A Dynamic Protocol to Explore NLRP3 Inflammasome Activation in Cerebral Organoids
Abstract
1. Introduction
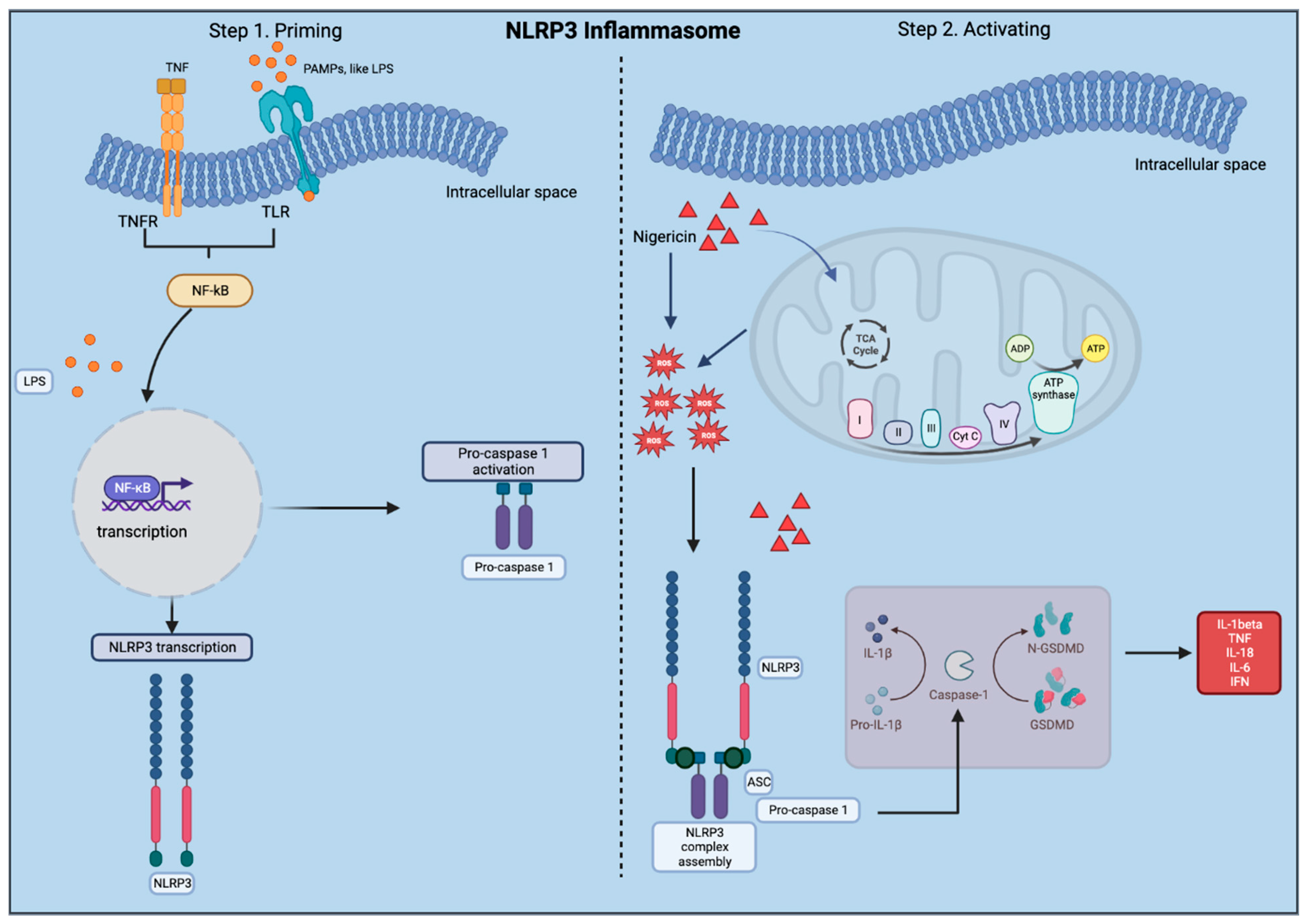
1.1. The Nod-Like Receptor Pyrin Containing 3 (NLRP3)
1.2. Neuropsychiatric Diseases and the NLRP3 Inflammasome
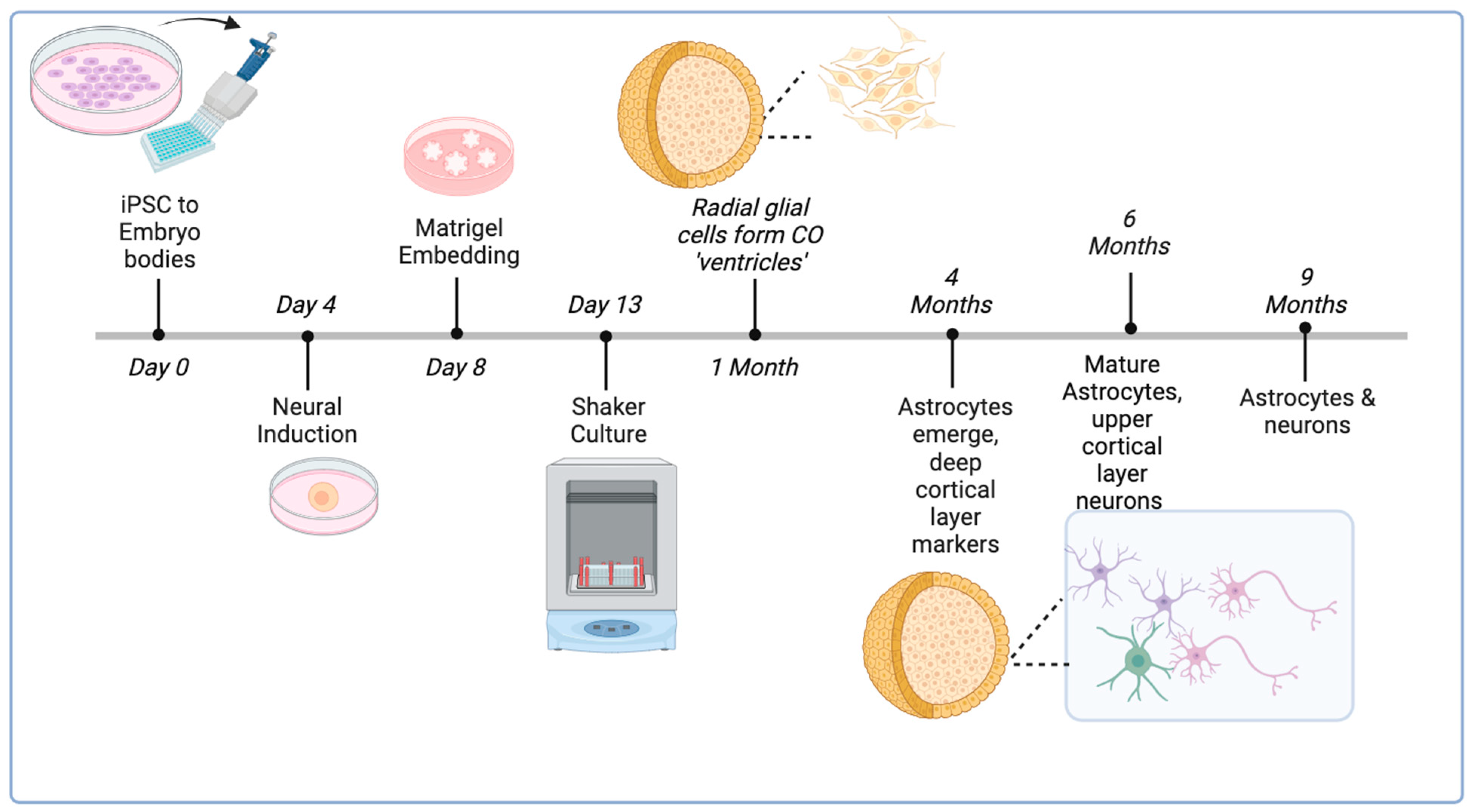
1.3. Use of Cerebral Organoids to Study the NLRP3 Inflammasome
1.4. The NLRP3 Inflammasome: Astrocytes and Microglia
2. Materials and Methods
2.1. Methodological Experimental Design
2.2. Protocol to Activate the NLRP3 Inflammasome in COs
2.2.1. Generation and Long-Term Culture of COs
- Cerebral organoids (COs) were generated from H9 ESCs using an established protocol at the Applied Organoid Core Facility (ApOC) at the Donnelly Centre, University of Toronto) [32].
- COs were cultured on an orbital shaker in six-well non-tissue culture plates using 3 mL of maturation medium which was changed twice a week.
- Once CO size exceeded 2 mm in diameter, a maximum of four COs were placed in each well and allowed to grow to 6 months of age (24 weeks).
2.2.2. Slicing COs with the Vibratome
- COs were placed in plastic base molds, and excess media was removed to allow the CO to dry.
- Low-melting-point 4% agarose was warmed in PBS to 37 °C, and the CO was embedded in agarose and carefully centered in the base mold.
- Base molds were placed on ice for 10 min.
- Once solidified, the CO embedded in agarose was scooped out and cut into a 1 cm ±× 1 cm cube.
- A drop of glue was placed on the vibratome’s stage block, and the CO cube was carefully placed on it.
- The vibratome’s stage was submerged in the block filled with cold PBS+/+ (with calcium and magnesium). This helped keep the slice alive and reduce tissue damage.
- The vibratome was then lowered and moved to allow the blade to meet the CO for slicing into thin sections.
- Each CO was sliced at a speed of 1 mm/sec and an amplitude of 0.8 to generate 400 µm thick sections. This size allows for better drug penetration to all cell types in the CO while maintaining CO structure and integrity.
- Sliced sections were collected from the vibratome block using a transfer pipette and were placed in a six-well plate containing PBS and 1% penicillin/streptomycin.
- After slicing, the plate was placed in the biosafety cabinet (BSC), and the CO slices were transferred to a new six-well plate pre-filled with 2 mL CO maturation media with 1% penicillin/streptomycin. This avoids contamination as the CO slices had been exposed to nonsterile conditions during slicing.
2.2.3. Activating the NLRP3 Inflammasome
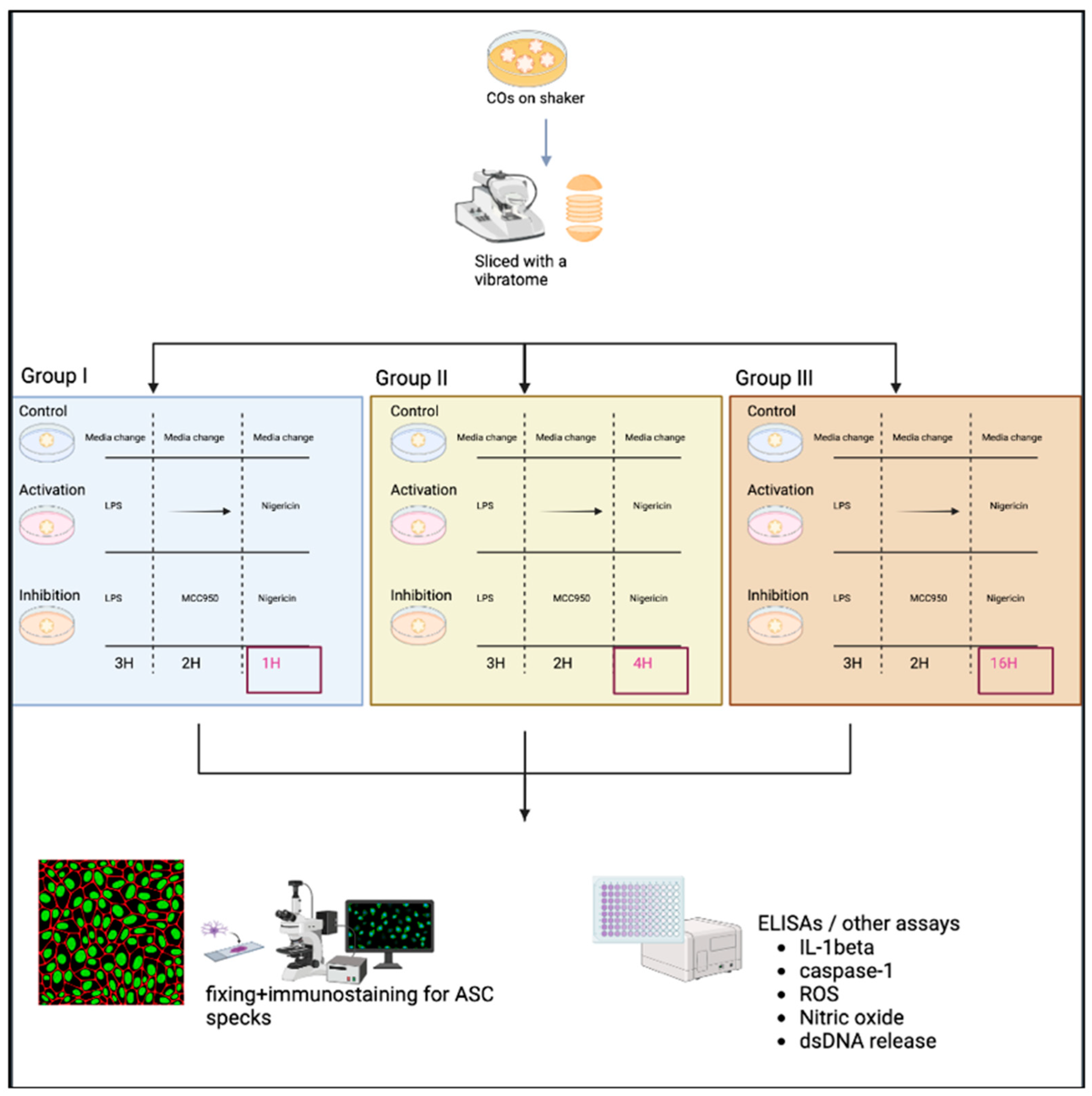
- Note: Three groups were used to generate a time-response curve of the NLRP3 activation protocol as depicted in Figure 3.
- The original protocol (used for PBMCs) involved priming using 100 ng/mL LPS (Invivogen, San Diego, CA, USA, TLRL-3PELPS LPS) for 3 h, followed by pre-treatment with an NLRP3 inhibitor at 100 nM MCC950 (Invivogen, San Diego, CA, USA, 210826-40-7) for 2 h and then 10 µM of nigericin (Sigma, St. Louis, MO, USA, N7143), an NLRP3 activator for 1 h.
- Here, the LPS and MCC950 incubation times were consistent with the original protocol; however, the nigericin incubation time was altered. All treatments were performed using a shaker during the incubations.
- Slices were primed with 100 ng/mL LPS for 3 h at 37 °C.
- Media was removed, and slices were then incubated at 37 °C with 100 nM MCC950 for 2 h as a pre-treatment.
- Media was removed, and slices were incubated with 10 µM nigericin for 1, 4, and 16 h for the time-response curve.
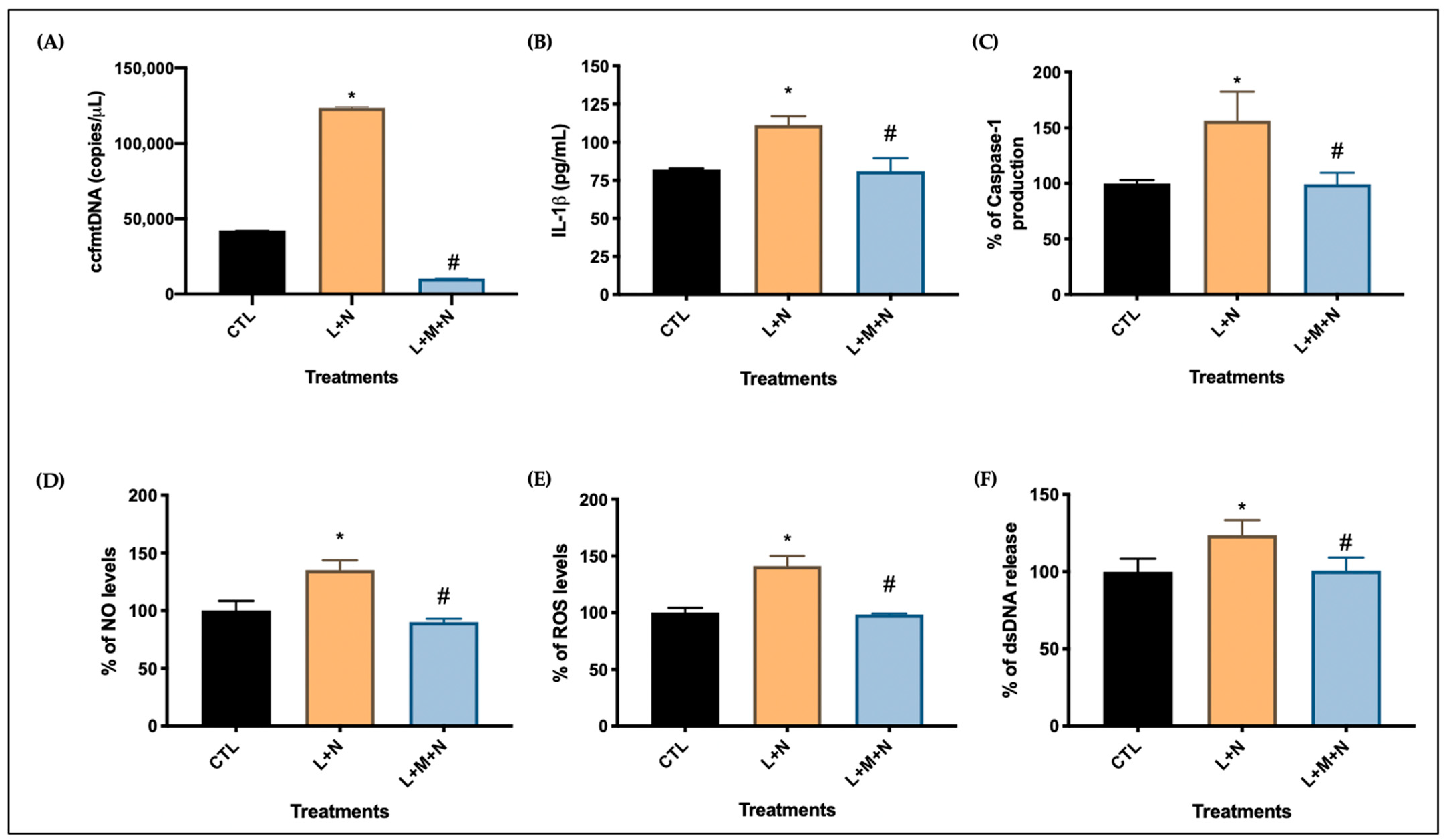
- Following nigericin incubation, the supernatants were collected and used to assess the levels of NO, ROS, dsDNA release, and IL-1β and caspase 1 expression as described in Section 2.3.
2.2.4. Fixing of CO Slices and Immunostaining
- After the removal of supernatants, slices were fixed with 4% paraformaldehyde (PFA) overnight, then transferred to 30% sucrose for another overnight incubation, and finally embedded in OCT gel at −80 °C and then cryosectioned at 20 µm thick for immunofluorescence staining.
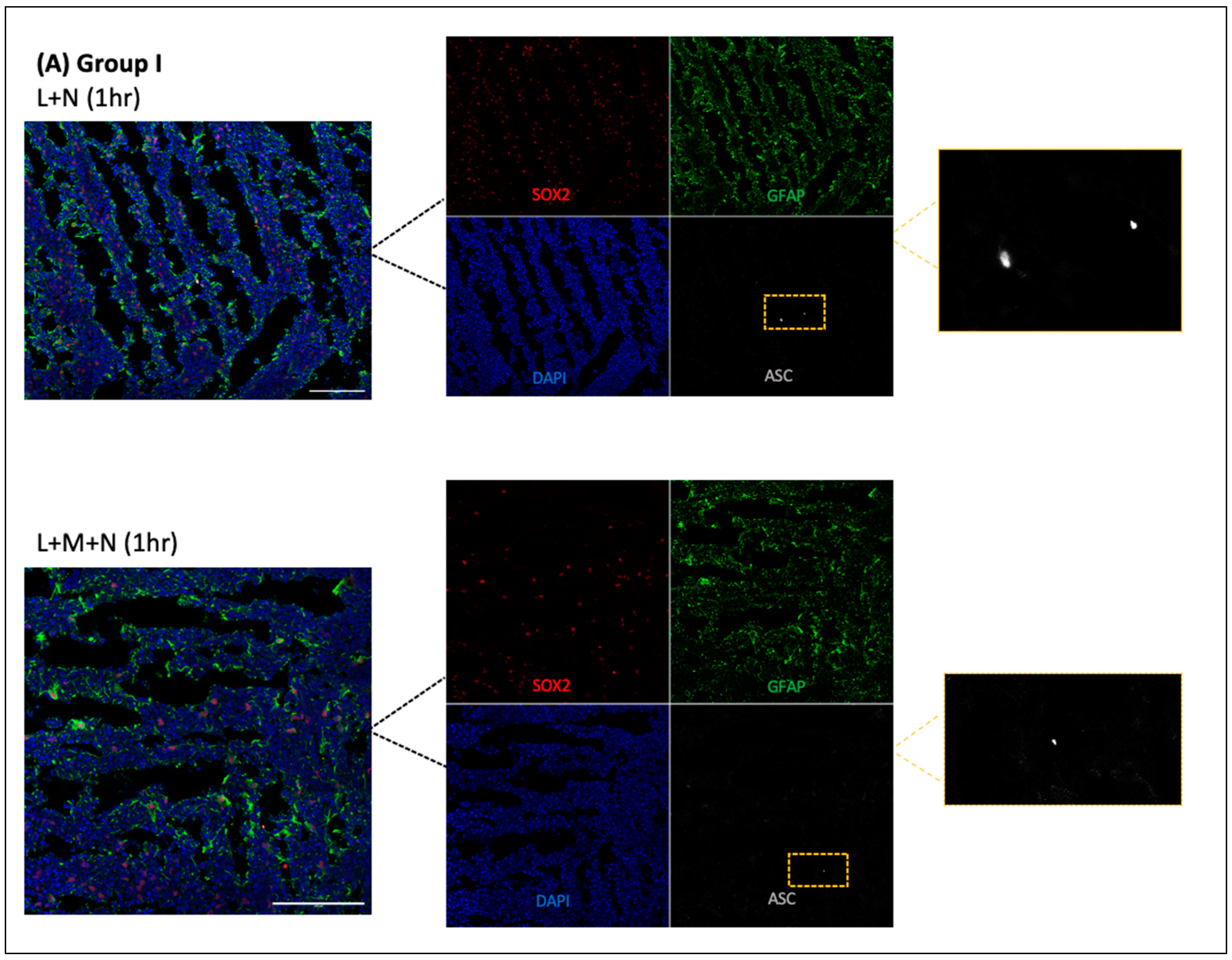
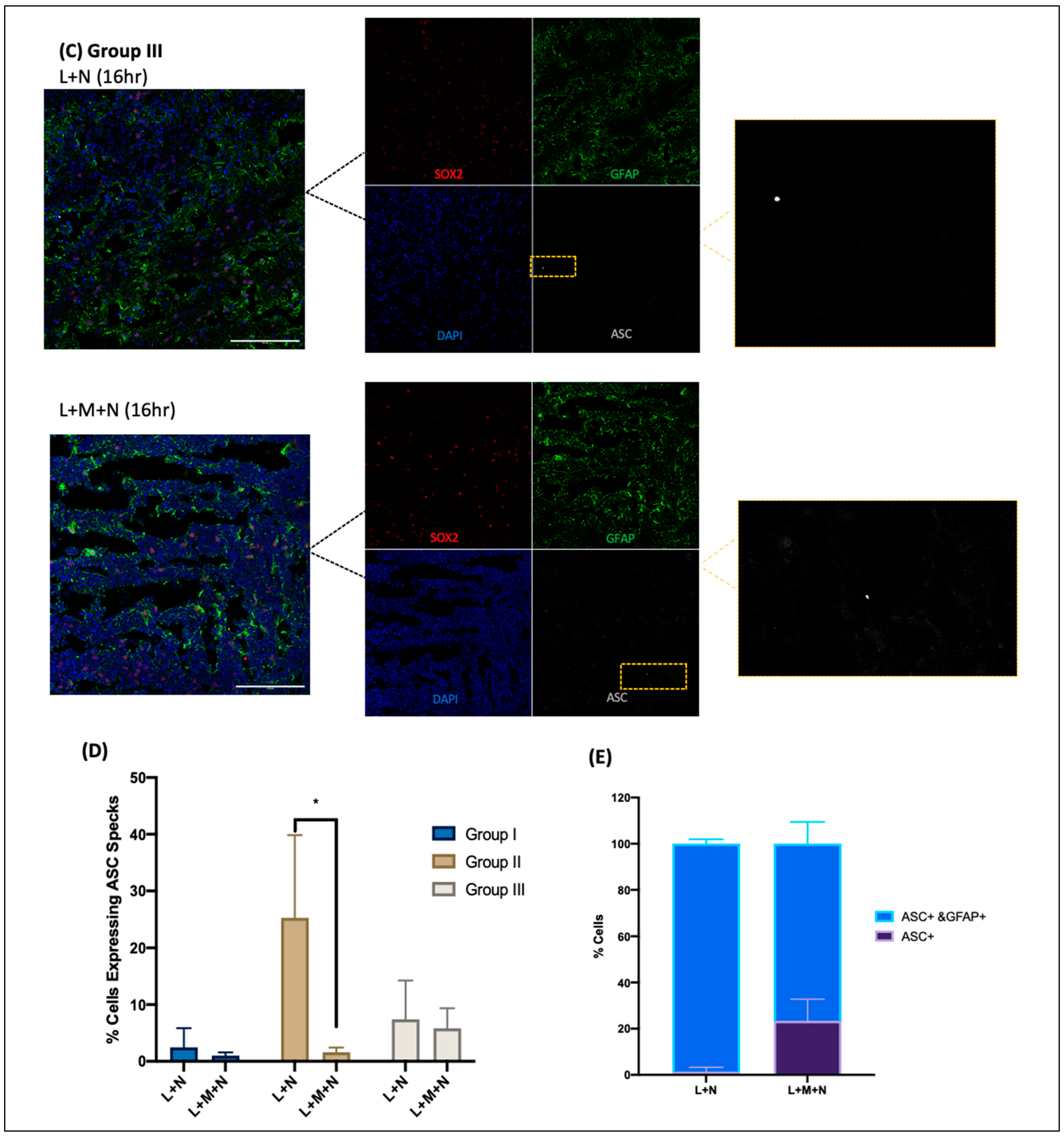
- Cryosections were stained as previously described by Duong et al. (2021) using GFAP for astrocytes (Abcam, Cambridge, UK, # ab4674), MAP2 for neurons (I3-1500), SOX2 for neural progenitors (MAB2018), and ASC for ASC specks (AL177) antibodies to examine ASC speck formation in astrocytes within each CO slice [41]. Secondary antibodies used were Goat anti-Mouse [IgG] [H + L] (Abcam, #Ab97035) secondary antibody Cy3, Donkey anti-Rabbit IgG [H + L] secondary antibody Alexa Fluor Plus 647, (Invitrogen, #A32795), and Donkey anti-Chicken IgY [H + L] secondary antibody Alexa Fluor 488 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA, #703-546-155). All antibodies were diluted using 0.5% BSA, and following staining, coverslips were mounted using ProLong Gold Antifade Mountant with DAPI (Invitrogen, Waltham, MA, USA, P36935), and samples were imaged at the Microscopy Imaging Laboratory, University of Toronto, Canada, using the LSM 880 Elyra Superresolution confocal microscope.
2.3. Developed Assays of Parameters Related to Inflammatory Activation
2.3.1. Indirect Measurement of Nitric Oxide
2.3.2. Semi-Quantitative Analysis of Total Levels of Reactive Oxygen Species
2.3.3. Measurement of Extracellular dsDNA
2.3.4. Interleukin-1β and Caspase-1 Protein Production
2.3.5. Circulating Cell-Free Mitochondrial DNA Measurement
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
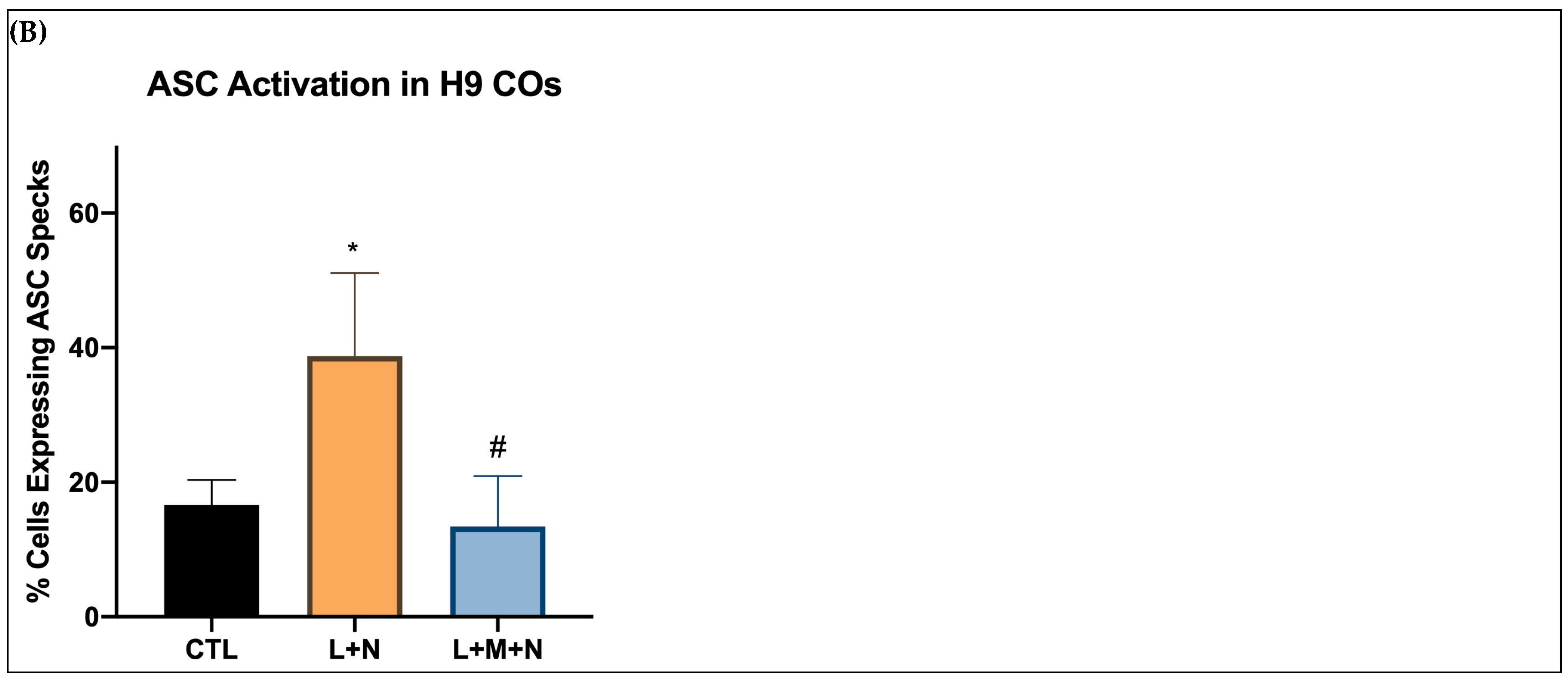
3.1. Results of Time-Response Curve (Groups I, II, and III)
3.2. Validating NLRP3 Inflammasome Activation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Boucher, D.; Monteleone, M.; Coll, R.C.; Chen, K.W.; Ross, C.M.; Teo, J.L.; Gomez, G.A.; Holley, C.L.; Bierschenk, D.; Stacey, K.J.; et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 2018, 215, 827–840. [Google Scholar] [CrossRef]
- Bryan, N.B.; Dorfleutner, A.; Kramer, S.J.; Yun, C.; Rojanasakul, Y.; Stehlik, C. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J. Inflamm. 2010, 7, 23. [Google Scholar] [CrossRef]
- Weber, A.N.R.; Bittner, Z.A.; Shankar, S.; Liu, X.; Chang, T.-H.; Jin, T.; Tapia-Abellán, A. Recent insights into the regulatory networks of NLRP3 inflammasome activation. J. Cell Sci. 2020, 133, jcs248344. [Google Scholar] [CrossRef]
- Zito, G.; Buscetta, M.; Cimino, M.; Dino, P.; Bucchieri, F.; Cipollina, C. Cellular Models and Assays to Study NLRP3 Inflammasome Biology. Int. J. Mol. Sci. 2020, 21, 4294. [Google Scholar] [CrossRef]
- Song, N.; Liu, Z.-S.; Xue, W.; Bai, Z.-F.; Wang, Q.-Y.; Dai, J.; Liu, X.; Huang, Y.-J.; Cai, H.; Zhan, X.-Y.; et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell 2017, 68, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.-J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Urwanisch, L.; Luciano, M.; Horejs-Hoeck, J. The NLRP3 Inflammasome and Its Role in the Pathogenicity of Leukemia. Int. J. Mol. Sci. 2021, 22, 1271. [Google Scholar] [CrossRef]
- Brinkschulte, R.; Fußhöller, D.M.; Hoss, F.; Rodríguez-Alcázar, J.F.; Lauterbach, M.A.; Kolbe, C.-C.; Rauen, M.; Ince, S.; Herrmann, C.; Latz, E.; et al. ATP-binding and hydrolysis of human NLRP3. Commun. Biol. 2022, 5, 1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Andreazza, A.C.; Elmi, N.; Chen, W.; Young, L.T. Nod-like receptor pyrin containing 3 (NLRP3) in the post-mortem frontal cortex from patients with bipolar disorder: A potential mediator between mitochondria and immune-activation. J. Psychiatr. Res. 2016, 72, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Feng, L.-X.; Zhu, X.-J.; Liu, Q.; Wang, H.-S.; Wu, X.; Yan, P.; Duan, X.-J.; Xiao, Y.-Q.; Cheng, W.; et al. Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats. Biomed. Pharmacother. 2020, 121, 109662. [Google Scholar] [CrossRef] [PubMed]
- Cadoná, F.C.; de Souza, D.V.; Fontana, T.; Bodenstein, D.F.; Ramos, A.P.; Sagrillo, M.R.; Salvador, M.; Mota, K.; Davidson, C.B.; Ribeiro, E.E.; et al. Açaí (Euterpe oleracea Mart.) as a Potential Anti-neuroinflammatory Agent: NLRP3 Priming and Activating Signal Pathway Modulation. Mol. Neurobiol. 2021, 58, 4460–4476. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory Microenvironment of Skin Wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef] [PubMed]
- Ginckels, P.; Holvoet, P. Oxidative Stress and Inflammation in Cardiovascular Diseases and Cancer: Role of Non-coding RNAs. Yale J. Biol. Med. 2022, 95, 129–152. [Google Scholar] [PubMed]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef]
- Machado, A.K.; Pan, A.Y.; da Silva, T.M.; Duong, A.; Andreazza, A.C. Upstream Pathways Controlling Mitochondrial Function in Major Psychosis: A Focus on Bipolar Disorder. Can J Psychiatry 2016, 61, 446–456. [Google Scholar] [CrossRef]
- Eratne, D.; Loi, S.M.; Farrand, S.; Kelso, W.; Velakoulis, D.; Looi, J.C. Alzheimer’s disease: Clinical update on epidemiology, pathophysiology and diagnosis. Australas. Psychiatry 2018, 26, 347–357. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, R.; Liu, Y.; Ruan, Y.; Lin, Z.; Xue, N.; Chen, Y.; Zhang, B.; Pu, J. Parkin regulates microglial NLRP3 and represses neurodegeneration in Parkinson’s disease. Aging Cell 2023, 22, e13834. [Google Scholar] [CrossRef]
- Yan, Y.-Q.; Fang, Y.; Zheng, R.; Pu, J.-L.; Zhang, B.-R. NLRP3 Inflammasomes in Parkinson’s disease and their Regulation by Parkin. Neuroscience 2020, 446, 323–334. [Google Scholar] [CrossRef]
- Podlesniy, P.; Figueiro-Silva, J.; Antonell, A.; Sanchez-Valle, R.; Alcolea, D.; Lleo, A.; Molinuevo, J.L.; Serra, N.; Trullas, R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann. Neurol. 2013, 74, 655–668. [Google Scholar] [CrossRef]
- Zhou, X.; Fernando, S.M.; Pan, A.Y.; Laposa, R.; Cullen, K.R.; Klimes-Dougan, B.; Andreazza, A.C. Characterizing the NLRP3 Inflammasome in Mood Disorders: Overview, Technical Development, and Measures of Peripheral Activation in Adolescent Patients. Int. J. Mol. Sci. 2021, 22, 12513. [Google Scholar] [CrossRef]
- Crews, F.T.; Qin, L.; Sheedy, D.; Vetreno, R.P.; Zou, J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol. Psychiatry 2013, 73, 602–612. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Comeau, S.; Rachalski, A.; Bo, G.D.; Cruceanu, C.; Turecki, G.; Giros, B.; Mechawar, N. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflamm. 2014, 11, 12. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Ren, X.; Bhaumik, R.; Dwivedi, Y. Toll-like receptors in the depressed and suicide brain. J. Psychiatr. Res. 2014, 53, 62–68. [Google Scholar] [CrossRef]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Elahi, L.S.; Pașca, A.M.; Marton, R.M.; Gordon, A.; Revah, O.; Miura, Y.; Walczak, E.M.; Holdgate, G.M.; Fan, H.C.; et al. Reliability of human cortical organoid generation. Nat. Methods 2019, 16, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Sivitilli, A.A.; Gosio, J.T.; Ghoshal, B.; Evstratova, A.; Trcka, D.; Ghiasi, P.; Hernandez, J.J.; Beaulieu, J.M.; Wrana, J.L.; Attisano, L. Robust production of uniform human cerebral organoids from pluripotent stem cells. Life Sci. Alliance 2020, 3, e202000707. [Google Scholar] [CrossRef] [PubMed]
- Sivitilli, A.; Ghiasi, P.; Attisano, L. Production of Phenotypically Uniform Human Cerebral Organoids from Pluripotent Stem Cells. Bio-Protocol 2021, 11, e3985. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Zhang, Y.; Liang, P.; He, Y.; Peng, B.; Liu, W.; Han, S.; Yin, J.; He, X. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Heitzer, M.; Kanagaratnam, M.; Goswami, A.; Rizo, T.; Weis, J.; Troost, D.; Beyer, C. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 2015, 63, 2260–2273. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Ji, H.; Shangguan, X.; Qu, S.; Cui, Y.; Xu, J. NLRP3 inflammasome of microglia promotes A1 astrocyte transformation, neo-neuron decline and cognition impairment in endotoxemia. Biochem. Biophys. Res. Commun. 2022, 602, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, B.; Zhang, Y.; Liu, J.; Lin, Y.; Wang, P.; Wei, Z.; Saribas, S.; Zhu, Y.; Li, F.; Wang, X.; et al. Novel Scalable and Simplified System to Generate Microglia-Containing Cerebral Organoids From Human Induced Pluripotent Stem Cells. Front. Cell. Neurosci. 2021, 15, 682272. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.E.; Aggarwal, N.; Deerhake, M.E.; Inoue, M.; Nonaka, T.; Nozaki, K.; Luzum, N.A.; Miao, E.A.; Shinohara, M.L. The AIM2 inflammasome is activated in astrocytes during the late phase of EAE. J. Clin. Investig. 2022, 7, e155563. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P.-Y. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef]
- Li, H.; Guan, Y.; Liang, B.; Ding, P.; Hou, X.; Wei, W.; Ma, Y. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 2022, 928, 175091. [Google Scholar] [CrossRef]
- Duong, A.; Evstratova, A.; Sivitilli, A.; Hernandez, J.J.; Gosio, J.; Wahedi, A.; Sondheimer, N.; Wrana, J.L.; Beaulieu, J.-M.; Attisano, L.; et al. Characterization of mitochondrial health from human peripheral blood mononuclear cells to cerebral organoids derived from induced pluripotent stem cells. Sci. Rep. 2021, 11, 4523. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.P.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539. [Google Scholar] [CrossRef]
- He, Y.; Varadarajan, S.; Muñoz-Planillo, R.; Burberry, A.; Nakamura, Y.; Núñez, G. 3,4-Methylenedioxy-β-nitrostyrene Inhibits NLRP3 Inflammasome Activation by Blocking Assembly of the Inflammasome. J. Biol. Chem. 2014, 289, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q.; et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017, 214, 3219–3238. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Abellán, A.; Angosto-Bazarra, D.; Martínez-Banaclocha, H.; de Torre-Minguela, C.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 2019, 15, 560–564. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019, 15, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.; Yang, Y.; Tao, J.; Deng, X.; Liang, G.; Zhang, H.; Jiang, W.; et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10, e8689. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Kim, W.M.; Lee, H.G.; Kim, Y.O.; Yoon, M.H. Role of neuronal nitric oxide synthase in the antiallodynic effects of intrathecal EGCG in a neuropathic pain rat model. Neurosci. Lett. 2012, 510, 53–57. [Google Scholar] [CrossRef]
- Costa, F.; Dornelles, E.; Mânica-Cattani, M.F.; Algarve, T.D.; Filho, O.C.d.S.; Sagrillo, M.R.; Garcia, L.F.M.; da Cruz, I.B.M. Influence of Val16Ala SOD2 polymorphism on the in-vitro effect of clomiphene citrate in oxidative metabolism. Reprod. Biomed. Online 2012, 24, 474–481. [Google Scholar] [CrossRef]
- Ahn, S.J.; Costa, J.; Emanuel, J.R. PicoGreen quantitation of DNA: Effective evaluation of samples pre- or post-PCR. Nucleic Acids Res. 1996, 24, 2623–2625. [Google Scholar] [CrossRef]
- Lu, R.X.Z.; Rafatian, N.; Zhao, Y.; Wagner, K.T.; Beroncal, E.L.; Li, B.; Lee, C.; Chen, J.; Churcher, E.; Vosoughi, D.; et al. Cardiac tissue model of immune-induced dysfunction reveals the role of free mitochondrial DNA and the therapeutic effects of exosomes. Sci. Adv. 2024, 10, eadk0164. [Google Scholar] [CrossRef] [PubMed]
- Fenini, G.; Grossi, S.; Contassot, E.; Biedermann, T.; Reichmann, E.; French, L.E.; Beer, H.-D. Genome Editing of Human Primary Keratinocytes by CRISPR/Cas9 Reveals an Essential Role of the NLRP1 Inflammasome in UVB Sensing. J. Investig. Dermatol. 2018, 138, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Rozario, P.; Pinilla, M.; Gorse, L.; Vind, A.C.; Robinson, K.S.; Toh, G.A.; Firdaus, M.J.; Martínez, J.F.; Kerk, S.K.; Lin, Z.; et al. Mechanistic basis for potassium efflux–driven activation of the human NLRP1 inflammasome. Proc. Natl. Acad. Sci. USA 2024, 121, e2309579121. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Kanneganti, T.-D. Advances in Understanding Activation and Function of the NLRC4 Inflammasome. Int. J. Mol. Sci. 2021, 22, 1048. [Google Scholar] [CrossRef]
- Vong, C.T.; Tseng, H.H.L.; Yao, P.; Yu, H.; Wang, S.; Zhong, Z.; Wang, Y. Specific NLRP3 inflammasome inhibitors: Promising therapeutic agents for inflammatory diseases. Drug Discov. Today 2021, 26, 1394–1408. [Google Scholar] [CrossRef]
- de Souza, D.V.; Pappis, L.; Bandeira, T.T.; Sangoi, G.G.; Fontana, T.; Rissi, V.B.; Sagrillo, M.R.; Duarte, M.M.; Duarte, T.; Bodenstein, D.F.; et al. Açaí (Euterpe oleracea Mart.) presents anti-neuroinflammatory capacity in LPS-activated microglia cells. Nutr. Neurosci. 2022, 25, 1188–1199. [Google Scholar] [CrossRef]


| PCR Primers for ccf mtDNA | |
|---|---|
| ND4 F | F1 5′-CCATTCTCCTCCTATCCCTCAAC-3′ |
| ND4 R | 5′-ACAATCTGATGTTTTGGTTAAACTATATTT-3′ |
| ND4 Probe | 5′-FAM/CCGACATCA/ZEN/TTACCGGGTTTTCCTCTTG/3IABkFQ/-3′ |
| ND1 F | 5′-CCCTAAAACCCGCCACATCT-3′ |
| ND1 R | 5′-GAGCGATGGTGAGAGCTAAGGT-3′ |
| ND1 Probe | 5′-HEX/CCATCACCC/ZEN/TCTACATCACCGCCC/3IABkFQ/-3′ |
| ND4 + ND1 geneblock | CACGAGAAAACACCCTCATGTTCATACACCTATCCCCCATTCTCCTCCTATC CCTCAACCCCGACATCATTACCGGGTTTTCCTCTTGTAAATATAGTTTAACCA AAACATCAGATTGTGAATCTGACAACAGAGGCTCTCTTCACCAAAGAGCCC CTAAAACCCGCCACATCTACCATCACCCTCTACATCACCGCCCCGACCTTAG CTCTCACCATCGCTCTTCTACT ATGAACCCCCCTCCCCATACCCAA-3′ |
| B2M F594 | 5′-TGCTGTCTCCATGTTTGATGTATCT-3′ |
| B2M R679 | 5′-TCTCTGCTCCCCACCTCTAAGT-3′ |
| B2M-Probe | 5′-FAM/TTGCTCCAC/ZEN/AGGTAGCTCTAGGAGG/3IABkFQ/-3′ |
| PPIA-F | 5′-GTGGCGGATTTGATCATTTGG-3′ |
| PPIA-R | 5′-CAAGACTGAGATGCACAAGTG-3′ |
| PPIA Probe | 5′-/56-FAM/AAT TCA CGC/ZEN/AGA AGGA ACC AGA CAG T/3IABkFQ/-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Soufi El Sabbagh, D.; Attisano, L.; Andreazza, A.C.; Machado, A.K. A Dynamic Protocol to Explore NLRP3 Inflammasome Activation in Cerebral Organoids. Int. J. Mol. Sci. 2024, 25, 6335. https://doi.org/10.3390/ijms25126335
El Soufi El Sabbagh D, Attisano L, Andreazza AC, Machado AK. A Dynamic Protocol to Explore NLRP3 Inflammasome Activation in Cerebral Organoids. International Journal of Molecular Sciences. 2024; 25(12):6335. https://doi.org/10.3390/ijms25126335
Chicago/Turabian StyleEl Soufi El Sabbagh, Dana, Liliana Attisano, Ana Cristina Andreazza, and Alencar Kolinski Machado. 2024. "A Dynamic Protocol to Explore NLRP3 Inflammasome Activation in Cerebral Organoids" International Journal of Molecular Sciences 25, no. 12: 6335. https://doi.org/10.3390/ijms25126335
APA StyleEl Soufi El Sabbagh, D., Attisano, L., Andreazza, A. C., & Machado, A. K. (2024). A Dynamic Protocol to Explore NLRP3 Inflammasome Activation in Cerebral Organoids. International Journal of Molecular Sciences, 25(12), 6335. https://doi.org/10.3390/ijms25126335





