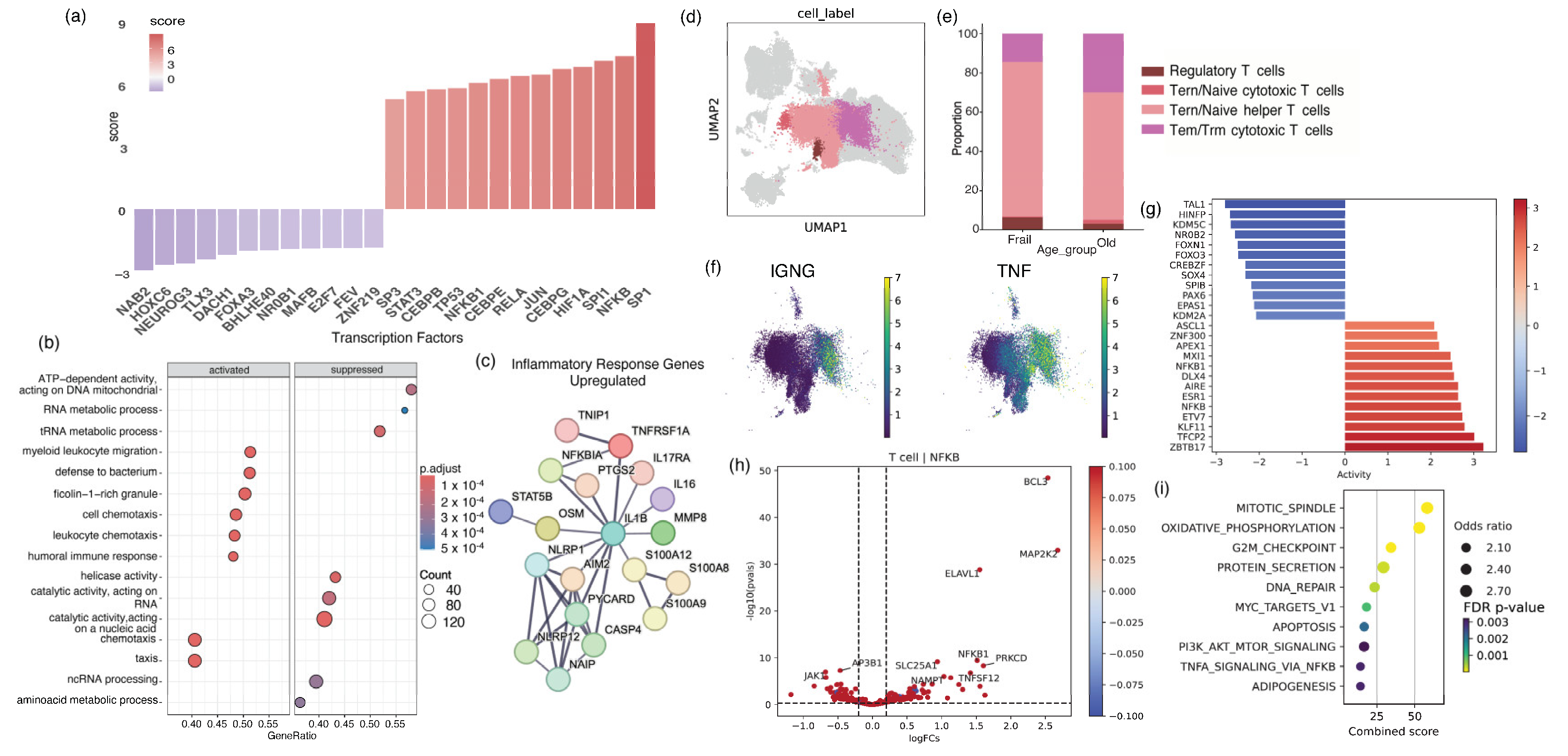
2.1. Older Adults with Frailty Possess Elevated Levels of Peripheral Inflammation and a Pathological CD4 Landscape Compared to Healthy Older Adults
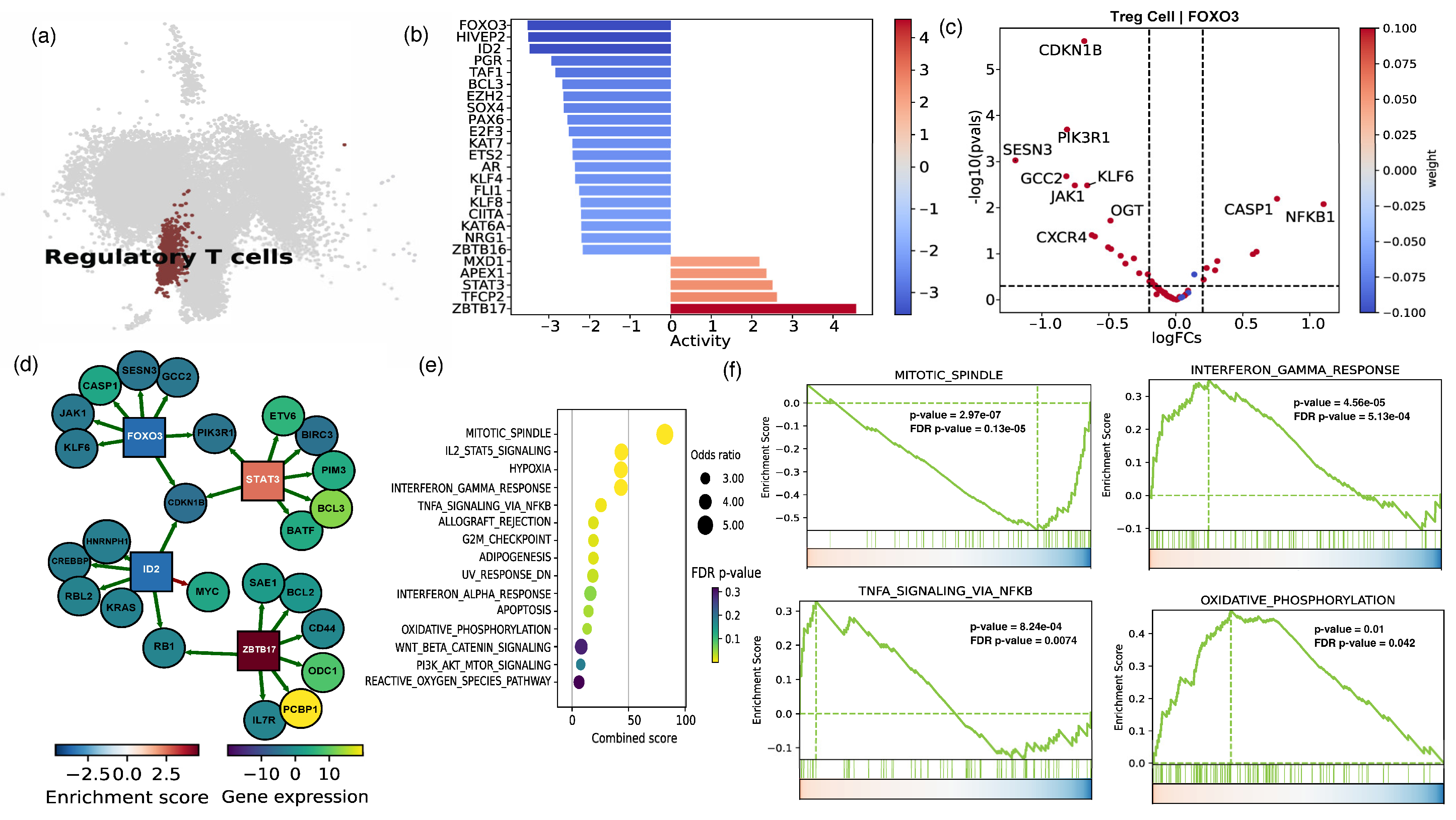
2.2. The Cellular and Functional Diversity of FOXP3+ Treg Cell Subtypes in Frail and Healthy Older Adults
2.3. Assessing Cell–Cell Interactions Associated with Treg Response in Frail Older Adults
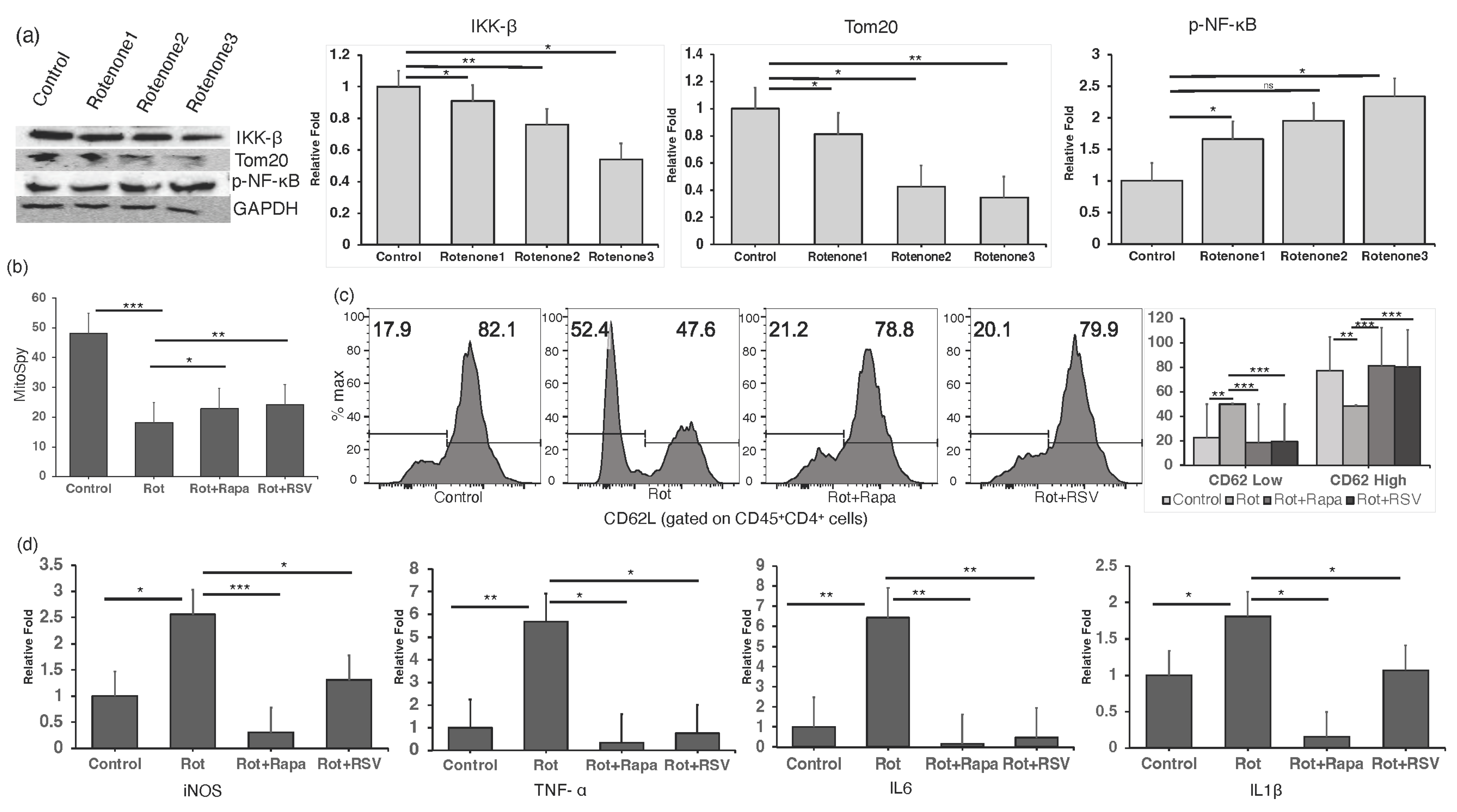
2.4. Restoring Mitochondrial Oxidative Stress Alleviates Inflammation in In Vitro CD4+ T Cells
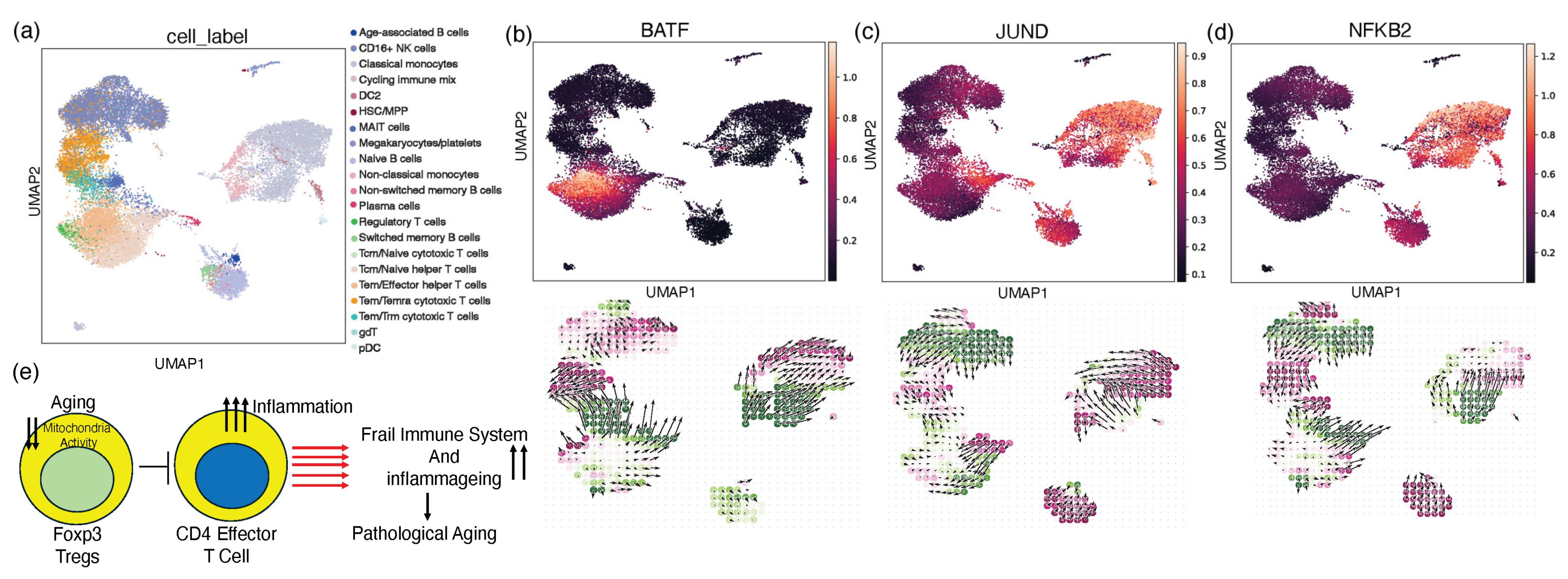
2.5. In Silico Knockout Modulating the Perturbation Score for the Treg Gene Signature
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Flow Cytometry Analysis
4.3. RNA Isolation and qPCR
4.4. Western Blot
4.5. Bulk RNA-Seq Data Analysis
4.6. Single-Cell RNA-Seq Data Processing
4.7. Single-Cell Data Cell-Type Annotation
4.8. Single-Cell Data Differential Gene Expression Analysis, Transcription Factor Inference, and Pathway Analysis
4.9. Cell–Cell Communication Analysis
4.10. Gene Signature and Perturbation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goronzy, J.J.; Weyand, C.M. Successful and Maladaptive T Cell Aging. Immunity 2017, 46, 364–378. [Google Scholar] [CrossRef]
- Suo, C.; Dann, E.; Goh, I.; Jardine, L.; Kleshchevnikov, V.; Park, J.-E.; Botting, R.A.; Stephenson, E.; Engelbert, J.; Tuong, Z.K.; et al. Mapping the Developing Human Immune System across Organs. Science 2022, 376, eabo0510. [Google Scholar] [CrossRef]
- Ponnappan, S.; Ponnappan, U. Aging and Immune Function: Molecular Mechanisms to Interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef]
- Fabian, D.K.; Fuentealba, M.; Dönertaş, H.M.; Partridge, L.; Thornton, J.M. Functional Conservation in Genes and Pathways Linking Ageing and Immunity. Immun. Ageing 2021, 18, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, P.; Guo, K.; Schirrick, G.; Gill, J.S.; Weis, J.; Lund Da Costa, A.; Rahman, M.; Mehta, H.; Fleecs, J.; et al. Age-Related Dysregulation of Intestinal Epithelium Fucosylation Is Linked to an Increased Risk of Colon Cancer. JCI Insight 2024, 9, e167676. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, G.; Wu, B.; Chou, W.-C.; Cheng, L.; Zhou, C.; Lou, J.; Wu, D.; Su, L.; Zheng, J.; et al. DCAF1 Regulates Treg Senescence via the ROS Axis during Immunological Aging. J. Clin. Investig. 2020, 130, 5893–5908. [Google Scholar] [CrossRef]
- Winger, M.E.; Caserotti, P.; Cauley, J.A.; Boudreau, R.M.; Piva, S.R.; Cawthon, P.M.; Orwoll, E.S.; Ensrud, K.E.; Kado, D.M.; Strotmeyer, E.S.; et al. Lower Leg Power and Grip Strength Are Associated with Increased Fall Injury Risk in Older Men: The Osteoporotic Fractures in Men Study. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Feyt, C.; Chapurlat, R. High Risk of Fall, Poor Physical Function, and Low Grip Strength in Men with Fracture-the STRAMBO Study. J. Cachexia Sarcopenia Muscle 2016, 7, 299–311. [Google Scholar] [CrossRef]
- Nagai, T.; Okano, I.; Ishikawa, K.; Kuroda, T.; Oshita, Y.; Tsuchiya, K.; Tani, S.; Okamura, H.; Sakamoto, K.; Inagaki, K. The Serum 25(OH)D Level and Hand Grip Strength for Fall Risk Assessment among Osteoporotic Elderly Japanese Women. Arch. Osteoporos. 2021, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Mawarikado, Y.; Inagaki, Y.; Fujii, T.; Kubo, T.; Kido, A.; Tanaka, Y. Relationship between Fall History and Toe Grip Strength in Older Adults with Knee Osteoarthritis in Japan: A Cross-Sectional Study. PLoS ONE 2023, 18, e0282944. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hou, Y.; Li, H.; Lin, J. Influencing Factors of Weak Grip Strength and Fall: A Study Based on the China Health and Retirement Longitudinal Study (CHARLS). BMC Public Health 2022, 22, 2337. [Google Scholar] [CrossRef] [PubMed]
- Tatsunami, S.; Mimaya, J.; Meguro, T.; Kuwabara, R.; Yago, N.; Yamada, K. Analysis of Clinical AIDS-free Interval after CD4+ Cell Counts Fall below 200 × 106 L−1 in Japanese Haemophiliacs Infected with HIV-1. Haemophilia 1998, 4, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Nuvor, S.V.; van der Sande, M.; Rowland-Jones, S.; Whittle, H.; Jaye, A. Natural Killer Cell Function Is Well Preserved in Asymptomatic Human Immunodeficiency Virus Type 2 (HIV-2) Infection but Similar to That of HIV-1 Infection When CD4 T-Cell Counts Fall. J. Virol. 2006, 80, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.; Wang, Y.; Cumberland, W.G.; Detels, R.; Bozorgmehri, M.; Fahey, J.L. Serum Beta2-Microglobulin Level Increases in HIV Infection. AIDS 1990, 4, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rivas, M.; Jiménez-Sousa, M.Á.; Rallón, N.; Jiménez, J.L.; Restrepo, C.; León, A.; Montero-Alonso, M.; González-García, J.; Muñoz-Fernández, M.Á.; Benito, J.M.; et al. High Plasma Levels of sTNF-R1 and CCL11 Are Related to CD4+ T-Cells Fall in Human Immunodeficiency Virus Elite Controllers with a Sustained Virologic Control. Front. Immunol. 2018, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An Inflammatory Aging Clock (iAge) Based on Deep Learning Tracks Multimorbidity, Immunosenescence, Frailty and Cardiovascular Aging. Nat. Aging 2021, 1, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, K.S.; Burka, K.; Kulaga, A.Y.; Vorobyeva, N.S.; Kennedy, B.K. Aging Biomarkers: From Functional Tests to Multi-Omics Approaches. Proteomics 2020, 20, 1900408. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Elyahu, Y.; Hekselman, I.; Eizenberg-Magar, I.; Berner, O.; Strominger, I.; Schiller, M.; Mittal, K.; Nemirovsky, A.; Eremenko, E.; Vital, A.; et al. Aging Promotes Reorganization of the CD4 T Cell Landscape toward Extreme Regulatory and Effector Phenotypes. Sci. Adv. 2019, 5, eaaw8330. [Google Scholar] [CrossRef]
- Lages, C.S.; Suffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional Regulatory T Cells Accumulate in Aged Hosts and Promote Chronic Infectious Disease Reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.-C.; Stolberg, V.R.; Zhang, H.; Chensue, S.W. Increased Foxp3+ Treg Cell Activity Reduces Dendritic Cell Co-Stimulatory Molecule Expression in Aged Mice. Mech. Ageing Dev. 2007, 128, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Lenaers, G.; Bonneau, D.; Delneste, Y.; Papon, N. Dysfunctional T Cell Mitochondria Lead to Premature Aging. Trends Mol. Med. 2020, 26, 799–800. [Google Scholar] [CrossRef]
- Joseph, A.-M.; Adhihetty, P.J.; Wawrzyniak, N.R.; Wohlgemuth, S.E.; Picca, A.; Kujoth, G.C.; Prolla, T.A.; Leeuwenburgh, C. Dysregulation of Mitochondrial Quality Control Processes Contribute to Sarcopenia in a Mouse Model of Premature Aging. PLoS ONE 2013, 8, e69327. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Hall, J.A.; Kroehling, L.; Wu, L.; Najar, T.; Nguyen, H.H.; Lin, W.-Y.; Yeung, S.T.; Silva, H.M.; Li, D.; et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 2020, 180, 79–91.e16. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Fanelli, G.; Tan, N.; Nova-Lamperti, E.; McGregor, R.; Lechler, R.I.; Lombardi, G.; Scottà, C. Expanded Regulatory T Cells Induce Alternatively Activated Monocytes with a Reduced Capacity to Expand T Helper-17 Cells. Front. Immunol. 2018, 9, 1625. [Google Scholar] [CrossRef]
- Barbi, J.; Pardoll, D.M.; Pan, F. Treg Functional Stability and Its Responsiveness to the Microenvironment. Immunol. Rev. 2014, 259, 115–139. [Google Scholar] [CrossRef]
- Korn, T.; Muschaweckh, A. Stability and Maintenance of Foxp3+ Treg Cells in Non-Lymphoid Microenvironments. Front. Immunol. 2019, 10, 2634. [Google Scholar] [CrossRef] [PubMed]
- Baixauli, F.; Acín-Pérez, R.; Villarroya-Beltrí, C.; Mazzeo, C.; Nuñez-Andrade, N.; Gabandé-Rodriguez, E.; Ledesma, M.D.; Blázquez, A.; Martin, M.A.; Falcón-Pérez, J.M.; et al. Mitochondrial Respiration Controls Lysosomal Function during Inflammatory T Cell Responses. Cell Metab. 2015, 22, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Gonzalez-Freire, M.; Dunn, C.A.; Singh, A.K.; Macian, F.; Cuervo, A.M.; Sen, R.; Ferrucci, L. Age-Associated Changes in Human CD4+ T Cells Point to Mitochondrial Dysfunction Consequent to Impaired Autophagy. Aging 2019, 11, 9234–9263. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Wang, H.; Ho, P.-C. Mitochondrial Control and Guidance of Cellular Activities of T Cells. Front. Immunol. 2017, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Iparraguirre, L.; Alberro, A.; Iñiguez, S.G.; Muñoz-Culla, M.; Vergara, I.; Matheu, A.; Otaegui, D. Blood RNA-Seq Profiling Reveals a Set of Circular RNAs Differentially Expressed in Frail Individuals. Immun. Ageing 2023, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Luo, O.J.; Lei, W.; Zhu, G.; Ren, Z.; Xu, Y.; Xiao, C.; Zhang, H.; Cai, J.; Luo, Z.; Gao, L.; et al. Multidimensional Single-Cell Analysis of Human Peripheral Blood Reveals Characteristic Features of the Immune System Landscape in Aging and Frailty. Nat. Aging 2022, 2, 348–364. [Google Scholar] [CrossRef]
- Fehérvari, Z.; Sakaguchi, S. CD4+ Tregs and Immune Control. J. Clin. Investig. 2004, 114, 1209–1217. [Google Scholar] [CrossRef]
- Valencia, X.; Lipsky, P.E. CD4+CD25+FoxP3+ Regulatory T Cells in Autoimmune Diseases. Nat. Clin. Pract. Rheumatol. 2007, 3, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2011, 2012, e646354. [Google Scholar] [CrossRef]
- Heo, Y.J.; Choi, S.-E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Visfatin Induces Inflammation and Insulin Resistance via the NF-κB and STAT3 Signaling Pathways in Hepatocytes. J. Diabetes Res. 2019, 2019, 4021623. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, K.; Wang, S.; Ansari, A.R.; Niu, X.; Yang, W.; Lu, M.; Yang, Z.; Rehman, Z.U.; Zou, W.; et al. Visfatin Is a Multifaceted Molecule That Exerts Regulation Effects on Inflammation and Apoptosis in RAW264.7 Cells and Mice Immune Organs. Front. Immunol. 2022, 13, 1018973. [Google Scholar] [CrossRef]
- Tseng, W.-Y.; Stacey, M.; Lin, H.-H. Role of Adhesion G Protein-Coupled Receptors in Immune Dysfunction and Disorder. Int. J. Mol. Sci. 2023, 24, 5499. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.A.; Hattiangady, B.; Upadhya, D.; Bates, A.; Attaluri, S.; Shuai, B.; Kodali, M.; Shetty, A.K. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front. Mol. Neurosci. 2017, 10, 182. [Google Scholar] [CrossRef]
- Danieli, M.G.; Antonelli, E.; Piga, M.A.; Cozzi, M.F.; Allegra, A.; Gangemi, S. Oxidative Stress, Mitochondrial Dysfunction, and Respiratory Chain Enzyme Defects in Inflammatory Myopathies. Autoimmun. Rev. 2023, 22, 103308. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative Stress–Induced Mitochondrial Dysfunction Drives Inflammation and Airway Smooth Muscle Remodeling in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M. Energy Crisis: The Role of Oxidative Phosphorylation in Acute Inflammation and Sepsis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, e1452696. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Nicol, C.J.B.; Lo, S.-S.; Hung, S.-W.; Wang, C.-J.; Lin, C.-H. Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture. Int. J. Mol. Sci. 2022, 23, 11678. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, K.; Stringa, B.; Hoffmann, C.M.; Jindal, K.; Solnica-Krezel, L.; Morris, S.A. Dissecting Cell Identity via Network Inference and in Silico Gene Perturbation. Nature 2023, 614, 742–751. [Google Scholar] [CrossRef]
- Betz, B.C.; Jordan-Williams, K.L.; Wang, C.; Kang, S.G.; Liao, J.; Logan, M.R.; Kim, C.H.; Taparowsky, E.J. Batf Coordinates Multiple Aspects of B and T Cell Function Required for Normal Antibody Responses. J. Exp. Med. 2010, 207, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Arvey, A.; Josefowicz, S.Z.; Peng, X.; Reynolds, A.; Sandstrom, R.; Neph, S.; Sabo, P.; Kim, J.M.; Liao, W.; et al. Foxp3 Exploits a Pre-Existent Enhancer Landscape for Regulatory T Cell Lineage Specification. Cell 2012, 151, 153–166. [Google Scholar] [CrossRef]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4+ T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef]
- Zheng, Y.; Rudensky, A.Y. Foxp3 in Control of the Regulatory T Cell Lineage. Nat. Immunol. 2007, 8, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Dastrange, M.; Oukka, M. Foxp3 Interacts with Nuclear Factor of Activated T Cells and NF-Kappa B to Repress Cytokine Gene Expression and Effector Functions of T Helper Cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5138–5143. [Google Scholar] [CrossRef]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the Immune System: Focus on Inflammation and Vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Beier, U.H.; Angelin, A.; Akimova, T.; Wang, L.; Liu, Y.; Xiao, H.; Koike, M.A.; Hancock, S.A.; Bhatti, T.R.; Han, R.; et al. Essential Role of Mitochondrial Energy Metabolism in Foxp3+ T-Regulatory Cell Function and Allograft Survival. FASEB J. 2015, 29, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Howie, D.; Cobbold, S.P.; Adams, E.; Ten Bokum, A.; Necula, A.S.; Zhang, W.; Huang, H.; Roberts, D.J.; Thomas, B.; Hester, S.S.; et al. Foxp3 Drives Oxidative Phosphorylation and Protection from Lipotoxicity. JCI Insight 2017, 2, e89160. [Google Scholar] [CrossRef] [PubMed]
- Assadiasl, S.; Mooney, N.; Mohebbi, B.; Fatahi, Y.; Soleimanifar, N. Sirtuin 1: A Dilemma in Transplantation. J. Transplant. 2020, 2020, e9012980. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, S.-T.; Zhang, X.-Y.; Ding, H.-R.; Yuan, Y.; He, J.-J.; Wang, M.-S.; Yang, B.; Li, Y.-B. The Evolution of Single-Cell RNA Sequencing Technology and Application: Progress and Perspectives. Int. J. Mol. Sci. 2023, 24, 2943. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, R.; Fu, Y.; Zhu, Q.; Hong, L.; Wu, A.; Wang, H. Unveiling Aging Dynamics in the Hematopoietic System Insights from Single-Cell Technologies. Brief. Funct. Genom. 2024, elae019. [Google Scholar] [CrossRef]
- Zaro, B.W.; Noh, J.J.; Mascetti, V.L.; Demeter, J.; George, B.; Zukowska, M.; Gulati, G.S.; Sinha, R.; Flynn, R.A.; Banuelos, A.; et al. Proteomic Analysis of Young and Old Mouse Hematopoietic Stem Cells and Their Progenitors Reveals Post-Transcriptional Regulation in Stem Cells. eLife 2020, 9, e62210. [Google Scholar] [CrossRef]
- Piergallini, T.J.; Scordo, J.M.; Allué-Guardia, A.; Pino, P.A.; Zhang, H.; Cai, H.; Wang, Y.; Schlesinger, L.S.; Torrelles, J.B.; Turner, J. Acute Inflammation Alters Lung Lymphocytes and Potentiates Innate-like Behavior in Young Mouse Lung CD8 T Cells, Resembling Lung CD8 T Cells from Old Mice. J. Leukoc. Biol. 2023, 114, 237–249. [Google Scholar] [CrossRef]
- Khalyfa, A.; Klinge, C.M.; Hall, W.C.; Zhao, X.; Miller, M.M.; Wang, E. Transcription Profiling of Estrogen Target Genes in Young and Old Mouse Uterus. Exp. Gerontol. 2003, 38, 1087–1099. [Google Scholar] [CrossRef]
- Basisty, N.; Dai, D.-F.; Gagnidze, A.; Gitari, L.; Fredrickson, J.; Maina, Y.; Beyer, R.P.; Emond, M.J.; Hsieh, E.J.; MacCoss, M.J.; et al. Mitochondrial-Targeted Catalase Is Good for the Old Mouse Proteome, but Not for the Young: “Reverse” Antagonistic Pleiotropy? Aging Cell 2016, 15, 634–645. [Google Scholar] [CrossRef]
- Jasiulionis, M.G. Abnormal Epigenetic Regulation of Immune System during Aging. Front. Immunol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.; Zhu, Y.; Liu, X.; Gu, Y.; Dai, X.; Li, B. Transcriptional and Posttranslational Regulation of Th17/Treg Balance in Health and Disease. Eur. J. Immunol. 2021, 51, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial Dysfunction and Oxidative Stress Activate Inflammasomes: Impact on the Aging Process and Age-Related Diseases. Cell. Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Pallardó, F.V.; Viña, J. The Role of Mitochondrial Oxidative Stress in Aging. Free Radic. Biol. Med. 2003, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ye, J.; Dean, J.W.; Bostick, J.W.; Weinberg, S.E.; Xiong, L.; Oliff, K.N.; Chen, Z.E.; Avram, D.; Chandel, N.S.; et al. Requirement of Mitochondrial Transcription Factor A in Tissue-Resident Regulatory T Cell Maintenance and Function. Cell Rep. 2019, 28, 159–171.e4. [Google Scholar] [CrossRef] [PubMed]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T Cells with Dysfunctional Mitochondria Induce Multimorbidity and Premature Senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- Yadav, M.; Bluestone, J.A.; Stephan, S. Peripherally Induced Tregs—Role in Immune Homeostasis and Autoimmunity. Front. Immunol. 2013, 4, 232. [Google Scholar] [CrossRef] [PubMed]
- Smigiel, K.S.; Srivastava, S.; Stolley, J.M.; Campbell, D.J. Regulatory T Cell Homeostasis: Steady-State Maintenance and Modulation during Inflammation. Immunol. Rev. 2014, 259, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Badia-i-Mompel, P.; Vélez Santiago, J.; Braunger, J.; Geiss, C.; Dimitrov, D.; Müller-Dott, S.; Taus, P.; Dugourd, A.; Holland, C.H.; Ramirez Flores, R.O.; et al. decoupleR: Ensemble of Computational Methods to Infer Biological Activities from Omics Data. Bioinform. Adv. 2022, 2, vbac016. [Google Scholar] [CrossRef] [PubMed]
- Müller-Dott, S.; Tsirvouli, E.; Vazquez, M.; Ramirez Flores, R.O.; Badia-I-Mompel, P.; Fallegger, R.; Türei, D.; Lægreid, A.; Saez-Rodriguez, J. Expanding the Coverage of Regulons from High-Confidence Prior Knowledge for Accurate Estimation of Transcription Factor Activities. Nucleic Acids Res. 2023, 51, 10934–10949. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, A.; Lopez, R.; Xing, G.; Boyeau, P.; Valiollah Pour Amiri, V.; Hong, J.; Wu, K.; Jayasuriya, M.; Mehlman, E.; Langevin, M.; et al. A Python Library for Probabilistic Analysis of Single-Cell Omics Data. Nat. Biotechnol. 2022, 40, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Conde, C.; Xu, C.; Jarvis, L.B.; Rainbow, D.B.; Wells, S.B.; Gomes, T.; Howlett, S.K.; Suchanek, O.; Polanski, K.; King, H.W.; et al. Cross-Tissue Immune Cell Analysis Reveals Tissue-Specific Features in Humans. Science 2022, 376, eabl5197. [Google Scholar] [CrossRef]
- Franzén, O.; Gan, L.-M.; Björkegren, J.L.M. PanglaoDB: A Web Server for Exploration of Mouse and Human Single-Cell RNA Sequencing Data. Database J. Biol. Databases Curation 2019, 2019, baz046. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An Updated Mitochondrial Proteome Now with Sub-Organelle Localization and Pathway Annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.-H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and Analysis of Cell-Cell Communication Using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, J.S.; Bansal, B.; Guo, K.; Huang, F.; Singh, H.; Hur, J.; Khan, N.; Mathur, R. Mitochondrial Oxidative Stress Regulates FOXP3+ T-Cell Activity and CD4-Mediated Inflammation in Older Adults with Frailty. Int. J. Mol. Sci. 2024, 25, 6235. https://doi.org/10.3390/ijms25116235
Gill JS, Bansal B, Guo K, Huang F, Singh H, Hur J, Khan N, Mathur R. Mitochondrial Oxidative Stress Regulates FOXP3+ T-Cell Activity and CD4-Mediated Inflammation in Older Adults with Frailty. International Journal of Molecular Sciences. 2024; 25(11):6235. https://doi.org/10.3390/ijms25116235
Chicago/Turabian StyleGill, Jappreet Singh, Benu Bansal, Kai Guo, Fang Huang, Harpreet Singh, Junguk Hur, Nadeem Khan, and Ramkumar Mathur. 2024. "Mitochondrial Oxidative Stress Regulates FOXP3+ T-Cell Activity and CD4-Mediated Inflammation in Older Adults with Frailty" International Journal of Molecular Sciences 25, no. 11: 6235. https://doi.org/10.3390/ijms25116235
APA StyleGill, J. S., Bansal, B., Guo, K., Huang, F., Singh, H., Hur, J., Khan, N., & Mathur, R. (2024). Mitochondrial Oxidative Stress Regulates FOXP3+ T-Cell Activity and CD4-Mediated Inflammation in Older Adults with Frailty. International Journal of Molecular Sciences, 25(11), 6235. https://doi.org/10.3390/ijms25116235






