Development of Loop-Mediated Isothermal Amplification (LAMP) Assays for the Rapid Detection of Toxigenic Aspergillus flavus and A. carbonarius in Nuts
Abstract
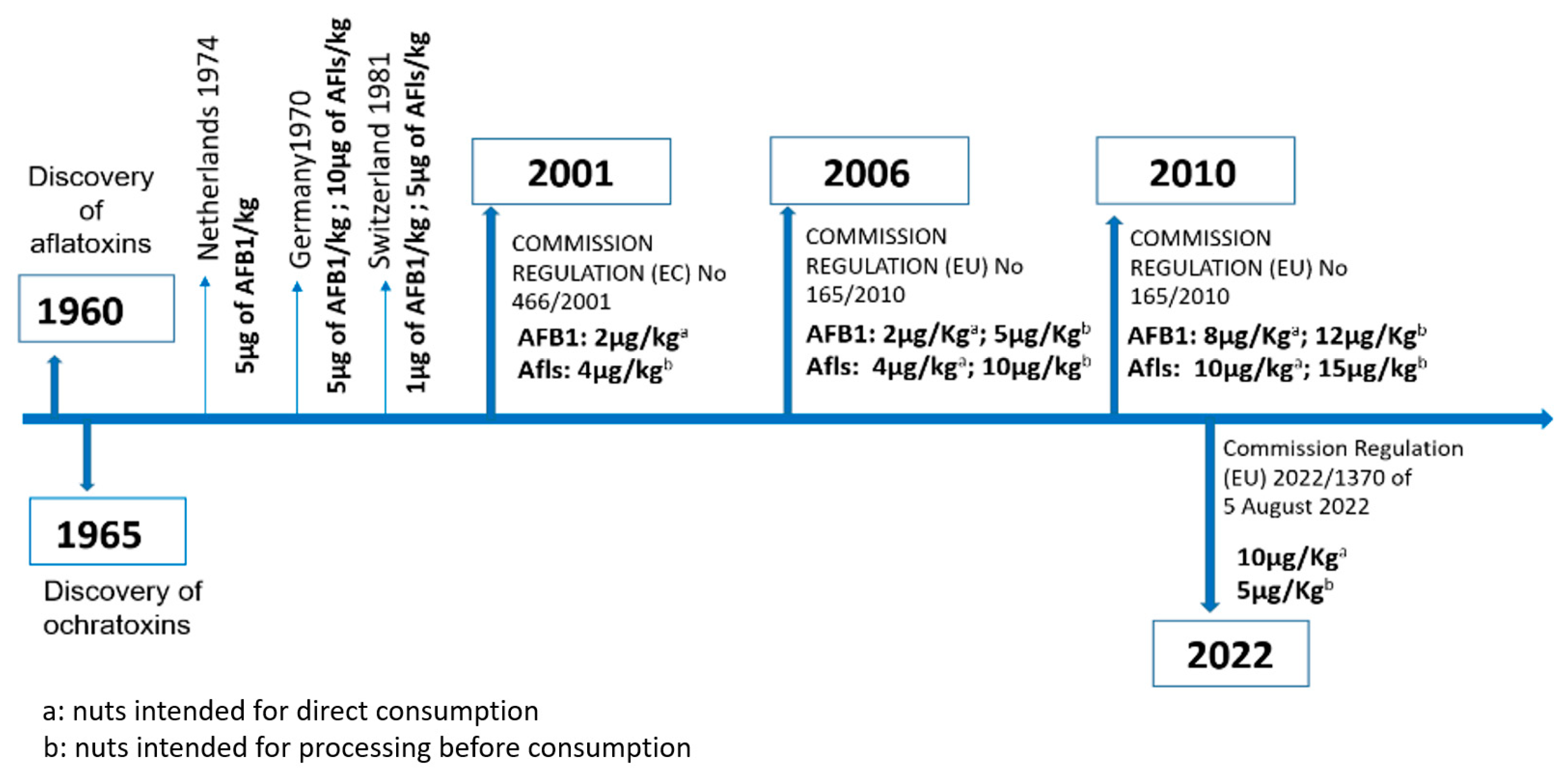
1. Introduction
2. Results
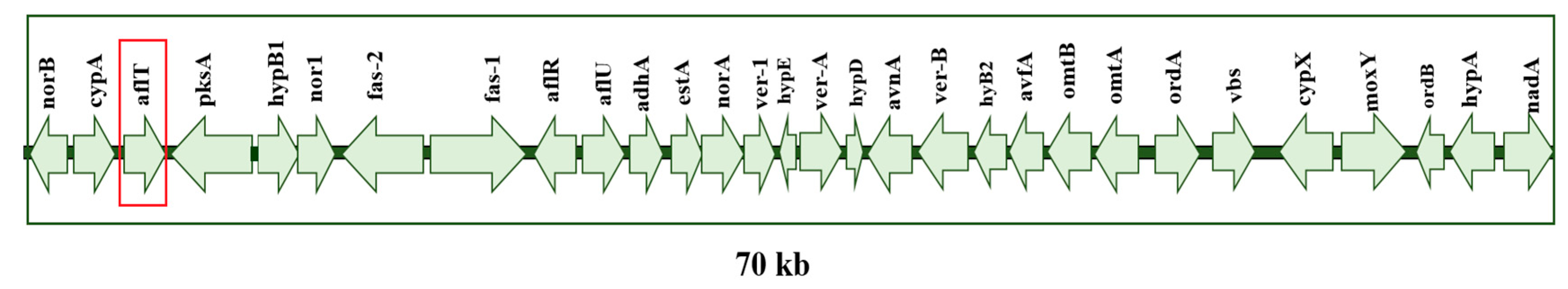
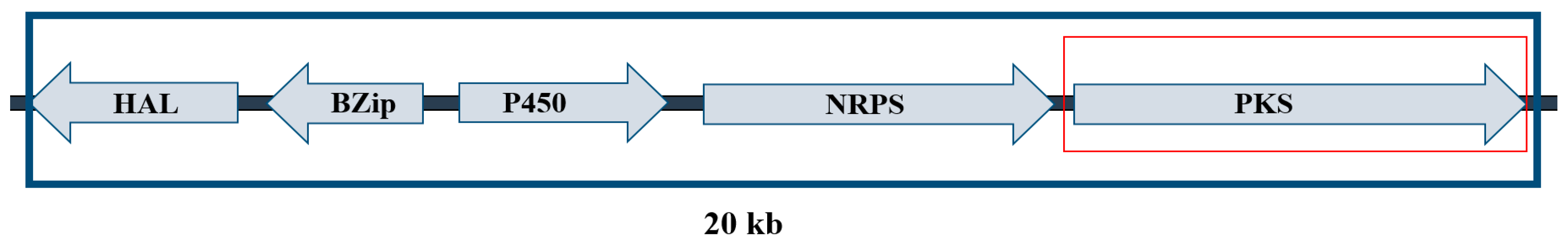
2.1. LAMP Primer Design
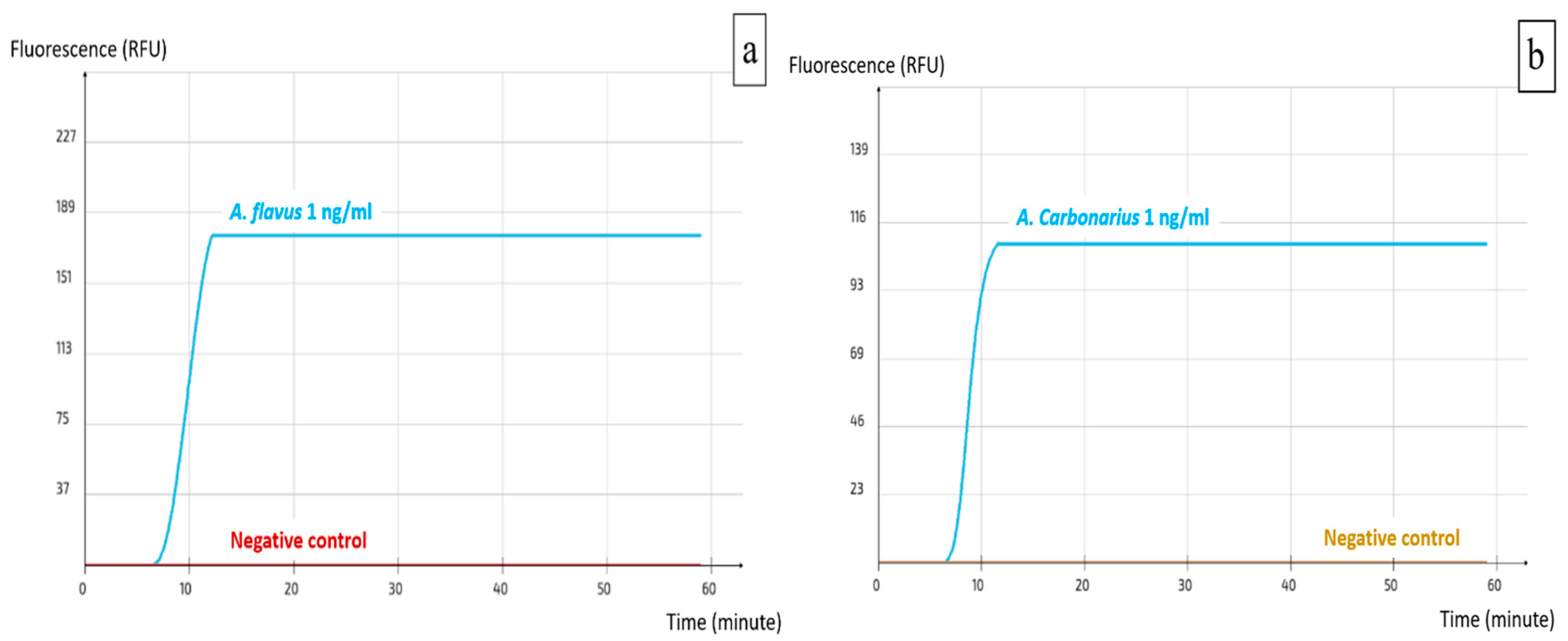
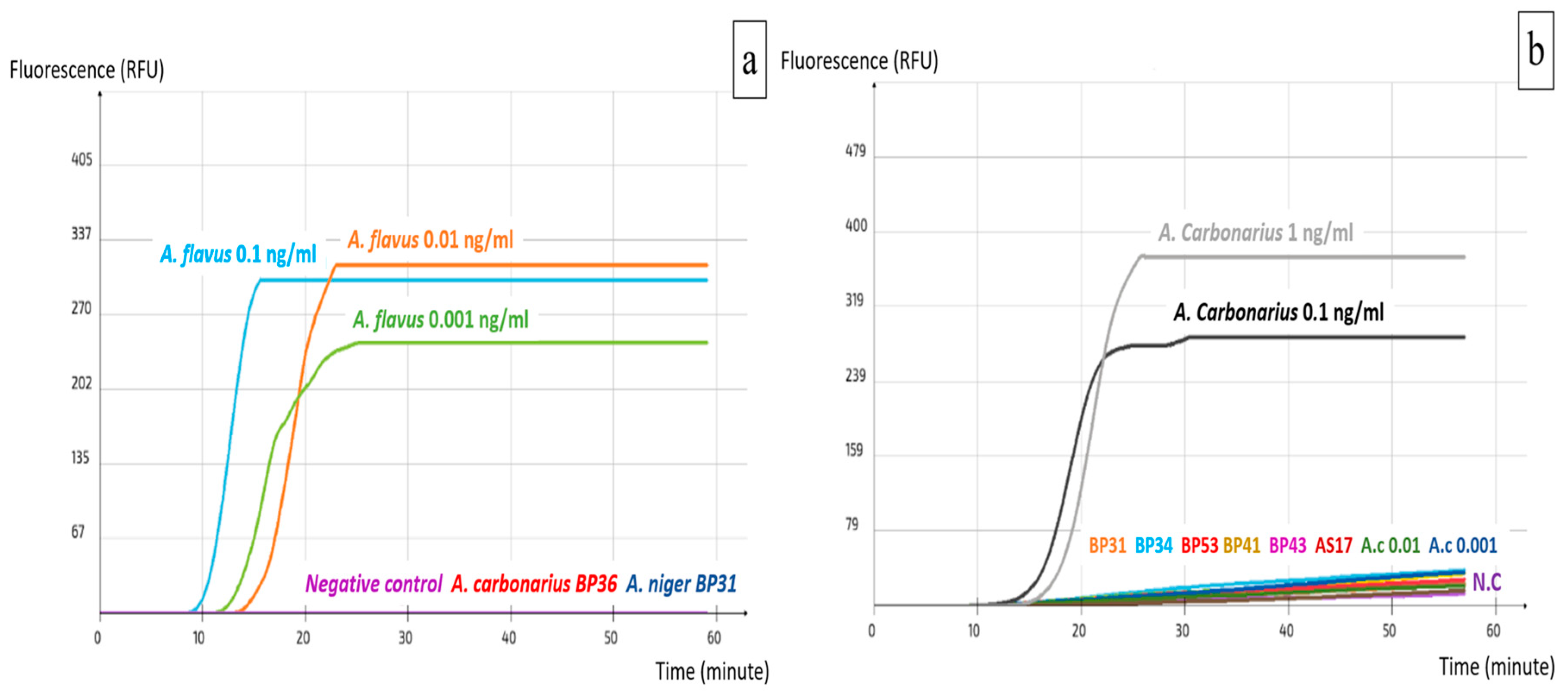
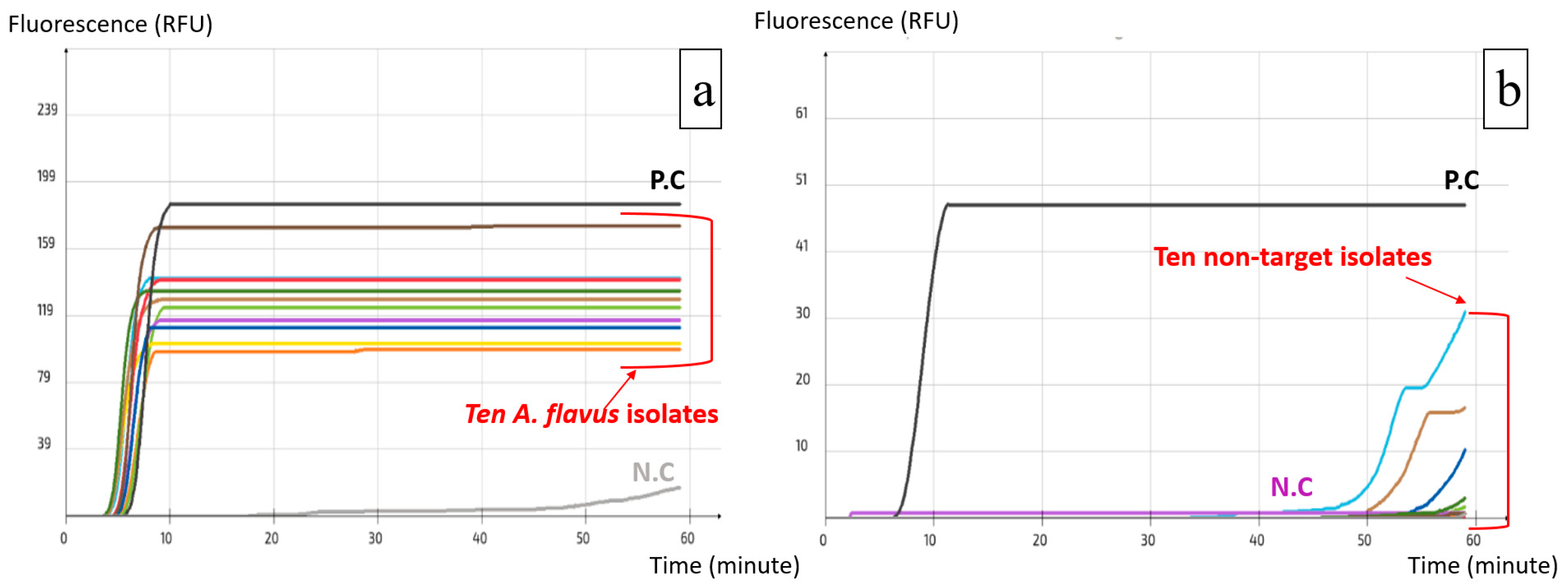
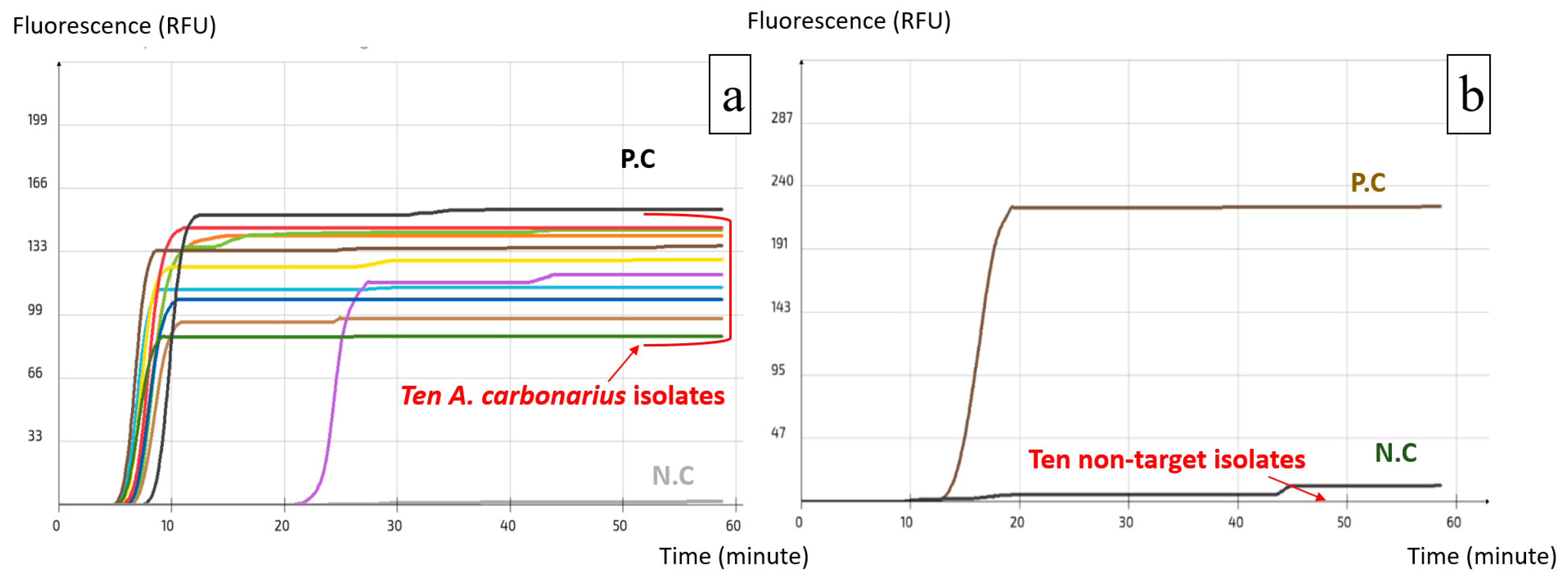
2.2. Specificity and Sensitivity
2.3. Evaluation of the LAMP Assays on Food Material
2.4. Validation
2.5. Production of LAMP Kits
3. Discussion
4. Material and Methods
4.1. Fungal Strains
4.2. DNA Extraction
4.3. Crude DNA Extraction
4.4. Primer Design
4.5. LAMP Reaction and Result Visualization
4.6. Optimization of the LAMP Assay Conditions
4.7. Specificity and Sensitivity of the Primers
4.8. Primer Dehydration
4.9. Evaluation of the Primer Sets on Food Material
4.10. Validation of LAMP Assays
4.11. Sensitivity Test
4.12. Relative Level of Detection (RLOD) Test
4.13. Selectivity
4.14. Production of Prototype LAMP Kits for the Detection of A. flavus and A. carbonarius
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Novotna, E. Toxicity of the mycotoxin ochratoxin A in the light of recent data. Toxin Rev. 2013, 32, 19–33. [Google Scholar] [CrossRef]
- Solcan, C.; Pavel, G.; Floristean, V.C.; Chiriac, I.S.; Şlencu, B.G.; Solcan, G. Effect of ochratoxin A on the intestinal mucosa and mucosa-associated lymphoid tissues in broiler chickens. Acta. Vet. Hung. 2015, 63, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Ruprich, J. Producers and Important Dietary Sources of Ochratoxin A and Citrinin. Toxins 2013, 5, 1574–1586. [Google Scholar] [CrossRef]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, Occurrence, Detection and Detoxification of Aflatoxins in Foods and Feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention (Review). Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Van Egmond, H.P. Current situation on regulations for mycotoxins. Overview of tolerances and status of standard methods of sampling and analysis. Food Addit. Contam. 1989, 6, 139–188. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple mycotoxin analysis in nut products: Occurrence and risk characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Sharifnabi, B.; Bahar, M. Detection of Aflatoxin in Aspergillus Species Isolated from Pistachio in Iran. J. Phythopathol. 2007, 156, 15–20. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Luque, M.I.; Martín, A.; Córdoba, J.J. Real-time PCR assays for detection and quantification of aflatoxin-producing molds in foods. Int. J. Food Microbiol. 2012, 31, 89–99. [Google Scholar] [CrossRef]
- Ortega, S.F.; Siciliano, I.; Prencipe, S.; Gullino, M.L.; Spadaro, D. Development of PCR, LAMP and qPCR Assays for the Detection of Aflatoxigenic Strains of Aspergillus flavus and A. parasiticus in Hazelnut. Toxins 2020, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Kanitkar, Y.H.; Stedtfeld, R.D.; Hatzinger, P.B.; Hashsham, S.A.; Cupples, A.M. Development and application of a rapid, user-friendly, and inexpensive method to detect Dehalococcoides sp. reductive dehalogenase genes from groundwater. Appl. Microbiol. Biotechnol. 2017, 101, 4827–4835. [Google Scholar] [CrossRef]
- Niessen, L.; Luo, J.; Denschlag, C.; Vogel, R.F. The application of loop-mediated isothermal amplification (LAMP) in food testing for bacterial pathogens and fungal contaminants. Food Microbiol, 2013; 36, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Mellikeche, W.; Casini, G.; Ricelli, A.; Colelli, G.; Gallo, M.; D’Onghia, A.M. Detection of post-harvest pathogens by loop-mediated isothermal amplification: A review. Phytopathol. Mediterr. 2022, 61, 531–547. [Google Scholar] [CrossRef]
- Luo, J.; Vogel, R.F.; Niessen, L. Development and application of a loop-mediated isothermal amplification assay for rapid identification of aflatoxigenic molds and their detection in food samples. Int. J. Food Microbiol. 2012, 159, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Storari, M.; Rohr, R.; Pertot, I.; Gessler, C.; Broggini, G.A.L. Identification of ochratoxin A producing Aspergillus carbonarius and A. niger clade isolated from grapes using the loop-mediated isothermal amplification (LAMP) reaction. J. Appl. Microbiol. 2013, 114, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Vogel, R.F.; Niessen, L. Rapid detection of aflatoxin producing fungi in food by real-time quantitative loop-mediated isothermal amplification. Food Microbiol. 2014, 44, 142–148. [Google Scholar] [CrossRef]
- Niessen, L.; Bechtner, J.; Fodil, S.; Taniwaki, M.H.; Vogel, R.F. LAMP-based group specific detection of aflatoxin producers within Aspergillus section Flavi in food raw materials, spices, and dried fruit using neutral red for visible-light signal detection. Int. J. Food Microbiol. 2018, 266, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lee, S.Y.; Back, C.G.; Ten, L.N.; Jung, H.Y. Loop-Mediated Isothermal Amplification for the Detection of Xanthomonas arboricola pv. Pruni in Peaches. Plant Pathol. J. 2019, 35, 635–643. [Google Scholar] [CrossRef]
- Carlucci, A.; Raimondo, M.A.; Cibelli, F.; Phillips, A.J.L.; Lops, F. Pleurostomophora richardsiae, Neofusicoccum parvum and Phaeoacremonium aleophilum Associated with a Decline of Olives in Southern Italy. Phytopathol. Mediterr. 2013, 52, 517–527. [Google Scholar] [CrossRef]
- Gallo, A.; Perrone, G.; Solfrizzo, M.; Epifani, F.; Abbas, A.; Dobson, A.D.W.; Mulè, G. Characterisation of a pks gene which is expressed during ochratoxin A production by Aspergillus carbonarius. Int. J. Food Microbiol. 2009, 129, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.P.; Oswald, I.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- ISO 16140-1:2016; Microbiology of the Food Chain-Method Validation. International Organization for Standardization: Geneva, Switzerland, 2016.

| Target | Primer Name | Sequence | T (°C) | bp |
|---|---|---|---|---|
| A. carbonariuspks | ACWM02-F3 | GCGCAATGCGGCTTCA | 55.18 | 17 |
| ACWM02-B3 | AATAGGACGCGGGTTCTG | 56.75 | 18 | |
| ACWM02-FIP | AGCGTGTGACACAGGTCGTTttttGCATCGCATCTGGAGTCG | 62.68/58.38 | 42 | |
| ACWM02-BIP | GAGAAGAGGACCCGGTTCGATttttAGTGAATTGCGCAGACTGTC | 61.3/59.13 | 45 | |
| ACWM02-LF | CATCCGAGCCCGTCATC | 57.16 | 17 | |
| ACWM02-LB | GCAGCTACCATGTGCCT | 56.83 | 17 | |
| A. flavus aflT | AFWM03-F3 | CCAACCGGAGTACATGGAC | 59.11 | 19 |
| AFWM03-B3 | AGACAAAGTTAGCCGGGTAC | 58.90 | 20 | |
| AFWM03-FIP | GCAAATTAGGGCCACCTCAATTTTCAGGTGCTGGTGGCATAT | 60.24/59.76 | 42 | |
| AFWM03-BIP | TCTTAGCATTCTCGGCGCCTTTTCGGTCGTTACCTGTTCTGC | 61.76/59.88 | 42 | |
| AFWM03-LF | TCCAGCAACGCGGCATT | 61.95 | 17 | |
| AFWM03-LB | TCGGGATCGAATGGAGAAGC | 61.26 | 20 |
| Reference Method | Alternative Method |
|---|---|
| Isolation of the pathogen from nuts seeded on PDA medium for 48 h at 25 °C Observation of morphological characteristics Pure DNA extraction Molecular identification by PCR using calmodulin gene | Pre-enrichment of 20 g of contaminated nuts Crude DNA extraction Molecular identification by LAMP assay |
| Sensitivity of the alternative method | SEalt = (PA + PD)/(PA + ND + PD) × 100% |
| Sensitivity of the reference method | SEref = (PA + ND)/(PA + ND + PD) × 100% |
| Relative accuracy | RT = (PA + NA)/N × 100% |
| Ratio of false positive results for the alternative method after confirmation | FPR = FP/(PD + NA) × 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mellikeche, W.; Ricelli, A.; Casini, G.; Gallo, M.; Baser, N.; Colelli, G.; D’Onghia, A.M. Development of Loop-Mediated Isothermal Amplification (LAMP) Assays for the Rapid Detection of Toxigenic Aspergillus flavus and A. carbonarius in Nuts. Int. J. Mol. Sci. 2024, 25, 3809. https://doi.org/10.3390/ijms25073809
Mellikeche W, Ricelli A, Casini G, Gallo M, Baser N, Colelli G, D’Onghia AM. Development of Loop-Mediated Isothermal Amplification (LAMP) Assays for the Rapid Detection of Toxigenic Aspergillus flavus and A. carbonarius in Nuts. International Journal of Molecular Sciences. 2024; 25(7):3809. https://doi.org/10.3390/ijms25073809
Chicago/Turabian StyleMellikeche, Wanissa, Alessandra Ricelli, Giulia Casini, Marilita Gallo, Nuray Baser, Giancarlo Colelli, and Anna Maria D’Onghia. 2024. "Development of Loop-Mediated Isothermal Amplification (LAMP) Assays for the Rapid Detection of Toxigenic Aspergillus flavus and A. carbonarius in Nuts" International Journal of Molecular Sciences 25, no. 7: 3809. https://doi.org/10.3390/ijms25073809
APA StyleMellikeche, W., Ricelli, A., Casini, G., Gallo, M., Baser, N., Colelli, G., & D’Onghia, A. M. (2024). Development of Loop-Mediated Isothermal Amplification (LAMP) Assays for the Rapid Detection of Toxigenic Aspergillus flavus and A. carbonarius in Nuts. International Journal of Molecular Sciences, 25(7), 3809. https://doi.org/10.3390/ijms25073809







