Optimizing Messenger RNA Analysis Using Ultra-Wide Pore Size Exclusion Chromatography Columns
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Chromatographic Conditions
2.1.1. Optimal Pore Size for mRNA Analysis
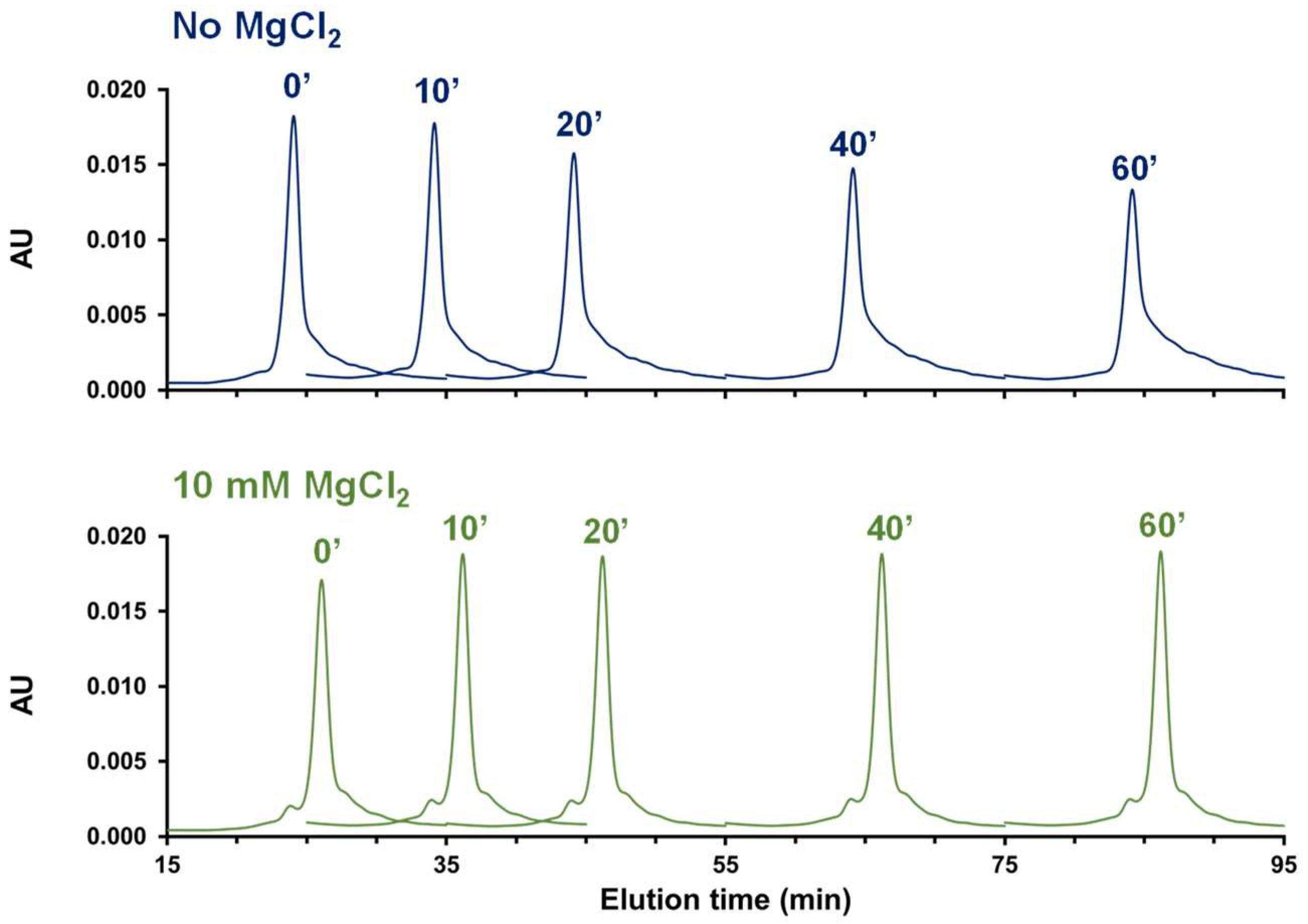
2.1.2. Influence of Buffer and Mobile Phase pH
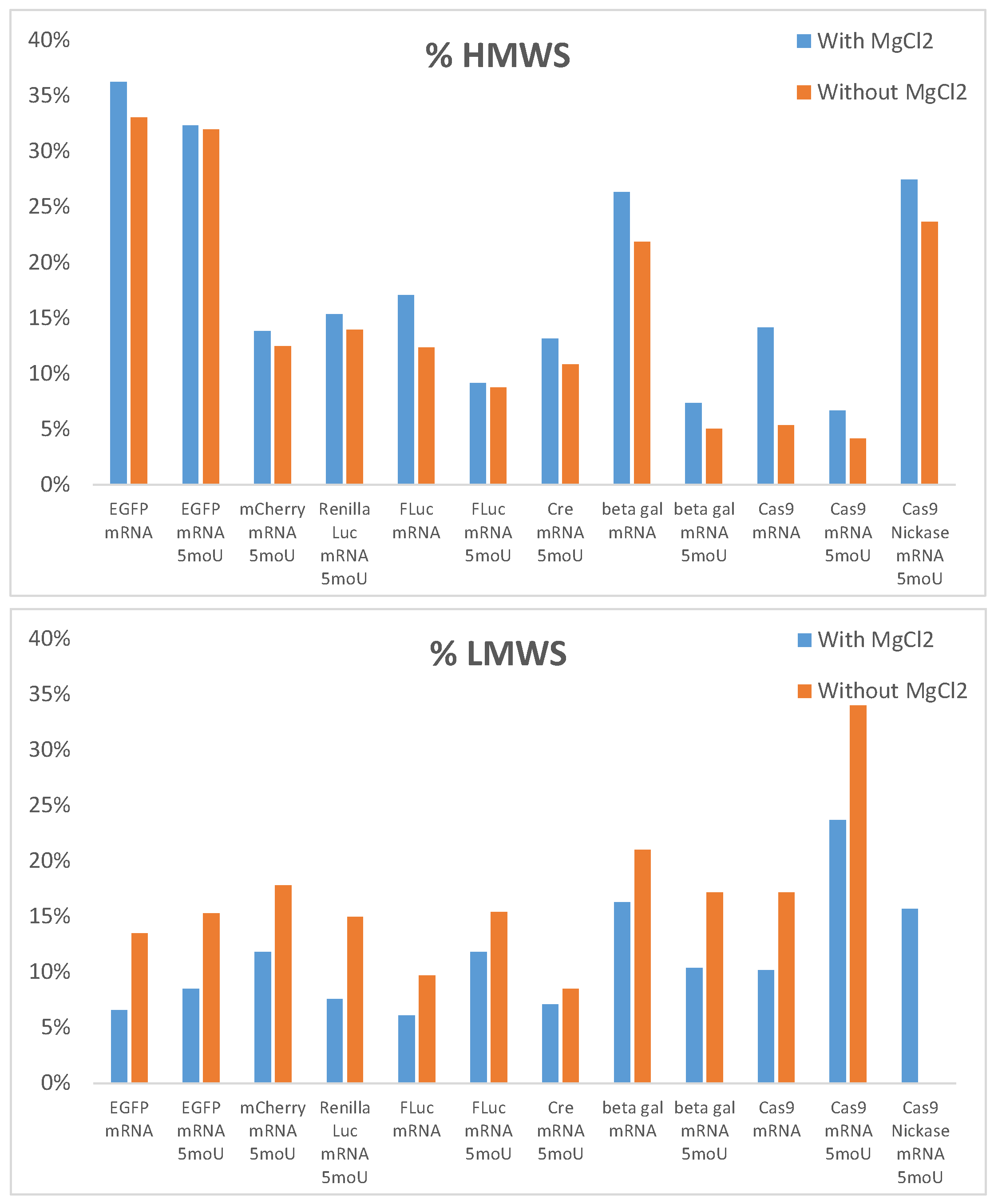
2.1.3. Use of Additives in the Mobile Phase
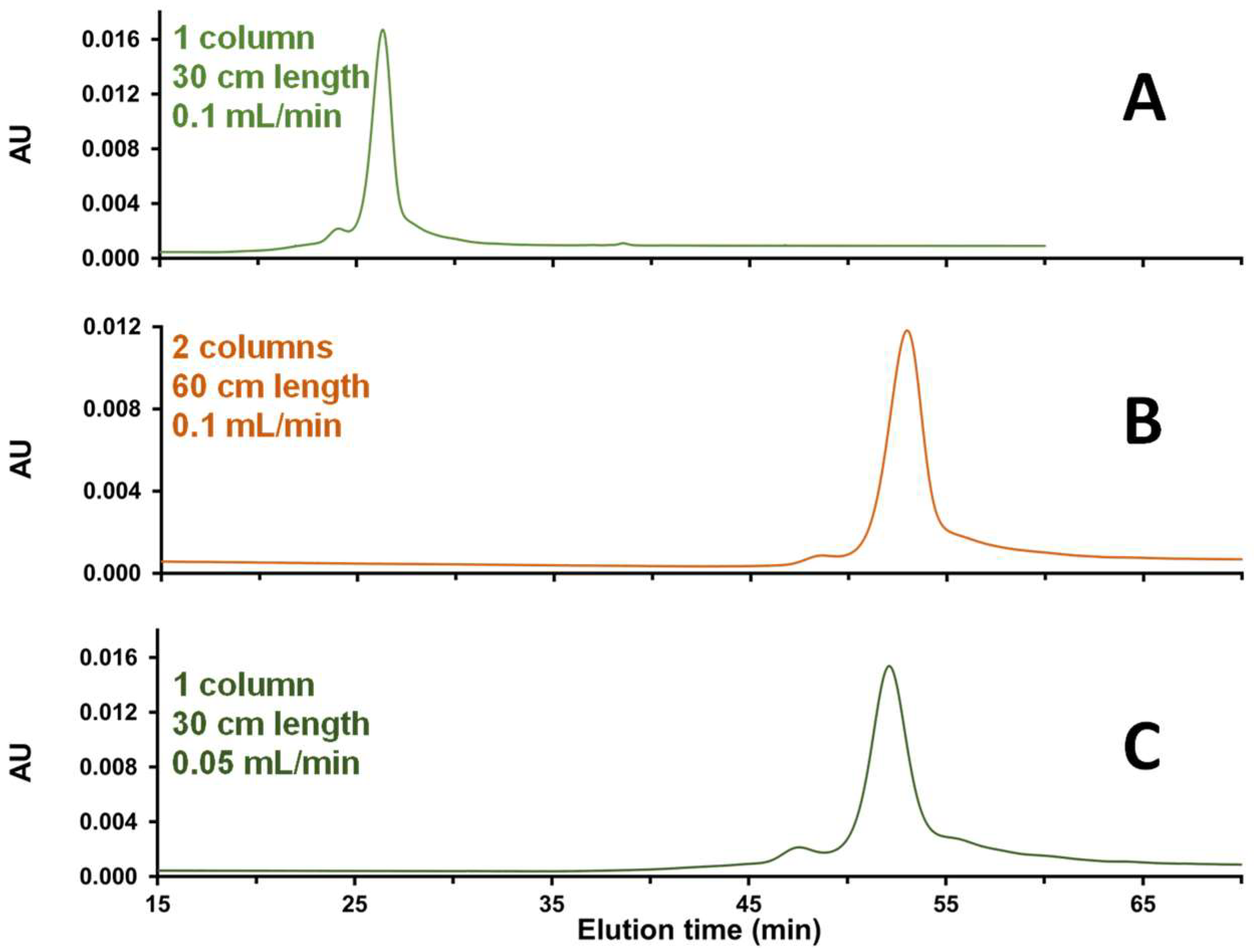
2.1.4. Tuning Column Length and Mobile Phase Flow Rate in SEC
2.1.5. Residence Time in the SEC Column
2.2. Application to the Analysis of mRNA Samples
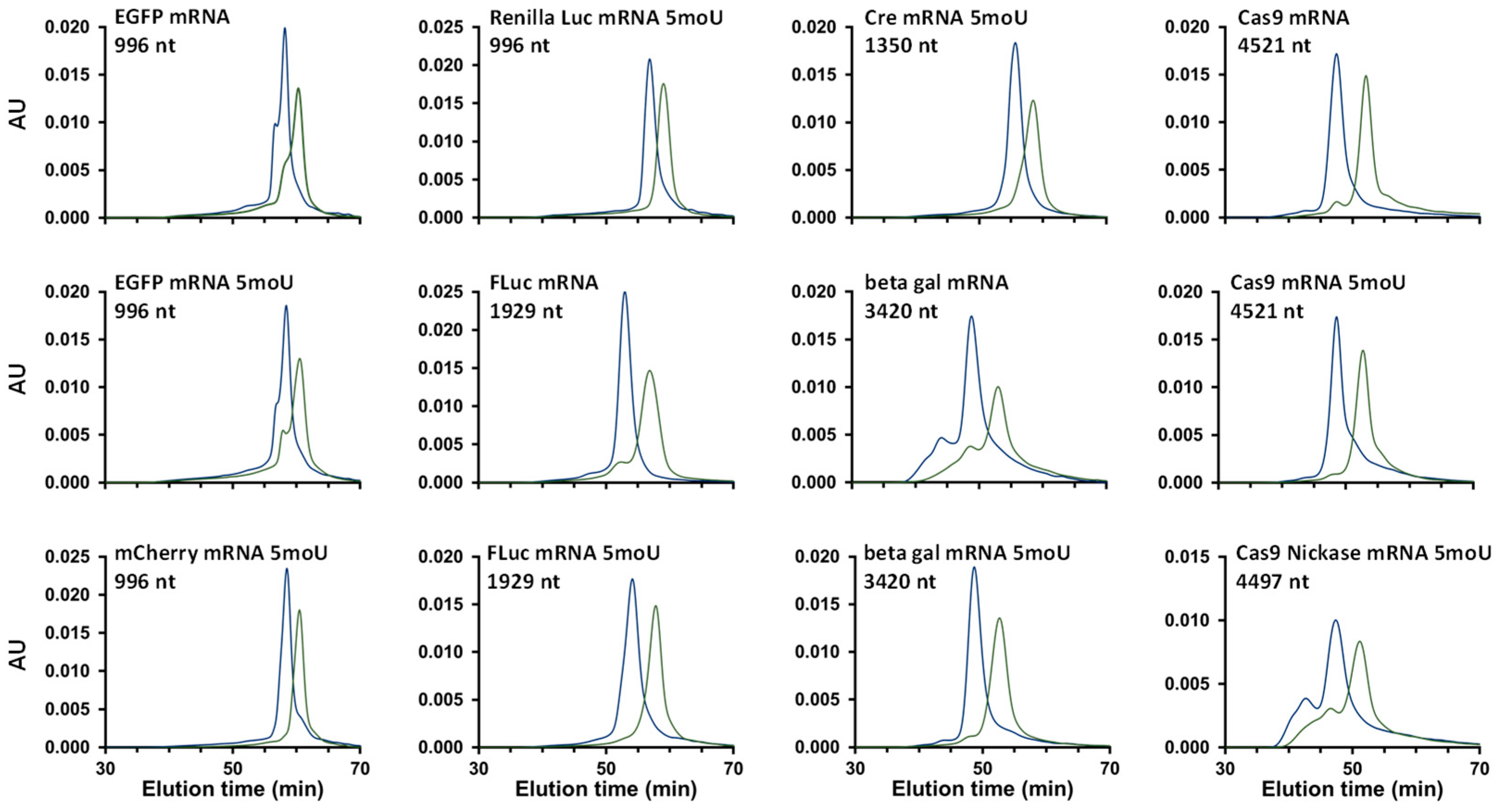
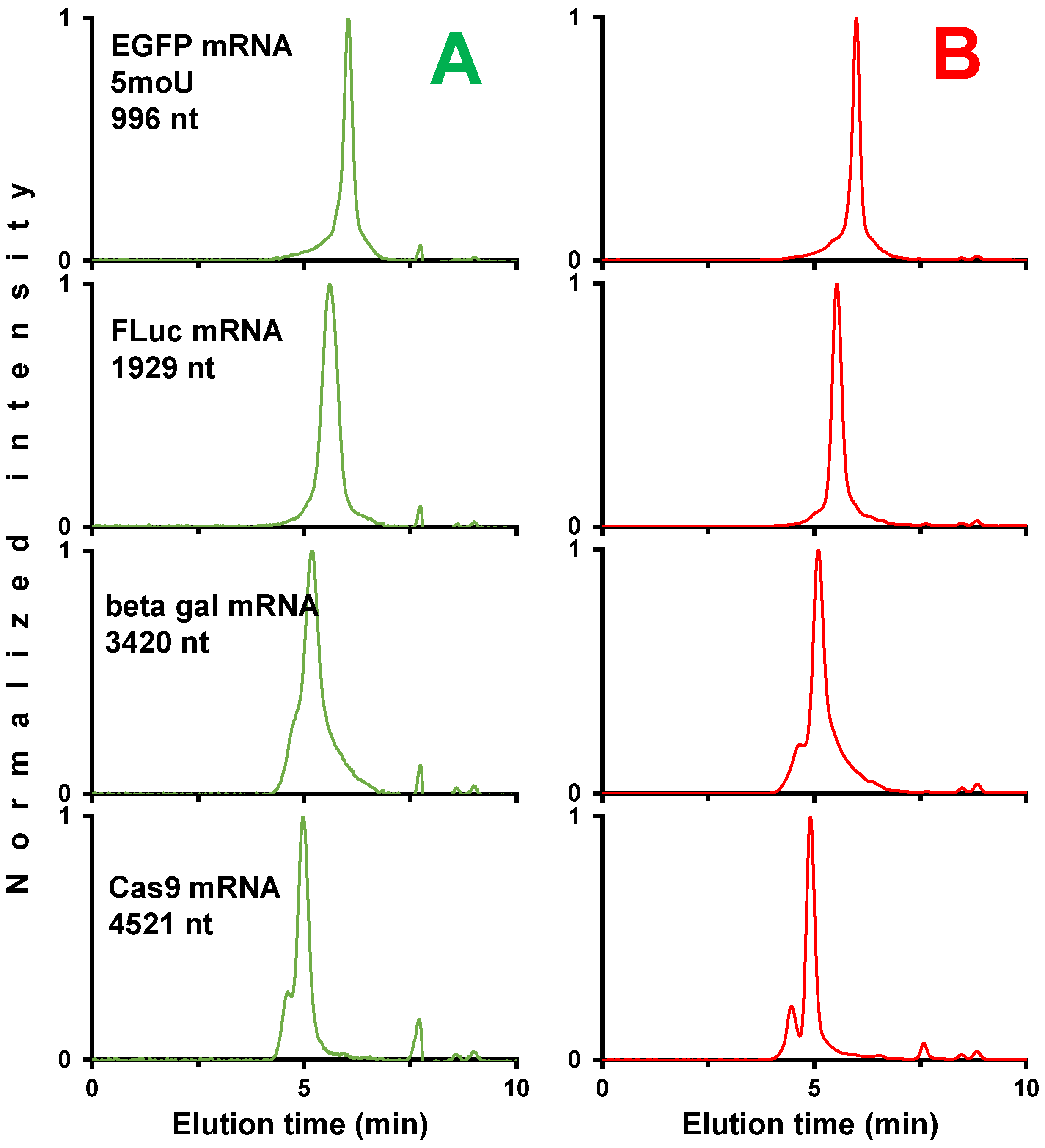
2.2.1. Analysis of a Wide Range of mRNA Samples
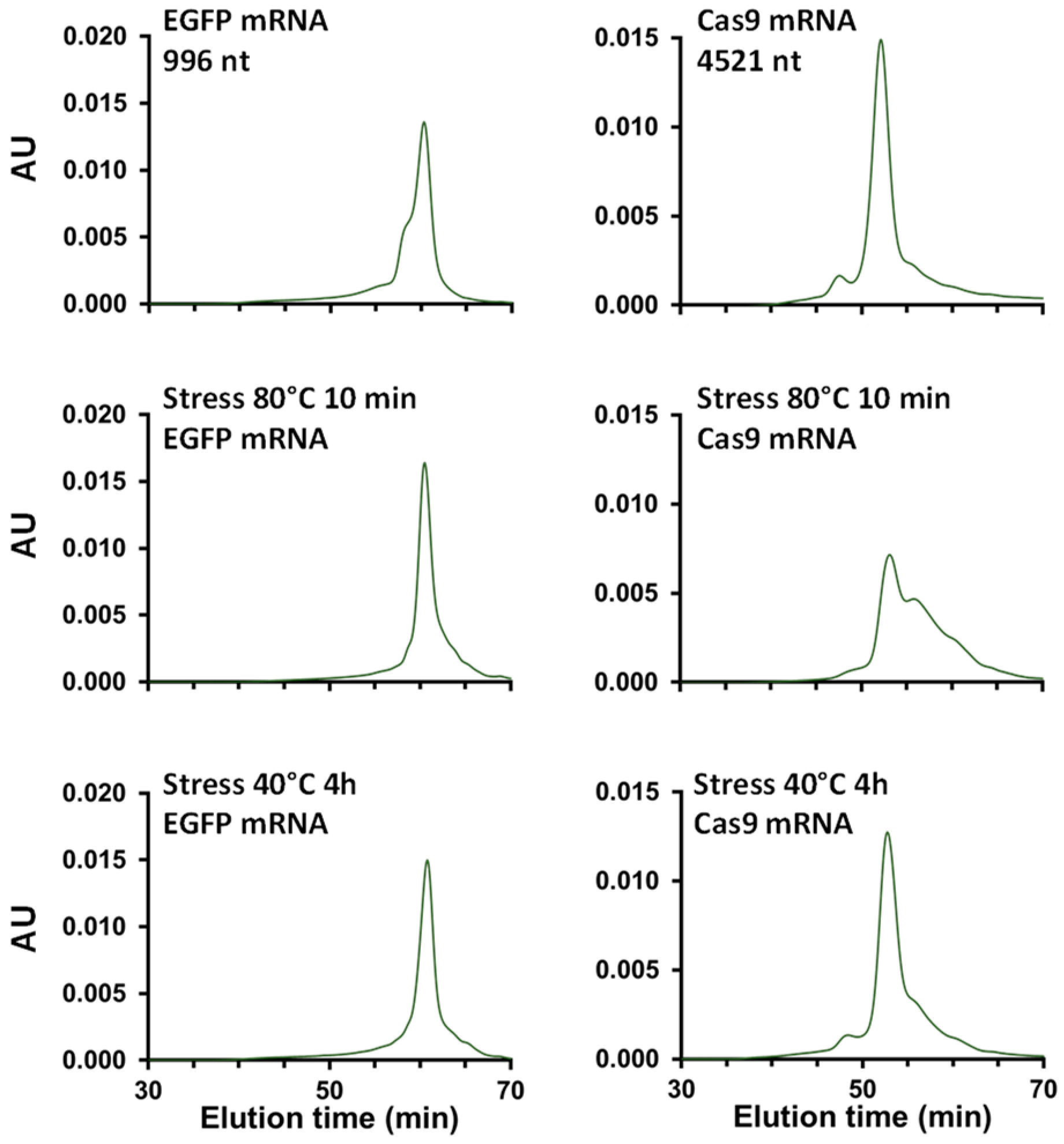
2.2.2. Is SEC a Stability-Indicating Method for mRNA?
2.2.3. Fast Analysis of mRNA Samples in SEC
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Instrumentation and Columns
3.3. SEC Chromatographic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Nobel Prize in Physiology or Medicine 2023. Press Release. NobelPrize.Org. Nobel Prize Outreach AB 2024. 2023. Available online: https://www.nobelprize.org/prizes/medicine/2023/press-release/ (accessed on 2 May 2024).
- Ichihashi, Y.; Fukushima, A.; Shibata, A.; Shirasu, K. High Impact Gene Discovery: Simple Strand-Specific mRNA Library Construction and Differential Regulatory Analysis Based on Gene Co-Expression Network. In Plant Transcription Factors; Yamaguchi, N., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1830, pp. 163–189. ISBN 978-1-4939-8656-9. [Google Scholar]
- Xie, W.; Chen, B.; Wong, J. Evolution of the Market for mRNA Technology. Nat. Rev. Drug Discov. 2021, 20, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Hu, T.; Zhu, Q.; Jin, X.; Chi, F.; Chen, X. How Far Are the New Wave of mRNA Drugs from Us? mRNA Product Current Perspective and Future Development. Front. Immunol. 2022, 13, 974433. [Google Scholar] [CrossRef] [PubMed]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The Promise of mRNA Vaccines: A Biotech and Industrial Perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Peptide Therapeutics Market—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2023–2031. 2023. Available online: https://www.transparencymarketresearch.com/peptide-therapeutics-market.html (accessed on 2 May 2024).
- Lu, T.; Klein, L.J.; Ha, S.; Rustandi, R.R. High-Resolution Capillary Electrophoresis Separation of Large RNA under Non-Aqueous Conditions. J. Chromatogr. A 2020, 1618, 460875. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Doneanu, C.; Addepalli, B.; Gaye, M.; Nguyen, J.; Alden, B.; Birdsall, R.; Han, D.; Isaac, G.; Lauber, M. Challenges and Emerging Trends in Liquid Chromatography-Based Analyses of mRNA Pharmaceuticals. J. Pharm. Biomed. Anal. 2023, 224, 115174. [Google Scholar] [CrossRef] [PubMed]
- Vanhinsbergh, C.J.; Criscuolo, A.; Sutton, J.N.; Murphy, K.; Williamson, A.J.K.; Cook, K.; Dickman, M.J. Characterization and Sequence Mapping of Large RNA and mRNA Therapeutics Using Mass Spectrometry. Anal. Chem. 2022, 94, 7339–7349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. Research Advances on the Stability of mRNA Vaccines. Viruses 2023, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, J.; Loughney, J.W.; Rustandi, R.R. Development of a Microchip Capillary Electrophoresis Method for Determination of the Purity and Integrity of mRNA in Lipid Nanoparticle Vaccines. Electrophoresis 2022, 43, 1101–1106. [Google Scholar] [CrossRef]
- Camperi, J.; Lippold, S.; Ayalew, L.; Roper, B.; Shao, S.; Freund, E.; Nissenbaum, A.; Galan, C.; Cao, Q.; Yang, F.; et al. Comprehensive Impurity Profiling of mRNA: Evaluating Current Technologies and Advanced Analytical Techniques. Anal. Chem. 2024, 96, 3886–3897. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Taoka, M.; Nobe, Y.; Izumikawa, K.; Takahashi, N.; Nakayama, H.; Isobe, T. Denaturing Reversed Phase Liquid Chromatographic Separation of Non-Coding Ribonucleic Acids on Macro-Porous Polystyrene-Divinylbenzene Resins. J. Chromatogr. A 2013, 1312, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Spivak, V.B.; Shahrokh, Z.; Issa, W.J. Analysis of mRNA Heterogeneity and Stability. U.S. Patent 2016/0017313, 21 January 2016. [Google Scholar]
- Currie, J.; Dahlberg, J.R.; Eriksson, J.; Schweikart, F.; Nilsson, G.A.; Örnskov, E. Stability Indicating Ion-Pair Reversed-Phase Liquid Chromatography Method for Modified mRNA. J. Pharm. Biomed. Anal. 2021, 245, 116144. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Tang, S.; Fekete, S.; Nguyen, D.; Hofmann, K.; Wang, S.; Shatz-Binder, W.; Fernandez, K.I.; Hecht, E.S.; Lauber, M.; et al. Separation of Plasmid DNA Topological Forms, Messenger RNA, and Lipid Nanoparticle Aggregates Using an Ultrawide Pore Size Exclusion Chromatography Column. Anal. Chem. 2023, 95, 15017–15024. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.; Morreel, K.; Alvarez, P.; Vanluchene, H.; Vankeirsbilck, R.; Sandra, P.; Sandra, K. Evaluation of Size-Exclusion Chromatography, Multi-Angle Light Scattering Detection and Mass Photometry for the Characterization of mRNA. J. Chromatogr. A 2024, 1719, 464756. [Google Scholar] [CrossRef] [PubMed]
- Hawe, A.; Hulse, W.L.; Jiskoot, W.; Forbes, R.T. Taylor Dispersion Analysis Compared to Dynamic Light Scattering for the Size Analysis of Therapeutic Peptides and Proteins and Their Aggregates. Pharm. Res. 2011, 28, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Malburet, C.; Leclercq, L.; Cotte, J.-F.; Thiebaud, J.; Bazin, E.; Garinot, M.; Cottet, H. Taylor Dispersion Analysis to Support Lipid-Nanoparticle Formulations for mRNA Vaccines. Gene Ther. 2023, 30, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; D’Atri, V.; Bobaly, B.; Wagner-Rousset, E.; Beck, A.; Fekete, S.; Guillarme, D. Protocols for the Analytical Characterization of Therapeutic Monoclonal Antibodies. I—Non-Denaturing Chromatographic Techniques. J. Chromatogr. B 2017, 1058, 73–84. [Google Scholar] [CrossRef]
- Malburet, C.; Carboni, A.; Guinamand, S.; Naik, H.; Fertier-Prizzon, S. mRNA Extraction from Lipid Nanoparticles. J. Chromatogr. A 2024, 1714, 464545. [Google Scholar] [CrossRef]
- Vandeventer, P.E.; Lin, J.S.; Zwang, T.J.; Nadim, A.; Johal, M.S.; Niemz, A. Multiphasic DNA Adsorption to Silica Surfaces under Varying Buffer, pH, and Ionic Strength Conditions. J. Phys. Chem. B 2012, 116, 5661–5670. [Google Scholar] [CrossRef]
- Guth-Metzler, R.; Mohamed, A.M.; Cowan, E.T.; Henning, A.; Ito, C.; Frenkel-Pinter, M.; Wartell, R.M.; Glass, J.B.; Williams, L.D. Goldilocks and RNA: Where Mg2+ Concentration Is Just Right. Nucleic Acids Res. 2023, 51, 3529–3539. [Google Scholar] [CrossRef]
- Bowman, J.C.; Lenz, T.K.; Hud, N.V.; Williams, L.D. Cations in Charge: Magnesium Ions in RNA Folding and Catalysis. Curr. Opin. Struct. Biol. 2012, 22, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-H.; De Vivo, M.; Dal Peraro, M.; Klein, M.L. Understanding the Effect of Magnesium Ion Concentration on the Catalytic Activity of Ribonuclease H through Computation: Does a Third Metal Binding Site Modulate Endonuclease Catalysis? J. Am. Chem. Soc. 2010, 132, 13702–13712. [Google Scholar] [CrossRef] [PubMed]
- Morrill, G.A.; Reiss, M.M. Inhibition of Enzymatic Degradation of RNA by Bound Calcium and Magnesium. Biochim. Biophys. Acta 1969, 179, 43–49. [Google Scholar] [CrossRef]
- Kankia, B.I. Mg2+-Induced Triplex Formation of an Equimolar Mixture of Poly(rA) and Poly(rU). Nucleic Acids Res. 2003, 31, 5101–5107. [Google Scholar] [CrossRef] [PubMed]
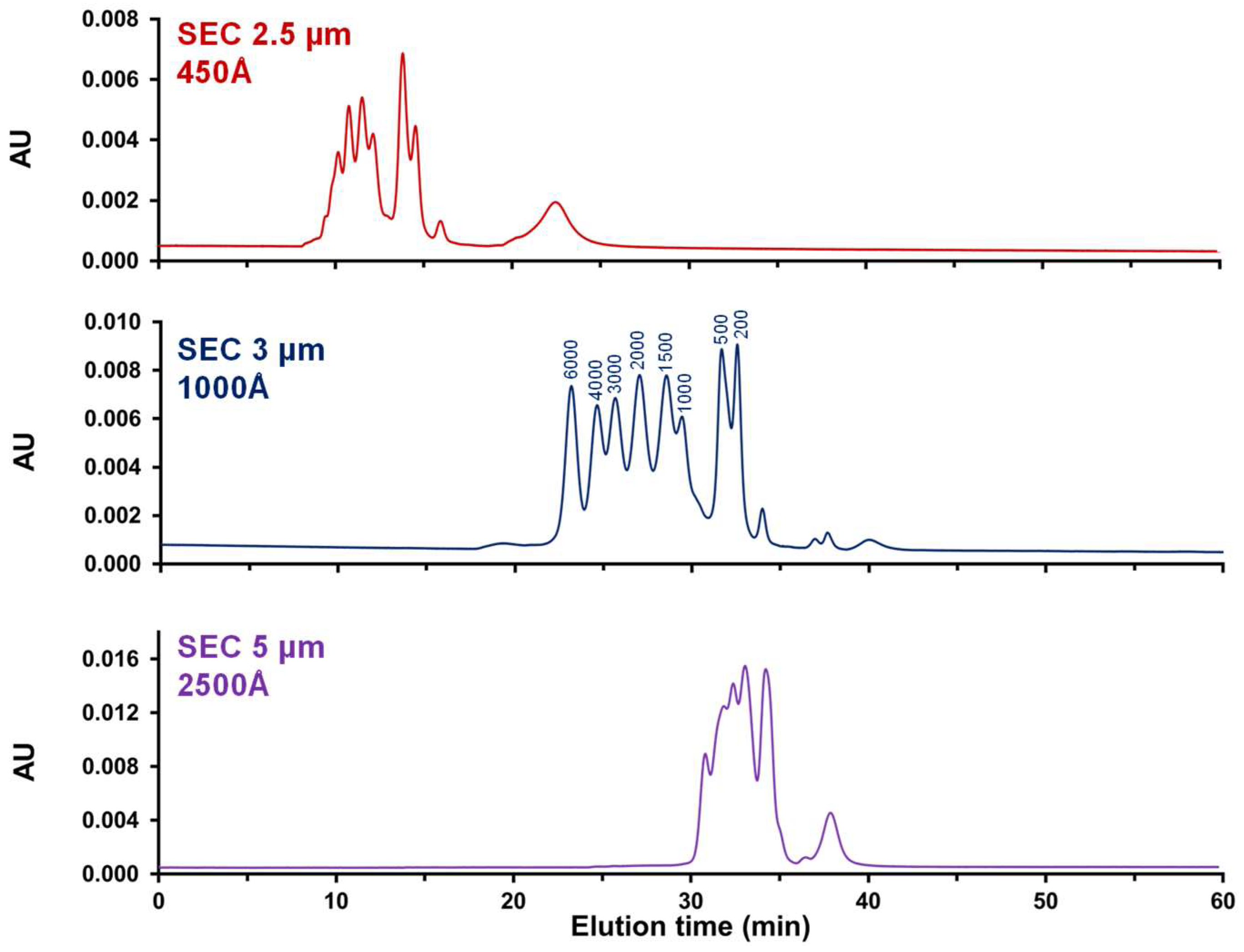


| Nominal Pore Size (Å) | Median (Å) | Mode (Å) | Pore Size Range (Å) | Pore Volume, cc/g |
|---|---|---|---|---|
| 450 | 427 | 453 | 190–650 | 1.07 |
| 1000 | 965 | 884 | 400–2000 | 0.79 |
| 2500 | 2458 | 2578 | 1500–4500 | 0.86 |
| Sample | % HMWS | % mRNA | % LMWS |
|---|---|---|---|
| Cas9 mRNA | 14% | 75% | 10% |
| Cas9 mRNA stressed 80 °C 10 min | 5% | 40% | 53% |
| Cas9 mRNA stressed 40 °C 4 h | 11% | 62% | 26% |
| EGFP mRNA | 36% | 57% | 6% |
| EGFP mRNA Stressed 80 °C 10 min | 15% | 65% | 19% |
| EGFP mRNA Stressed 40 °C 4 h | 22% | 62% | 15% |
| Cas9 mRNA Provider A | 14% | 75% | 10% |
| Cas9 mRNA Provider B | 27% | 68% | 4% |
| EGFP mRNA Provider A | 36% | 57% | 6% |
| EGFP mRNA Provider B | 36% | 59% | 4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Atri, V.; Lardeux, H.; Goyon, A.; Imiołek, M.; Fekete, S.; Lauber, M.; Zhang, K.; Guillarme, D. Optimizing Messenger RNA Analysis Using Ultra-Wide Pore Size Exclusion Chromatography Columns. Int. J. Mol. Sci. 2024, 25, 6254. https://doi.org/10.3390/ijms25116254
D’Atri V, Lardeux H, Goyon A, Imiołek M, Fekete S, Lauber M, Zhang K, Guillarme D. Optimizing Messenger RNA Analysis Using Ultra-Wide Pore Size Exclusion Chromatography Columns. International Journal of Molecular Sciences. 2024; 25(11):6254. https://doi.org/10.3390/ijms25116254
Chicago/Turabian StyleD’Atri, Valentina, Honorine Lardeux, Alexandre Goyon, Mateusz Imiołek, Szabolcs Fekete, Matthew Lauber, Kelly Zhang, and Davy Guillarme. 2024. "Optimizing Messenger RNA Analysis Using Ultra-Wide Pore Size Exclusion Chromatography Columns" International Journal of Molecular Sciences 25, no. 11: 6254. https://doi.org/10.3390/ijms25116254





