The Key Role of Plant Hormone Signaling Transduction and Flavonoid Biosynthesis Pathways in the Response of Chinese Pine (Pinus tabuliformis) to Feeding Stimulation by Pine Caterpillar (Dendrolimus tabulaeformis)
Abstract
:1. Introduction
2. Results
2.1. Differences in Treatment Modes and Time after Treatment Impact on Plant Hormones
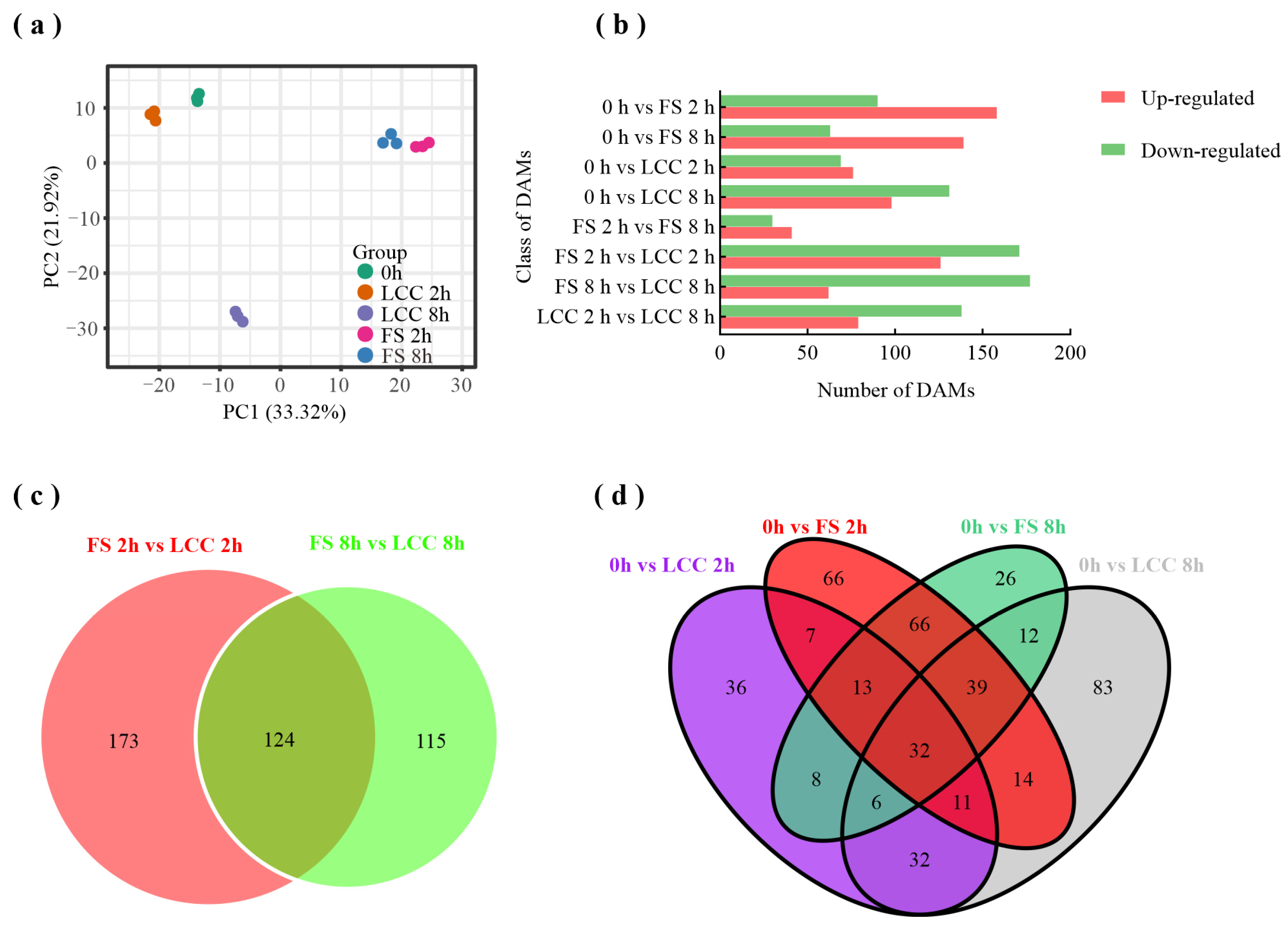
2.2. Analysis of FS-Induced Metabolites
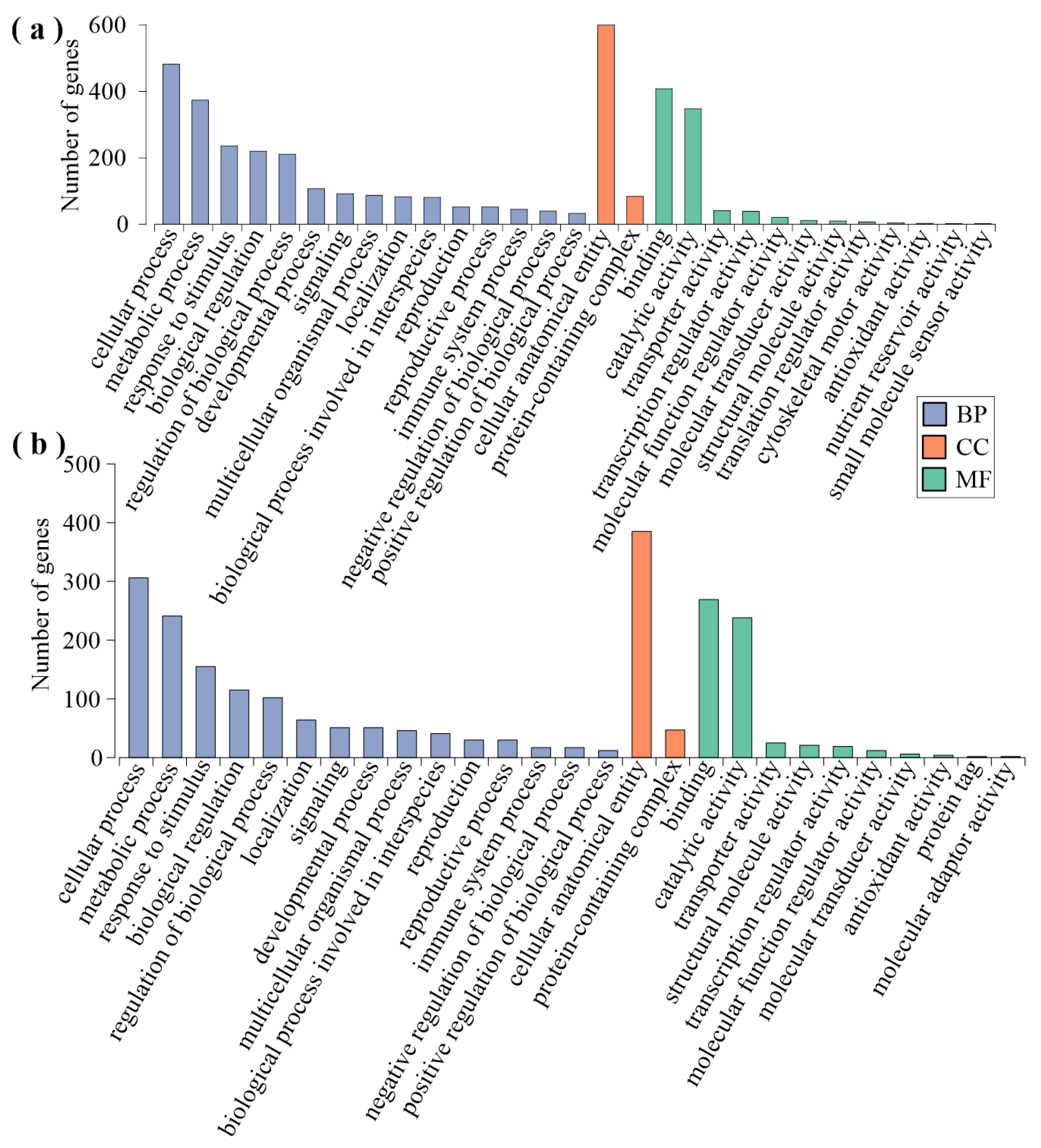
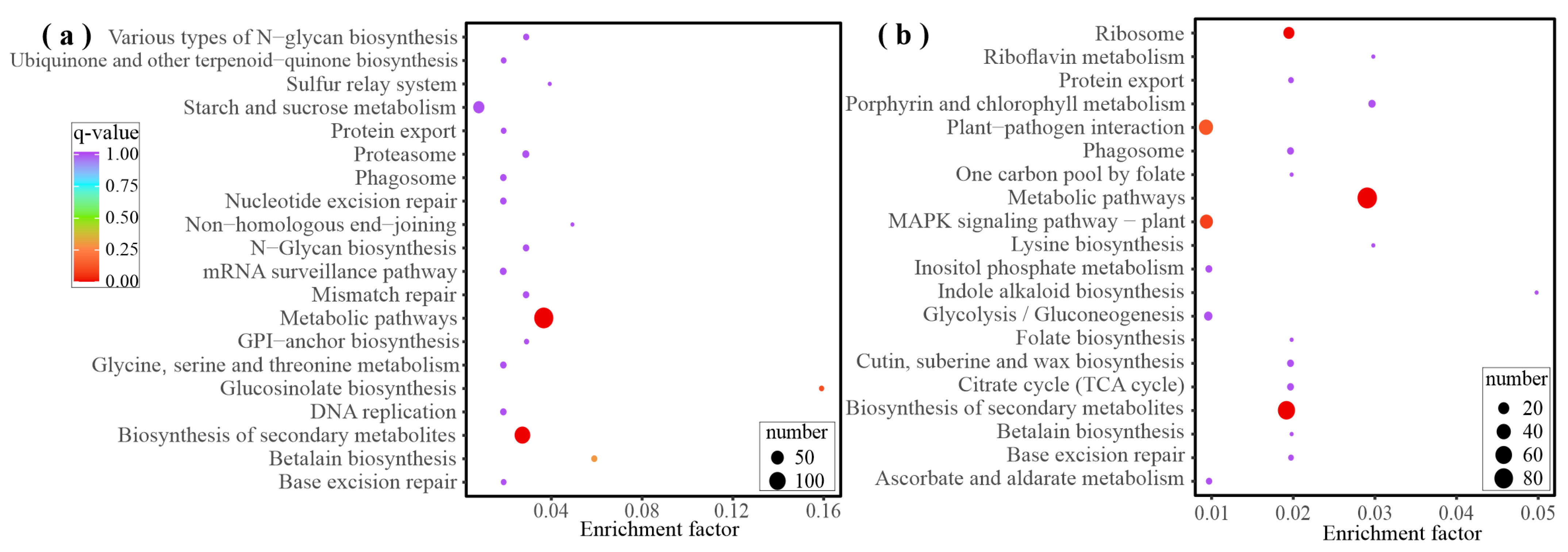
2.3. Analysis of FS-Induced Genes
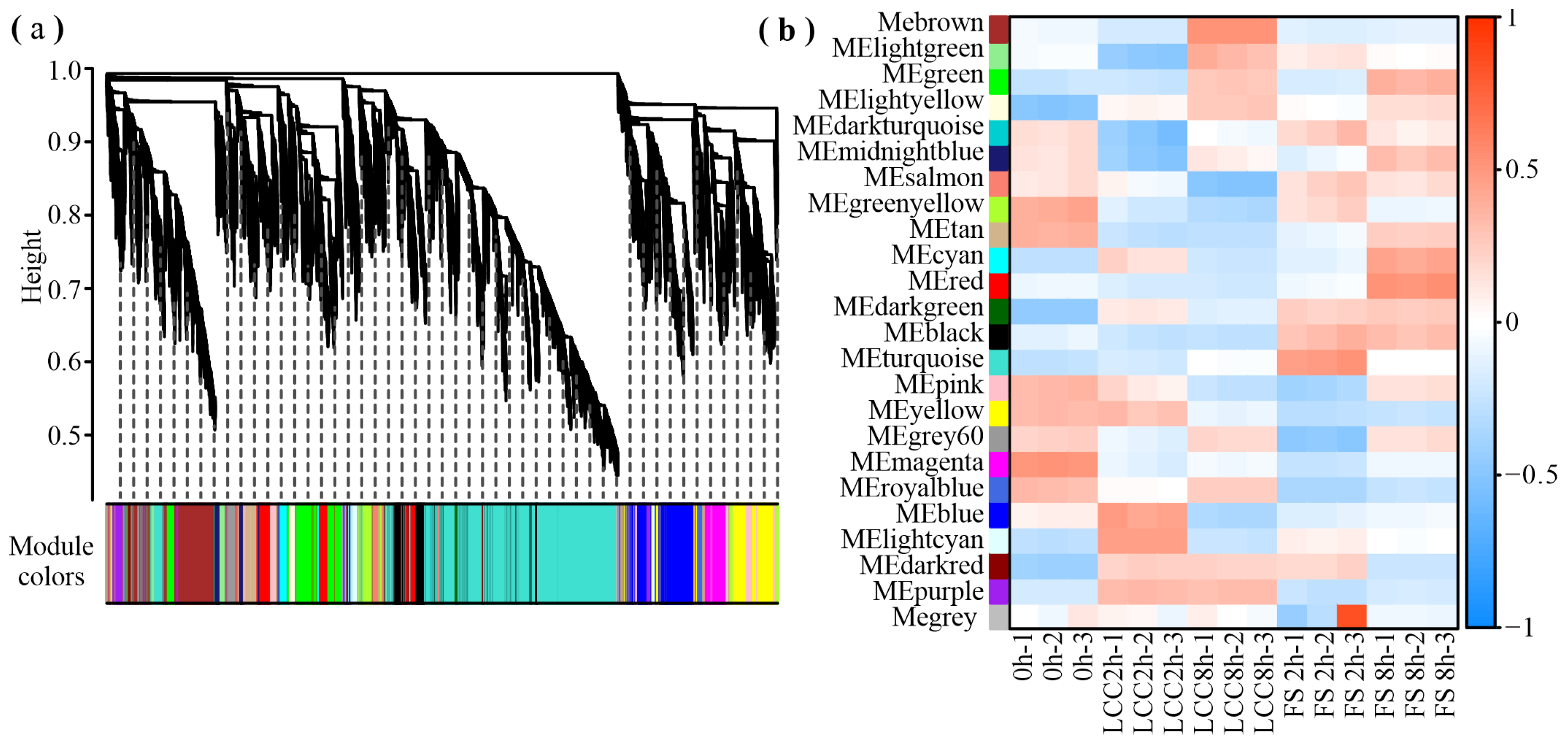
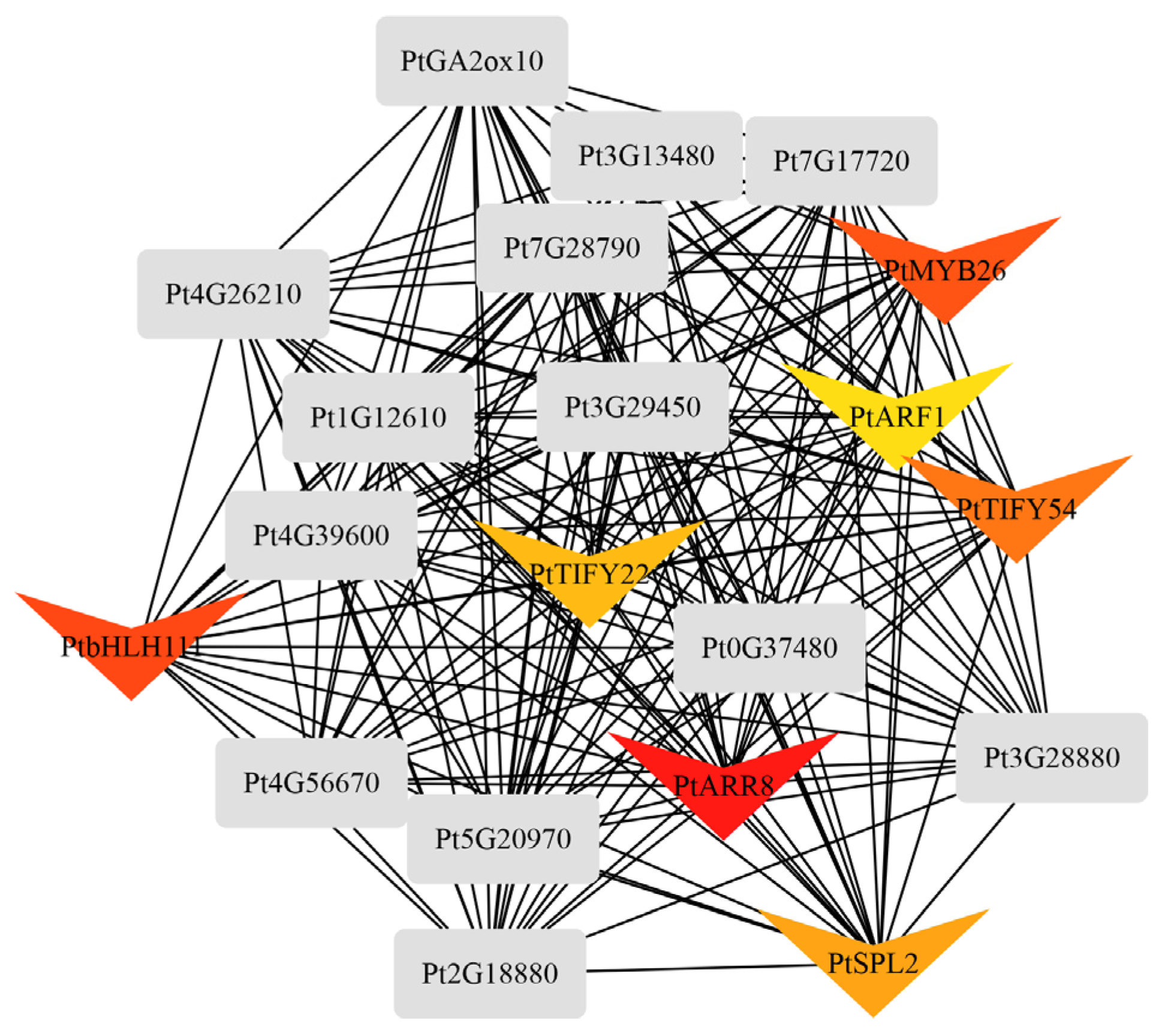
2.4. Differential Co-Expression of Genes and Metabolites Induced by Feeding Stimulation
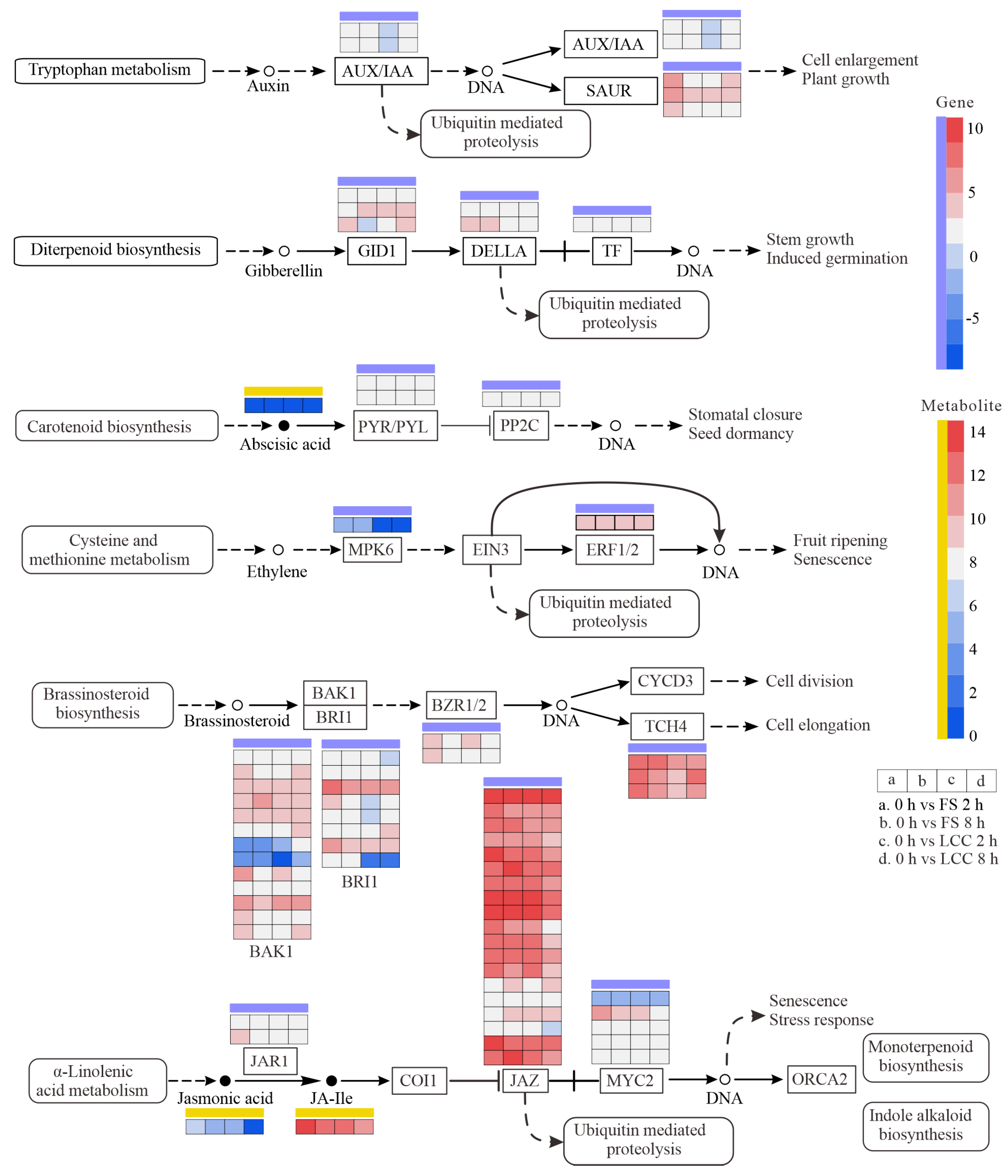
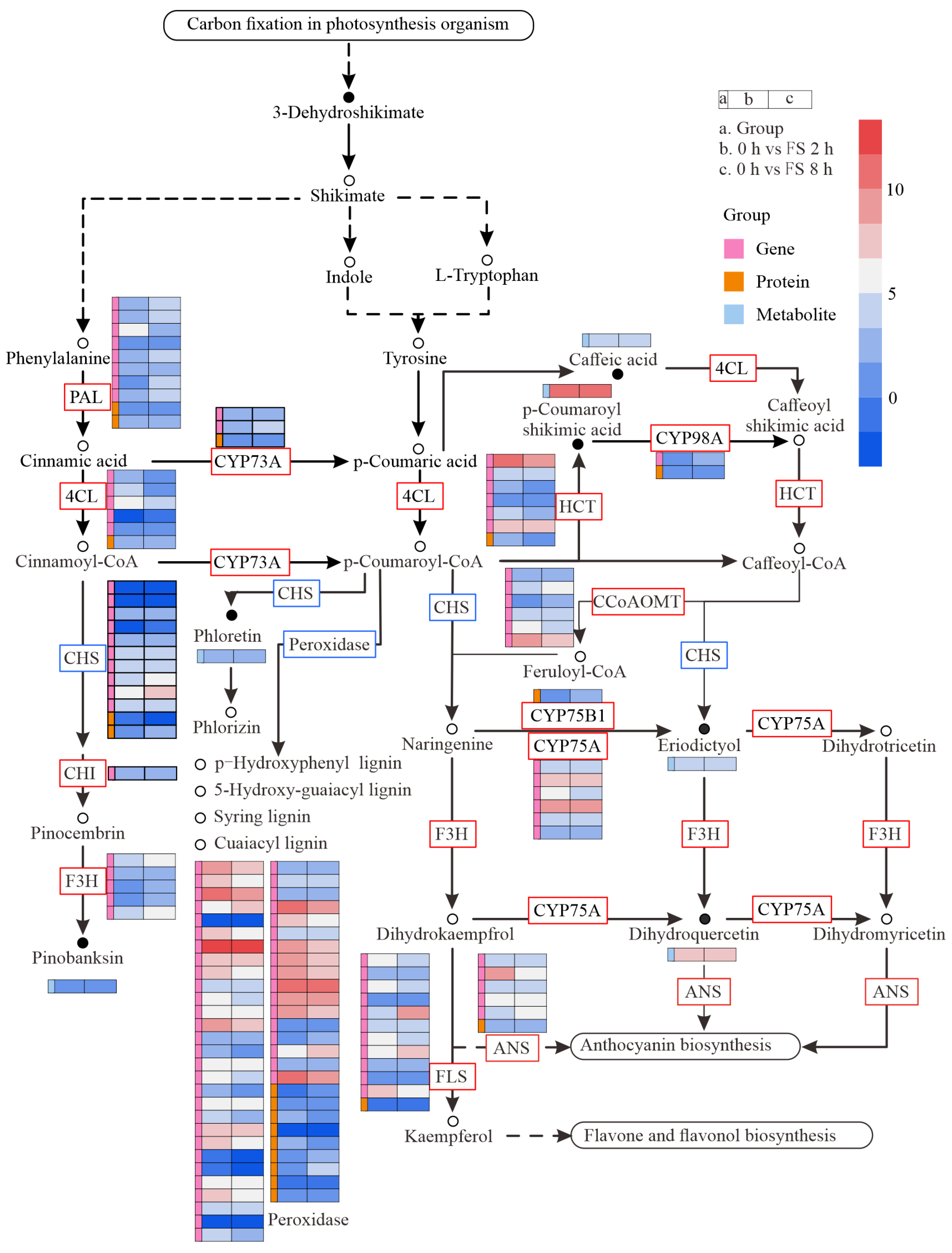
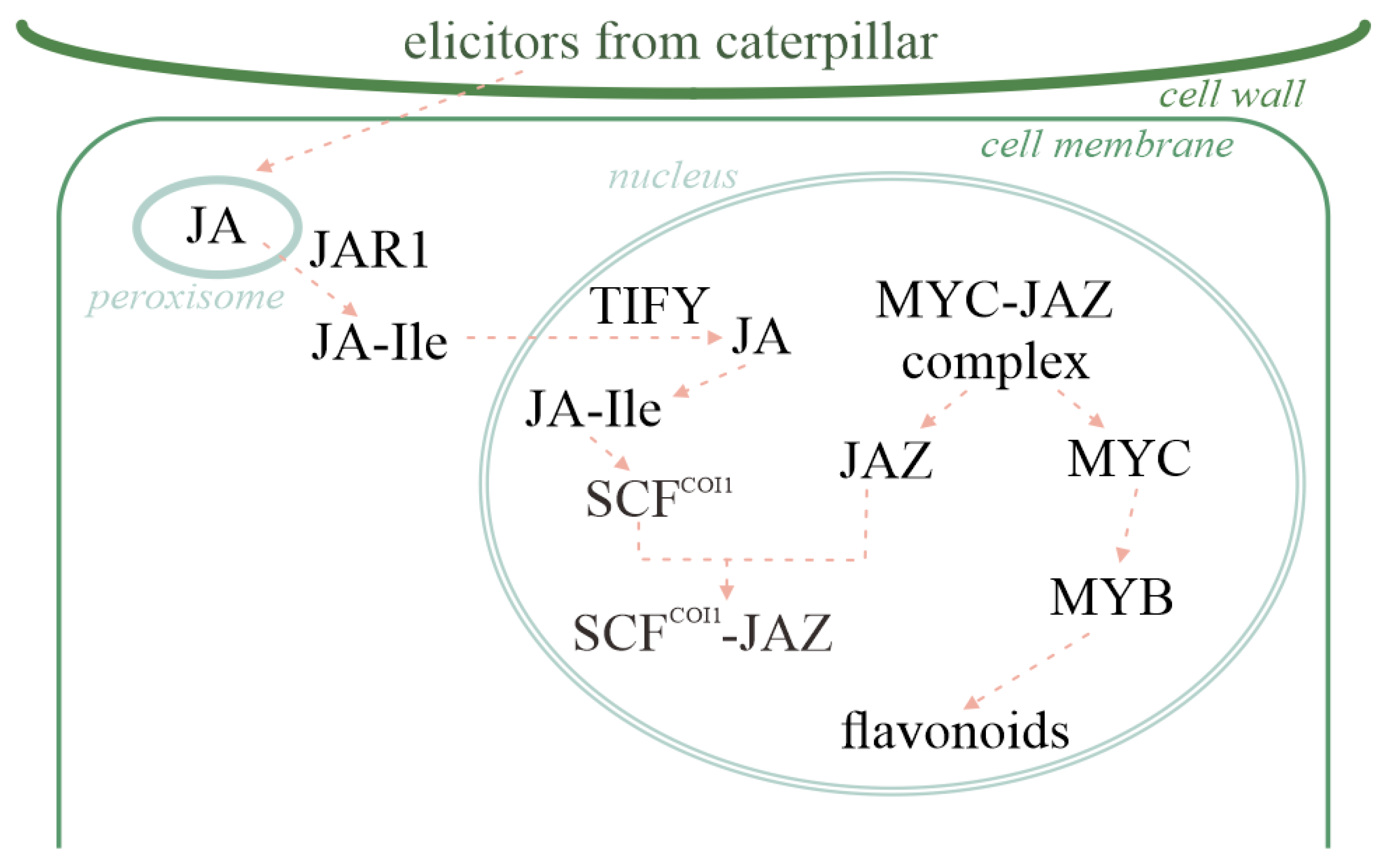
2.5. JA Activates Flavonoid Expression
3. Discussion
3.1. Different Responses Induced by Feeding Stimulation and Leaf Clipping Control
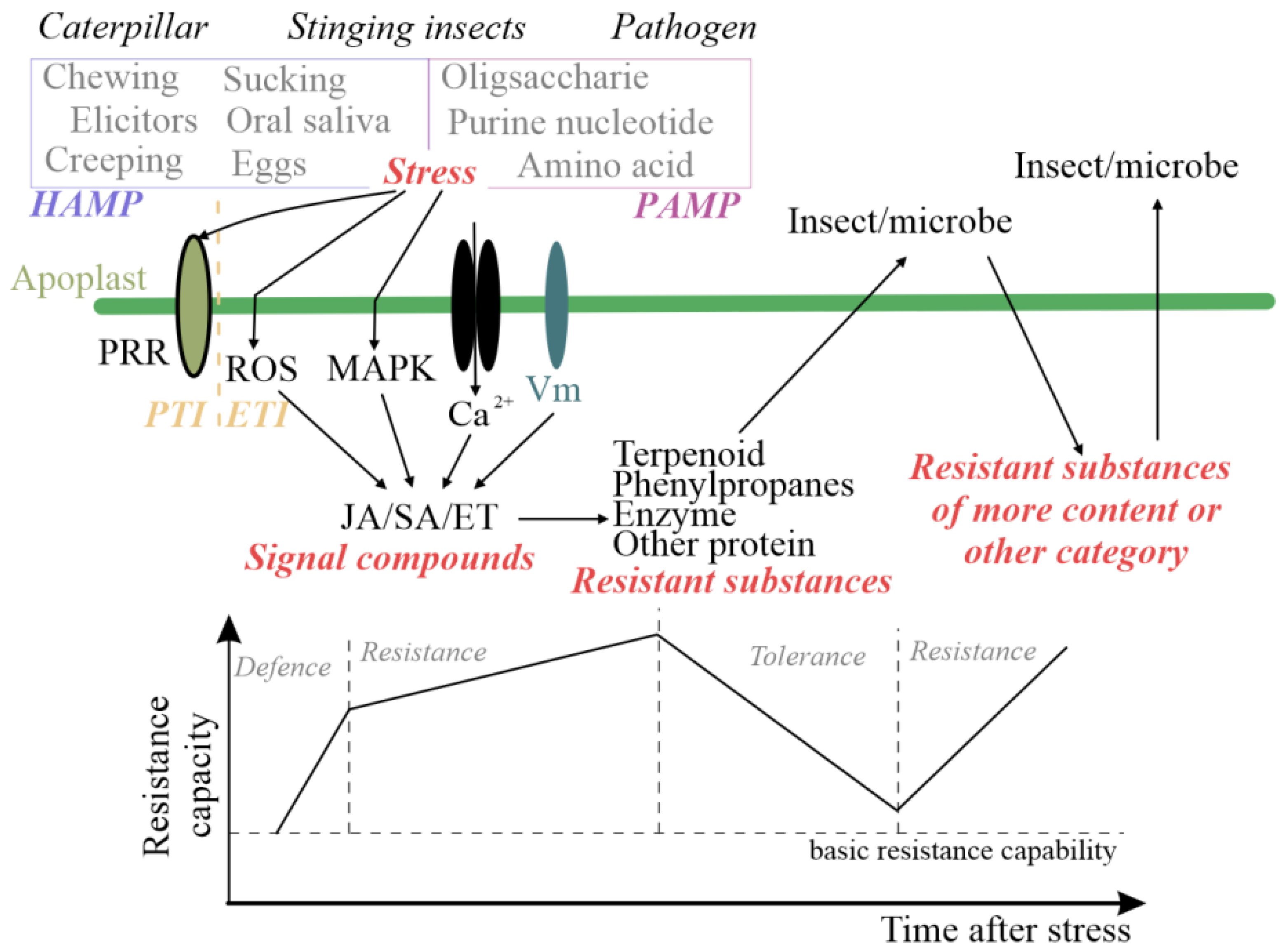
3.2. The Role of Plant Hormones in Signal Transduction Related to the Induction of Plant Resistance to Insects
3.3. Flavonoid Biosynthesis Involved in Plant-Induced Resistance to Insects
4. Materials and Methods
4.1. Study Area and Experiment Setting
4.2. Quantifications of JA and SA
4.3. RNA Preparation
4.4. Transcriptome Assay
4.5. Metabolome Assay
4.6. q-PCR Analysis
4.7. Statistic Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, L.N.; Li, Y.T.; Wu, Y.Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vazvani, M.G.; Kennedy, J.F. Beta-glucan-induced disease resistance in plants: A review. Int. J. Biol. Macromol. 2023, 253, 127043. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Pappas, M.L.; Broekgaarden, C.; Broufas, G.D.; Kant, M.R.; Messelink, G.J.; Steppuhn, A.; Wäckers, F.; Van, D.N.M. Induced plant defences in biological control of arthropod pests: A double-edged sword. Pest. Manag. Sci. 2017, 73, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Arnaiz, A.; Gonzalez-melendi, P.; Martinez, M.; Diaz, I. Plant Perception and Short-Term Responses to Phytophagous Insects and Mites. Int. J. Mol. Sci. 2018, 19, 1356. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chen, H.; Liu, F.; Fu, Z.Q. PTI and ETI: Convergent pathways with diverse elicitors. Trends Plant Sci. 2022, 27, 113–115. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Delplace, F.; Huard-chauveau, C.; Berthome, R.; Roby, D. Network organization of the plant immune system: From pathogen perception to robust defense induction. Plant J. 2022, 109, 447–470. [Google Scholar] [CrossRef]
- Malook, S.U.; Maqbool, S.; Hafeez, M.; Karunarathna, S.C.; Suwannarach, N. Molecular and Biochemical Mechanisms of Elicitors in Pest Resistance. Life 2022, 12, 844. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, S.; Guayazan-palacios, N.; Steinbrenner, A.D. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell 2022, 34, 1497–1513. [Google Scholar] [CrossRef]
- Furstenberg-hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Modesto, I.; Mendes, A.; Carrasquinho, I.; Miguel, C.M. Molecular Defense Response of Pine Trees (Pinus spp.) to the Parasitic Nematode Bursaphelenchus xylophilus. Cells 2022, 11, 3208. [Google Scholar] [CrossRef]
- Nantongo, J.S.; Potts, B.M.; Frickey, T.; Telfer, E.; Dungey, H.; Fitzgerald, H.; O’Reilly-Wapstra, J.M. Analysis of the transcriptome of the needles and bark of Pinus radiata induced by bark stripping and methyl jasmonate. BMC Genom. 2022, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Whitehill, J.G.A.; Bohlmann, J. A molecular and genomic reference system for conifer defence against insects. Plant Cell Environ. 2019, 42, 2844–2859. [Google Scholar] [CrossRef]
- Whitehill, J.G.A.; Yuen, M.M.S.; Henderson, H.; Madilao, L.; Kshatriya, K.; Bryan, J.; Jaquish, B.; Bohlmann, J. Functions of stone cells and oleoresin terpenes in the conifer defense syndrome. New Phytol. 2019, 221, 1503–1517. [Google Scholar] [CrossRef]
- Niu, S.; Li, J.; Bo, W. The Chinese pine genome and methylome unveil key features of conifer evolution. Cell 2022, 185, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, W.; Li, Y. Chinese pine (Pinus tabuliformis Carr.). Trends Genet. 2022, 38, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Han, F.X.; Wang, P.Y.; Chen, X.; Zhao, H.; Zhu, Q.; Song, Y.; Nie, Y.; Li, Y.; Guo, M.; Niu, S. An ethylene-induced NAC transcription factor acts as a multiple abiotic stress responsor in conifer. Hortic. Res. 2023, 10, uhad130. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Berger, S.; Schaller, A.; Stintzi, A. Jasmonate-dependent induction of polyphenol oxidase activity in tomato foliage is important for defense against but not against. BMC Plant Biol. 2014, 14, 257. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.M.; Besomi, G.; Glauser, G.; Turlings, T.C.J. Caterpillar-Induced Volatile Emissions in Cotton: The Relative Importance of Damage and Insect-Derived Factors. Front. Plant Sci. 2021, 12, 709858. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Halitschke, R.; Gase, K.; Leddy, S.M.; Schuman, M.C.; Rodde, N.; Baldwin, I.T. A persistent major mutation in canonical jasmonate signaling is embedded in an herbivory-elicited gene networ. Proc. Natl. Acad. Sci. USA 2023, 120, e2308500120. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Q.; Zhou, W.W.; Pottinger, S.; Baldwin, I.T. Herbivore associated elicitor-induced defences are highly specific among closely related Nicotiana species. BMC Plant Biol. 2015, 151, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, W.; Gu, Z.; Wu, S.; E, Y.; Zhou, W.; Lin, J.; Xu, L. Roles of the wound hormone jasmonate in plant regeneration. J. Exp. Bot. 2021, 74, 1198–1206. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef]
- Li, C.Y.; Zha, W.J.; Li, W.; Wang, J.; You, A. Advances in the Biosynthesis of Terpenoids and Their Ecological Functions in Plant Resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Ton, J.; D’Alessandro, M.; Jourdie, V.; Jakab, G.; Karlen, D.; Held, M.; Mauch-Mani, B.; Turlings, T.C. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007, 49, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Song, S.; Xu, W.P.; Zhang, C.X.; Ma, C.; Wang, L. Shiping Wang. Evolution of volatile compounds during the development of Muscat grape ‘Shine Muscat’ (Vitis labrusca x V. vinifera). Food Chem. 2020, 309, 125778. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, S.; Adamczyk, B.; Kitunen, V.; Aino, S. Monoterpenes and higher terpenes may inhibit enzyme activities in boreal forest soil. Soil. Biol. Biochem. 2015, 87, 59–66. [Google Scholar] [CrossRef]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Verne, S.; Jaquish, B.; White, R.; Ritland, C.; Ritland, K. Global transcriptome analysis of constitutive resistance to the white pine weevil in spruce. Genome Biol. Evol. 2011, 3, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.M.; Huang, D.Z.; Cao, Y.X.; Kong, X.B.; Zhang, Z. Relationship between Resin Monoterpenes and Resistance of Chinese pine to red Turpentine bettle. J. Northeast. For. Univ. 2008, 1, 48–50. (In Chinese) [Google Scholar]
- Gaquerel, E.; Stitz, M. Insect Resistance: An Emerging Molecular Framework Linking Plant Age and JA Signaling. Mol. Plant 2017, 10, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Liu, Y.Q.; Chen, D.Y.; Chen, F.Y.; Fang, X.; Hong, G.J.; Wang, L.J.; Wang, J.W.; Chen, X.Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, E.; Stahl, E.; Glauser, G.; Bellani, E.; Raaymakers, T.M.; Van, D.A.G.; Zeier, J.; Reymond, P. Insect eggs trigger systemic acquired resistance against a fungal and an oomycete pathogen. New Phytol. 2021, 232, 2491–2505. [Google Scholar] [CrossRef]
- Zhang, Y.; Bouwmeester, H.J.; Kappers, I.F. Combined transcriptome and metabolome analysis identifies defence responses in spider mite-infested pepper (Capsicum annuum). J. Exp. Bot. 2020, 71, 330–343. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, W.; Zhao, Q. Salicylic acid biosynthesis is not from phenylalanine in Arabidopsis. J. Integr. Plant Biol. 2022, 65, 881–887. [Google Scholar] [CrossRef]
- Yan, S.; Dong, X. Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 2014, 20, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ahammed, G.J. Hormonal regulation of anthocyanin biosynthesis for improved stress tolerance in plants. Plant Physiol. Biochem. 2023, 201, 107835. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.L.; Li, W.J.; Hong, Y.S.; Zhou, Y.M.; Tian, L.; Zhang, X.G.; Liu, F.L.; Liu, P. TMT based proteomic profiling of Sophora alopecuroides leaves reveal flavonoid biosynthesis processes in response to salt stress. J. Proteom. 2021, 253, 104457. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Yang, Y. Anthocyanin-mediated arsenic tolerance in plants. Environ. Pollut. 2022, 292, 118475. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, S.; Liu, C.; Zhao, L.; Cong, B.; Zhang, Z. Transcriptomics Integrated with Metabolomics Reveal the Effects of Ultraviolet-B Radiation on Flavonoid Biosynthesis in Antarctic Moss. Front. Plant Sci. 2021, 12, 788377. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, L.; Chang, P.; Chen, Z.; Zhang, X.; Yin, W.; Fan, Y.; Wang, X. The Impact of Elsinoë ampelina Infection on Key Metabolic Properties in Vitis vinifera ‘Red Globe’ Berries via Multiomics Approaches. Mol. Plant Microbe Interact. 2022, 35, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yan, X.; Sun, T.; Shen, Y.; Shi, Q.; Wang, W.; Bao, M.; Luo, H.; Nian, F.; Ning, G. Homeostatic regulation of flavonoid and lignin biosynthesis in phenylpropanoid pathway of transgenic tobacco. Gene 2022, 809, 146017. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Z.; Niu, J. Phytotoxic effect and molecular mechanism induced by graphene towards alfalfa (Medicago sativa L.) by integrating transcriptomic and metabolomics analysis. Chemosphere 2021, 290, 133368. [Google Scholar] [CrossRef]
- Wang, J.D.; Chen, L.F.; Lin, D.J.; Zhang, J.S.; Zhao, J.H.; Xiao, D.; Wang, R.; Wang, R.; Gao, S.J. Molecular cloning, characterization and functional analysis of GluCl from the oriental armyworm, Mythimna separata Walker. Pestic. Biochem. Physiol. 2019, 156, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W. Resistance Evaluation and Mechanism of Camellia sinensis Response to Ectropis obliqua. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Niu, Y.; Sun, H.; Stevens, M. Plant Camouflage: Ecology, Evolution, and Implications. Trends Ecol. Evol. 2018, 33, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Deenamo, N.; Kuyyogsuy, A.; Khompatara, K.; Chanwun, T.; Ekchaweng, K.; Churngchow, N. Salicylic Acid Induces Resistance in Rubber Tree against Phytophthora palmivora. Int. J. Mol. Sci. 2018, 19, 1883. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic Profiling Reveals Differentially Expressed Genes Associated with Pine Wood Nematode Resistance in Masson Pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysen, G. The tify family previously known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; Vanholme, B.; Pauwels, L.; Plovie, E.; Inzé, D.; Gheysen, G.; Goossens, A. Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin. EMBO Rep. 2009, 10, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.B.; Gu, M.F. Transcriptome analysis and differential gene expression profiling of two contrasting quinoa genotypes in response to salt stress. BMC Plant Biol. 2020, 20, 568. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.; Fukui, K.; Seto, Y.; Takaoka, Y.; Okamoto, M. Ligand-receptor interactions in plant hormone signaling. Plant J. 2021, 105, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Xu, J.; Lu, X.; Liu, Y. Anthocyanin Biosynthesis Induced by MYB Transcription Factors in Plants. Int. J. Mol. Sci. 2022, 23, 11701. [Google Scholar] [CrossRef]
- Liu, J.Y.; Osbourn, A.; Ma, P.D. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.C.; Song, S.S.; Ren, Q.C.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-Domain Proteins Interact with the WD-Repeat/bHLH/MYB Complexes to Regulate Jasmonate-Mediated Anthocyanin Accumulation and Trichome Initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Su, L.T.; Lv, A.M.; Wen, W.W.; Fan, N.; Li, J.; Gao, L.; Zhou, P.; An, Y. MsMYB741 is involved in alfalfa resistance to aluminum stress by regulating flavonoid biosynthesis. Plant J. 2022, 112, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Wang, Y.X.; Li, H.; Liu, Z.W.; Cui, X.; Zhuang, J. Two MYB transcription factors (CsMYB2 and CsMYB26) are involved in flavonoid biosynthesis in tea plant [Camellia sinensis (L.) O. Kuntze]. BMC Plant Biol. 2019, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.K.Y.; Li, L.; Liu, H.; Yan, X.; Ma, Y.; Li, Y.; Chen, T.; Wang, C.; Xie, L.; Hao, X.; et al. AaMYB15, an R2R3-MYB TF in Artemisia annua, acts as a negative regulator of artemisinin biosynthesis. Plant Sci. 2021, 308, 110920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.Y.; Zhang, Y.F.; Zhang, C.; Li, X.; Liu, X.; Wang, C.L. PpyMYB144 transcriptionally regulates pear fruit skin russeting by activating the cytochrome P450 gene PpyCYP86B1. Planta 2023, 257, 69. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Rathinam, M.; Dokka, N.; Chaudhary, N.; Satish, L.; Dash, P.K.; Shasany, A.K.; Sreevathsa, R. Cajanus platycarpus Flavonoid 3’5’ Hydroxylase_2 (CpF3’5’H_2) Confers Resistance to Helicoverpa armigera by Modulating Total Polyphenols and Flavonoids in Transgenic Tobacco. Int. J. Mol. Sci. 2023, 24, 1755. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, M.; Mishra, P.; Mahato, A.K.; Singh, N.K.; Rao, U.; Sreevathsa, R. Comparative transcriptome analyses provide novel insights into the differential response of Pigeonpea (Cajanus cajan L.) and its wild relative (Cajanus platycarpus (Benth.) Maesen) to herbivory by Helicoverpa armigera (Hubner). Plant Mol. Biol. 2019, 101, 163–182. [Google Scholar] [CrossRef]
- Sozen, C.; Schenk, S.T.; Boudsocq, M.; Chardin, C.; Almeida-Trapp, M.; Krapp, A.; Hirt, H.; Mithöfer, A.; Colcombet, J. Wounding and Insect Feeding Trigger Two Independent MAPK Pathways with Distinct Regulation and Kinetics. Plant Cell 2020, 32, 1988–2003. [Google Scholar] [CrossRef]
- Smythers, A.L.; Bhatnagar, N.; Ha, C.; Majumdar, P.; McConnell, E.W.; Mohanasundaram, B.; Hicks, L.M.; Pandey, S. Abscisic acid-controlled redox proteome of Arabidopsis and its regulation by heterotrimeric Gbeta protein. New Phytol. 2022, 236, 447–463. [Google Scholar] [CrossRef]
- Behr, M.; Neutelings, G.E.l.; Jaziri, M.; Baucher, M. You Want it Sweeter: How Glycosylation Affects Plant Response to Oxidative Stress. Front. Plant Sci. 2020, 11, 571399. [Google Scholar] [CrossRef]
- Aerts, N.; Mendes, M.P.; Van, W.S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, J.P.; Zhang, Q.; Shi, S.; Guan, W.; Zhou, C.; Chen, R.; Du, B.; Zhu, L.; He, G. Necessity of rice resistance to planthoppers for OsEXO70H3 regulating SAMSL excretion and lignin deposition in cell walls. New Phytol. 2022, 234, 1031–1046. [Google Scholar] [CrossRef]
- Soderberg, D.N.; Kyre, B.; Bonello, P.; Bentz, B.J. Lignin concentrations in phloem and outer bark are not associated with resistance to mountain pine beetle among high elevation pines. PLoS ONE 2021, 16, e0250395. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.Q.; Yuan, D.Y. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Sheng, L.P.; Zhang, H.R.; Du, X.; An, C.; Xia, X.; Chen, F.; Jiang, J.; Chen, S. CmMYB19 Over-Expression Improves Aphid Tolerance in Chrysanthemum by Promoting Lignin Synthesis. Int. J. Mol. Sci. 2017, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Y.; Tian, C.; Wang, X.; Zhou, L.; Jiang, J.; Wang, L.; Chen, F.; Chen, S. Molecular module of CmMYB15-like-Cm4CL2 regulating lignin biosynthesis of chrysanthemum (Chrysanthemum morifolium) in response to aphid (Macrosiphoniella sanborni) feeding. New Phytol. 2023, 237, 1776–1793. [Google Scholar] [CrossRef]
- Yao, X.W.; Liang, X.; Chen, Q.; Liu, Y.; Wu, C.; Wu, M.; Shui, J.; Qiao, Y.; Zhang, Y.; Geng, Y. MePAL6 regulates lignin accumulation to shape cassava resistance against two-spotted spider mite. Front. Plant Sci. 2023, 13, 1067695. [Google Scholar] [CrossRef]
- Whitehill, J.G.A.; Henderson, H.; Schuetz, M.; Skyba, O.; Yuen, M.M.; King, J.; Samuels, A.L.; Mansfield, S.D.; Bohlmann, J. Histology and cell wall biochemistry of stone cells in the physical defence of conifers against insects. Plant Cell Environ. 2016, 39, 1646–1661. [Google Scholar] [CrossRef]
- Yu, Y.B.; Yu, Y.; Cui, N.; Ma, L.; Tao, R.; Ma, Z.; Meng, X.; Fan, H. Lignin biosynthesis regulated by CsCSE1 is required for Cucumis sativus defence to Podosphaera xanthii. Plant Physiol. Biochem. 2022, 186, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mafa, M.S.; Rufetu, E.; Alexander, O.; Kemp, G.; Mohase, L. Cell-wall structural carbohydrates reinforcements are part of the defence mechanisms of wheat against Russian wheat aphid (Diuraphis noxia) infestation. Plant Physiol. Biochem. 2022, 179, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Dowd, P.F.; Sattler, S.E. Helicoverpa zea (Lepidoptera: Noctuidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae) Responses to Sorghum bicolor (Poales: Poaceae) Tissues from Lowered Lignin Lines. J. Insect Sci. 2015, 15, 162. [Google Scholar] [CrossRef]
- Grover, S.; Shinde, S.; Puri, H.; Palmer, N.; Sarath, G.; Sattler, S.E.; Louis, J. Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm. Front. Plant Sci. 2022, 13, 1019266. [Google Scholar] [CrossRef]
- Hu, Q.; Min, L.; Yang, X.Y. Laccase GhLac1 Modulates Broad-Spectrum Biotic Stress Tolerance via Manipulating Phenylpropanoid Pathway and Jasmonic Acid Synthesis. Plant Physiol. 2018, 176, 1808–1823. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.P. Hydrolyzable tannins as “quantitative defenses”: Limited impact against Lymantria dispar caterpillars on hybrid poplar. J. Insect Physiol. 2009, 55, 297–304. [Google Scholar] [CrossRef]
- Lu, J.N.; Shi, Y.Z.; Li, W.J.; Chen, S.; Wang, Y.; He, X.; Yin, X. RcPAL, a key gene in lignin biosynthesis in Ricinus communis L. BMC Plant Biol. 2019, 19, 181. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Chrysafi, D.; Avtzis, D.N.; Moreira, X. Photosynthetic and defensive responses of two Mediterranean oaks to insect leaf herbivory. Tree Physiol. 2022, 42, 2282–2293. [Google Scholar] [CrossRef] [PubMed]
- Lamara, M.; Parent, G.J.; Giguere, I.; Beaulieu, J.; Bousquet, J.; MacKay, J.J. Association genetics of acetophenone defence against spruce budworm in mature white spruce. BMC Plant Biol. 2018, 18, 231. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef]
- Rehman, E.; Ikram, M.; Feng, M.T.; Rehman, S. Sectoral-based CO2 emissions of Pakistan: A novel Grey Relation Analysis (GRA) approach. Environ. Sci. Pollut. Res. Int. 2020, 27, 29118–29129. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, Y.C.; Lu, Y.C.; Chen, C.I. Application of Grey Relational Analysis to Predict Dementia Tendency by Cognitive Function, Sleep Disturbances, and Health Conditions of Diabetic Patients. Brain Sci. 2022, 12, 1642. [Google Scholar] [CrossRef]
- Yao, Y.; Han, R.; Gong, Z.; Zheng, C.; Zhao, Y. RNA-seq Analysis Reveals Gene Expression Profiling of Female Fertile and Sterile Ovules of Pinus tabulaeformis Carr. during Free Nuclear Mitosis of the Female Gametophyte. Int. J. Mol. Sci. 2018, 19, 2246. [Google Scholar] [CrossRef]
- Mo, J.X.; Xu, J.; Jin, W.J.; Yang, L.W. Identification of Reference Genes for Quantitative Gene Expression Studies in Pinus massoniana and Its Introgression Hybrid. Forests 2019, 10, 787. [Google Scholar] [CrossRef]




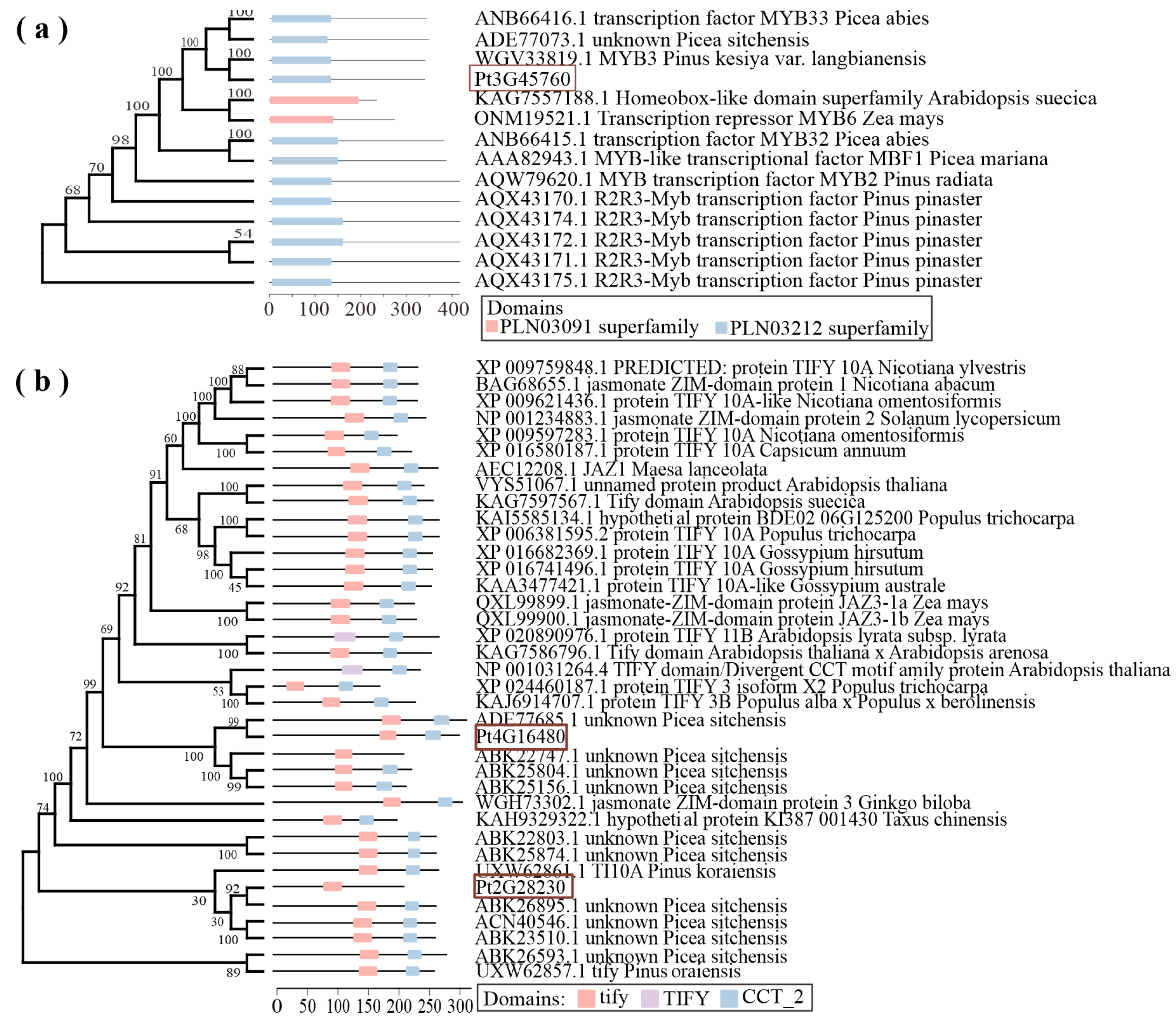
| Plant Hormone | Evaluation Items | Correlation Degree | Rank |
|---|---|---|---|
| JA | Treatment mode | 0.745 | 1 |
| Time after treatments | 0.595 | 2 | |
| SA | Treatment mode | 0.822 | 1 |
| Time after treatments | 0.5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Sun, T.; Liu, J.; Zhang, R.; Yu, Y.; Zhou, G.; Liu, J.; Gao, B. The Key Role of Plant Hormone Signaling Transduction and Flavonoid Biosynthesis Pathways in the Response of Chinese Pine (Pinus tabuliformis) to Feeding Stimulation by Pine Caterpillar (Dendrolimus tabulaeformis). Int. J. Mol. Sci. 2024, 25, 6354. https://doi.org/10.3390/ijms25126354
Zhao Y, Sun T, Liu J, Zhang R, Yu Y, Zhou G, Liu J, Gao B. The Key Role of Plant Hormone Signaling Transduction and Flavonoid Biosynthesis Pathways in the Response of Chinese Pine (Pinus tabuliformis) to Feeding Stimulation by Pine Caterpillar (Dendrolimus tabulaeformis). International Journal of Molecular Sciences. 2024; 25(12):6354. https://doi.org/10.3390/ijms25126354
Chicago/Turabian StyleZhao, Yanan, Tianhua Sun, Jie Liu, Ruibo Zhang, Yongjie Yu, Guona Zhou, Junxia Liu, and Baojia Gao. 2024. "The Key Role of Plant Hormone Signaling Transduction and Flavonoid Biosynthesis Pathways in the Response of Chinese Pine (Pinus tabuliformis) to Feeding Stimulation by Pine Caterpillar (Dendrolimus tabulaeformis)" International Journal of Molecular Sciences 25, no. 12: 6354. https://doi.org/10.3390/ijms25126354





