Ethanol Extract of the Microalga Phaeodactylum tricornutum Shows Hepatoprotective Effects against Acetaminophen-Induced Acute Liver Injury in Mice
Abstract
:1. Introduction
2. Results
2.1. Liver Injury Protection Effect of PT Extract against Acetaminophen-Induced Acute Liver Failure
2.2. Liver Injury Protection Effect of PT Extract against D-GalN/LPS-Induced Acute Liver Failure
2.3. Preventive Effect of PT on Acetaminophen- or D-GalN/LPS-Induced Liver Injury Models
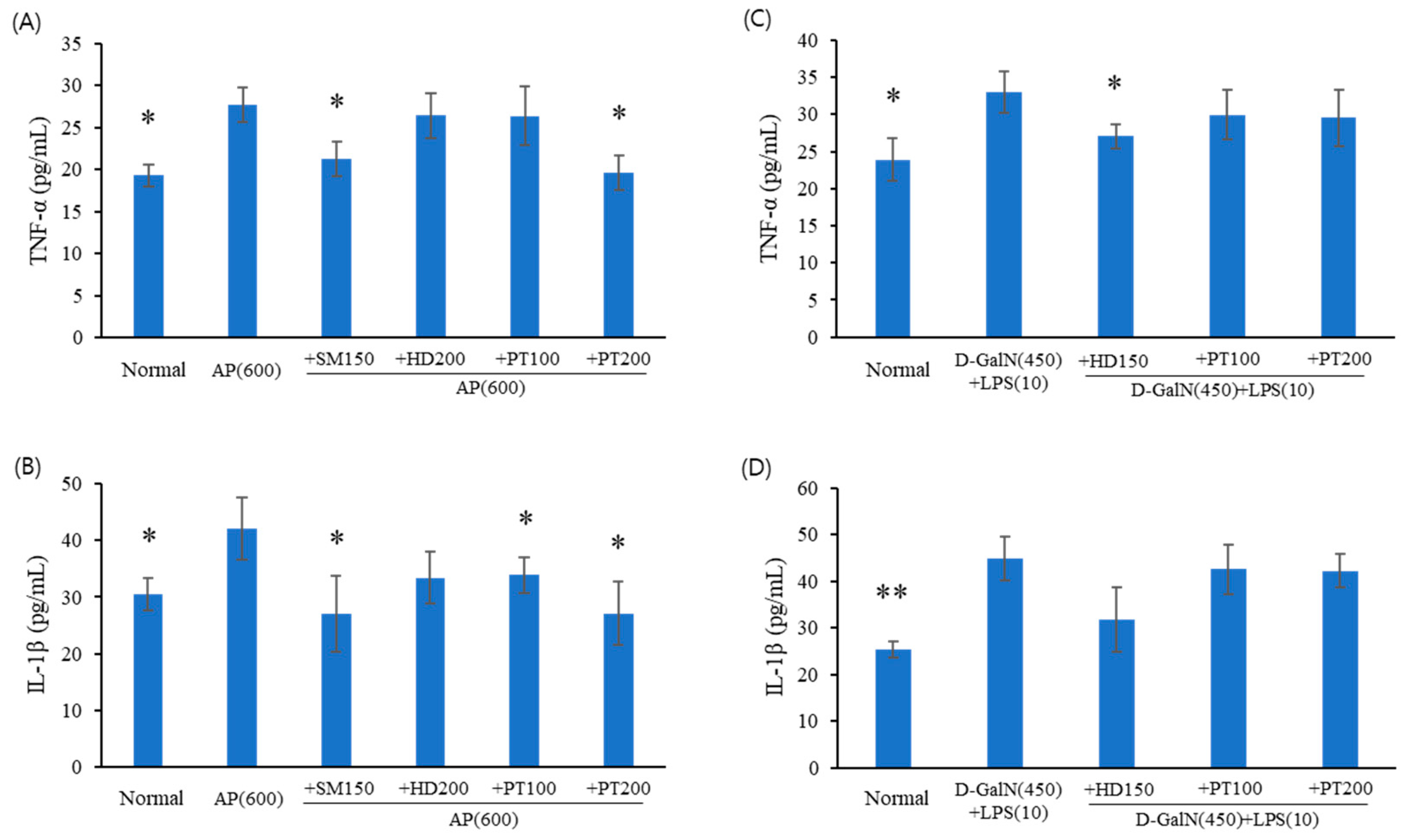
2.4. Expression Levels of Inflammatory Cytokines in Acute Liver Failure Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Culture of Phaeodactylum tricornutum (PT)
4.3. Preparation of PT Extract
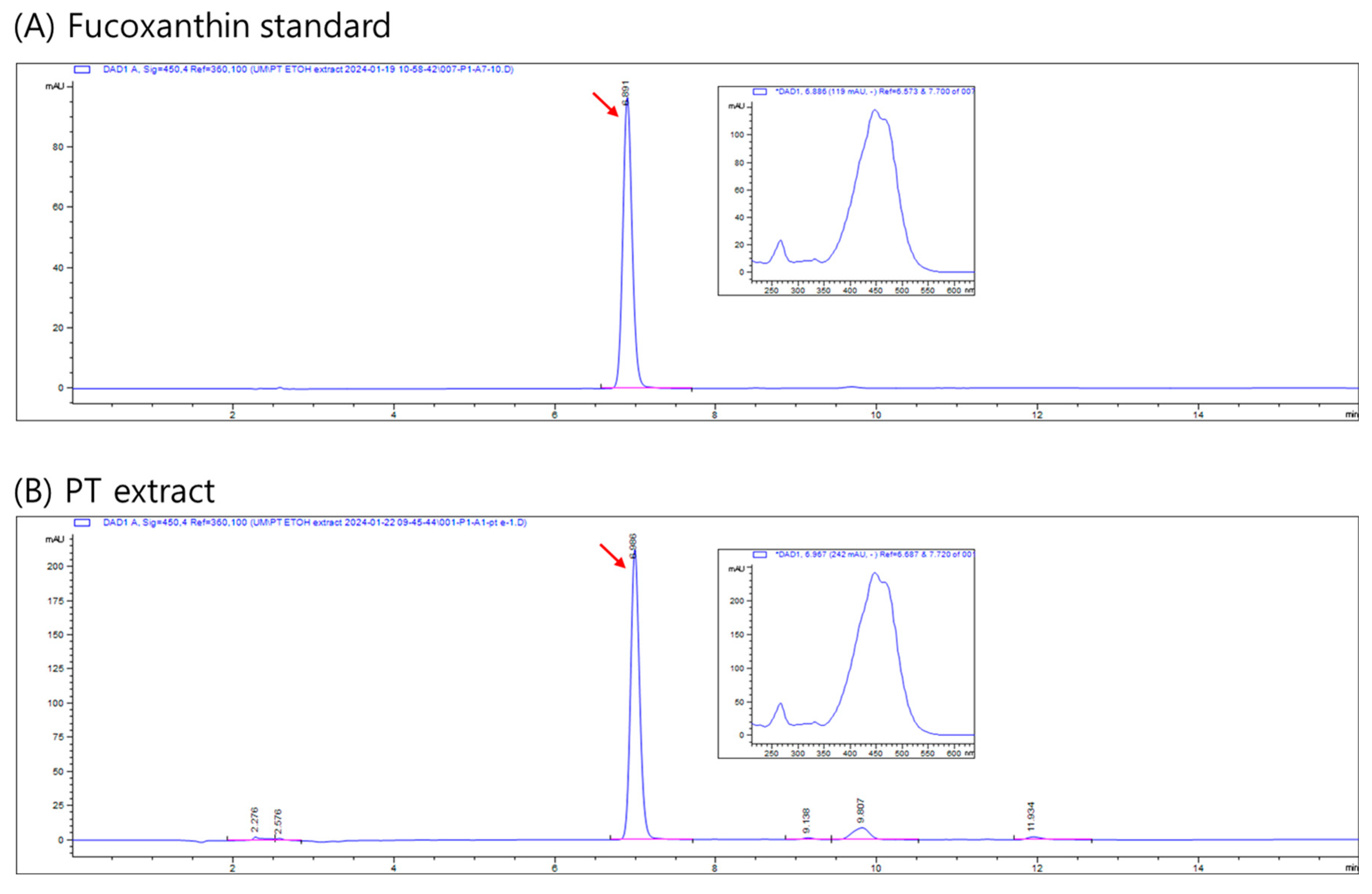
4.4. Characterization of the PT Extract
4.5. Analysis of Fucoxanthin
4.6. Animals
4.7. Acetaminophen-Induced Acute Liver Injury Model
4.8. D-GalN- and LPS-Induced Acute Liver Injury Model
4.9. Histological Analysis
4.10. Enzyme Immunoassay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Zheng, C.; Xue, J.; Chen, X.; Yang, W.; Liu, J.; Bai, W.; Li, H. Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J. Agric. Food Chem. 2014, 62, 8773–8776. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Muylaert, K.; Eeckhout, M.; Ruyssen, T.; Foubert, I. Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J. Agric. Food Chem. 2011, 59, 11063–11069. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewic, R.; Niedźwiedzka-Rystwej, P. Etiology of viral induced acute liver failure and defensins as potential therapeutic agents in ALF treatment. Front. Immunol. 2023, 14, 1153528. [Google Scholar] [CrossRef]
- Hassan, H.M.; Li, J. Prospect of Animal Models for Acute-on-chronic Liver Failure: A Mini-review. J. Clin. Transl. Hepatol. 2022, 10, 995–1003. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Animal models of drug-induced liver injury. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1031–1039. [Google Scholar] [CrossRef]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Suda, C.; Horie, T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J. Hepatol. 2005, 42, 110–116. [Google Scholar] [CrossRef]
- Xia, X.; Su, C.; Fu, J.; Zhang, P.; Jiang, X.; Xu, D.; Hu, L.; Song, E.; Song, Y. Role of α-lipoic acid in LPS/d-GalN induced fulminant hepatic failure in mice: Studies on oxidative stress, inflammation and apoptosis. Int. Immunopharmacol. 2014, 22, 293–302. [Google Scholar] [CrossRef]
- Xia, X.; Fu, J.; Song, X.; Shi, Q.; Su, C.; Song, E.; Song, Y. Neohesperidin dihydrochalcone down-regulates MyD88-dependent and -independent signaling by inhibiting endotoxin-induced trafficking of TLR4 to lipid rafts. Free Radic. Biol. Med. 2015, 89, 522–532. [Google Scholar] [CrossRef]
- Rahman, T.M.; Hodgson, H.J. Animal models of acute hepatic failure. Int. J. Exp. Pathol. 2000, 81, 145–157. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, S.M.; Jeong, S.-M.; Choi, H.-N.; Jang, Y.-H.; Kim, J.-I. Antioxidant effect of Phaeodactylum tricornutum in mice fed high-fat diet. Food Sci. Biotechnol. 2013, 22, 107–113. [Google Scholar] [CrossRef]
- Mayer, C.; Côme, M.; Ulmann, L.; Zittelli, G.C.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive Effects of the Marine Microalga Phaeodactylum tricornutum, Used as a Food Supplement, on Risk Factors Associated with Metabolic Syndrome in Wistar Rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ji, J.; Zhang, B.; Hu, L.; Cui, X.; Wang, H. Protective effects and possible molecular mechanism of Hovenia dulcis Thunb. extract on acetaminophen-induced hepatotoxicity. Pharmazie 2018, 73, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Holubek, W.J.; Kalman, S.; Hoffman, R.S. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2006, 43, 880, author reply 882. [Google Scholar] [CrossRef] [PubMed]
- Budnitz, D.S.; Lovegrove, M.C.; Crosby, A.E. Emergency department visits for overdoses of acetaminophen-containing products. Am. J. Prev. Med. 2011, 40, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Manthripragada, A.D.; Zhou, E.H.; Budnitz, D.S.; Lovegrove, M.C.; Willy, M.E. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol. Drug Saf. 2011, 20, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Mossanen, J.C.; Tacke, F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg. Nutr. 2014, 3, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar] [CrossRef] [PubMed]
- Bajt, M.L.; Knight, T.R.; Lemasters, J.J.; Jaeschke, H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: Protection by N-acetyl cysteine. Toxicol. Sci. 2004, 80, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.R.; Laskin, J.D.; Dambach, D.M.; Chiu, H.; Durham, S.K.; Zhou, P.; Bruno, M.; Gerecke, D.R.; Gordon, M.K.; Laskin, D.L. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol. Appl. Pharmacol. 2003, 192, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M.E.; Wilmer, J.L.; Holladay, S.D.; Wilson, R.E.; Luster, M.I. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 1995, 133, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Blazka, M.E.; Elwell, M.R.; Holladay, S.D.; Wilson, R.E.; Luster, M.I. Histopathology of acetaminophen-induced liver changes: Role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol. Pathol. 1996, 24, 181–189. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.; Lee, W.; Han, S.; Park, K. Protective effect of melittin on inflammation and apoptosis in acute liver failure. Apoptosis 2012, 17, 61–69. [Google Scholar] [CrossRef]
- Tsutsui, S.; Hirasawa, K.; Takeda, M.; Itagaki, S.; Kawamura, S.; Maeda, K.; Mikami, T.; Doi, K. Apoptosis of murine hepatocytes induced by high doses of galactosamine. J. Vet. Med. Sci. 1997, 59, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Jirillo, E.; Caccavo, D.; Magrone, T.; Piccigallo, E.; Amati, L.; Lembo, A.; Kalis, C.; Gumenscheimer, M. The role of the liver in the response to LPS: Experimental and clinical findings. J. Endotoxin. Res. 2002, 8, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-obesity effect of standardized extract of microalga Phaeodactylum tricornutum containing fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Mossanen, J.C.; Krenkel, O.; Ergen, C.; Govaere, O.; Liepelt, A.; Puengel, T.; Heymann, F.; Kalthoff, S.; Lefebvre, E.; Eulberg, D.; et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology 2016, 64, 1667–1682. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Tacke, F. Acetaminophen-induced acute liver injury in mice. Lab. Anim. 2015, 49, 30–36. [Google Scholar] [CrossRef]
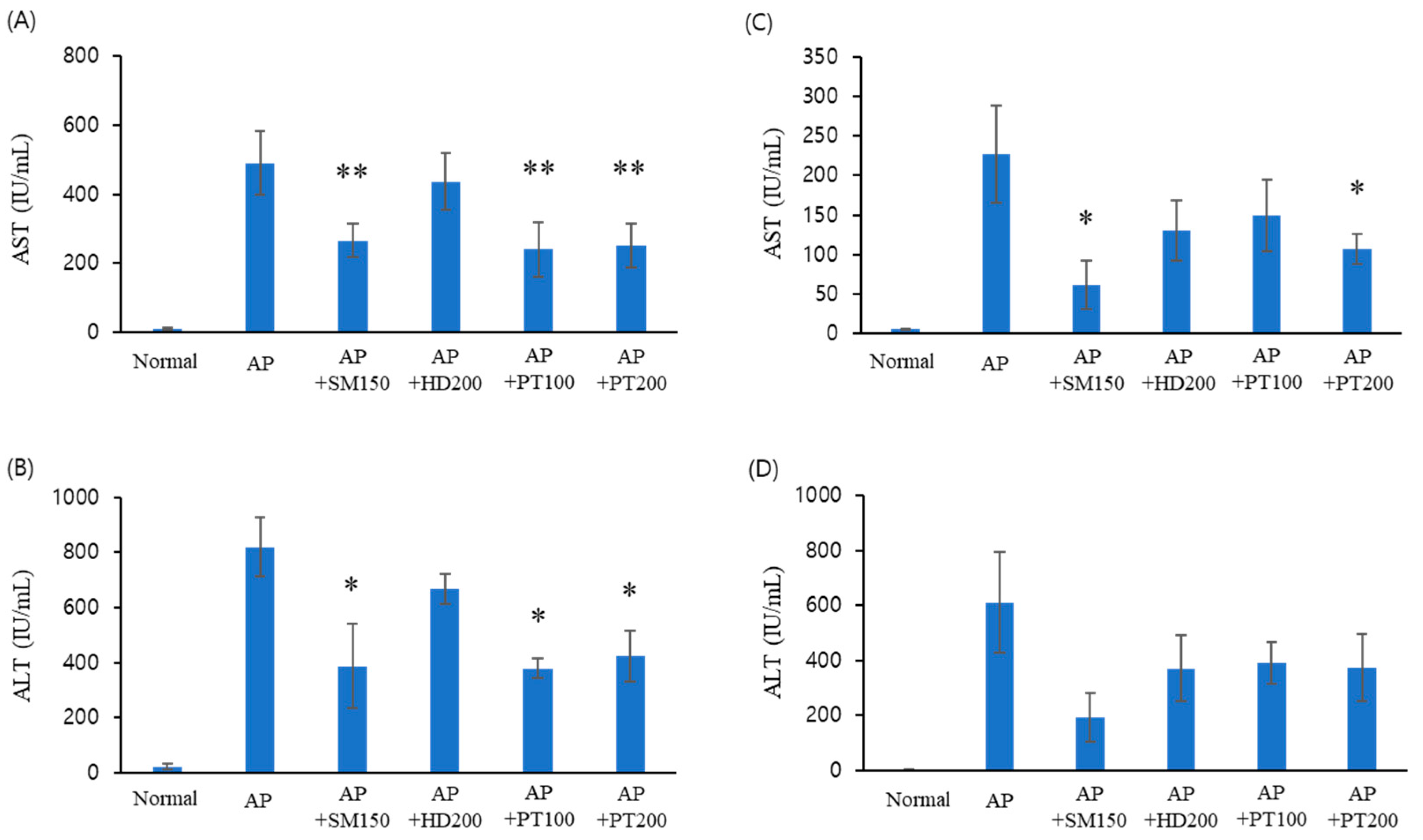
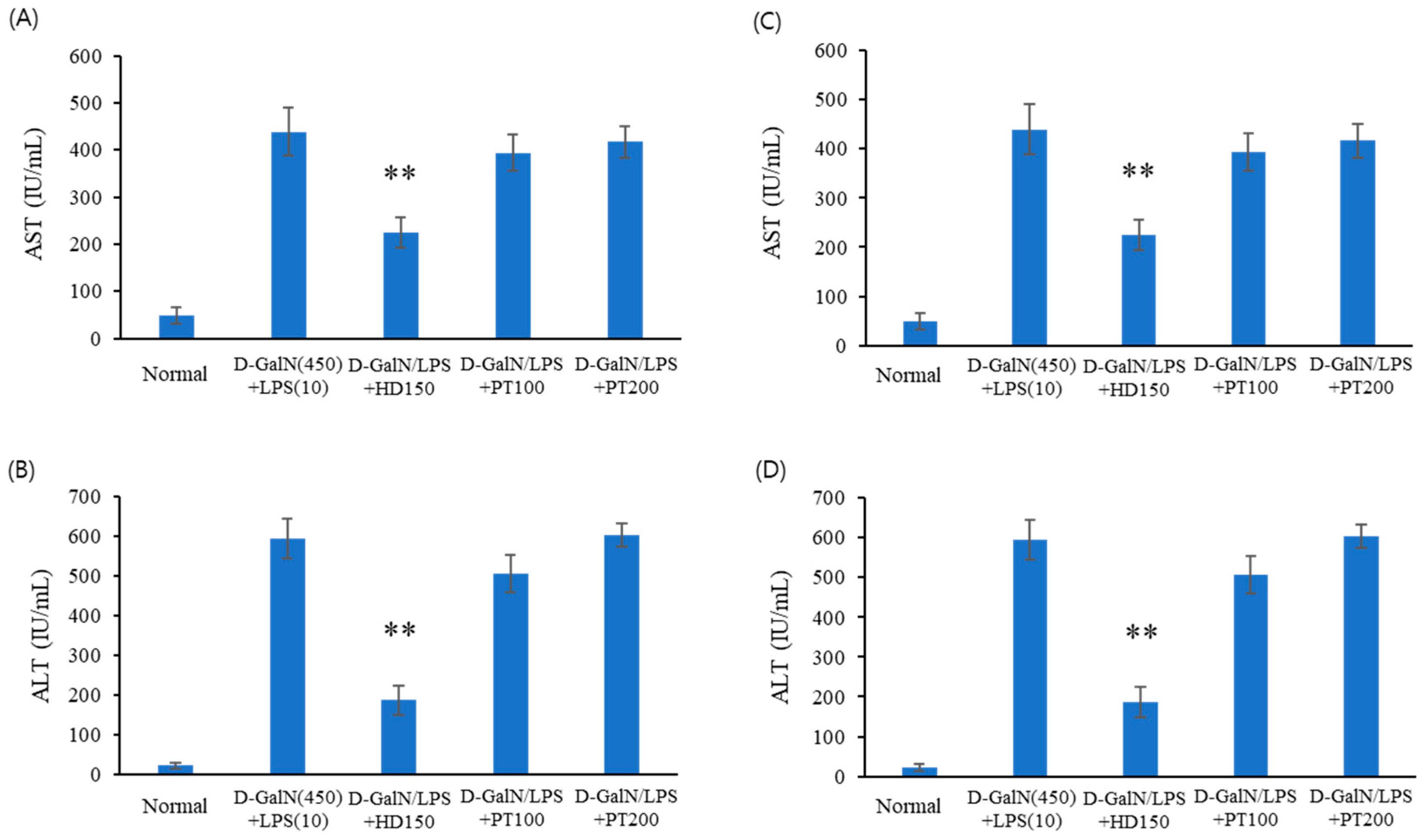
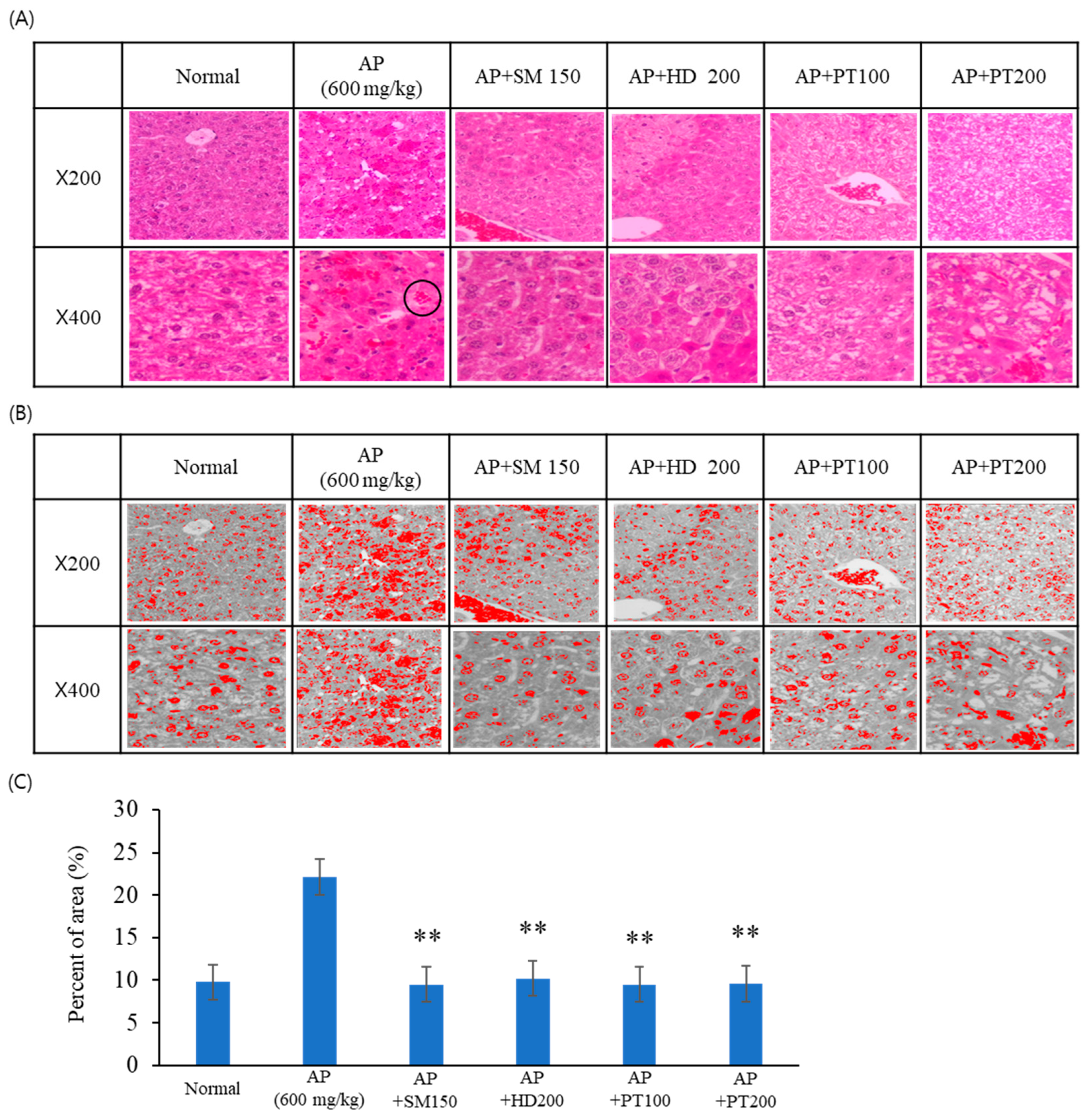
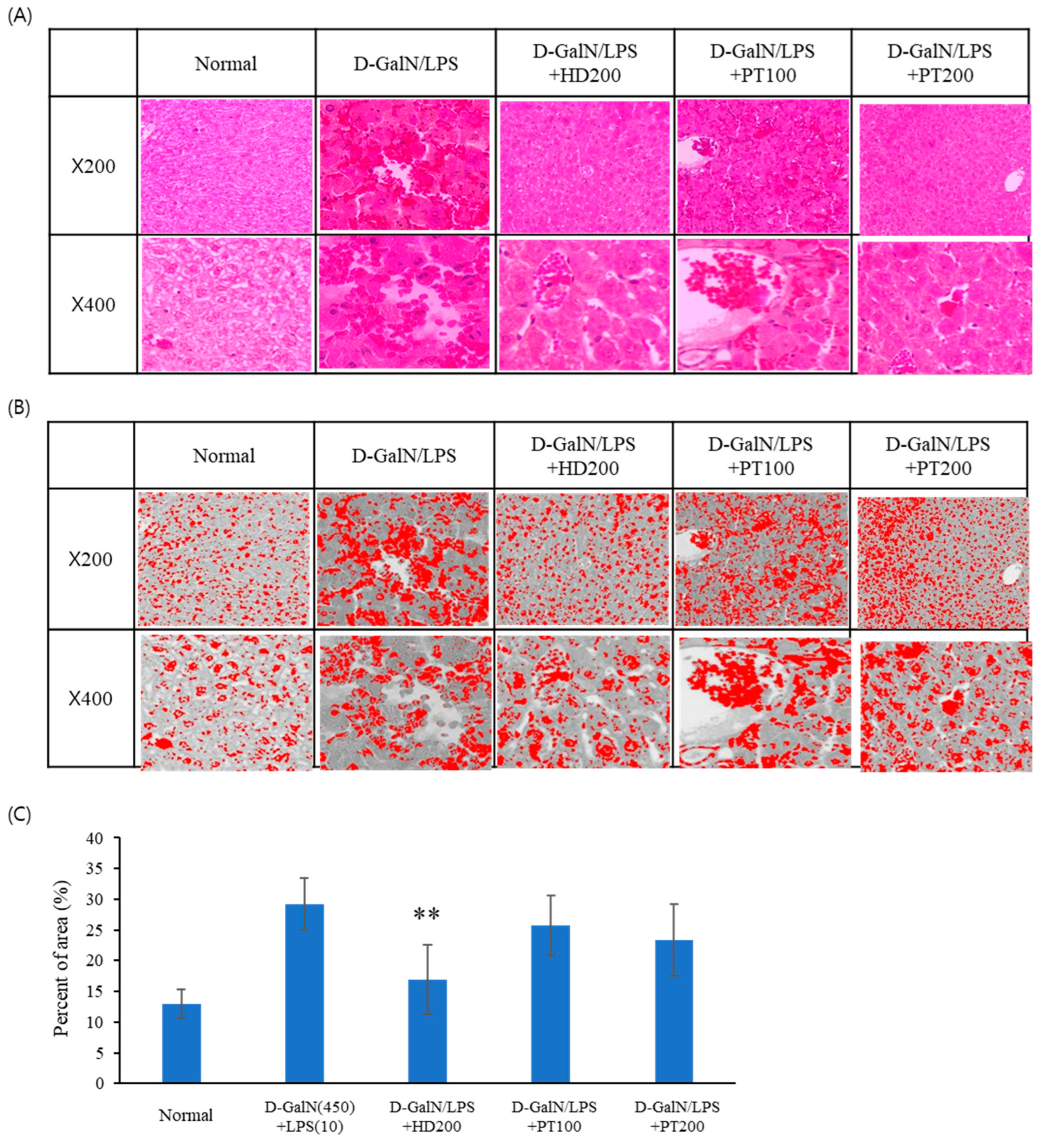

| Number | Group | Explanation |
|---|---|---|
| 1 | Normal | Untreated control group, without AP injection |
| 2 | AP | 600 mg/kg AP injection (IP) |
| 3 | AP + SM | Oral administration of 150 mg/kg Silybum marianum Seed extract after AP injection. |
| 4 | AP + HD | Oral administration of 200 mg/kg Hovenia dulcis Thunb. extract after AP injection. |
| 5 | AP + PT100 | Oral administration of 100 mg/kg PT extract after AP injection. |
| 6 | AP + PT200 | Oral administration of 200 mg/kg PT extract after AP injection. |
| Number | Group | Explanation |
|---|---|---|
| 1 | Normal | Untreated control group, without D-GalN/LPS injection |
| 2 | D-GalN/LPS | IP injection of D-GalN/LPS |
| 3 | D-GalN/LPS + SM | Oral administration of 150 mg/kg Silybum marianum Seed extract after D-GalN/LPS injection. |
| 4 | D-GalN/LPS + HD | Oral administration of 200 mg/kg Hovenia dulcis Thunb. extract after D-GalN/LPS injection. |
| 5 | D-GalN/LPS + PT100 | Oral administration of 100 mg/kg PT extract after D-GalN/LPS injection. |
| 6 | D-GalN/LPS + PT200 | Oral administration of 200 mg/kg PT extract after D-GalN/LPS injection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Park, H.J.; Eom, J.-I.; Han, C.-H.; Pan, C.-H.; Lee, J.K. Ethanol Extract of the Microalga Phaeodactylum tricornutum Shows Hepatoprotective Effects against Acetaminophen-Induced Acute Liver Injury in Mice. Int. J. Mol. Sci. 2024, 25, 6247. https://doi.org/10.3390/ijms25116247
Kim DY, Park HJ, Eom J-I, Han C-H, Pan C-H, Lee JK. Ethanol Extract of the Microalga Phaeodactylum tricornutum Shows Hepatoprotective Effects against Acetaminophen-Induced Acute Liver Injury in Mice. International Journal of Molecular Sciences. 2024; 25(11):6247. https://doi.org/10.3390/ijms25116247
Chicago/Turabian StyleKim, Dae Yoon, Hui Jin Park, Jae-In Eom, Cheol-Ho Han, Cheol-Ho Pan, and Jae Kwon Lee. 2024. "Ethanol Extract of the Microalga Phaeodactylum tricornutum Shows Hepatoprotective Effects against Acetaminophen-Induced Acute Liver Injury in Mice" International Journal of Molecular Sciences 25, no. 11: 6247. https://doi.org/10.3390/ijms25116247






