Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement
Abstract
1. Introduction
- Personalized medicine [10] takes into account individual disease characteristics, as well as the genotype and phenotype of the patient, and environmental factors.
- Generic clinical definition protocols such as the ICD-11 taxonomy, which includes approximately 85,000 categories, and the MalaCards integrated database, which lists about 20,000 disease names [11].
- Evaluations of pathologies based on models of general pathological processes, including various types of canonical and non-classical inflammation. These models classify the pathogenesis of diseases into broader disease categories.
- Conceptual syndromes serve as surrogate forms of general pathological processes and clinical definitions. They temporarily bridge gaps in general pathology knowledge to support clinical practice needs. For example, the syndrome of systemic inflammatory response preceded the adoption of Sepsis-3 [12] and the current clinical interpretations of “cytokine storm” and systemic inflammation. “Post-COVID syndrome” may also be categorized under such marginal definitions.
- Provide a general characterization of LC (Section 2).
- Summarize methodological approaches and limitations (Section 3).
- Discuss the main theories of typical pathological processes and the role of cellular and tissue stress as a foundation for pathogenetic models of various human diseases (Section 4).
- Describe low-grade inflammation from the perspective of general pathology and its pathogenetic role in viral infections (Section 5).
- Define the role of low-grade inflammation in various clinical manifestations of LC (Section 6).
- Summarize the study’s findings (Section 7).
2. General Characterization of Long COVID
- Viral Persistence and Detection: The isolation of SARS-CoV-2 viral RNA can persist for up to 3 months in the upper respiratory tract, 2 months in serum, and 126 days in stool samples. Individual LC symptoms have been noted to last up to 20 months, indicating the long-term impact of the virus [24,25].
- Post-Hospitalization Health Outcomes: Comparisons show that patients post-COVID-19 hospitalization report their health as slightly worse than the previous year, especially when compared to patients recovering from non-COVID pneumonia. The severity of the initial infection correlates with poorer long-term outcomes and quality of life, a pattern that supports the link between acute-phase tissue damage and long-term LC symptoms [6,26,27,28].
- Cognitive, Neurological Complaints, Chronic Fatigue, and Viral Activation: Evidence supports the development of numerous symptoms of chronic fatigue syndrome and other cognitive and neurological disorders in LC. These complex changes may partly relate to the activation of Epstein-Barr virus (EBV) and other herpesviruses following COVID-19 [34]. Some of the most persistent and commonly reported complaints of LC are cognitive in nature, often subjectively described as “brain fog.” These are objectively measured by deficits in executive function, working memory, attention, and information processing speed [35,36]. There is also a correlation between chronic fatigue in long COVID (LC) and latent microcirculatory disorders, which can be verified through imaging of the retinal microvascular network [37].
- Mitochondrial Dysfunction and Neuropsychiatric Effects: Elevated total mitochondrial protein levels in extracellular vesicles during acute SARS-CoV-2 infection are predictive of a high risk of LC and indicate neuropsychiatric manifestations once LC is established [38]. Additionally, LC is characterized by the prolonged presence of the SARS-CoV-2 nucleocapsid (N) protein in non-cellular vesicles [38].
- Blood–Brain Barrier Integrity and Endothelial Damage: Studies have shown that the Spike (S) protein of SARS-CoV-2 can damage the endothelium in an animal model, disrupt the integrity of an in vitro model of the blood–brain barrier (BBB), and penetrate the BBB, leading to perivascular inflammation in the CNS [39].
- Non-structural proteins of SARS-CoV-2 act as pathogenicity factors and are critical for virus survival. These proteins induce various cellular pro-inflammatory stress processes, including autophagy, oxidative and mitochondrial stress, and endoplasmic reticulum stress (ERS) [40,41,42]. Furthermore, SARS-CoV-2, including through the effects of its structural proteins S, E, and N, can induce pro-inflammatory cellular stress in various cell types via activation of the NLRP3 inflammasome [43,44,45].
- Gastrointestinal Impact and Microbiome Disruption: The long-term persistence of SARS-CoV-2 as potential bacteriophages in the intestinal microflora and the sustained disruption of the intestinal microbiome in LC have been demonstrated. Additionally, dysbiosis and intestinal barrier disruption contribute to elevated levels of lipopolysaccharides (LPSs) and other intestinal toxins, which in turn, increase pro-inflammatory status at the systemic level [46,47,48]. An increase in the relative abundance of opportunistic microorganisms in feces, accompanied by a decrease in anti-inflammatory taxa, is positively correlated with LC symptoms [49].
- Immune System Dysfunction: SARS-CoV-2-induced immune system dysfunction can potentially contribute to other acute infections or exacerbations of chronic infections, including viral infections. Moreover, LC-associated immune dysfunction may promote the reactivation of herpesviruses (CMV, EBV, HHV-6), mimicking many of the symptoms of LC [50,51,52,53,54,55].
- Autoimmune Responses: SARS-CoV-2-related autoimmune processes are clinically usually latent or cofactorial to other causes of immune inflammation but, in some cases, initiate the onset of canonical autoimmune diseases [58,59,60,61,62,63]. Meanwhile, latent autoimmune processes subclinical to the development of canonical autoimmune diseases may act as pathogenetic mechanisms of LC [50,64]. Specific autoimmune response mechanisms may include molecular mimicry between SARS-CoV-2 antigens and potentially autoimmune CNS autoantigens, as well as the formation of chimeric host-virus proteins. The long-term presence of antinuclear antibodies (ANAs), antineutrophil antibodies (ANCAs), anticardiolipin (aCL), and anti-beta-2-glycoprotein-1 (anti-β2GP1) antibodies, as well as antibodies to citrullinated proteins, have been reported in LC, along with the presence of ANA and antiphospholipid autoantibodies associated with vasculitides [65,66,67,68,69].
- Several meta-analyses have demonstrated the protective role of vaccinations both before SARS-CoV-2 infection and after recovery from the disease [32,70,71,72,73,74,75,76,77,78,79]. This protection extends not only to the primary and recurrent incidence of COVID-19 but also to a reduced risk of developing long COVID (LC) [70,71,72]. This vaccine effect has been shown in elderly patients [32] as well as adolescents [73]. At the same time, contradictory data have been noted [74,75], likely due to a lack of randomized controlled trials [76]. Overall, there is a need for additional research to study the primary mechanisms of the protective effects of vaccines against LC [76,77,78].
3. Cellular and Tissue Stress: Relationship to Inflammation and Other General Pathological Processes
3.1. Summary of General Pathological Processes
- Stereotypy: presence of typical features irrespective of the cause or location.
- Universality: these processes characterize the pathogenesis of many nosological entities.
- Polyetiology: the general pathological process does not depend on a single specific etiological factor.
- Self-development: the capacity to progress independently, even after the cessation of the etiological factor.
- Functionality and Dysfunctionality: Any genetically determined process is aimed at a beneficial outcome for the organism; when this is unachievable, dysfunctional systems form. These systems encompass both adaptive and pathological mechanisms. Examples include low-grade inflammation (parainflammation) and systemic hyperinflammation, whereas acute, rapidly resolving canonical inflammation typically represents a functional system.
- Phylogenetics: General pathological processes are usually not limited by evolutionary species constraints and manifest to varying degrees as general biological processes. Para-inflammatory processes, such as the encapsulation of parasites by phagocytes without the microvascular component of inflammation, are prevalent across various invertebrate phyla [80,81]. Conversely, canonical (classical) inflammation is specific to vertebrates, requiring an advanced blood microcirculation system, as well as sophisticated neuroendocrine and immune systems [82,83]. Systemic hyperinflammation associated with shock, on the other hand, seems to manifest fully only in mammals [83].
- Internal Inconsistency: pathological processes include mechanisms aimed at both maintaining and altering homeostasis, encompassing functionally divergent mechanisms that render the process manageable and balanced.
- Organizational Systematicity: As complex systems, general pathological processes are comprised of interrelated subprocesses (subsystems) and may also be components of more highly organized supersystems. These integrate various general pathological processes at the level of the whole organism, with the most comprehensive including abstract models of tumor growth regularities, canonical inflammation, and basic variants of non-classical inflammation.
3.2. A Brief Characterization of Canonical Inflammation and the Main Non-Classical Variants of Inflammation from the Perspective of Pathological Process Theory
3.3. Cellular Proinflammatory Stress as an Elementary but Integral Unit of Pathological Processes and Tissue Stress as a Common Basis of Typical Pathological Processes
4. Low-Grade Inflammation in Long COVID and Other Viral Infections
4.1. Viral Infections Associated with Low-Grade Inflammation
- Post-Acute Infectious Syndromes (PAIS): This relatively new medical term refers to symptoms of long-term consequences of acute infections caused by numerous pathogenic agents, including viruses such as SARS-CoV-2 [144]. The main manifestations of PAIS encompass general poor functional status, exercise intolerance, debilitating fatigue, signs of depression, cognitive and sensory impairments, dysautonomia, musculoskeletal complaints, flu-like symptoms, as well as disturbances in gut microbiota and various immunologic dysfunctions [144,145]. Besides SARS-CoV-2, other viruses like Ebola, Dengue, Polio, SARS (SARS-CoV-1), Chikungunya, EBV, West Nile virus, and potentially alphaviruses, Ross River virus, and VZV have also been implicated in initiating PAIS [144]. Notably, PAIS following mild to moderate COVID-19 shares many characteristics with chronic diseases triggered by other pathogens, which remain poorly understood. Various causes of PAIS have been proposed, including intestinal dysbiosis, autoimmunity, microvascular endothelial damage, and long-term persistence of pathogens and their pathogenic factors [146,147,148].
- Slow Viral Diseases: These diseases manifest after a prolonged latent period with a slow, progressive course that often lasts several months to years and generally progresses until the end of life. The most well-known slow viruses include HIV and HPV [149].
- Latent Viral Infections: This concept encompasses a phase in the life cycle of certain viruses where the spread of viral particles halts following initial infection. However, the viral genome remains intact and can reactivate without reinfection, persisting indefinitely within the host. This latent stage is typical of all herpes viruses and many oncoviruses, including HIV, HPV, HBV, and Hepatitis C virus (HCV) [150,151,152,153]. The latency allows viruses to evade not only the immune system’s antiviral effects but also the protective mechanisms against cellular and tissue stress in infected tissues. Nonetheless, with prolonged persistence of viruses or their components, the distinction between the latent period and the emergence of systemic parainflammatory signs can become blurred and subtle.
4.2. The Relationship of Long COVID to Systemic Low-Grade Inflammation
4.3. The Association of HIV and Herpesviruses with Systemic Low-Grade Inflammation
5. Local Phenomena of Low-Grade Inflammation in LC and Other Viral Infections
5.1. Possible Role of Low-Grade Inflammation in CNS Dysfunction in LC and Other Viral Infections
5.1.1. The Physiological Role of Cellular and Tissue Stress in the Central Nervous System and the Path of Its Pathological Transformation
5.1.2. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
- A significant reduction in the ability to engage in pre-illness levels of activity that persists for more than six months, accompanied by fatigue.
- Post-exertional malaise, where physical or cognitive exertions trigger a worsening of symptoms.
- Unrefreshing sleep, alongside either cognitive impairment or orthostatic intolerance.
5.1.3. Long COVID and PTSD
5.2. The Problem of Arthritis and Long-Term Arthralgia Associated with Long COVID
5.3. Osteomyopathy as an LC Phenomenon
5.4. Kidney Pathology in LC
5.5. Liver Pathology in LC
5.6. Cardiovascular Pathology in LC
5.7. Pathology of the Respiratory System in LC
6. NSAIDs and Their Role in Subclinical Inflammation and Long COVID
7. Conclusions
8. Materials and Methods
8.1. General Characterization of the Study
8.2. Principles of Inclusion and Exclusion
8.3. Operational Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gusev, E. Molecular Mechanisms of Pathogenesis, Prevention, and Therapy of COVID-19: Summarizing the Results of 2022. Int. J. Mol. Sci. 2023, 24, 16073. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Ancochea, J. On the new post COVID-19 condition. Arch. Bronconeumol. 2021, 57, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M. Chronic COVID syndrome: Need for an appropriate medical terminology for Long COVID and COVID long-haulers. J. Med. Virol. 2021, 93, 2555–2556. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C.; Boland, J.M.; Johnson, T.F.; Aubry, M.C.; Lo, Y.C.; Butt, Y.M.; Maleszewski, J.J.; Larsen, B.T.; Tazelaar, H.D.; Khoor, A.; et al. Late Complications of COVID-19. Arch. Pathol. Lab. Med. 2022, 146, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Nath, A. Long-Haul COVID. Neurology 2020, 95, 559–560. [Google Scholar] [CrossRef]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B.; RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86002. [Google Scholar] [CrossRef]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Rappaport, N.; Twik, M.; Plaschkes, I.; Nudel, R.; Iny Stein, T.; Levitt, J.; Gershoni, M.; Morrey, C.P.; Safran, M.; Lancet, D. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017, 45, D877–D887. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L. Addressing the Long-term Effects of COVID-19. JAMA 2022, 328, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Marjenberg, Z.; Leng, S.; Tascini, C.; Garg, M.; Misso, K.; El Guerche Seblain, C.; Shaikh, N. Risk of long COVID main symptoms after SARS-CoV-2 infection: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 15332. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Boufidou, F.; Medić, S.; Lampropoulou, V.; Siafakas, N.; Tsakris, A.; Anastassopoulou, C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int. J. Mol. Sci. 2023, 24, 12962. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.K.; Kim, J.H.; Han, M.Y. Long COVID in children and adolescents: Prevalence, clinical manifestations, and management strategies. Clin. Exp. Pediatr. 2023, 66, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. Millions of US Children Experience Range of Long COVID Effects. JAMA 2024, 331, 726. [Google Scholar] [CrossRef] [PubMed]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Vahlne, A.; Nikolich, J. Biological mechanisms underpinning the development of long COVID. iScience 2023, 26, 106935. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect. 2022, 28, e9–e611. [Google Scholar] [CrossRef]
- Huerne, K.; Filion, K.B.; Grad, R.; Ernst, P.; Gershon, A.S.; Eisenberg, M.J. Epidemiological and clinical perspectives of long COVID syndrome. Am. J. Med. Open. 2023, 9, 100033. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Nontawong, N.; Saengsiwaritt, W.; Panthan, B.; Jiaranai, P.; Thongchompoo, N.; Santon, S.; Runcharoen, C.; Sensorn, I.; Jittikoon, J.; et al. Host genetic polymorphisms involved in long-term symptoms of COVID-19. Emerg Microbes Infect. 2023, 12, 2239952. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Julg, B.; Mohandas, S.; Bradfute, S.B. RECOVER Mechanistic Pathways Task Force. Viral persistence, reactivation, and mechanisms of long COVID. eLife 2023, 12, e86015. [Google Scholar] [CrossRef] [PubMed]
- O’ Mahony, L.; Buwalda, T.; Blair, M.; Forde, B.; Lunjani, N.; Ambikan, A.; Neogi, U.; Barrett, P.; Geary, E.; O’Connor, N.; et al. Impact of Long COVID on health and quality of life. HRB Open Res. 2022, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Elkan, M.; Dvir, A.; Zaidenstein, R.; Keller, M.; Kagansky, D.; Hochman, C.; Koren, R. Patient-Reported Outcome Measures After Hospitalization During the COVID-19 Pandemic: A Survey Among COVID-19 and Non-COVID-19 Patients. Int. J. Gen. Med. 2021, 14, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Wang, Z.; Abdel-Mohsen, M.; Chen, X.; Montaner, L.J. Tissue injury and leukocyte changes in post-acute sequelae of SARS-CoV-2: Review of 2833 post-acute patient outcomes per immune dysregulation and microbial translocation in long COVID. J. Leukoc. Biol. 2023, 113, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Haidar, M.A.; Shakkour, Z.; Reslan, M.A.; Al-Haj, N.; Chamoun, P.; Habashy, K.; Kaafarani, H.; Shahjouei, S.; Farran, S.H.; Shaito, A.; et al. SARS-CoV-2 involvement in central nervous system tissue damage. Neural. Regen. Res. 2022, 17, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Guo, G.; Gibson, D.S.; Bjourson, A.J.; Rai, T.S. Role of Senescence and Aging in SARS-CoV-2 Infection and COVID-19 Disease. Cells 2021, 10, 3367. [Google Scholar] [CrossRef]
- Nguyen, D.; Jeon, H.M.; Lee, J. Tissue factor links inflammation, thrombosis, and senescence in COVID-19. Sci. Rep. 2022, 12, 19842. [Google Scholar] [CrossRef]
- Adjaye-Gbewonyo, D.; Vahratian, A.; Perrine, C.G.; Bertolli, J. Long COVID in Adults: United States, 2022. NCHS Data Brief. 2023, 480, 1–8. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and older people. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Detrimental effects of COVID-19 in the brain and therapeutic options for long COVID: The role of Epstein-Barr virus and the gut-brain axis. Mol. Psychiatry 2023, 28, 4968–4976. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Frase, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Nouraeinejad, A. Brain fog as a Long-term Sequela of COVID-19. SN Compr. Clin. Med. 2023, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Schlick, S.; Lucio, M.; Wallukat, G.; Bartsch, A.; Skornia, A.; Hoffmanns, J.; Szewczykowski, C.; Schröder, T.; Raith, F.; Rogge, L.; et al. Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int. J. Mol. Sci. 2022, 23, 13683. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Yao, P.J.; Kapogiannis, D. Prediction of Post-Acute-Sequelae of COVID-19 by Cargo Protein Biomarkers of Blood Total Extracellular Vesicles in Acute COVID-19. Am. J. Med. 2023, 136, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Li, W.; Zhai, J.; Li, X.; Zheng, C. The role of SARS-CoV-2 ORF7a in autophagy flux disruption: Implications for viral infection and pathogenesis. Autophagy 2024, 1–3. [Google Scholar] [CrossRef]
- De la Cruz-Enríquez, J.; Rojas-Morales, E.; Ruíz-García, M.G.; Tobón-Velasco, J.C.; Jiménez-Ortega, J.C. SARS-CoV-2 induces mitochondrial dysfunction and cell death by oxidative stress/inflammation in leukocytes of COVID-19 patients. Free Radic. Res. 2021, 55, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Wang, T.; Wang, J.; Jiang, Y.; Wang, X.; Qiu, Z.; Feng, N.; Sun, W.; Li, C.; et al. ORF8 Protein Induces Endoplasmic Reticulum Stress-like Responses and Facilitates Virus Replication by Triggering Calnexin: An Unbiased Study. J. Virol. 2023, 97, e0001123. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, E.A.; Amarilla, A.A.; Modhiran, N.; Parker, S.; Li, X.X.; Wijesundara, D.K.; Aguado, J.; Zamora, A.P.; McMillan, C.L.D.; Liang, B.; et al. SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein. Mol. Psychiatry 2023, 28, 2878–2893. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Hu, D.; Zheng, F.; Chen, W.; Hu, K.; Liu, J.; Yao, C.; Li, H.; Wei, Y. Investigating the Nexus of NLRP3 Inflammasomes and COVID-19 Pathogenesis: Unraveling Molecular Triggers and Therapeutic Strategies. Viruses 2024, 16, 213. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, F.; Chang, W.; Zhang, Y.; Zhang, L.; Li, P. Inflammasomes: A rising star on the horizon of COVID-19 pathophysiology. Front. Immunol. 2023, 14, 1185233. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Brogna, B.; Bisaccia, D.R.; Lauritano, F.; Marino, G.; Montano, L.; Cristoni, S.; Prisco, M.; Piscopo, M. Could SARS-CoV-2 Have Bacteriophage Behavior or Induce the Activity of Other Bacteriophages? Vaccines 2022, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Pang, X.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut microbiota in COVID-19: New insights from inside. Gut Microbes 2023, 15, 2201157. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Modrego, J.; Gómez-Garre, D.; Manucha, W.; de las Heras, N. Gut Microbiota Dysbiosis in COVID-19: Modulation and Approaches for Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 12249. [Google Scholar] [CrossRef] [PubMed]
- Alenazy, M.F.; Aljohar, H.I.; Alruwaili, A.R.; Daghestani, M.H.; Alonazi, M.A.; Labban, R.S.; El-Ansary, A.K.; Balto, H.A. Gut Microbiota Dynamics in Relation to Long COVID -19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites 2022, 12, 912. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Ryan, F.J.; Hope, C.M.; Masavuli, M.G.; Lynn, M.A.; Mekonnen, Z.A.; Yeow, A.E.L.; Garcia-Valtanen, P.; Al-Delfi, Z.; Gummow, J.; Ferguson, C.; et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. Could long COVID be linked to herpes viruses? Early data offer a hint. Nature 2022, 601, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zubchenko, S.; Kril, I.; Nadizhko, O.; Matsyura, O.; Chopyak, V. Herpesvirus infections and post-COVID-19 manifestations: A pilot observational study. Rheumatol. Int. 2022, 42, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef] [PubMed]
- Berentschot, J.C.; Drexhage, H.A.; Aynekulu Mersha, D.G.; Wijkhuijs, A.J.M.; Geurtsvan Kessel, C.H.; Koopmans, M.P.G.; Voermans, J.J.C.; Hendriks, R.W.; Nagtzaam, N.M.A.; de Bie, M.; et al. Immunological profiling in long COVID: Overall low-grade inflammation and T-lymphocyte senescence and increased monocyte activation correlating with increasing fatigue severity. Front. Immunol. 2023, 14, 1254899. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.A. SARS-CoV-2 triggering autoimmune diseases. Cytokine 2022, 154, 155873. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tamiazzo, S.; Stobbione, P.; Agatea, L.; De Gaspari, P.; Stecca, A.; Lauritano, E.C.; Roveta, A.; Tozzoli, R.; Guaschino, R.; et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin. Transl. Sci. 2021, 14, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Li, X.; Yang, D.; Chan, S.C.W.; Zhou, J.; Wan, E.Y.F.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Chan, E.W.Y.; et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: A population-based cohort study. eClinicalMedicine 2023, 63, 102154. [Google Scholar] [CrossRef] [PubMed]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Morrone, M.C.; Patrono, C.; Santoro, M.G.; Schiaffino, S.; Remuzzi, G.; Bussolati, G. Long Covid: Where we stand and challenges ahead. Cell Death Differ. 2022, 29, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Gutman, E.G.; Fernandes, R.A.; Raposo-Vedovi, J.V.; Salvio, A.L.; Duarte, L.A.; Tardim, C.F.; Costa, V.G.C.; Pereira, V.C.S.R.; Bahia, P.R.V.; da Silva, M.M.; et al. Molecular Mimicry between SARS-CoV-2 Proteins and Human Self-Antigens Related with Autoimmune Central Nervous System (CNS) Disorders. Microorganisms 2023, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Hughes, L. Towards Understanding Long COVID: SARS-CoV-2 Strikes the Host Cell Nucleus. Pathogens 2023, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur. Respir. J. 2023, 61, 2200970. [Google Scholar] [CrossRef]
- Vlachoyiannopoulos, P.G.; Magira, E.; Alexopoulos, H.; Jahaj, E.; Theophilopoulou, K.; Kotanidou, A.; Tzioufas, A.G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum. Dis. 2020, 79, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Lerma, L.A.; Chaudhary, A.; Bryan, A.; Morishima, C.; Wener, M.H.; Fink, S.L. Prevalence of autoantibody responses in acute coronavirus disease 2019 (COVID-19). J. Transl. Autoimmun. 2020, 3, 100073. [Google Scholar] [CrossRef]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef]
- Ceban, F.; Kulzhabayeva, D.; Rodrigues, N.B.; Di Vincenzo, J.D.; Gill, H.; Subramaniapillai, M.; Lui, L.M.W.; Cao, B.; Mansur, R.B.; Ho, R.C.; et al. COVID-19 vaccination for the prevention and treatment of long COVID: A systematic review and meta-analysis. Brain Behav. Immun. 2023, 111, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Forrest, C.B.; Hirabayashi, K.; Wu, Q.; Allen, A.J.; Rao, S.; Chen, Y.; Bunnell, H.T.; Chrischilles, E.A.; Cowell, L.G.; et al. Vaccine Effectiveness Against Long COVID in Children. Pediatrics 2024, 153, e2023064446. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Nemati, M.; Shahisavandi, M.; Nemati, H.; Karimi, A.; Jafari, A.; Nasiri, S.; Mohammadi, S.S.; Rahimian, Z.; Bayat, H.; et al. How does COVID-19 vaccination affect long-COVID symptoms? PLoS ONE 2024, 19, e0296680. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of COVID-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2022, 19, 12422. [Google Scholar] [CrossRef] [PubMed]
- Sebők, S.; Gyires, K. Long COVID and possible preventive options. Inflammopharmacology 2023, 31, 2807–2817. [Google Scholar] [CrossRef]
- Cho, K.; Park, S.; Kim, E.Y.; Koyanagi, A.; Jacob, L.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Radua, J.; Elena, D.; et al. Immunogenicity of COVID-19 vaccines in patients with diverse health conditions: A comprehensive systematic review. J. Med. Virol. 2022, 94, 4144–4155. [Google Scholar] [CrossRef]
- Goto, A.; Ji, S.; Chtarbanova, S.; Kuraishi, T. Editorial: Inflammatory and inflammatory-like responses in insects. Front. Immunol. 2023, 18, 1184429. [Google Scholar] [CrossRef]
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zhuravleva, Y.A.; Zotova, N.V. Correlation of the evolution of immunity and inflammation in vertebrates. Biol. Bull. Rev. 2019, 9, 358–372. [Google Scholar] [CrossRef]
- Zotova, N.V.; Chereshnev, V.A.; Gusev, E.Y. Systemic Inflammation: Methodological Approaches to Identification of the Common Pathological Process. PLoS ONE 2016, 11, e0155138. [Google Scholar] [CrossRef] [PubMed]
- Brazhnikov, A.; Zotova, N.; Solomatina, L.; Sarapultsev, A.; Spirin, A.; Gusev, E. Shock-Associated Systemic Inflammation in Amniotic Fluid Embolism, Complicated by Clinical Death. Pathophysiology 2023, 30, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. Int. J. Mol. Sci. 2023, 24, 1144. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zotova, N.V.; Chereshnev, V.A. Sepsis-3: New edition—old problems. Analysis from the perspective of general pathology. Russ. J. Infect. Immun. 2021, 11, 649–662. [Google Scholar] [CrossRef]
- Ippoliti, F.; Canitano, N.; Businaro, R. Stress and obesity as risk factors in cardiovascular diseases: A neuroimmune perspective. J. Neuroimmune Pharmacol. 2013, 8, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, K.; Patel, S.; Gupta, A.; Varshney, S.; Gupta, S.; Rajan, S.; Srivastava, A.; Vishwakarma, A.L.; Gaikwad, A.N. Chronic hyperinsulinemia promotes meta-inflammation and extracellular matrix deposition in adipose tissue: Implications of nitric oxide. Mol. Cell Endocrinol. 2018, 477, 15–28. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.J.; Wang, E.K.; Benayoun, B.A. Functional genomics of inflamm-aging and immunosenescence. Brief Funct. Genomics. 2022, 21, 43–55. [Google Scholar] [CrossRef]
- Vetrani, C.; Di Nisio, A.; Paschou, S.A.; Barrea, L.; Muscogiuri, G.; Graziadio, C.; Savastano, S.; Colao, A. On Behalf of The Obesity Programs of Nutrition Education Research and Assessment Opera Group. From Gut Microbiota through Low-Grade Inflammation to Obesity: Key Players and Potential Targets. Nutrients 2022, 14, 2103. [Google Scholar] [CrossRef]
- Dantzer, R.; Walker, A.K. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural Transm. 2014, 121, 925–932. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Interplay of G-Proteins and Serotonin in the Neuroimmunoinflammatory Model of Chronic Stress and Depression: A Narrative Review. Curr. Pharm. Des. 2024, 30, 180–214. [Google Scholar] [CrossRef]
- Sublette, M.E.; Postolache, T.T. Neuroinflammation and depression: The role of indoleamine 2,3-dioxygenase (IDO) as a molecular pathway. Psychosom. Med. 2012, 74, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Borráz-León, J.I.; Krams, I. Schizophrenia: The new etiological synthesis. Neurosci. Biobehav. Rev. 2022, 142, 104894. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Rönnbäck, C.; Hansson, E. The Importance and Control of Low-Grade Inflammation Due to Damage of Cellular Barrier Systems That May Lead to Systemic Inflammation. Front. Neurol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Zotova, N.V.; Zhuravleva, Y.A.; Chereshnev, V.A. Physiological and Pathogenic Role of Scavenger Receptors in Humans. Med. Immunol. 2020, 22, 7–48. [Google Scholar] [CrossRef]
- Wenzel, P.; Kossmann, S.; Münzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Steven, S.; Vujacic-Mirski, K.; Kalinovic, S.; Oelze, M.; Di Lisa, F.; Münzel, T. Regulation of Vascular Function and Inflammation via Cross Talk of Reactive Oxygen and Nitrogen Species from Mitochondria or NADPH Oxidase-Implications for Diabetes Progression. Int. J. Mol. Sci. 2020, 21, 3405. [Google Scholar] [CrossRef] [PubMed]
- Cassini-Vieira, P.; Araújo, F.A.; da Costa Dias, F.L.; Russo, R.C.; Andrade, S.P.; Teixeira, M.M.; Barcelos, L.S. iNOS Activity Modulates Inflammation, Angiogenesis, and Tissue Fibrosis in Polyether-Polyurethane Synthetic Implants. Mediat. Inflamm. 2015, 2015, 138461. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A. Nitric oxide in shock. Kidney Int. 2007, 72, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Vrettou, C.S.; Keskinidou, C.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Endotheliopathy in Acute COVID-19 and Long COVID. Int. J. Mol. Sci. 2023, 24, 8237. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, D.; Gratacòs, M.; Franch-Nadal, J. Diabetic microvascular disease in non-classical beds: The hidden impact beyond the retina, the kidney, and the peripheral nerves. Cardiovasc. Diabetol. 2023, 22, 314. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Engedal, T.S.; Moreton, F.; Hansen, M.B.; Wardlaw, J.M.; Dalkara, T.; Markus, H.S.; Muir, K.W. Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J. Cereb. Blood Flow. Metab. 2016, 36, 302–325. [Google Scholar] [CrossRef]
- Tomanek, R.J. The coronary capillary bed and its role in blood flow and oxygen delivery: A review. Anat. Rec. 2022, 305, 3199–3211. [Google Scholar] [CrossRef]
- Gusev, E.; Solomatina, L.; Zhuravleva, Y.; Sarapultsev, A. The Pathogenesis of End-Stage Renal Disease from the Standpoint of the Theory of General Pathological Processes of Inflammation. Int. J. Mol. Sci. 2021, 22, 11453. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Gorlé, N.; Vandendriessche, C.; Van Imschoot, G.; Van Wonterghem, E.; Van Cauwenberghe, C.; Parthoens, E.; Van Hamme, E.; Lippens, S.; Van Hoecke, L.; et al. Low-grade peripheral inflammation affects brain pathology in the AppNL-G-Fmouse model of Alzheimer’s disease. Acta. Neuropathol. Commun. 2021, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A. Inflammation and neurodegeneration: Chronicity matters. Aging 2018, 11, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal. Transduct. Target Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Lüscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef] [PubMed]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, Low-Grade Inflammation and Systemic Health: A Scoping Review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Leng, S.X. Chronic Low-grade Inflammatory Phenotype (CLIP) and Senescent Immune Dysregulation. Clin. Ther. 2019, 41, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr Pharm Des. 2019, 25, 251–297. [Google Scholar] [CrossRef]
- Yao, X.; Meng, G. Inflammasomes in the Gut Mucosal Homeostasis. Adv. Exp. Med. Biol. 2017, 1024, 133–151. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Madianos, P.N. Low-grade inflammation in chronic infectious diseases: Paradigm of periodontal infections. Ann. N. Y. Acad. Sci. 2006, 1088, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Trinh, Q.D. Recent Research in Cell Stress and Microbial Infection. Microorganisms 2022, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Valadão, A.L.; Aguiar, R.S.; de Arruda, L.B. Interplay between Inflammation and Cellular Stress Triggered by Flaviviridae Viruses. Front. Microbiol. 2016, 7, 1233. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, Y.; Fu, X.; Bai, G.; Li, X.; Mao, J.; Yan, Y.; Hu, L. Multiple functions of stress granules in viral infection at a glance. Front. Microbiol. 2023, 14, 1138864. [Google Scholar] [CrossRef]
- Wan, Q.; Song, D.; Li, H.; He, M.L. Stress proteins: The biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct. Target. Ther. 2020, 5, 125. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Prasad, V.; Greber, U.F. The endoplasmic reticulum unfolded protein response—Homeostasis, cell death and evolution in virus infections. FEMS Microbiol. Rev. 2021, 45, fuab016. [Google Scholar] [CrossRef] [PubMed]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy, and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Gioia, U.; Tavella, S.; Martínez-Orellana, P.; Cicio, G.; Colliva, A.; Ceccon, M.; Cabrini, M.; Henriques, A.C.; Fumagalli, V.; Paldino, A.; et al. SARS-CoV-2 infection induces DNA damage, through CHK1 degradation and impaired 53BP1 recruitment, and cellular senescence. Nat. Cell Biol. 2023, 25, 550–564. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, P.; Fiorillo Moreno, O.; Zarate Peñata, E.; Calderon-Villalba, A.; Pacheco Lugo, L.; Acosta Hoyos, A.; Villarreal Camacho, J.L.; Navarro Quiroz, R.; Pacheco Londoño, L.; Aroca Martinez, G.; et al. From Cell to Symptoms: The Role of SARS-CoV-2 Cytopathic Effects in the Pathogenesis of COVID-19 and Long COVID. Int. J. Mol. Sci. 2023, 24, 8290. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Reyes, A.; Villegas-Valverde, C.A. Implications of Low-grade Inflammation in SARS-CoV-2 Immunopathology. MEDICC Rev. 2021, 23, 42. [Google Scholar] [CrossRef]
- Chiappetta, S.; Sharma, A.M.; Bottino, V.; Stier, C. COVID-19 and the role of chronic inflammation in patients with obesity. Int. J. Obes. 2020, 44, 1790–1792. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Bettini, S.; Boschetti, M.; Barrea, L.; Savastano, S.; Colao, A. Obesity Programs of nutrition, education, research and evaluation (OPERA) group. Low-grade inflammation, COVID-19, and obesity: Clinical aspects and molecular insights in childhood and adulthood. Int. J. Obes. 2022, 46, 1254–1261. [Google Scholar] [CrossRef]
- Lv, B.; Huang, S.; Huang, H.; Niu, N.; Liu, J. Endothelial Glycocalyx Injury in SARS-CoV-2 Infection: Molecular Mechanisms and Potential Targeted Therapy. Mediat. Inflamm. 2023, 2023, 6685251. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Remuzzi, G. SARS-CoV-2 and the spike protein in endotheliopathy. Trends Microbiol. 2024, 32, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.A.; Kathuria, A.; Al Mahmeed, W.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; Cosentino, F.; et al. Cardiometabolic Panel of International experts on Syndemic COVID-19 (CAPISCO). Post-COVID syndrome, inflammation, and diabetes. J. Diabetes Complicat. 2022, 36, 108336. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Carrera Boada, C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 and diabetes: A bidirectional relationship. Clin. Investig. Arterioscler. 2021, 33, 151–157. [Google Scholar] [CrossRef]
- Faruqi, J.; Balasubramanyam, A. COVID-19 and diabetes mellitus: A review of the incidence, pathophysiology and management of diabetes during the pandemic. Expert. Rev. Endocrinol. Metab. 2023, 18, 167–179. [Google Scholar] [CrossRef]
- Espín, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. Cellular and molecular biomarkers of long COVID: A scoping review. eBioMedicine 2023, 91, 104552. [Google Scholar] [CrossRef]
- Wu, X.; Xiang, M.; Jing, H.; Wang, C.; Novakovic, V.A.; Shi, J. Damage to endothelial barriers and its contribution to long COVID. Angiogenesis 2024, 27, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kümpers, P.; Mohr, M.; Rovas, A. Persistent capillary rarefaction in long COVID syndrome. Angiogenesis. 2023, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Quickert, S.; Puta, C.; Reuken, P.A. The gastrointestinal microbiota in the development of ME/CFS: A critical view and potential perspectives. Front. Immunol. 2024, 15, 1352744. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yi, B.; Wu, J.; Lu, J. The microbiome in post-acute infection syndrome (PAIS). Comput. Struct. Biotechnol. J. 2023, 21, 3904–3911. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Adorjan, K.; Behrends, U.; Ertl, G.; Suttorp, N.; Lehmann, C. Post-COVID Syndrome. Dtsch. Arztebl. Int. 2023, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Putrino, D. Why we need a deeper understanding of the pathophysiology of long COVID. Lancet Infect. Dis. 2023, 23, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Levinson, W.; Chin-Hong, P.; Joyce, E.A.; Nussbaum, J.; Schwartz, B. Slow Viruses & Prions. In Review of Medical Microbiology & Immunology: A Guide to Clinical Infectious Diseases, 16th ed.; McGraw Hill: New York, NY, USA, 2020. [Google Scholar]
- Persaud, D.; Zhou, Y.; Siliciano, J.M.; Siliciano, R.F. Latency in human immunodeficiency virus type 1 infection: No easy answers. J. Virol. 2003, 77, 1659–1665. [Google Scholar] [CrossRef]
- Doorbar, J. The human Papillomavirus twilight zone—Latency, immune control and subclinical infection. Tumor Virus Res. 2023, 16, 200268. [Google Scholar] [CrossRef]
- Marusawa, H.; Uemoto, S.; Hijikata, M.; Ueda, Y.; Tanaka, K.; Shimotohno, K.; Chiba, T. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology 2000, 31, 488–495. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Paul, S. Viral hepatitis: Past, present, and future. World J. Gastroenterol. 2022, 28, 1405–1429. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends. Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhao, Y.; Zeng, N.; Liu, X.; Zheng, Y.; Sun, J.; Zhong, Y.; Wu, S.; Ni, S.; Gong, Y.; et al. Epidemiology, clinical presentation, pathophysiology, and management of long COVID: An update. Mol. Psychiatry 2023, 28, 4056–4069. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Zhou, J.; Han, S.; Kang, Z.; Chuang, H.; Fan, H.; Zhao, H.; Wang, L.; Ning, Y.; et al. Next-Generation Sequencing and Proteomics of Cerebrospinal Fluid From COVID-19 Patients with Neurological Manifestations. Front. Immunol. 2021, 12, 782731. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.T.; Anzurez, A.; Nakayama-Hosoya, K.; Miki, S.; Yamashita, K.; de Souza, M.; Matano, T.; Kawana-Tachikawa, A. Breadth and Durability of SARS-CoV-2-Specific T Cell Responses following Long-Term Recovery from COVID-19. Microbiol. Spectr. 2023, 11, e0214323. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.A.; Lim, E.Y.; Mactavous, L.; NIHR BioResource Team; Lyons, P.A.; Doffinger, R.; Bradley, J.R.; Smith, K.G.C.; Sinclair, J.; Matheson, N.J.; et al. Evidence of previous SARS-CoV-2 infection in seronegative patients with long COVID. EBioMedicine 2022, 81, 104129. [Google Scholar] [CrossRef] [PubMed]
- Fogh, K.; Larsen, T.G.; Hansen, C.B.; Hasselbalch, R.B.; Eriksen, A.R.R.; Bundgaard, H.; Frikke-Schmidt, R.; Hilsted, L.M.; Østergaard, L.; Johansen, I.S.; et al. Self-Reported Long COVID and Its Association with the Presence of SARS-CoV-2 Antibodies in a Danish Cohort up to 12 Months after Infection. Microbiol. Spectr. 2022, 10, e0253722. [Google Scholar] [CrossRef]
- Kervevan, J.; Staropoli, I.; Slama, D.; Jeger-Madiot, R.; Donnadieu, F.; Planas, D.; Pietri, M.P.; Loghmari-Bouchneb, W.; Alaba Tanah, M.; Robinot, R.; et al. Divergent adaptive immune responses define two types of long COVID. Front. Immunol. 2023, 14, 1221961. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Haunhorst, S.; Bloch, W.; Javelle, F.; Krüger, K.; Baumgart, S.; Drube, S.; Lemhöfer, C.; Reuken, P.; Stallmach, A.; Müller, M.; et al. A scoping review of regulatory T cell dynamics in convalescent COVID-19 patients—Indications for their potential involvement in the development of long-term COVID? Front. Immunol. 2022, 13, 1070994. [Google Scholar] [CrossRef] [PubMed]
- Saheb Sharif-Askari, F.; Ali Hussain Alsayed, H.; Tleyjeh, I.; Saheb Sharif-Askari, N.; Al Sayed Hussain, A.; Saddik, B.; Hamid, Q.; Halwani, R. Sotrovimab Lowers the Risk of COVID-19 Related Hospitalization or Death in a Large Population Cohort in the United Arab Emirates. Clin. Pharmacol. Ther. 2022, 112, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Whalley, J.P.; Knight, J.C.; Wicker, L.S.; Todd, J.A.; Ferreira, R.C. SARS-CoV-2 infection induces a long-lived pro-inflammatory transcriptional profile. Genome Med. 2023, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef] [PubMed]
- Sarıçam, E.; Dursun, A.D.; Türkmen Sarıyıldız, G.; Can, N.; Bozkurt, E.; Gönüllü, U.; Basay, N.; Türkmen, M.; Denli, A.; Ünlü, M. Laboratory and Imaging Evaluation of Cardiac Involvement in Patients with Post-Acute COVID-19. Int. J. Gen. Med. 2021, 14, 4977–4985. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.M.R.; Brito, A.C.S.; Manfro, W.F.P.; Ribeiro-Alves, M.; Ribeiro, R.S.A.; da Cal, M.S.; Lisboa, V.D.C.; Abreu, D.P.B.; Castilho, L.D.R.; Porto, L.C.M.S.; et al. High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-term-COVID-19 patients. PLoS ONE 2023, 18, e0283983. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef]
- Sumantri, S.; Rengganis, I. Immunological dysfunction and mast cell activation syndrome in long COVID. Asia Pac. Allergy 2023, 13, 50–53. [Google Scholar] [CrossRef]
- Maamar, M.; Artime, A.; Pariente, E.; Fierro, P.; Ruiz, Y.; Gutiérrez, S.; Tobalina, M.; Díaz-Salazar, S.; Ramos, C.; Olmos, J.M.; et al. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: A cross-sectional study. Curr. Med. Res. Opin. 2022, 38, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Talla, A.; Vasaikar, S.V.; Szeto, G.L.; Lemos, M.P.; Czartoski, J.L.; MacMillan, H.; Moodie, Z.; Cohen, K.W.; Fleming, L.B.; Thomson, Z.; et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat. Commun. 2023, 14, 3417. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; Campbell, T.B. Inflammation in Chronic HIV Infection: What Can We Do? J. Infect. Dis. 2015, 212, 339–342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crowe, S.M.; Westhorpe, C.L.; Mukhamedova, N.; Jaworowski, A.; Sviridov, D.; Bukrinsky, M. The macrophage: The intersection between HIV infection and atherosclerosis. J. Leukoc. Biol. 2010, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O. A characterization of low-grade inflammation and metabolic complications in HIV-infected patients. Dan. Med. J. 2016, 63, B5291. [Google Scholar] [PubMed]
- Bourgeois, C.; Gorwood, J.; Olivo, A.; Le Pelletier, L.; Capeau, J.; Lambotte, O.; Béréziat, V.; Lagathu, C. Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated with HIV Infection and Its Treatment. Front. Immunol. 2021, 12, 670566. [Google Scholar] [CrossRef] [PubMed]
- Guerville, F.; Vialemaringe, M.; Cognet, C.; Duffau, P.; Lazaro, E.; Cazanave, C.; Bonnet, F.; Leleux, O.; Rossignol, R.; Pinson, B.; et al. Mechanisms of systemic low-grade inflammation in HIV patients on long-term suppressive antiretroviral therapy: The inflammasome hypothesis. AIDS 2023, 37, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Eastburn, A.; Scherzer, R.; Zolopa, A.R.; Benson, C.; Tracy, R.; Do, T.; Bacchetti, P.; Shlipak, M.; Grunfeld, C.; Tien, P.C. Association of low-level viremia with inflammation and mortality in HIV-infected adults. PLoS ONE 2011, 6, e26320. [Google Scholar] [CrossRef] [PubMed]
- Hanser, S.; Mphekgwana, P.M.; Moraba, M.M.; Erasmus, L.; van Staden, M. Increased endothelial biomarkers are associated with HIV antiretroviral therapy and C-reactive protein among an African rural population in Limpopo Province, South Africa. Front. Public Health 2022, 10, 980754. [Google Scholar] [CrossRef]
- Parikh, R.V.; Ma, Y.; Scherzer, R.; Heringer, A.S.; Macgregor, J.S.; Martin, J.N.; Deeks, S.G.; Ganz, P.; Hsue, P.Y. Endothelin-1 Predicts Hemodynamically Assessed Pulmonary Arterial Hypertension in HIV Infection. PLoS ONE 2016, 11, e0146355. [Google Scholar] [CrossRef]
- Wang, R.; Underwood, M.; Llibre, J.M.; Bernal Morell, E.; Brinson, C.; Sanz Moreno, J.; Scholten, S.; Moore, R.; Saggu, P.; Oyee, J.; et al. Very-Low-Level Viremia, Inflammatory Biomarkers, and Associated Baseline Variables: Three-Year Results of the Randomized TANGO Study. Open Forum Infect. Dis. 2023, 11, ofad626. [Google Scholar] [CrossRef] [PubMed]
- Babu, H.; Ambikan, A.T.; Gabriel, E.E.; Svensson Akusjärvi, S.; Palaniappan, A.N.; Sundaraj, V.; Mupanni, N.R.; Sperk, M.; Cheedarla, N.; Sridhar, R.; et al. Systemic Inflammation and the Increased Risk of Inflamm-Aging and Age-Associated Diseases in People Living with HIV on Long Term Suppressive Antiretroviral Therapy. Front. Immunol. 2019, 10, 1965. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Glaser, R.; Malarkey, W.B.; Beversdorf, D.Q.; Peng, J.; Kiecolt-Glaser, J.K. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav. Immun. 2012, 26, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.T.; Haan, M.N.; Dowd, J.B.; Aiello, A.E. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol. 2010, 172, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Waldman, W.J.; Williams, M.V., Jr.; Lemeshow, S.; Binkley, P.; Guttridge, D.; Kiecolt-Glaser, J.K.; Knight, D.A.; Ladner, K.J.; Glaser, R. Epstein-Barr virus-encoded dUTPase enhances proinflammatory cytokine production by macrophages in contact with endothelial cells: Evidence for depression-induced atherosclerotic risk. Brain Behav. Immun. 2008, 22, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Fuente Mde, L. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 2016, 158, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Firth, C.M.; Phillips, A.C.; Moss, P.; Baylis, D.; Syddall, H.; Sayer, A.A.; Cooper, C.; Lord, J.M. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell 2012, 11, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Kasimir, F.; Toomey, D.; Liu, Z.; Kaiping, A.C.; Ariza, M.E.; Prusty, B.K. Tissue specific signature of HHV-6 infection in ME/CFS. Front. Mol. Biosci. 2022, 9, 1044964. [Google Scholar] [CrossRef] [PubMed]
- Lareau, C.A.; Yin, Y.; Maurer, K.; Sandor, K.D.; Daniel, B.; Yagnik, G.; Peña, J.; Crawford, J.C.; Spanjaart, A.M.; Gutierrez, J.C.; et al. Latent human herpesvirus 6 is reactivated in CAR T cells. Nature 2023, 623, 608–615. [Google Scholar] [CrossRef]
- Santpere, G.; Telford, M.; Andrés-Benito, P.; Navarro, A.; Ferrer, I. The Presence of Human Herpesvirus 6 in the Brain in Health and Disease. Biomolecules 2020, 10, 1520. [Google Scholar] [CrossRef]
- Sumala, S.; Ekalaksananan, T.; Pientong, C.; Buddhisa, S.; Passorn, S.; Duangjit, S.; Janyakhantikul, S.; Suktus, A.; Bumrungthai, S. The Association of HHV-6 and the TNF-α (-308G/A) Promotor with Major Depressive Disorder Patients and Healthy Controls in Thailand. Viruses 2023, 15, 1898. [Google Scholar] [CrossRef] [PubMed]
- Mozhgani, S.H.; Rajabi, F.; Qurbani, M.; Erfani, Y.; Yaslianifard, S.; Moosavi, A.; Pourrostami, K.; Baradaran Bagheri, A.; Soleimani, A.; Behzadian, F.; et al. Human Herpesvirus 6 Infection and Risk of Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis. Intervirology 2022, 65, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Boquet, A.; Boulay, G.; Hautin, E.; Mottard, N. Septic shock complicated by disseminated herpes simplex virus-1 infection: A case report. J. Med. Case. Rep. 2021, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Mularoni, A.; Cona, A.; Ribeiro Dias, L.; Bulati, M.; Busà, R.; Castelbuono, S.; Lo Porto, D.; Pietrosi, G.; Liotta, R.; Conaldi, P.G.; et al. Cytokine storm and severe hepatitis in pregnancy due to herpes simplex virus 2. Infection 2024, 52, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Watling, M.; Imbimbo, B.P. Time to test antibacterial therapy in Alzheimer’s disease. Brain 2019, 142, 2905–2929. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Yang, Y.X.; Kuo, K.; Li, H.Q.; Shen, X.N.; Chen, S.D.; Cui, M.; Tan, L.; Dong, Q.; Yu, J.T. Herpesvirus infections and Alzheimer’s disease: A Mendelian randomization study. Alzheimers Res. Ther. 2021, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, J.; Luo, L.; Xu, Z.; Zou, X. Multigenomics Reveals the Causal Effect of Herpes Simplex Virus in Alzheimer’s Disease: A Two-Sample Mendelian Randomization Study. Front. Genet. 2022, 12, 773725. [Google Scholar] [CrossRef] [PubMed]
- Mazzaro, C.; Quartuccio, L.; Adinolfi, L.E.; Roccatello, D.; Pozzato, G.; Nevola, R.; Tonizzo, M.; Gitto, S.; Andreone, P.; Gattei, V. A Review on Extrahepatic Manifestations of Chronic Hepatitis C Virus Infection and the Impact of Direct-Acting Antiviral Therapy. Viruses 2021, 13, 2249. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.; Cacoub, P. Extrahepatic manifestations in chronic hepatitis C virus carriers. Lupus 2015, 24, 469–482. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT signaling in inflammation and stress-related diseases: Implications for therapeutic interventions. Mol. Biomed. 2023, 4, 40. [Google Scholar] [CrossRef]
- Blackhurst, B.M.; Funk, K.E. Viral pathogens increase the risk of neurodegenerative disease. Nat. Rev. Neurol. 2023, 19, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, L.; Larionov, A.; Lannes, N. The Influence of Virus Infection on Microglia and Accelerated Brain Aging. Cells 2021, 10, 1836. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.C.; Guest, P.C.; Dobrowolny, H.; Steiner, J. Inflammation and viral infection as disease modifiers in schizophrenia. Front. Psychiatry 2023, 14, 1231750. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, S.; Wu, W.; Chang, H.; Shan, P.; Yang, L.; Zhang, W.; Wang, X. Exploring New Mechanism of Depression from the Effects of Virus on Nerve Cells. Cells 2023, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Mudra Rakshasa-Loots, A.; Whalley, H.C.; Vera, J.H.; Cox, S.R. Neuroinflammation in HIV-associated depression: Evidence and future perspectives. Mol. Psychiatry 2022, 27, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, M. Magnetic Resonance Imaging Studies of Neurodegenerative Disease: From Methods to Translational Research. Neurosci. Bull. 2023, 39, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Bohnert, S.; Bouchard, M.; Vair, C.; Farrell, J.S.; Teskey, G.C.; Mikler, J.; Dunn, J.F. Quantitative T2 MRI is predictive of neurodegeneration following organophosphate exposure in a rat model. Sci. Rep. 2020, 10, 13007. [Google Scholar] [CrossRef] [PubMed]
- Thomasson, M.; Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Lalive, P.H.; Lövblad, K.O.; Braillard, O.; et al. Markers of limbic system damage following SARS-CoV-2 infection. Brain Commun. 2023, 5, fcad177. [Google Scholar] [CrossRef]
- Llana, T.; Mendez, M.; Zorzo, C.; Fidalgo, C.; Juan, M.C.; Mendez-Lopez, M. Anosmia in COVID-19 could be associated with long-term deficits in the consolidation of procedural and verbal declarative memories. Front. Neurosci. 2022, 16, 1082811. [Google Scholar] [CrossRef]
- Hafiz, R.; Gandhi, T.K.; Mishra, S.; Prasad, A.; Mahajan, V.; Di, X.; Natelson, B.H.; Biswal, B.B. Higher limbic and basal ganglia volumes in surviving COVID-negative patients and the relations to fatigue. Neuroimage Rep. 2022, 2, 100095. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.; Palladini, M.; Mazza, M.G.; Colombo, F.; Vai, B.; Rovere-Querini, P.; Falini, A.; Poletti, S.; Benedetti, F. Brain correlates of subjective cognitive complaints in COVID-19 survivors: A multimodal magnetic resonance imaging study. Eur. Neuropsychopharmacol. 2023, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pintos-Pascual, I.; Moreno-Torres, V.; Ibánez-Estéllez, F.; Corrales-Rodriguez, P.; Trevino, A.; Corpas, M.; Corral, O.; Soriano, V.; de Mendoza, C. Is SARS-CoV-2 the only cause of Long COVID? AIDS Rev. 2022, 24, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.H.; Azimi, H.; Hassani Moghaddam, M.; Ebrahimi, V.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.R.; Forouzesh, M.; Boroujeni, M.E.; Nariman, Z.; et al. COVID-19 causes neuronal degeneration and reduces neurogenesis in human hippocampus. Apoptosis 2022, 27, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Toogood, P.L.; Clauw, D.J.; Phadke, S.; Hoffman, D. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Where will the drugs come from? Pharmacol. Res. 2021, 165, 105465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Julin, P.; Li, T.Q. Limbic Perfusion Is Reduced in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Tomography 2021, 7, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Saury, J.M. The role of the hippocampus in the pathogenesis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Med. Hypotheses 2016, 86, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Sato, W.; Son, C.G. Brain-regional characteristics and neuroinflammation in ME/CFS patients from neuroimaging: A systematic review and meta-analysis. Autoimmun. Rev. 2023, 23, 103484. [Google Scholar] [CrossRef] [PubMed]
- Hatziagelaki, E.; Adamaki, M.; Tsilioni, I.; Dimitriadis, G.; Theoharides, T.C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Metabolic Disease or Disturbed Homeostasis due to Focal Inflammation in the Hypothalamus? J. Pharmacol. Exp. Ther. 2018, 367, 155–167. [Google Scholar] [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Dubbo Infection Outcomes Study Group. Post-infectious and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef]
- Hanson, M.R. The viral origin of myalgic encephalomyelitis/chronic fatigue syndrome. PLoS Pathog. 2023, 19, e1011523. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Campen, C.L.C.V.; Rowe, P.C.; Visser, F.C. Orthostatic Symptoms and Reductions in Cerebral Blood Flow in Long-Haul COVID-19 Patients: Similarities with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Medicina 2021, 58, 28. [Google Scholar] [CrossRef] [PubMed]
- Retornaz, F.; Rebaudet, S.; Stavris, C.; Jammes, Y. Long-term neuromuscular consequences of SARS-CoV-2 and their similarities with myalgic encephalomyelitis/chronic fatigue syndrome: Results of the retrospective CoLGEM study. J. Transl. Med. 2022, 20, 429. [Google Scholar] [CrossRef] [PubMed]
- Tokumasu, K.; Honda, H.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Yamamoto, K.; Nakano, Y.; Hasegawa, T.; Yamamoto, Y.; Otsuka, Y.; et al. Clinical Characteristics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Diagnosed in Patients with Long COVID. Medicina 2022, 58, 850. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Hartle, M.; Sullivan, K.; Bell, J.; Abbaszadeh, S.; Unutmaz, D.; Bateman, L. Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work 2023, 74, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Son, C.G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2020, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Aenlle, K.K.; Cohen, J.; Mathew, A.; Isler, D.; Pangeni, R.P.; Nathanson, L.; Theoharides, T.C.; Klimas, N.G. COVID-19 and Long COVID: Disruption of the Neurovascular Unit, Blood-Brain Barrier, and Tight Junctions. Neuroscientist. 2023, 11, 10738584231194927. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, T.; Cai, J.; Huang, C.; Zhan, S.; Liu, J. Bioinformatics and systems biology approach to identify the pathogenetic link of Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Immunol. 2022, 13, 952987. [Google Scholar] [CrossRef]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the Brain, Nerves, and Cognitive Function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; Fernandes, G.G.; Gutman, E.G.; De Lima, E.V.; Antonio, L.S.; Hammerle, M.B.; Mota-Araujo, H.P.; Colodeti, L.C.; Araújo, S.M.B.; Froz, G.M.; et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 2023, 42, 112189. [Google Scholar] [CrossRef] [PubMed]
- Golzari-Sorkheh, M.; Weaver, D.F.; Reed, M.A. COVID-19 as a Risk Factor for Alzheimer’s Disease. J. Alzheimers Dis. 2023, 91, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Y.; Wang, Y.; Ke, Q.; Cui, L. The COVID-19 pandemic and Alzheimer’s disease: Mutual risks and mechanisms. Transl. Neurodegener. 2022, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Tiwari, N.; Singh, P.; Singh, A.K.; Mishra, D.; Imran, M.; Singh, S.; Hooshmandi, E.; Vamanu, E.; Singh, S.K.; et al. Exploring the Paradox of COVID-19 in Neurological Complications with Emphasis on Parkinson’s and Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 3012778. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Stigliano, E.; Micera, A. COVID-19 and Alzheimer’s Disease Share Common Neurological and Ophthalmological Manifestations: A Bidirectional Risk in the Post-Pandemic Future. Cells 2023, 12, 2601. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Zovi, A.; Mas, I.M.; Langella, R.; Trama, U.; Boccellino, M.; Vitiello, A. Long COVID could become a widespread post-pandemic disease? A debate on the organs most affected. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, B. Role of Tau protein in long COVID and potential therapeutic targets. Front. Cell. Infect. Microbiol. 2023, 13, 1280600. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Olff, M. Sex and gender differences in post-traumatic stress disorder: An update. Eur. J. Psychotraumatol. 2017, 8, 1351204. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Tsokos, G.C.; Chrousos, G.P.; Sfikakis, P.P. Protracted stress-induced hypocortisolemia may account for the clinical and immune manifestations of Long COVID. Clin. Immunol. 2022, 245, 109133. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Anunciacion-Llunell, A.; Esteve-Valverde, E.; Morales-Pérez, S.; Rivero-Santana, S.; Trapé, J.; González-García, L.; Ruiz, D.; Marques-Soares, J.; Miro-Mur, F. Pituitary-Adrenal Axis and Peripheral Immune Cell Profile in Long COVID. Biomedicines 2024, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Capatina, C.; Poiana, C.; Fleseriu, M. Pituitary and SARS-CoV-2: An unremitting conundrum. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101752. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Leow, M.K.; Kwek, D.S.; Ng, A.W.; Ong, K.C.; Kaw, G.J.; Lee, L.S. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin. Endocrinol. 2005, 63, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Afzali, A.; Hatef, B.; Sahraei, H.; Meftahi, G.H.; Khaleghi, A.; Jahromi, G.P. Changes in psychological and cognitive variables as well as cortisol levels in recovered COVID-19 patients: A longitudinal study. Curr. Psychol. 2023, 43, 12159–12168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, Y.; Joung, J.Y.; Son, C.G. Clinical and Laboratory Characteristics of Fatigue-dominant Long COVID subjects: A Cross-Sectional Study. Am. J. Med. 2024; in press. [Google Scholar] [CrossRef]
- Schein, J.; Houle, C.; Urganus, A.; Cloutier, M.; Patterson-Lomba, O.; Wang, Y.; King, S.; Levinson, W.; Guérin, A.; Lefebvre, P.; et al. Prevalence of post-traumatic stress disorder in the United States: A systematic literature review. Curr. Med. Res. Opin. 2021, 37, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Marks, J.L. Viral arthritis. Clin. Med. 2016, 16, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Bergman, M.J. Viral Arthritis. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Suhrbier, A.; Mahalingam, S. The immunobiology of viral arthritides. Pharmacol. Ther. 2009, 124, 301–308. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res. Ther. 2006, 8, 20. [Google Scholar] [CrossRef]
- Hoong, C.W.S.; Amin, M.N.M.E.; Tan, T.C.; Lee, J.E. Viral arthralgia a new manifestation of COVID-19 infection? A cohort study of COVID-19-associated musculoskeletal symptoms. Int. J. Infect. Dis. 2021, 104, 363–369. [Google Scholar] [CrossRef]
- Ciaffi, J.; Vanni, E.; Mancarella, L.; Brusi, V.; Lisi, L.; Pignatti, F.; Naldi, S.; Assirelli, E.; Neri, S.; Reta, M.; et al. Post-Acute COVID-19 Joint Pain and New Onset of Rheumatic Musculoskeletal Diseases: A Systematic Review. Diagnostics 2023, 13, 1850. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Á Steig, B.; Gaini, S.; Strøm, M.; Weihe, P. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin. Infect. Dis. 2021, 73, e4058–e4063. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Grigolo, B. COVID-19 and rheumatic diseases: A mini-review. Front. Med. 2022, 9, 997876. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, H.R.; Nune, A. Long COVID from rheumatology perspective—A narrative review. Clin. Rheumatol. 2022, 41, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Au, M.; Yuan, S.; Wen, C. COVID-19 in Joint Aging and Osteoarthritis: Current Status and Perspectives. Int. J. Mol. Sci. 2022, 23, 720. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Visconti, V.V.; Bonanni, R.; Gatti, A.; Marcozzi, M.; Calabrò, D.; Cariati, I. Osteosarcopenia and Long COVID: A dangerous combination. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221130485. [Google Scholar] [CrossRef]
- Freund, O.; Eviatar, T.; Bornstein, G. Concurrent myopathy and inflammatory cardiac disease in COVID-19 patients: A case series and literature review. Rheumatol. Int. 2022, 42, 905–912. [Google Scholar] [CrossRef]
- Aschman, T.; Stenzel, W. COVID-19 associated myopathy. Curr. Opin. Neurol. 2022, 35, 622–628. [Google Scholar] [CrossRef]
- Hejbøl, E.K.; Harbo, T.; Agergaard, J.; Madsen, L.B.; Pedersen, T.H.; Østergaard, L.J.; Andersen, H.; Schrøder, H.D.; Tankisi, H. Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: Evidence of skeletal muscle histopathology. Eur. J. Neurol. 2022, 29, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Legarra-Gorgoñon, G.; Oscoz-Ochandorena, S.; García-Alonso, Y.; García-Alonso, N.; Oteiza, J.; Ernaga Lorea, A.; Correa-Rodriguez, M.; Izquierdo, M. Reduced muscle strength in patients with Long COVID-19 syndrome is mediated by limb muscle mass. J. Appl. Physiol. 2023, 134, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tankisi, H.; Ochala, J. Myopathy in acute and long-term COVID-19. Clin. Neurophysiol. 2022, 134, 141–142. [Google Scholar] [CrossRef]
- Agergaard, J.; Yamin Ali Khan, B.; Engell-Sørensen, T.; Schiøttz-Christensen, B.; Østergaard, L.; Hejbøl, E.K.; Schrøder, H.D.; Andersen, H.; Blicher, J.U.; Holm Pedersen, T.; et al. MULTICOV Consortium. Myopathy as a cause of Long COVID fatigue: Evidence from quantitative and single fiber EMG and muscle histopathology. Clin. Neurophysiol. 2023, 148, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bocci, T.; Bertini, A.; Campiglio, L.; Botta, S.; Libelli, G.; Guidetti, M.; Priori, A. Not myopathic, but autonomic changes in patients with Long COVID syndrome: A case series. Neurol. Sci. 2023, 44, 1147–1153. [Google Scholar] [CrossRef]
- Sapra, L.; Saini, C.; Garg, B.; Gupta, R.; Verma, B.; Mishra, P.K.; Srivastava, R.K. Long-term implications of COVID-19 on bone health: Pathophysiology and therapeutics. Inflamm. Res. 2022, 71, 1025–1040. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.N.; Coffey, L.L.; Haudenschild, D.R. Acute bone loss following SARS-CoV-2 infection in mice. J. Orthop. Res. 2023, 41, 1945–1952. [Google Scholar] [CrossRef]
- Buccino, F.; Zagra, L.; Longo, E.; D’Amico, L.; Banfi, G.; Berto, F.; Tromba, G.; Vergani, L.M. Osteoporosis and COVID-19: Detected similarities in bone lacunar-level alterations via combined AI and advanced synchrotron testing. Mater. Des. 2023, 231, 112087. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, W.; Zhang, X.; Pang, R.; Liang, X.; Xu, Q.; Xu, C.; Wan, X.; Cui, W.; Li, D.; et al. Causal relationships between COVID-19 and osteoporosis: A two-sample Mendelian randomization study in European population. Front. Public Health 2023, 11, 1122095. [Google Scholar] [CrossRef]
- Martin de Francisco, A.; Fernández Fresnedo, G. Long COVID-19 renal disease: A present medical need for nephrology. Nephrologia 2023, 43, 1–5. [Google Scholar] [CrossRef]
- Kudose, S.; Santoriello, D.; Bomback, A.S.; Sekulic, M.; Batal, I.; Stokes, M.B.; Ghavami, I.A.; Kim, J.S.; Marasa, M.; Xu, K.; et al. Longitudinal Outcomes of COVID-19-Associated Collapsing Glomerulopathy and Other Podocytopathies. J. Am. Soc. Nephrol. 2021, 32, 2958–2969. [Google Scholar] [CrossRef]
- Jansen, J.; Reimer, K.C.; Nagai, J.S.; Varghese, F.S.; Overheul, G.J.; de Beer, M.; Roverts, R.; Daviran, D.; Fermin, L.A.S.; Willemsen, B.; et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 2022, 29, 217–231.e8. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Garrett, R.E.; Palacio, C.H.; Bar-Or, D. Long COVID: Is there a kidney link? Front. Med. 2023, 10, 1138644. [Google Scholar] [CrossRef]
- Grootemaat, A.E.; Wiersma, N.; van der Niet, S.; Schimmel, I.M.; Florquin, S.; Reits, E.A.; Miller, S.E.; van der Wel, N.N. Nucleocapsid protein accumulates in renal tubular epithelium of a post-COVID-19 patient. Microbiol. Spectr. 2023, 11, e0302923. [Google Scholar] [CrossRef]
- de Lima, I.C.; de Menezes, D.C.; Uesugi, J.H.E.; Bichara, C.N.C.; da Costa Vasconcelos, P.F.; Quaresma, J.A.S.; Falcão, L.F.M. Liver Function in Patients with Long-Term Coronavirus Disease 2019 of up to 20 Months: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 5281. [Google Scholar] [CrossRef]
- Fassan, M.; Mescoli, C.; Sbaraglia, M.; Guzzardo, V.; Russo, F.P.; Fabris, R.; Trevenzoli, M.; Pelizzaro, F.; Cattelan, A.M.; Basso, C.; et al. Liver histopathology in COVID-19 patients: A mono-Institutional series of liver biopsies and autopsy specimens. Pathol. Res. Pract. 2021, 221, 153451. [Google Scholar] [CrossRef]
- Backer, S.; Khanna, D. The Lasting Effects of COVID-19 on the Progression of Metabolic Dysfunction-Associated Steatic Liver Disease (MASLD). Cureus 2023, 15, e45231. [Google Scholar] [CrossRef]
- Roth, N.C.; Kim, A.; Vitkovski, T.; Xia, J.; Ramirez, G.; Bernstein, D.; Crawford, J.M. Post-COVID-19 Cholangiopathy: A Novel Entity. Am. J. Gastroenterol. 2021, 116, 1077–1082. [Google Scholar] [CrossRef]
- Spengler, U.; Fischer, H.P.; Caselmann, W.H. Liver Disease Associated with Viral Infections. Zakim Boyer’s Hepatol. 2012, 629–643. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Goes-Neto, A.; et al. Long COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Tanne, J.H. COVID-19: Even mild infections can cause long term heart problems, large study finds. BMJ 2022, 376, o378. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gil, I.J.; Feltes, G.; Viana-Llamas, M.C.; Raposeiras-Roubin, S.; Romero, R.; Alfonso-Rodriguez, E.; Uribarri, A.; Santoro, F.; Becerra-Muñoz, V.; HOPE-2 Investigators; et al. Post-COVID-19 Symptoms and Heart Disease: Incidence, Prognostic Factors, Outcomes and Vaccination: Results from a Multi-Center International Prospective Registry (HOPE 2). J. Clin. Med. 2023, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. The COVID Heart-One Year After SARS-CoV-2 Infection, Patients Have an Array of Increased Cardiovascular Risks. JAMA 2022, 327, 1113–1114. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, F.; Miyashita, S.; Yoo, T.K.; Lee, P.T.; Foster, G.P. An Insight into Pathophysiology, Epidemiology, and Management of Cardiovascular Complications of SARS-CoV-2 Infection, Post-acute COVID Syndrome, and COVID Vaccine. Crit. Pathw Cardiol. 2022, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Bayry, J. High risk of autoimmune diseases after COVID-19. Nat. Rev. Rheumatol. 2023, 19, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Chen, Y.-T.; Wang, S.I.; Hung, Y.M.; Chen, H.Y.; Wei, C.J. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. eClinicalMedicine 2023, 56, 101783. [Google Scholar] [CrossRef] [PubMed]
- Tesch, F.; Ehm, F.; Vivirito, A.; Wende, D.; Batram, M.; Loser, F.; Menzer, S.; Jacob, J.; Roessler, M.; Seifert, M.; et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: A matched cohort study. Clin. Rheumatol. 2023, 42, 2905–2914. [Google Scholar] [CrossRef]
- Daines, L.; Zheng, B.; Pfeffer, P.; Hurst, J.R.; Sheikh, A. A clinical review of Long COVID with a focus on the respiratory system. Curr. Opin. Pulm. Med. 2022, 28, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Zhao, Y.M.; Yan, W.; Li, C.; Lu, Q.D.; Liu, L.; Ni, S.Y.; Mei, H.; Yuan, K.; Shi, L.; et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action. Mol. Psychiatry 2023, 28, 423–433. [Google Scholar] [CrossRef]
- Cevenini, E.; Caruso, C.; Candore, G.; Capri, M.; Nuzzo, D.; Duro, G.; Rizzo, C.; Colonna-Romano, G.; Lio, D.; Di Carlo, D.; et al. Age-related inflammation: The contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr. Pharm. Des. 2010, 16, 609–618. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease-potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.; Mander, A.; Ugoni, A.; Vajda, F.; Christophidis, N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology 1999, 53, 197–201. [Google Scholar] [CrossRef] [PubMed]
- ADAPT Research Group; Lyketsos, C.G.; Breitner, J.C.; Green, R.C.; Martin, B.K.; Meinert, C.; Piantadosi, S.; Sabbagh, M. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007, 68, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Green, R.C.; Schneider, L.S.; Amato, D.A.; Beelen, A.P.; Wilcock, G.; Swabb, E.A.; Zavitz, K.H.; Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA 2009, 302, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, P.; Bonomini, C.; Dal Forno, G.; Paulon, L.; Sinforiani, E.; Marra, C.; Zanetti, O.; Rossini, P.M. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin. Exp. Res. 2009, 21, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease Anti-inflammatory Prevention Trial Research Group. Results of a follow-up study to the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT). Alzheimers Dement. 2013, 9, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Shrank, W.H.; Patrick, A.R.; Brookhart, M.A. Healthy user and related biases in observational studies of preventive interventions: A primer for physicians. J. Gen. Intern. Med. 2011, 26, 546–550. [Google Scholar] [CrossRef]
- Martyn, C. Anti-inflammatory drugs and Alzheimer’s disease. BMJ 2003, 327, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Peluso, M.J.; Rodgers, K.; Aberg, J.A.; Patterson, T.F.; Tamburro, R.; Baizer, L.; Goldman, J.D.; Rouphael, N.; Deitchman, A.; et al. Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 2023, 14, 1129459. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef]
- Bellucci, P.N.; González Bagnes, M.F.; Di Girolamo, G.; González, C.D. Potential Effects of Nonsteroidal Anti-Inflammatory Drugs in the Prevention and Treatment of Type 2 Diabetes Mellitus. J. Pharm. Pract. 2017, 30, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Reginster, J.Y.; Hochberg, M.; Branco, J.; Bruyère, O.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; Herrero-Beaumont, G.; et al. Type 2 diabetes mellitus and osteoarthritis. Semin. Arthritis Rheum. 2019, 49, 9–19. [Google Scholar] [CrossRef]
- Ribeiro, H.; Rodrigues, I.; Napoleão, L.; Lira, L.; Marques, D.; Veríssimo, M.; Andrade, J.P.; Dourado, M. Non-steroidal anti-inflammatory drugs (NSAIDs), pain and aging: Adjusting prescription to patient features. Biomed. Pharmacother. 2022, 150, 112958. [Google Scholar] [CrossRef]
- McCarberg, B.H. NSAIDs in the older patient: Balancing benefits and harms. Pain Med. 2013, 14 (Suppl. S1), S43–S44. [Google Scholar] [CrossRef]
- LaForge, J.M.; Urso, K.; Day, J.M.; Bourgeois, C.W.; Ross, M.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Non-steroidal Anti-inflammatory Drugs: Clinical Implications, Renal Impairment Risks, and AKI. Adv. Ther. 2023, 40, 2082–2096. [Google Scholar] [CrossRef]
- Kushner, P.; McCarberg, B.H.; Grange, L.; Kolosov, A.; Haveric, A.L.; Zucal, V.; Petruschke, R.; Bissonnette, S. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in COVID-19. NPJ Prim. Care Respir. Med. 2022, 32, 35. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.T.; Coleman, B.; Chan, L.; Blau, H.; Callahan, T.J.; Cappelletti, L.; Fontana, T.; Bradwell, K.R.; Harris, N.L.; Casiraghi, E.; et al. NSAID use and clinical outcomes in COVID-19 patients: A 38-center retrospective cohort study. Virol. J. 2022, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Laughey, W.; Lodhi, I.; Pennick, G.; Smart, L.; Sanni, O.; Sandhu, S.; Charlesworth, B. Ibuprofen, other NSAIDs and COVID-19: A narrative review. Inflammopharmacology 2023, 31, 2147–2159. [Google Scholar] [CrossRef]
- Adler, U.C. Low-grade inflammation in chronic diseases: An integrative pathophysiology anticipated by homeopathy? Med. Hypotheses 2011, 76, 622–626. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhou, M.; Ma, H.L.; Qiao, Y.B.; Li, Q.S. The Role of Chronic Inflammation in Various Diseases and Anti-inflammatory Therapies Containing Natural Products. ChemMedChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Halim, A.; Halim, M.; Ming, L.C.; Goh, K.W.; Alfaresi, M.; AlShehail, B.M.; Al Fares, M.A.; Alissa, M.; Sulaiman, T.; et al. Experimental drugs in randomized controlled trials for long-COVID: What’s in the pipeline? A systematic and critical review. Expert Opin. Investig. Drugs 2023, 32, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Beloglazov, V.; Yatskov, I.; Dudchenko, L.; Dubuske, L. Effect of Rebamipide on Low-Grade Inflammation in Post COVID-19 Syndrome Patients. J. Allergy Clin. Immunol. 2024, 153, AB189. [Google Scholar] [CrossRef]
- Bohmwald, K.; Diethelm-Varela, B.; Rodríguez-Guilarte, L.; Rivera, T.; Riedel, C.A.; González, P.A.; Kalergis, A.M. Pathophysiological, immunological, and inflammatory features of long COVID. Front. Immunol. 2024, 15, 1341600. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Huang, Y.; Sun, H.; Yang, L. Research progress of post-acute sequelae after SARS-CoV-2 infection. Cell Death Dis. 2024, 15, 257. [Google Scholar] [CrossRef] [PubMed]
- Bogariu, A.M.; Dumitrascu, D.L. Digestive involvement in the Long-COVID syndrome. Med. Pharm. Rep. 2022, 95, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Frasier, K.M.; Gallagher-Poehls, C.; Cochrane, M.; Roy, D. Secondary Vasculitis Attributable to Post-COVID Syndrome. Cureus 2023, 15, e44119. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, M.; Kim, M.; Kim, H.J.; Lee, S.W.; Rhee, S.Y.; Koyanagi, A.; Smith, L.; Kim, M.S.; Lee, H.; et al. Incident allergic diseases in post-COVID-19 condition: Multinational cohort studies from South Korea, Japan and the UK. Nat. Commun. 2024, 15, 2830. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Drewitz, K.P.; Ulrich, A.; Siegels, D.; Deckert, S.; Sprenger, A.A.; Kuper, P.R.; Schmitt, J.; Munblit, D.; Apfelbacher, C. Allergic diseases as risk factors for Long-COVID symptoms: Systematic review of prospective cohort studies. Clin. Exp. Allergy 2023, 53, 1162–1176. [Google Scholar] [CrossRef]
- Fedorchenko, Y.; Zimba, O. Long COVID in autoimmune rheumatic diseases. Rheumatol. Int. 2023, 43, 1197–1207. [Google Scholar] [CrossRef]
- Lee, M.H.; Li, H.J.; Wasuwanich, P.; Kim, S.E.; Kim, J.Y.; Jeong, G.H.; Park, S.; Yang, J.W.; Kim, M.S.; Yon, D.K.; et al. COVID-19 susceptibility and clinical outcomes in inflammatory bowel disease: An updated systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2414. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.C.; Muleme, M.; Cooper, D.; McNamara, B.J.; Hussain, M.A.; Bartolo, C.; O’Brien, D.P.; Athan, E. Prevalence, risk factors and outcomes of secondary infections among hospitalised patients with COVID-19 or post-COVID-19 conditions in Victoria, 2020–2023. Int. J. Infect. Dis. 2024, 145, 107078. [Google Scholar] [CrossRef] [PubMed]
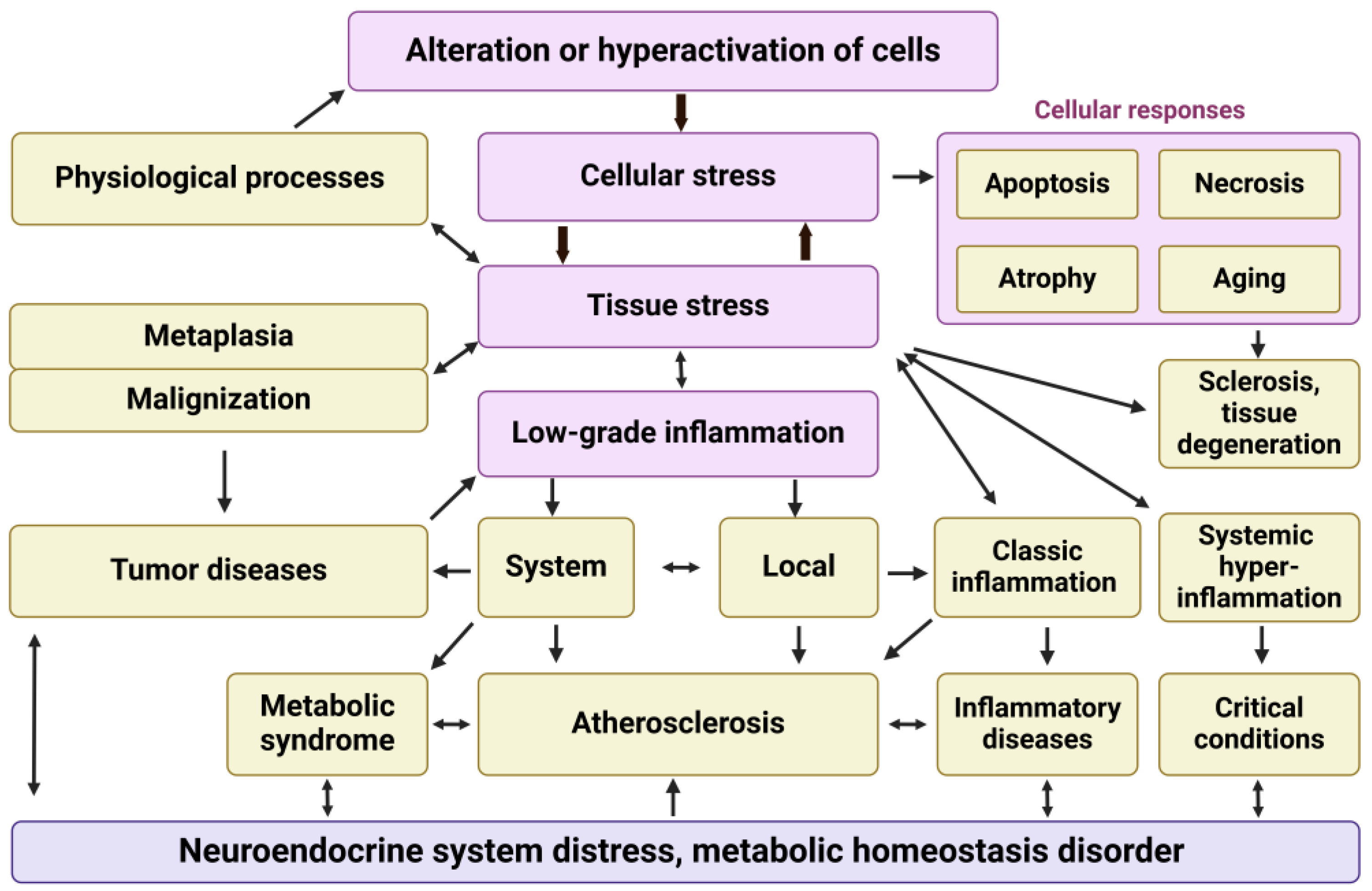

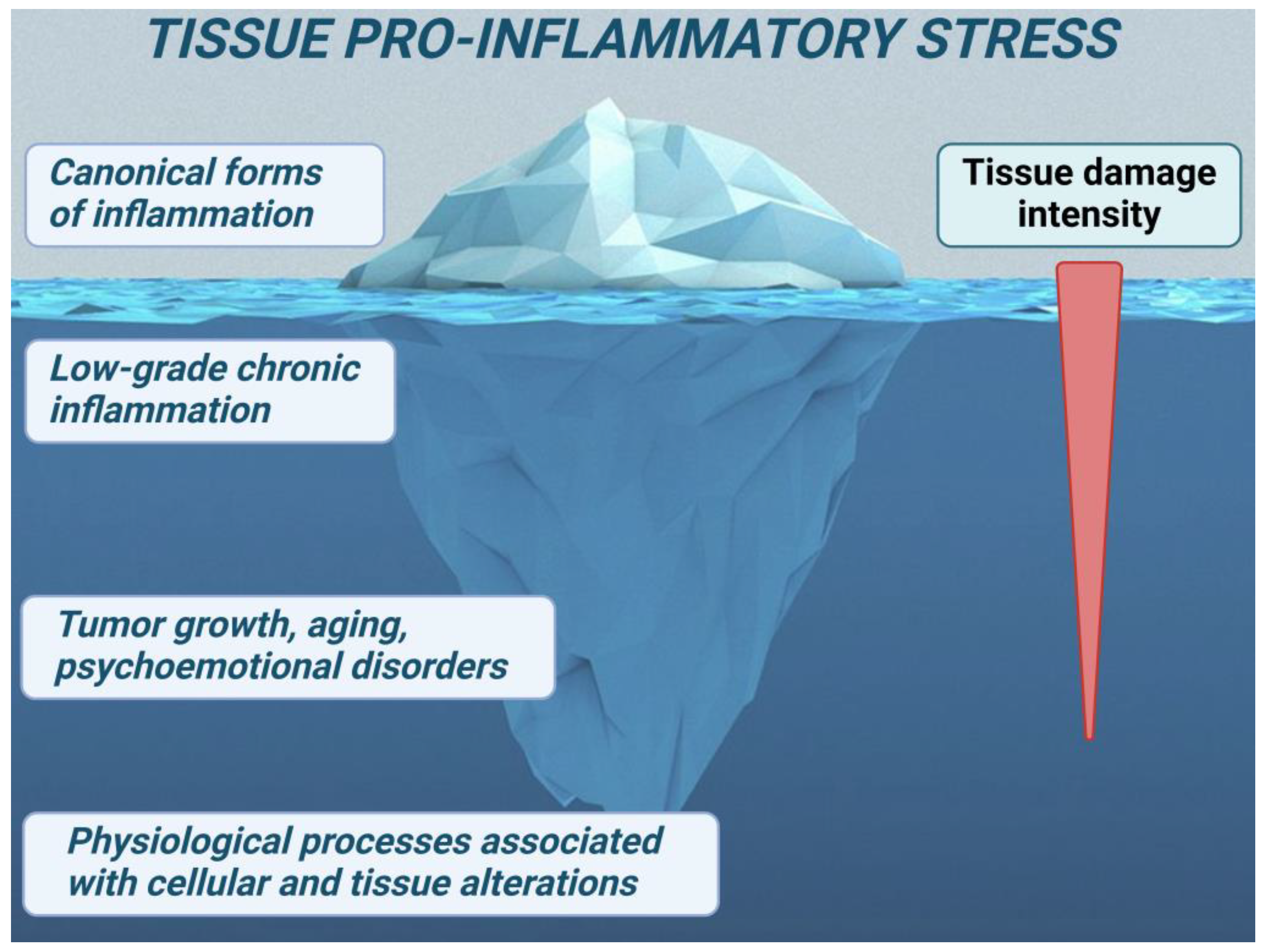
| Inflammation Type | Definition | Mechanisms and Agents | Primary Role | Key Features | Refs |
|---|---|---|---|---|---|
| Canonical Inflammation | A classic inflammatory response characterized by local signs and a barrier function. | - Response to local damage - Exudative-vascular reactions - Leukocyte migration - Triggered by local damage. The systemic inflammatory response is primarily aimed at providing resources to the site of inflammation. | Supports immune processes at the site of inflammation without systemic threat. Localizes and eliminates the damaging factor. Ends with tissue regeneration or repair (sclerosis) | - Local signs: redness, heat, swelling, pain, loss of function - Can become systemic in severe cases - Chronic progression may mimic low-grade inflammation With decompensation, it can transform into systemic hyperinflammation. | [82] |
| Systemic Hyperinflammation | A severe, systemic inflammatory response resulting in widespread tissue damage. | - Microcirculatory disorders - Significant systemic damage - Dysfunctional inflammatory response | Results from severe systemic alterations or inflammation dysfunction. | - Refractory shock - Resuscitation syndromes - Acute respiratory distress - Multi-organ failure | [84,85,86,87] |
| Low-Grade Inflammation (Parainflammation), can be systemic and local. Characteristic variants: meta-inflammation and inflamm-aging. | A chronic, mild inflammation associated with metabolic disorders and aging. | - Chronic exposure to mild damaging factors - Stromal macrophages - Degenerative changes in organs | Responds to conditions like obesity and type 2 diabetes; maintains stable allostasis. | - No barrier function - Generalizes within tissues and body over time - Leads to stable allostasis- Associated with morbid obesity, metabolic syndrome, type 2 diabetes, and other conditions | [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] |
| Endotheliosis. Key phenomenon Low-Grade Inflammation | A para-inflammatory dysfunction of endothelial cells leading to hypertension. | - Reduced nitric oxide (NO) - Increased vasopressors (e.g., angiotensin II, endothelin I), - impaired barrier function of the endothelial glycocalyx | Contributes to hypertension and atherosclerosis, latent microcirculatory disorders and thrombophilia. | - Spreads through the vascular network - Activates and thins the glycocalyx - Impairs barrier function in arteries - Markers: glycocalyx degradation, activated endotheliocytes, Ang-2, endocan, ET-1, soluble adhesion receptors | [102,103,104,105,106] |
| Endotheliitis The phenomenon of a focus of canonical inflammation and systemic hyperinflammation | Inflammation of endothelial cells characterized by microthrombosis of postcapillaries and “inflammatory microcirculation” | - Activation of inducible NO synthase - Overproduction of NO - Vasodilation and exudative-vascular response | Promotes vasodilation and localized responses, including inflammatory edema damaged fabrics; can lead to systemic disorders. | - Localized in postcapillary zones - Markers: glycocalyx degradation, - DIC markers, - various endothelial hyperstress products, including soluble adhesion receptors | [102,103,104,105,106,107] |
| Systemic Low-Grade Inflammation | A chronic systemic inflammation with extensive tissue involvement and dysfunction. | - Reduced capillary density - Microvascular occlusions - Dysfunction in vascular cells | Influences diseases like diabetic kidney disease, osteoarthritis, neurodegeneration. | - Ischemia - Sclerosis and tissue degeneration - Affects organs like kidney, liver, heart, brain, eyes, muscles, joints - Can transform from parainflammation to classical inflammation | [81,108,109,110,111,112,113,114,115,116,117] |
| Infectious Variants of Low-Grade Inflammation | Variants of low-grade inflammation driven by infectious agents. | - Persistent infection - Chronic immune activation, endotheliosis | Contributes to accelerated aging, sclerotic and degenerative changes in various organs. | - Requires markers of systemic response and endotheliosis - CRP levels (3 to 10 mg/L) insufficient alone - Persistent infection and chronic immune activation | [117,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusev, E.; Sarapultsev, A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. Int. J. Mol. Sci. 2024, 25, 6389. https://doi.org/10.3390/ijms25126389
Gusev E, Sarapultsev A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. International Journal of Molecular Sciences. 2024; 25(12):6389. https://doi.org/10.3390/ijms25126389
Chicago/Turabian StyleGusev, Evgenii, and Alexey Sarapultsev. 2024. "Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement" International Journal of Molecular Sciences 25, no. 12: 6389. https://doi.org/10.3390/ijms25126389
APA StyleGusev, E., & Sarapultsev, A. (2024). Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. International Journal of Molecular Sciences, 25(12), 6389. https://doi.org/10.3390/ijms25126389







