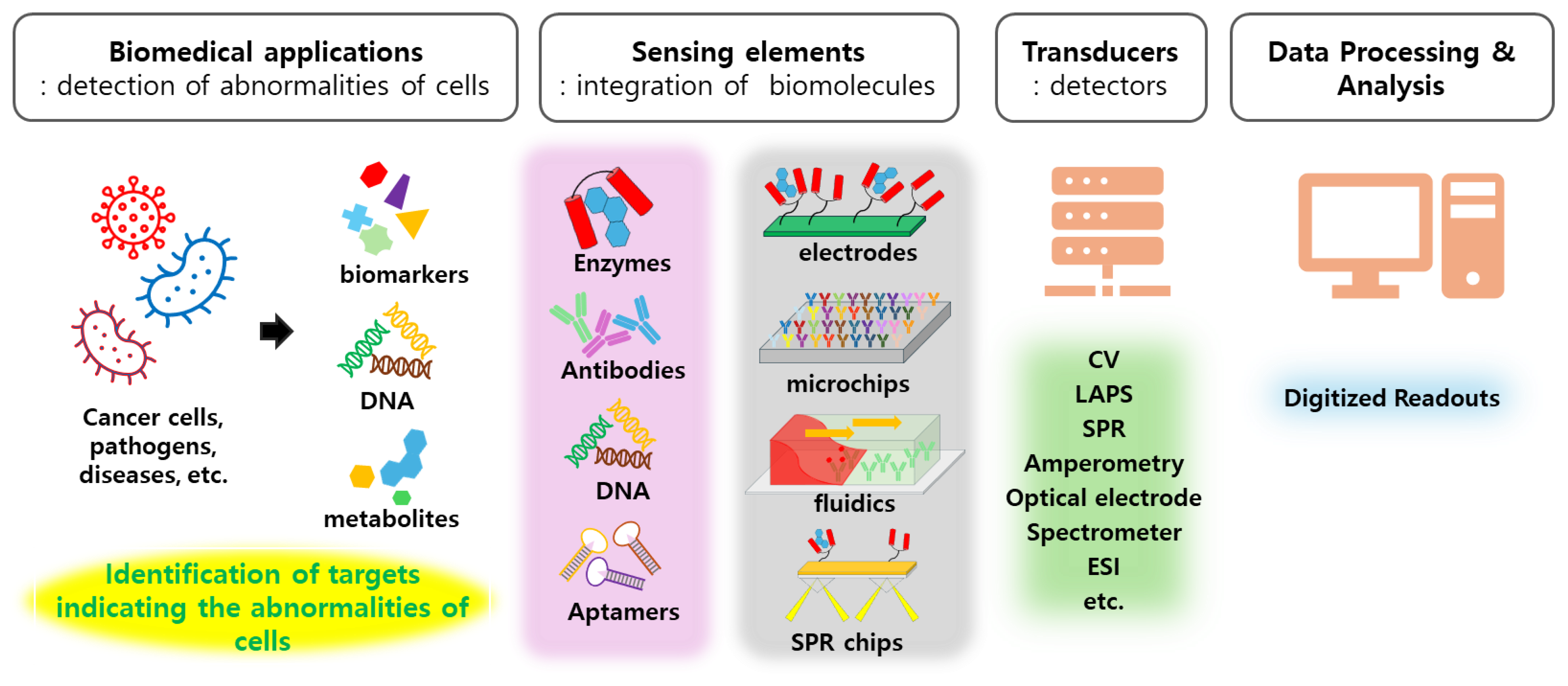
The Biomedical Applications of Biomolecule Integrated Biosensors for Cell Monitoring
Abstract
1. Introduction
2. Cell Monitoring for Biomedical Applications
3. Chemical Sensor Systems for Cell Monitoring
| Targets | Sensing Elements | Signal Transducers | LOD/ Linear Range | Targets/Application | Ref. |
|---|---|---|---|---|---|
| Acidification level | pH-sensitive hydrogel nanofiber | LAPS | 102 CFU/mL | E. coli/S. typhi | [28] |
| Acidification level | Silicon oxide/silicon nitride layer | LAPS- microphysiometer | - | Human breast cancer cells MCF-7 | [29] |
| Formaldehyde | TiO2/Au hybrid film | SPR | 0.2 ppm/ 0.2–1.8 ppm | Breast cancer | [33] |
| Alkaline phosphatase | ZnSe/ZnS QDs | Fluorescence spectrometry | 0.57 U/L 4–96 U/L | Chronic kidney disease | [34] |
| β-glucuronidase | Fluorescent nitrogen-doped CDs | Fluorescence spectrometry | 0.3 U/L 1 to 15 U/L | Early diagnosis of cancer | [35] |
| NAG β-galactosidase | Silicon nanoparticles (SiNPs) | fluorometric/colorimetric analysis | 0.66 U/L 13.1 U/L | Kidney diseases diagnosis | [42] |
| Isoprene | Prism/Au/air cavity/(GaN/SiO2)10 | Tamm plasmon resonance | 80 ppb 0–600 ppb | Chronic liver fibrosis | [43] |
| Norepinephrine | Pt surface electrodes on CMOS microchip | trans-impedance amplifier | 8–1024 µM | Electrochemical Imaging of live tissue | [44] |
| NO/nitric oxide | Au/RGO-TiO2-ITO electrode | CV | 5 nM/ 20–500 nM | HUVECs | [45] |
| H2O2/oxidative stress | Au-Pd alloy NPs/graphene QDs | Amperometry | 500 nM | Breast cancer cells | [46] |
| Glucose | Ni3C/Ni nanochain modified electrode | CV | 0.28 μM/ 1.0–6.5 μM | Biological fluids/clinical application | [47] |
| Cells | Au/Cr coated glass | EC-SPR | - | Monitoring liver cancer cell viability | [48] |
4. Biosensor Systems for Cell Monitoring
4.1. Chemical Sensor Systems as Platforms for Biosensors
4.2. Biomolecule Integrated Biosensor Systems
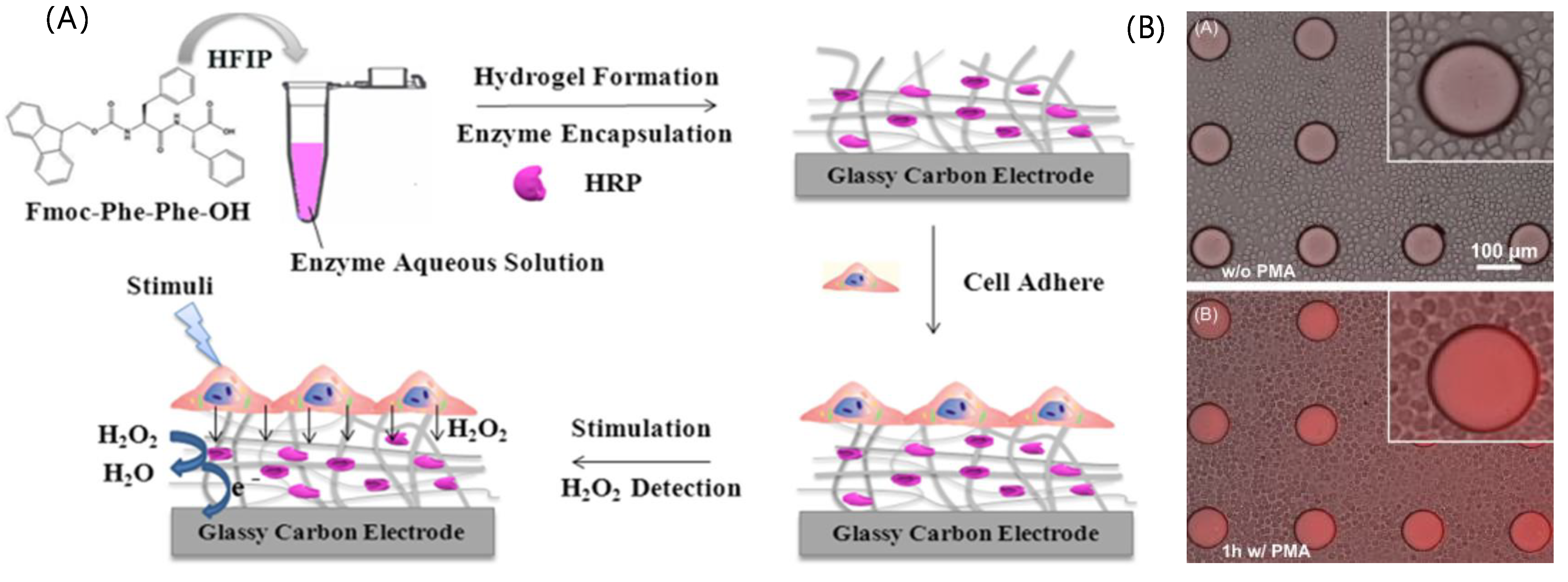
4.2.1. Enzyme-Based Biosensors
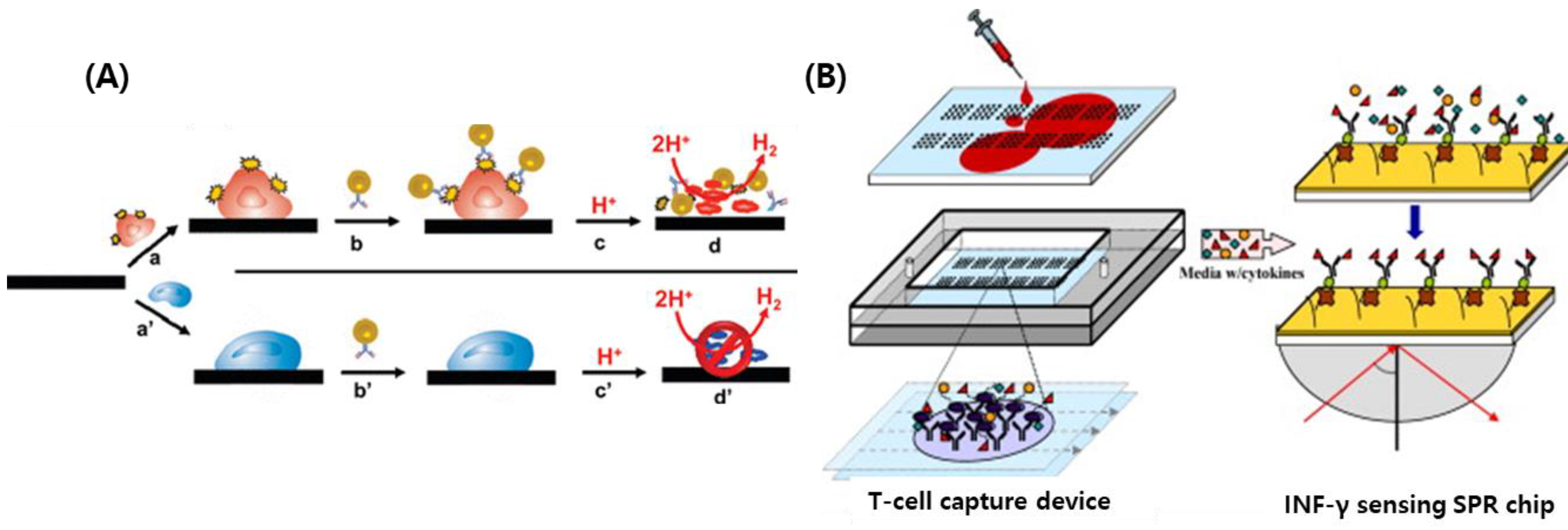
4.2.2. Antibody-Based Biosensors
4.2.3. DNA Hybridization-Based Biosensors
4.2.4. Aptamer-Based Biosensors
| Target | Sensing Elements | Signal Transducers | LOD/ Linear Range | Applications | Ref. | |
|---|---|---|---|---|---|---|
| Enzyme | H2O2 | HRP/Fmoc-FF modified electrode | CV | 18 nM | HeLa cells | [60] |
| H2O2 | HRP/PEG-hydrogels AuNP electrode | CV | 0.29–1.16 μM | Hepatocytes | [62] | |
| glucose | chitosan/Gox complex-Au electrode | SWV | 5 μM/ 5 μM–7 mM | Saliva samples | [65] | |
| glutathione | GSH-Px-GO/nafion/GCE | DPV | 1.5 nM/ 0.003–370.0 μM | Body fluids | [68] | |
| Glucose Lactate hypoxanthine | Oxidase/MXCeO2 | Electrodes | 0.49 μM 3.6 μM 1.7 μM | Artificial sweat | [69] | |
| Acetylcholine | ACHE-conjugated Au electrode | EIS | 5.5–550 μM | Rat brain slurry Rat whole blood | [118] | |
| L-MC-LR | MlrB-MWCNT/GCE | CV/EIS | 0.127 pg/mL 0.001–100 ng/mL | Water samples | [119] | |
| E. coli S. aureus | β-galactosidase-AuNPs | CV | 100 CFU/mL | Water samples | [120] | |
| antibody | Interleukin-10 | Antibody-SNW | Spectrometer | 100 μg/mL | Monitoring lung cancer | [73] |
| TDP-43 | Antibody-Au electrode | EIS | 11 ± 6 nM | Amyotrophic lateral sclerosis | [83] | |
| Hemoglobin | Antibody-microfluidics | Plate reader | 4.0 g/L for Hb A 5.0 g/L for Hb S | Monitoring sickle cell disease | [121] | |
| Biomarkers for cancers | Antibody-conjugated microchips | SPR | - | Diagnosis for diverse cancers | [77] | |
| COVID-19 Spike S1 | S1 antigen-RGO nanoflakes | CV | 2.8–16.9 fM | COVID diagnosis | [81] | |
| Cytokines | Antibody-ssDNA on chips | Plate reader | 1 fg/mL 1 fg/mL–1 ng/mL | Health monitoring | [122] | |
| Cells | Anti-EpCAM-GO-COOH | LAPS | 10 cells/mL | Circulating tumor cells | [123] | |
| E. coli O157 Salmonella | Antibody-coated graphite felt electrode | OSWV | 400 cells/mL | Detecting pathogens | [124] | |
| DNA | HER2, EpCAM, CD63 | Au@Ag Nanocubes on AuFON | SERS | 50 exosomes/mL | Human/bovine serum | [125] |
| COVID-19 cDNA | DNA-IDE | Impedance analyzer | 10 nM | COVID diagnosis | [89] | |
| Alpha-fetoprotein | Aptamer-AuNP | LAPS | 92.0 ng/mL 0.1–100 μg/mL | Diagnosis of liver cancer | [30] | |
| Salmonella | AuNP-aptamer | Spectrometer | 104 to 105 copies | Monitoring pathogens | [107] | |
| PSA | Aptamer-HCR-AuNP | Colorimetric | 30 pg/mL | Human serum | [126] | |
| PSA | PSA-Aptamer/TdT/T7 Exo/Taq12 | Fluorescence | 0.043 pg/mL | Human serum | [117] |
4.3. Recent Progresses in Biosensor Systems for Cell Monitoring
5. Current Challenges of Biosensing Systems for Biomedical Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraden, J. Physics, Designs, and Applications. In Handbook of Modern Sensors; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Kim, Y.; Jeon, Y.; Na, M.; Hwang, S.-J.; Yoon, Y. Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors 2024, 24, 431. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef]
- Kochmann, S.; Hirsch, T.; Wolfbeis, O.S. Graphenes in chemical sensors and biosensors. TrAC Trends Anal. Chem. 2012, 39, 87–113. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef]
- Fattahi, Z.; Hasanzadeh, M. Nanotechnology-assisted microfluidic systems for chemical sensing, biosensing, and bioanalysis. TrAC Trends Anal. Chem. 2022, 152, 116637. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. Surface plasmon resonance (SPR) for sensors and biosensors. In Nanocharacterization Techniques; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–200. [Google Scholar]
- Patil, P.O.; Pandey, G.R.; Patil, A.G.; Borse, V.B.; Deshmukh, P.K.; Patil, D.R.; Tade, R.S.; Nangare, S.N.; Khan, Z.G.; Patil, A.M. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: A review. Biosens. Bioelectron. 2019, 139, 111324. [Google Scholar] [CrossRef]
- Justino, C.I.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Kim, H.; Jang, G.; Yoon, Y. Specific heavy metal/metalloid sensors: Current state and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 907–914. [Google Scholar] [CrossRef]
- Oehme, I.; Wolfbeis, O.S. Optical sensors for determination of heavy metal ions. Microchim. Acta 1997, 126, 177–192. [Google Scholar] [CrossRef]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured Sensors for Detection of Heavy Metals: A Review; ACS Publication: Washington, DC, USA, 2013. [Google Scholar]
- Sugunan, A.; Thanachayanont, C.; Dutta, J.; Hilborn, J. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci. Technol. Adv. Mater. 2005, 6, 335. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Zhu, Y.; Yang, Z.; Yao, M.; Shi, X.; An, T.; Zhang, Q.; Huang, C.; Bi, X. An overview for monitoring and prediction of pathogenic microorganisms in the atmosphere. Fundam. Res. 2023, 4, 430–441. [Google Scholar] [CrossRef]
- Dhar, B.C.; Lee, N.Y. Lab-on-a-chip technology for environmental monitoring of microorganisms. BioChip J. 2018, 12, 173–183. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, X.-P.; Younis, M.R.; Li, Z.-Q.; Xia, X.-H.; Wang, C. Ultrasensitive capture, detection, and release of circulating tumor cells using a nanochannel–ion channel hybrid coupled with electrochemical detection technique. Anal. Chem. 2017, 89, 10957–10964. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, L.; Ding, S.; Yin, Y.; Ju, H. A Simple Electrochemical Cytosensor Array for Dynamic Analysis of Carcinoma Cell Surface Glycans. Angew. Chem. Int. Ed. 2009, 48, 6465–6468. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Guille, M.; Lemaitre, F. Electrochemical monitoring of single cell secretion: Vesicular exocytosis and oxidative stress. Chem. Rev. 2008, 108, 2585–2621. [Google Scholar] [CrossRef]
- Simon, E. Biological and chemical sensors for cancer diagnosis. Meas. Sci. Technol. 2010, 21, 112002. [Google Scholar] [CrossRef]
- De Kievit, T.R.; Iglewski, B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000, 68, 4839–4849. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Mirasoli, M.; Guardigli, M.; Michelini, E.; Roda, A. Recent advancements in chemical luminescence-based lab-on-chip and microfluidic platforms for bioanalysis. J. Pharm. Biomed. Anal. 2014, 87, 36–52. [Google Scholar] [CrossRef]
- Deshpande, A.S.; Muraoka, W.; Andreescu, S. Electrochemical sensors for oxidative stress monitoring. Curr. Opin. Electrochem. 2021, 29, 100809. [Google Scholar] [CrossRef]
- Yoshinobu, T.; Schöning, M.J. Light-addressable potentiometric sensors for cell monitoring and biosensing. Curr. Opin. Electrochem. 2021, 28, 100727. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable nanofiber-light addressable potentiometric sensor for rapid Escherichia coli detection in orange juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef]
- Hu, N.; Wu, C.; Ha, D.; Wang, T.; Liu, Q.; Wang, P. A novel microphysiometer based on high sensitivity LAPS and microfluidic system for cellular metabolism study and rapid drug screening. Biosens. Bioelectron. 2013, 40, 167–173. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Li, S.; Shi, X.; Liang, J.; Lai, J.; Zhou, Z. A novel aptasensor based on light-addressable potentiometric sensor for the determination of Alpha-fetoprotein. Biochem. Eng. J. 2020, 164, 107780. [Google Scholar] [CrossRef]
- Wang, J.; Du, L.; Krause, S.; Wu, C.; Wang, P. Surface modification and construction of LAPS towards biosensing applications. Sens. Actuators B Chem. 2018, 265, 161–173. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Q.; Wu, L.; Shi, X.; Yan, K.; Guo, F.; Zhou, Z.; Li, G. A GOX/RGO-CS-Fc/AuNPs nanosensing membrane in a light-addressable potentiometric biosensor for glucose specific detection. Microchem. J. 2024, 200, 110478. [Google Scholar] [CrossRef]
- Kim, J.; Hong, U.G.; Choi, Y.; Hong, S. Enhancing the evanescent field in TiO2/Au hybrid thin films creates a highly sensitive room-temperature formaldehyde gas biosensor. Colloids Surf. B Biointerfaces 2019, 182, 110303. [Google Scholar] [CrossRef]
- Hu, P.; Huang, R.; Xu, Y.; Li, T.; Yin, J.; Yang, Y.; Liang, Y.; Mao, X.; Ding, L.; Shu, C. A novel and sensitive ratiometric fluorescent quantum dot-based biosensor for alkaline phosphatase detection in biological samples via the inner-filter effect. RSC Adv. 2023, 13, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Sun, L.; Wang, F.; Liu, X.; Yan, Z.; Wang, M.; Zhang, L.; Tian, Z.; Liu, Z.; You, J. Highly fluorescent N-doped carbon dots with two-photon emission for ultrasensitive detection of tumor marker and visual monitor anticancer drug loading and delivery. Chem. Eng. J. 2019, 356, 994–1002. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Lisse, C.; Medawala, W.; Brady, P.N.; Bwambok, D.K.; Anum, D.; Alonge, T.; Taylor, M.E.; Baker, G.A.; Mehari, T.F. Fluorescent chemical sensors: Applications in analytical, environmental, forensic, pharmaceutical, biological, and biomedical sample measurement, and clinical diagnosis. Appl. Spectrosc. Rev. 2024, 59, 1–89. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Z.; Zhou, J.; Zhu, M.; Liu, J.; James, T.D. Recent progress in the development of fluorescent probes for imaging pathological oxidative stress. Chem. Soc. Rev. 2023, 52, 3873–3926. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Kukkar, D.; Kim, K.-H. Fluorescent nanomaterials for the detection of chronic kidney disease. TrAC Trends Anal. Chem. 2024, 173, 117572. [Google Scholar] [CrossRef]
- Qu, X.; Hu, Y.; Xu, C.; Li, Y.; Zhang, L.; Huang, Q.; Moshirian-Farahi, S.S.; Zhang, J.; Xu, X.; Liao, M. Optical sensors of volatile organic compounds for non-invasive diagnosis of diseases. Chem. Eng. J. 2024, 485, 149804. [Google Scholar] [CrossRef]
- Hosseini, S.-N.; Das, P.S.; Lazarjan, V.K.; Gagnon-Turcotte, G.; Bouzid, K.; Gosselin, B. Recent advances in CMOS electrochemical biosensor design for microbial monitoring: Review and design methodology. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tang, X.; Wu, T.; Zeng, W.; Zhu, X.; Hu, B.; Zhang, S. A review on current progress of Raman-based techniques in food safety: From normal Raman spectroscopy to SESORS. Food Res. Int. 2023, 169, 112944. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Gao, D.; Mu, X.; Wu, Q.; Ma, P.; Song, D. Dual-signal triple-mode optical sensing platform for assisting in the diagnosis of kidney disorders. Anal. Chem. 2023, 95, 4653–4661. [Google Scholar] [CrossRef]
- Mehaney, A.; Alrowaili, Z.; Elsayed, H.A.; Taha, T.; Ahmed, A.M. Theoretical investigations of Tamm plasmon resonance for monitoring of isoprene traces in the exhaled breath: Towards chronic liver fibrosis disease biomarkers. Phys. Lett. A 2021, 413, 127610. [Google Scholar] [CrossRef]
- Tedjo, W.; Nejad, J.E.; Feeny, R.; Yang, L.; Henry, C.S.; Tobet, S.; Chen, T. Electrochemical biosensor system using a CMOS microelectrode array provides high spatially and temporally resolved images. Biosens. Bioelectron. 2018, 114, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Q.; Liu, Y.L.; Wang, Q.; Duo, H.H.; Zhang, X.W.; Li, Y.T.; Huang, W.H. Photocatalytically Renewable Micro-electrochemical Sensor for Real-Time Monitoring of Cells. Angew. Chem. Int. Ed. 2015, 54, 14402–14406. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yuan, H.; Dong, X.; Zhang, Y.; Asif, M.; Dong, Z.; He, W.; Ren, J.; Sun, Y.; Xiao, F. Dual nanoenzyme modified microelectrode based on carbon fiber coated with AuPd alloy nanoparticles decorated graphene quantum dots assembly for electrochemical detection in clinic cancer samples. Biosens. Bioelectron. 2018, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Y.; Qiu, W.; Song, Z.; Wang, Q.; Niu, L. Ni3C/Ni nanochains for electrochemical sensing of glucose. ACS Appl. Nano Mater. 2021, 4, 8520–8529. [Google Scholar] [CrossRef]
- Wu, C.; Rehman, F.U.; Li, J.; Ye, J.; Zhang, Y.; Su, M.; Jiang, H.; Wang, X. Real-time evaluation of live cancer cells by an in situ surface plasmon resonance and electrochemical study. ACS Appl. Mater. Interfaces 2015, 7, 24848–24854. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Anal. Lett. 2001, 34, 635–659. [Google Scholar] [CrossRef]
- Noori, H.N.; Abdulameer, A.F. A Review of Biosensors; Definition, Classification, Properties, and Applications. Iraqi J. Sci. 2023, 64, 5665–5690. [Google Scholar] [CrossRef]
- Clark Jr, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.; Prazeres, D.M.F.; Cabral, J.M.; Fonseca, L.P. Ethanol biosensors based on alcohol oxidase. Biosens. Bioelectron. 2005, 21, 235–247. [Google Scholar] [CrossRef]
- Tran-Minh, C.; Pandey, P.; Kumaran, S. Studies on acetylcholine sensor and its analytical application based on the inhibition of cholinesterase. Biosens. Bioelectron. 1990, 5, 461–471. [Google Scholar] [CrossRef]
- Strehlitz, B.; Gründig, B.; Schumacher, W.; Kroneck, P.M.; Vorlop, K.-D.; Kotte, H. A nitrite sensor based on a highly sensitive nitrite reductase mediator-coupled amperometric detection. Anal. Chem. 1996, 68, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ispas, C.R.; Crivat, G.; Andreescu, S. Recent developments in enzyme-based biosensors for biomedical analysis. Anal. Lett. 2012, 45, 168–186. [Google Scholar] [CrossRef]
- Kucherenko, I.; Soldatkin, O.; Kucherenko, D.Y.; Soldatkina, O.; Dzyadevych, S. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef] [PubMed]
- Chhillar, A.K.; Rana, J.S. Enzyme nanoparticles and their biosensing applications: A review. Anal. Biochem. 2019, 581, 113345. [Google Scholar]
- Gong, C.; Fan, Y.; Zhao, H. Recent advances and perspectives of enzyme-based optical biosensing for organophosphorus pesticides detection. Talanta 2022, 240, 123145. [Google Scholar] [CrossRef]
- Nigam, V.K.; Shukla, P. Enzyme based biosensors for detection of environmental pollutants-a review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Chen, X.; Lu, Y.; Yang, W. Self-assembled peptide hydrogel as a smart biointerface for enzyme-based electrochemical biosensing and cell monitoring. ACS Appl. Mater. Interfaces 2016, 8, 25036–25042. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, Y.; Zhu, H.; Marcu, L.; Revzin, A. Enzyme-containing hydrogel micropatterns serving a dual purpose of cell sequestration and metabolite detection. Biosens. Bioelectron. 2009, 24, 2604–2610. [Google Scholar] [CrossRef] [PubMed]
- Matharu, Z.; Enomoto, J.; Revzin, A. Miniature enzyme-based electrodes for detection of hydrogen peroxide release from alcohol-injured hepatocytes. Anal. Chem. 2013, 85, 932–939. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Jouven, X.; Lemaître, R.N.; Rea, T.D.; Sotoodehnia, N.; Empana, J.-P.; Siscovick, D.S. Diabetes, glucose level, and risk of sudden cardiac death. Eur. Heart J. 2005, 26, 2142–2147. [Google Scholar] [CrossRef]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Rafin, G.; Majdoub, H.; Jaffrezic-Renault, N. Voltammetric glucose biosensor based on glucose oxidase encapsulation in a chitosan-kappa-carrageenan polyelectrolyte complex. Mater. Sci. Eng. C 2019, 95, 152–159. [Google Scholar] [CrossRef]
- Kim, J.H.; Jun, S.-A.; Kwon, Y.; Ha, S.; Sang, B.-I.; Kim, J. Enhanced electrochemical sensitivity of enzyme precipitate coating (EPC)-based glucose oxidase biosensors with increased free CNT loadings. Bioelectrochemistry 2015, 101, 114–119. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.-M.; Huang, S.-T.; Huang, T.-T.; Lin, C.-M.; Hwa, K.-Y.; Chen, T.-Y.; Chen, B.-J. Glucose biosensor based on glucose oxidase immobilized at gold nanoparticles decorated graphene-carbon nanotubes. Enzym. Microb. Technol. 2015, 78, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187. [Google Scholar] [CrossRef]
- Khan, R.; Andreescu, S. Catalytic MXCeO2 for enzyme based electrochemical biosensors: Fabrication, characterization and application towards a wearable sweat biosensor. Biosens. Bioelectron. 2024, 248, 115975. [Google Scholar] [CrossRef]
- Gizeli, E.; Lowe, C.R. Immunosensors. Curr. Opin. Biotechnol. 1996, 7, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors–a powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, J.; Seo, Y.; Gilad, A.A.; Choi, J. Optical immunosensors for the efficient detection of target biomolecules. Biotechnol. Bioprocess Eng. 2018, 23, 123–133. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Ramgir, N.S.; Joshi, R.K.; Bhansali, S. Selective growth of silica nanowires using an Au catalyst for optical recognition of interleukin-10. Nanotechnology 2008, 19, 245502. [Google Scholar] [CrossRef]
- de la Escosura-Muniz, A.; Sanchez-Espinel, C.; Díaz-Freitas, B.; Gonzalez-Fernandez, A.; Maltez-da Costa, M.; Merkoci, A. Rapid identification and quantification of tumor cells using an electrocatalytic method based on gold nanoparticles. Anal. Chem. 2009, 81, 10268–10274. [Google Scholar] [CrossRef] [PubMed]
- Stybayeva, G.; Kairova, M.; Ramanculov, E.; Simonian, A.L.; Revzin, A. Detecting interferon-gamma release from human CD4 T-cells using surface plasmon resonance. Colloids Surf. B Biointerfaces 2010, 80, 251–255. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Brimmo, A.T.; Qasaimeh, M.A.; Chen, P.; Chen, W. Recent Advances and Perspectives in Microfluidics-Based Single-Cell Biosensing Techniques. Small Methods 2017, 1, 1700192. [Google Scholar] [CrossRef]
- Washburn, A.L.; Shia, W.W.; Lenkeit, K.A.; Lee, S.-H.; Bailey, R.C. Multiplexed cancer biomarker detection using chip-integrated silicon photonic sensor arrays. Analyst 2016, 141, 5358–5365. [Google Scholar] [CrossRef] [PubMed]
- Bradley, Z.; Bhalla, N. Point-of-care diagnostics for sepsis using clinical biomarkers and microfluidic technology. Biosens. Bioelectron. 2023, 227, 115181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Y.; Weng, Z.; Yang, J.; Avery, L.; Dieckhaus, K.D.; Lai, R.Y.; Gao, X.; Zhang, Y. A point-of-care microfluidic biosensing system for rapid and ultrasensitive nucleic acid detection from clinical samples. Lab A Chip 2023, 23, 3862–3873. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, S.E.; Amiri, A.M. A brief overview of medical fiber optic biosensors and techniques in the modification for enhanced sensing ability. Diagnostics 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.J.; Panat, R. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Perez-Potti, A. Applications of nanomaterials for immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef]
- Wallace, W.; Tabobondung, M.; Esposto, J.; Martic, S. Antibody-based electrochemical sensor for detection of the full-length phosphorylated TDP-43 protein biomarker of amyotrophic lateral sclerosis. J. Electrochem. Soc. 2023, 170, 045502. [Google Scholar] [CrossRef]
- Watts, H.J.; Yeung, D.; Parkes, H. Real-time detection and quantification of DNA hybridization by an optical biosensor. Anal. Chem. 1995, 67, 4283–4289. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.; Fonseca, L. Trends in DNA biosensors. Talanta 2008, 77, 606–623. [Google Scholar] [CrossRef]
- Kowalczyk, A. Trends and perspectives in DNA biosensors as diagnostic devices. Curr. Opin. Electrochem. 2020, 23, 36–41. [Google Scholar] [CrossRef]
- Tosar, J.; Branas, G.; Laíz, J. Electrochemical DNA hybridization sensors applied to real and complex biological samples. Biosens. Bioelectron. 2010, 26, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Singh, S.G. Label-free electrochemical detection of DNA hybridization: A method for COVID-19 diagnosis. Trans. Indian Natl. Acad. Eng. 2020, 5, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Park, N.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.; Kim, N.Y.; Kim, J.H. Ultra-fast and recyclable DNA biosensor for point-of-care detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.M.; Cozma, I.; Mou, Q.; Brennan, J.D.; Lu, Y.; Li, Y. Biosensing with dnazymes. Chem. Soc. Rev. 2021, 50, 8954–8994. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef]
- Gerasimova, Y.V.; Kolpashchikov, D.M. Enzyme-assisted target recycling (EATR) for nucleic acid detection. Chem. Soc. Rev. 2014, 43, 6405–6438. [Google Scholar] [CrossRef]
- Wang, Z.-y.; Li, P.; Cui, L.; Qiu, J.-G.; Jiang, B.; Zhang, C.-Y. Integration of nanomaterials with nucleic acid amplification approaches for biosensing. TrAC Trends Anal. Chem. 2020, 129, 115959. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Wang, E. “Fitting” makes “sensing” simple: Label-free detection strategies based on nucleic acid aptamers. Acc. Chem. Res. 2013, 46, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Hianik, T.; Wang, J. Electrochemical aptasensors–recent achievements and perspectives. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1223–1235. [Google Scholar] [CrossRef]
- Sefah, K.; Shangguan, D.; Xiong, X.; O’donoghue, M.B.; Tan, W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Futane, A.; Narayanamurthy, V.; Jadhav, P.; Srinivasan, A. Aptamer-based rapid diagnosis for point-of-care application. Microfluid. Nanofluidics 2023, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.n.; Zhang, H.; Li, X.; Zhao, Y. Advances in Optical Fiber Aptasensor for Biochemical Sensing Applications. Adv. Mater. Technol. 2023, 8, 2300137. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [PubMed]
- Nooranian, S.; Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Kazemi Oskuee, R. Biosensors based on aptamer-conjugated gold nanoparticles: A review. Biotechnol. Appl. Biochem. 2022, 69, 1517–1534. [Google Scholar] [CrossRef] [PubMed]
- Mazaafrianto, D.N.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Recent microdevice-based aptamer sensors. Micromachines 2018, 9, 202. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, F.; Zhang, Y.; Xiong, Y.; Han, S. Recent advances in aptamer-based optical and electrochemical biosensors for detection of pesticides and veterinary drugs. Food Control 2022, 131, 108399. [Google Scholar] [CrossRef]
- Han, K.; Liang, Z.; Zhou, N. Design strategies for aptamer-based biosensors. Sensors 2010, 10, 4541–4557. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.M.; Nguyen, J.; Li, Y. Aptamer-based biosensors for environmental monitoring. Front. Chem. 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Kwon, Y.S.; Gu, M.B. Aptamer-based environmental biosensors for small molecule contaminants. Curr. Opin. Biotechnol. 2017, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Bai, Y.; Cui, Y.; Wang, D.; Shi, X. Identification and characterization of two high affinity aptamers specific for Salmonella Enteritidis. Food Control 2019, 106, 106719. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ranjbar, S. Aptamer immobilization on amino-functionalized metal–organic frameworks: An ultrasensitive platform for the electrochemical diagnostic of Escherichia coli O157: H7. Analyst 2018, 143, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.-R.; Sekhon, S.S.; Rhee, S.-K.; Ko, J.H.; Ahn, J.-Y.; Min, J.; Kim, Y.-H. Aptamer-based paper strip sensor for detecting Vibrio fischeri. ACS Comb. Sci. 2018, 20, 261–268. [Google Scholar] [CrossRef]
- Dhiman, A.; Kalra, P.; Bansal, V.; Bruno, J.G.; Sharma, T.K. Aptamer-based point-of-care diagnostic platforms. Sens. Actuators B Chem. 2017, 246, 535–553. [Google Scholar] [CrossRef]
- Khan, N.I.; Song, E. Lab-on-a-chip systems for aptamer-based biosensing. Micromachines 2020, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Zhang, Y.S.; Kim, D.-J.; Manbohi, A.; Avci, H.; Silvestri, A.; Aleman, J.; Hu, N.; Kilic, T.; Keung, W. Aptamer-based microfluidic electrochemical biosensor for monitoring cell-secreted trace cardiac biomarkers. Anal. Chem. 2016, 88, 10019–10027. [Google Scholar] [CrossRef]
- Crulhas, B.P.; Karpik, A.E.; Delella, F.K.; Castro, G.R.; Pedrosa, V.A. Electrochemical aptamer-based biosensor developed to monitor PSA and VEGF released by prostate cancer cells. Anal. Bioanal. Chem. 2017, 409, 6771–6780. [Google Scholar] [CrossRef]
- Liang, L.; Su, M.; Li, L.; Lan, F.; Yang, G.; Ge, S.; Yu, J.; Song, X. Aptamer-based fluorescent and visual biosensor for multiplexed monitoring of cancer cells in microfluidic paper-based analytical devices. Sens. Actuators B Chem. 2016, 229, 347–354. [Google Scholar] [CrossRef]
- Mo, T.; Liu, X.; Luo, Y.; Zhong, L.; Zhang, Z.; Li, T.; Gan, L.; Liu, X.; Li, L.; Wang, H. Aptamer-based biosensors and application in tumor theranostics. Cancer Sci. 2022, 113, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Liu, C.; Shi, G. Colorimetric enzyme-linked aptamer assay utilizing hybridization chain reaction for determination of bovine pregnancy-associated glycoproteins. Microchim. Acta 2020, 187, 316. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, Z.; Ma, C.; Yan, Y. A fluorometric aptamer based assay for prostate specific antigen based on enzyme-assisted target recycling. Sens. Actuators B Chem. 2020, 302, 127178. [Google Scholar] [CrossRef]
- Chen, J.; Lin, K.-C.; Prasad, S.; Schmidtke, D.W. Label free impedance based acetylcholinesterase enzymatic biosensors for the detection of acetylcholine. Biosens. Bioelectron. 2023, 235, 115340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Si, S.; Huang, F.; Wei, J.; Dong, S.; Yang, F.; Li, H.; Liu, S. Ultrasensitive label-free electrochemical biosensor for detecting linear microcystin-LR using degrading enzyme MlrB as recognition element. Bioelectrochemistry 2022, 144, 108000. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Z.; Ackerman, J.D.; Yazdani, M.; Hou, S.; Nugen, S.R.; Rotello, V.M. Electrochemical nanoparticle–enzyme sensors for screening bacterial contamination in drinking water. Analyst 2015, 140, 4991–4996. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Paniagua, M.C.; DiNello, R.K.; Panchal, A.; Geisberg, M. A rapid, inexpensive and disposable point-of-care blood test for sickle cell disease using novel, highly specific monoclonal antibodies. Br. J. Haematol. 2016, 175, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, F.; Goldys, E.M. A simple and versatile CRISPR/Cas12a-based immunosensing platform: Towards attomolar level sensitivity for small protein diagnostics. Talanta 2022, 246, 123469. [Google Scholar] [CrossRef]
- Gu, Y.; Ju, C.; Li, Y.; Shang, Z.; Wu, Y.; Jia, Y.; Niu, Y. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015, 66, 24–31. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Armstrong, C.M.; Lee, J.; Gehring, A.G. Detection of pathogenic bacteria in large volume food samples using an enzyme-linked immunoelectrochemical biosensor. Food Control 2021, 119, 107456. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Z.; Zong, S.; Liu, Y.; Yang, K.; Li, N.; Wang, Z.; Li, L.; Tang, H.; Cui, Y. Hydrophobic plasmonic nanoacorn array for a label-free and uniform SERS-based biomolecular assay. ACS Appl. Mater. Interfaces 2020, 12, 29917–29927. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.; Li, X.; Lin, C.; Wang, X.; Liu, J.; Ling, L.; Wang, J. CRISPR/Cas12a-based biosensor for colorimetric detection of serum prostate-specific antigen by taking nonenzymatic and isothermal amplification. Sens. Actuators B Chem. 2022, 354, 131228. [Google Scholar] [CrossRef]
- Li, Y.-C.E.; Lee, I.-C. The current trends of biosensors in tissue engineering. Biosensors 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas systems towards next-generation biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Kadam, U.S.; Cho, Y.; Park, T.Y.; Hong, J.C. Aptamer-based CRISPR-Cas powered diagnostics of diverse biomarkers and small molecule targets. Appl. Biol. Chem. 2023, 66, 13. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhao, R.T.; Welch, N.L.; Tan, E.K.W.; Zhong, Q.; Harzallah, N.S.; Ngambenjawong, C.; Ko, H.; Fleming, H.E.; Sabeti, P.C. CRISPR-Cas-amplified urinary biomarkers for multiplexed and portable cancer diagnostics. Nat. Nanotechnol. 2023, 18, 798–807. [Google Scholar] [CrossRef]
- Weng, Z.; You, Z.; Yang, J.; Mohammad, N.; Lin, M.; Wei, Q.; Gao, X.; Zhang, Y. CRISPR-Cas biochemistry and CRISPR-based molecular diagnostics. Angew. Chem. Int. Ed. 2023, 62, e202214987. [Google Scholar] [CrossRef] [PubMed]
- Amitai, G.; Sorek, R. CRISPR–Cas adaptation: Insights into the mechanism of action. Nat. Rev. Microbiol. 2016, 14, 67–76. [Google Scholar] [CrossRef]
- Aman, R.; Mahas, A.; Mahfouz, M. Nucleic acid detection using CRISPR/Cas biosensing technologies. ACS Synth. Biol. 2020, 9, 1226–1233. [Google Scholar] [CrossRef]
- Mao, S.; Ying, Y.; Wu, X.; Krueger, C.J.; Chen, A.K. CRISPR/dual-FRET molecular beacon for sensitive live-cell imaging of non-repetitive genomic loci. Nucleic Acids Res. 2019, 47, e131. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Rao, R.; Xiong, M.; He, B.; Zheng, B.; Jia, Y.; Li, Y.; Yang, Y. CRISPR-powered strategies for amplification-free diagnostics of infectious diseases. Anal. Chem. 2024, 96, 8091–8108. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Somoza, R.A.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the trans-cleavage activity of CRISPR-Cas12a (cpf1) for the development of a universal electrochemical biosensor. Angew. Chem. 2019, 131, 17560–17566. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Xu, F.; Yang, X. Powerful CRISPR-based biosensing techniques and their integration with microfluidic platforms. Front. Bioeng. Biotechnol. 2022, 10, 851712. [Google Scholar] [CrossRef] [PubMed]
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2010, 4, 1–10. [Google Scholar] [PubMed]
- Abpeikar, Z.; Alizadeh, A.A.; Rezakhani, L.; Ramezani, V.; Goodarzi, A.; Safaei, M. Advantages of Material Biofunctionalization Using Nucleic Acid Aptamers in Tissue Engineering and Regenerative Medicine. Mol. Biotechnol. 2023, 65, 1935–1953. [Google Scholar] [CrossRef]
- Solaimuthu, A.; Vijayan, A.N.; Murali, P.; Korrapati, P.S. Nano-biosensors and their relevance in tissue engineering. Curr. Opin. Biomed. Eng. 2020, 13, 84–93. [Google Scholar] [CrossRef]
- Liping, Q.; Tao, Z.; Jianhui, J.; Cuichen, W.; Guizhi, Z.; Mingxu, Y.; Xigao, C.; Liqin, Z.; Cheng, C.; Ruqin, Y. Cell Membrane-Anchored Biosensors for Real-Time Monitoring of the Cellular Microenvironment. J. Am. Chem. Soc. 2014, 136, 13090–13093. [Google Scholar]
- Zhao, W.; Schafer, S.; Choi, J.; Yamanaka, Y.J.; Lombardi, M.L.; Bose, S.; Carlson, A.L.; Phillips, J.A.; Teo, W.; Droujinine, I.A. Cell-surface sensors for real-time probing of cellular environments. Nat. Nanotechnol. 2011, 6, 524–531. [Google Scholar] [CrossRef]
- Yang, W.; Li, T.; Liao, S.; Zhou, J.; Huang, L. Organ-on-a-chip platforms integrated with biosensors for precise monitoring of the cells and cellular microenvironment. TrAC Trends Anal. Chem. 2024, 172, 117569. [Google Scholar] [CrossRef]
- Zimmerling, A.; Dahlan, N.A.; Zhou, Y.; Chen, X. Recent frontiers in biofabrication for respiratory tissue engineering. Bioprinting 2024, 40, e00342. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Han, M.; Long, M.; Li, T.; Hu, L.; Wang, L.; Huang, W.; Wu, Y. Engineering Cardiac Tissue for Advanced Heart-On-A-Chip Platforms. Adv. Healthc. Mater. 2024, 13, 2301338. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, N.; Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces 2000, 18, 197–219. [Google Scholar] [CrossRef]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.; Moulton, S.E.; Guijt, R.M.; Greene, G.W. Antifouling strategies for electrochemical biosensing: Mechanisms and performance toward point of care based diagnostic applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. Apmis 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Liu, B.; Huang, P.-J.J.; Zhang, X.; Wang, F.; Pautler, R.; Ip, A.C.F.; Liu, J. Parts-per-million of polyethylene glycol as a non-interfering blocking agent for homogeneous biosensor development. Anal. Chem. 2013, 85, 10045–10050. [Google Scholar] [CrossRef]
- Wang, W.; Han, R.; Chen, M.; Luo, X. Antifouling peptide hydrogel based electrochemical biosensors for highly sensitive detection of cancer biomarker HER2 in human serum. Anal. Chem. 2021, 93, 7355–7361. [Google Scholar] [CrossRef] [PubMed]
- Chaniotakis, N.A. Enzyme stabilization strategies based on electrolytes and polyelectrolytes for biosensor applications. Anal. Bioanal. Chem. 2004, 378, 89–95. [Google Scholar] [CrossRef]
- Demir, E.; Kırboga, K.K.; Işık, M. An overview of stability and lifetime of electrochemical biosensors. In Novel Nanostructured Materials for Electrochemical Bio-sensing Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 129–158. [Google Scholar]
- Watson, S.R.; Chang, Y.-F.; O’Connell, D.; Weigand, L.; Ringquist, S.; Parma, D.H. Anti-L-selectin aptamers: Binding characteristics, pharmacokinetic parameters, and activity against an intravascular target in vivo. Antisense Nucleic Acid Drug Dev. 2000, 10, 63–75. [Google Scholar] [CrossRef]
- Feagin, T.A.; Olsen, D.P.; Headman, Z.C.; Heemstra, J.M. High-throughput enantiopurity analysis using enantiomeric DNA-based sensors. J. Am. Chem. Soc. 2015, 137, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Wu, H.; Niu, Y.; Cai, J. Improving the stability of aptamers by chemical modification. Curr. Med. Chem. 2011, 18, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.N.; Rangel, A.E.; Heemstra, J.M. Enhancing aptamer function and stability via in vitro selection using modified nucleic acids. Methods 2016, 106, 29–36. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Hwang, S.-J.; Jeon, Y.; Yoon, Y. The Biomedical Applications of Biomolecule Integrated Biosensors for Cell Monitoring. Int. J. Mol. Sci. 2024, 25, 6336. https://doi.org/10.3390/ijms25126336
Song K, Hwang S-J, Jeon Y, Yoon Y. The Biomedical Applications of Biomolecule Integrated Biosensors for Cell Monitoring. International Journal of Molecular Sciences. 2024; 25(12):6336. https://doi.org/10.3390/ijms25126336
Chicago/Turabian StyleSong, Kyeongseok, Soon-Jin Hwang, Yangwon Jeon, and Youngdae Yoon. 2024. "The Biomedical Applications of Biomolecule Integrated Biosensors for Cell Monitoring" International Journal of Molecular Sciences 25, no. 12: 6336. https://doi.org/10.3390/ijms25126336
APA StyleSong, K., Hwang, S.-J., Jeon, Y., & Yoon, Y. (2024). The Biomedical Applications of Biomolecule Integrated Biosensors for Cell Monitoring. International Journal of Molecular Sciences, 25(12), 6336. https://doi.org/10.3390/ijms25126336








