Mortality Prediction by Kinetic Parameters of Lactate and S-Adenosylhomocysteine in a Cohort of Critically Ill Patients
Abstract
:1. Introduction
2. Results
2.1. Baseline and Clinical Characteristics of Study Participants
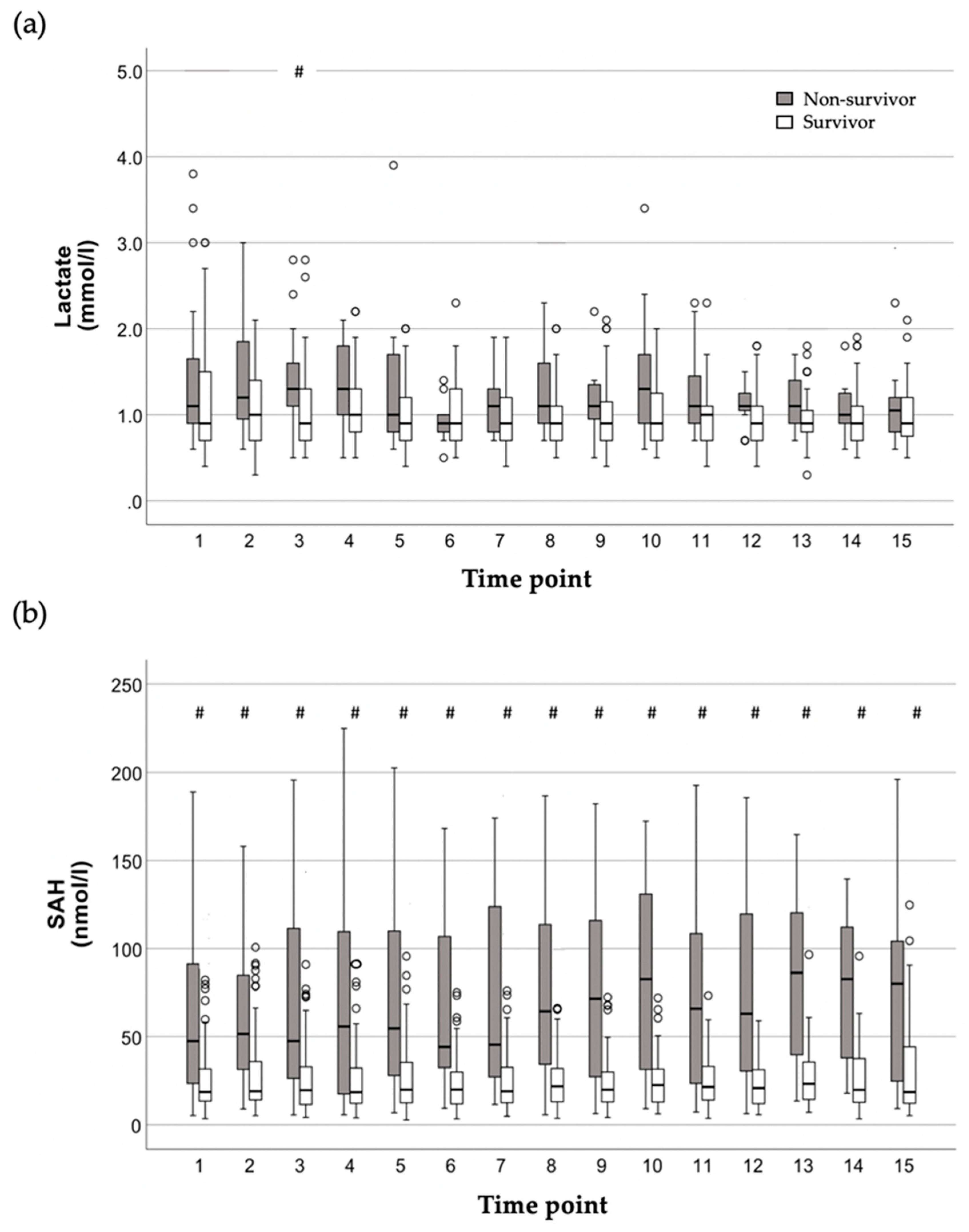
2.2. Time Course of Lactate and SAH Plasma Concentrations in Relation to Mortality
2.3. Correlations of Kinetic Parameters of Serially Determined Lactate and SAH Plasma Concentrations with Each Other as Well as Clinical Scores
2.4. Kinetic Parameters of Serially Determined Lactate and SAH Plasma Concentrations and Their Relation to Mortality
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Ethics
4.2. Study Population
4.3. Collection of Data and Blood Sampling
4.4. Determination of Plasma Concentrations of SAH
4.5. Calculation of Kinetic Parameters
- Early period: The 24 h following study inclusion, containing four measurements (time points 1 to 4) of lactate and SAH per patient, respectively.
- Total study period: Up to 15 measurements of lactate and SAH per patient over a span of up to five days (=112 h). Altogether, 1126 lactate and 1248 SAH measurements were analyzed for this period.
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gourd, N.M.; Nikitas, N. Multiple Organ Dysfunction Syndrome. J. Intensive Care Med. 2020, 35, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2018, 34, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.M. Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.; De Backer, D.; Shahla, M.; Vincent, J.L. Oxygen supply dependency can characterize septic shock. Intensive Care Med. 1998, 24, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Ronco, J.J.; Fenwick, J.C.; Tweeddale, M.G.; Wiggs, B.R.; Phang, P.T.; Cooper, D.J.; Cunningham, K.F.; Russell, J.A.; Walley, K.R. Identification of the critical oxygen delivery for anaerobic metabolism in critically ill septic and nonseptic humans. JAMA 1993, 270, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Schoonderbeek, F.J.; Sleeswijk Visser, S.J.; van der Klooster, J.M.; Lima, A.P.; Willemsen, S.P.; Bakker, J. Early lactate-guided therapy in intensive care unit patients: A multicenter, open-label, randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Bakker, J. Blood lactate monitoring in critically ill patients: A systematic health technology assessment. Crit. Care Med. 2009, 37, 2827–2839. [Google Scholar] [PubMed]
- Bakker, J. Lactate: May I have your votes please? Intensive Care Med. 2001, 27, 6–11. [Google Scholar] [CrossRef]
- Suetrong, B.; Walley, K.R. Lactic Acidosis in Sepsis: It’s Not All Anaerobic: Implications for Diagnosis and Management. Chest 2016, 149, 252–261. [Google Scholar] [CrossRef]
- Aduen, J.; Bernstein, W.K.; Khastgir, T.; Miller, J.; Kerzner, R.; Bhatiani, A.; Lustgarten, J.; Bassin, A.S.; Davison, L.; Chernow, B. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA 1994, 272, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Damiani, L.P.; Bakker, J.; Ospina-Tascón, G.A.; Castro, R.; Cavalcanti, A.B.; Hernandez, G. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels among Patients with Septic Shock. A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.B.; Skrede, S.; Perner, A.; Arnell, P.; Nekludov, M.; Bruun, T.; Karlsson, Y.; Hansen, M.B.; Polzik, P.; Hedetoft, M.; et al. Patient’s characteristics and outcomes in necrotising soft-tissue infections: Results from a Scandinavian, multicentre, prospective cohort study. Intensive Care Med. 2019, 45, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- James, J.H.; Luchette, F.A.; McCarter, F.D.; Fischer, J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999, 354, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, J.J.; Kirschning, T.; Hagmann, M.; Hahn, B.; Fairley, A.M.; Centner, F.S.; Schneider-Lindner, V.; Herrle, F.; Tzatzarakis, E.; Thiel, M.; et al. Maintaining oxygen delivery is crucial to prevent intestinal ischemia in critical ill patients. PLoS ONE 2021, 16, e0254352. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Gris, P.; Coffernils, M.; Kahn, R.J.; Vincent, J.L. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am. J. Surg. 1996, 171, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Manikis, P.; Jankowski, S.; Zhang, H.; Kahn, R.J.; Vincent, J.L. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am. J. Emerg. Med. 1995, 13, 619–622. [Google Scholar] [CrossRef]
- Khodashahi, R.; Sarjamee, S. Early lactate area scores and serial blood lactate levels as prognostic markers for patients with septic shock: A systematic review. Infect. Dis. 2020, 52, 451–463. [Google Scholar] [CrossRef]
- Chen, H.; Gong, S.R.; Yu, R.G. Association between normalized lactate load and mortality in patients with septic shock: An analysis of the MIMIC-III database. BMC Anesth. Anesthesiol. 2021, 21, 16. [Google Scholar] [CrossRef]
- Chen, H.; Gong, S.R.; Yu, R.G. Increased normalized lactate load is associated with higher mortality in both sepsis and non-sepsis patients: An analysis of the MIMIC-IV database. BMC Anesth. Anesthesiol. 2022, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Ha, E.J.; Jhang, W.K.; Park, S.J. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013, 39, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Yin, M.; Chen, X.M.; Ding, S.F.; Li, C.; Zhai, Q.; Li, Y.; Liu, H.; Wu, D.W. Combination of Acute Physiology and Chronic Health Evaluation II score, early lactate area, and N-terminal prohormone of brain natriuretic peptide levels as a predictor of mortality in geriatric patients with septic shock. J. Crit. Care 2015, 30, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yoo, S.J.; Lee, S.H.; Kim, J.S.; Jung, S.; Kim, Y.J.; Kim, W.Y.; Ryoo, S.M. Utility of the early lactate area score as a prognostic marker for septic shock patients in the emergency department. Acute Crit. Care 2019, 34, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ni, H. Normalized lactate load is associated with development of acute kidney injury in patients who underwent cardiopulmonary bypass surgery. PLoS ONE 2015, 10, e0120466. [Google Scholar] [CrossRef] [PubMed]
- Deussen, A.; Borst, M.; Kroll, K.; Schrader, J. Formation of S-adenosylhomocysteine in the heart. II: A sensitive index for regional myocardial underperfusion. Circ. Res. 1988, 63, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Deussen, A.; Smolenski, R.T. Adenosine is a sensitive oxygen sensor in the heart. Experientia 1990, 46, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Centner, F.S.; Schoettler, J.J.; Brohm, K.; Mindt, S.; Jäger, E.; Hahn, B.; Fuderer, T.; Lindner, H.A.; Schneider-Lindner, V.; Krebs, J.; et al. S-Adenosylhomocysteine Is a Useful Metabolic Factor in the Early Prediction of Septic Disease Progression and Death in Critically Ill Patients: A Prospective Cohort Study. Int. J. Mol. Sci. 2023, 24, 12600. [Google Scholar] [CrossRef] [PubMed]
- Goeman, J.J.; Solari, A. Multiple hypothesis testing in genomics. Stat. Med. 2014, 33, 1946–1978. [Google Scholar] [CrossRef]
- David, W.; Hosmer, J. Stanley Lemeshow. In Applied Logistic Regression, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Levy, B.; Gibot, S.; Franck, P.; Cravoisy, A.; Bollaert, P.E. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: A prospective study. Lancet 2005, 365, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.D.; Donnino, M.; Clardy, P.; Talmor, D.; Shapiro, N.I. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007, 33, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Desebbe, O.; Montemont, C.; Gibot, S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 2008, 30, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.; Constantin-Teodosiu, D.; Murton, A.J.; Gardiner, S.M.; Bennett, T.; Layfield, R.; Greenhaff, P.L. Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats. J. Physiol. 2008, 586, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Sterling, S.A.; Puskarich, M.A.; Jones, A.E. The effect of liver disease on lactate normalization in severe sepsis and septic shock: A cohort study. Clin. Exp. Emerg. Med. 2015, 2, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Wang, M.; Li, X.; Su, D.; Qiu, J.; Li, D.; Yang, Y.; Xia, M.; Ling, W. Plasma S-adenosylhomocysteine is associated with the risk of cardiovascular events in patients undergoing coronary angiography: A cohort study. Am. J. Clin. Nutr. 2013, 98, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Chen, X.; Chen, Y.; Pang, J.; Chen, Q.; Liu, Q.; Xue, H.; Zeng, Y.; Xiao, J.; Mi, J.; et al. Epigenetic modulation of Drp1-mediated mitochondrial fission by inhibition of S-adenosylhomocysteine hydrolase promotes vascular senescence and atherosclerosis. Redox Biol. 2023, 65, 102828. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liao, R.; Liu, C.; Liu, S.; Huang, H.; Liu, J.; Jin, T.; Guo, H.; Zheng, Z.; Xia, M.; et al. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021, 45, 102033. [Google Scholar] [CrossRef] [PubMed]
- Kruglova, M.P.; Ivanov, A.V.; Fedoseev, A.N.; Virus, E.D.; Stupin, V.A.; Parfenov, V.A.; Titova, S.A.; Lazareva, P.I.; Kubatiev, A.A.; Silina, E.V. The Diagnostic and Prognostic Roles Played by Homocysteine and Other Aminothiols in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 5653. [Google Scholar] [CrossRef]
- Kloor, D.; Delabar, U.; Mühlbauer, B.; Luippold, G.; Osswald, H. Tissue levels of S-adenosylhomocysteine in the rat kidney: Effects of ischemia and homocysteine. Biochem. Pharmacol. 2002, 63, 809–815. [Google Scholar] [CrossRef]
- Deussen, A.; Borst, M.; Schrader, J. Formation of S-adenosylhomocysteine in the heart. I: An index of free intracellular adenosine. Circ. Res. 1988, 63, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Wang, T.; Zhang, H.; Li, X.; Xu, J.; Zhang, Y.; Zhao, Z.; Yang, P.; Zhou, C.; et al. SAH and SAM/SAH ratio associate with acute kidney injury in critically ill patients: A case-control study. Clin. Chim. Acta 2024, 553, 117726. [Google Scholar] [CrossRef] [PubMed]
- Wexler, O.; Gough, M.S.; Morgan, M.A.M.; Mack, C.M.; Apostolakos, M.J.; Doolin, K.P.; Mooney, R.A.; Arning, E.; Bottiglieri, T.; Pietropaoli, A.P. Methionine Metabolites in Patients With Sepsis. J. Intensive Care Med. 2018, 33, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. Critical illness and flat batteries. Crit. Care 2017, 21 (Suppl. 3), 309. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. The New Sepsis Consensus Definitions (Sepsis-3): The Good, the Not-So-Bad, and the Actually-Quite-Pretty. Intensive Care Med. 2016, 42, 2027–2029. [Google Scholar] [CrossRef] [PubMed]
- Revelly, J.P.; Tappy, L.; Martinez, A.; Bollmann, M.; Cayeux, M.C.; Berger, M.M.; Chioléro, R.L. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit. Care Med. 2005, 33, 2235–2240. [Google Scholar] [CrossRef]
- Koliski, A.; Cat, I.; Giraldi, D.J.; Cat, M.L. Blood lactate concentration as prognostic marker in critically ill children. J. Pediatr. (Rio J.) 2005, 81, 287–292. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest J. 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Gellekink, H.; van Oppenraaij-Emmerzaal, D.; van Rooij, A.; Struys, E.A.; den Heijer, M.; Blom, H.J. Stable-isotope dilution liquid chromatography-electrospray injection tandem mass spectrometry method for fast, selective measurement of S-adenosylmethionine and S-adenosylhomocysteine in plasma. Clin. Chem. 2005, 51, 1487–1492. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]

| All (N = 99) | Survivors (S) (N = 75) | Non-Survivors (NS) (N = 24) | |||||
|---|---|---|---|---|---|---|---|
| n | n | n | S vs. NS | ||||
| Demographics | |||||||
| Age (years) | 99 | 63 (53–76) | 75 | 61 (49–74) | 24 | 73 (60–79) | 0.002 |
| Male (%) | 65 (66) | 46 (61) | 19 (79) | 0.109 | |||
| BMI [kg/m2] | 26.1 (24.2–29.4) | 25.8 (24–29.3) | 26.7 (24.6–30.6) | 0.246 | |||
| Primary diagnosis (%) | |||||||
| Major surgery | 11 (11) | 8 (11) | 3 (13) | 0.725 | |||
| Sepsis | 20 (20) | 12 (16) | 8 (33) | 0.082 | |||
| Cardiac arrest | 2 (2) | 2 (3) | |||||
| Polytrauma | 42 (42) | 36 (48) | 6 (25) | 0.047 | |||
| Major bleeding | 14 (14) | 9 (12) | 5 (21) | 0.318 | |||
| Resp. insuff./ARDS | 10 (10) | 8 (11) | 2 (8) | 1.000 | |||
| Comorbidities (%) | |||||||
| Cardiac | 35 (35) | 25 (33) | 10 (42) | 0.457 | |||
| Vascular 1 | 21 (21) | 11 (15) | 10 (42) | 0.005 | |||
| Arterial hypertension | 51 (52) | 36 (48) | 15 (63) | 0.216 | |||
| Pulmonary | 12 (12) | 8 (11) | 4 (17) | 0.478 | |||
| Renal | 20 (20) | 12 (16) | 8 (33) | 0.082 | |||
| Hepatic | 6 (6) | 4 (5) | 2 (8) | 0.630 | |||
| Diabetes mellitus | 17 (17) | 13 (17) | 4 (17) | 1.000 | |||
| Metabolic | 10 (10) | 8 (11) | 2 (8) | 1.000 | |||
| Cerebral | 11 (11) | 9 (12) | 2 (8) | 1.000 | |||
| Smoking | 7 (7) | 4 (5) | 3 (13) | 0.355 | |||
| Alcoholism | 6 (6) | 3 (4) | 3 (13) | 0.151 | |||
| Clinical course | |||||||
| Mechanical ventilation (%) | 86 (86.9) | 64 (85.3) | 22 (91.7) | 0.501 | |||
| Vasopressor therapy (%) | 68 (69) | 48 (64) | 20 (83) | 0.088 | |||
| Volume balance (mL) 2 | 97 | 22.8 (−106.6–128.0) | 74 | 31.5 (−83.6–130.1) | 23 | −12.4 (−162.4–129.2) | 0.309 |
| ICU-LOS (days) | 99 | 25 (16–47) | 75 | 27 (18–53) | 24 | 19 (13–34) | 0.028 |
| In-hospital mortality (%) | 24 (24) | ||||||
| Clinical chemistry at baseline | |||||||
| Creatinine (mg/dL) | 96 | 0.98 (0.73–1.50) | 72 | 0.88 (0.68–1.26) | 24 | 1.72 (1.05–2.72) | 0.003 |
| Urea (mg/dL) | 96 | 45.1 (33.4–63.8) | 72 | 42.2 (30.8–55.8) | 24 | 60.1 (43.7–81.7) | 0.007 |
| K+ (mmol/L) | 98 | 4.1 (3.8–4.3) | 74 | 4.0 (3.8–4.2) | 24 | 4.3 (4.1–4.5) | 0.041 |
| Bilirubin (mg/dL) | 93 | 0.61 (0.35–0.94) | 71 | 0.61 (0.34–0.97) | 22 | 0.625 (0.41–0.94) | 0.552 |
| AST (U/L) | 90 | 43 (27–90) | 69 | 42 (29–93) | 21 | 48 (26–78) | 0.854 |
| ALT (U/L) | 92 | 36.5 (20–85) | 70 | 39 (20–88) | 22 | 28 (20–82) | 0.857 |
| Lipase | 90 | 85.5 (61–207) | 69 | 87 (61–193) | 21 | 82 (61–357) | 0.854 |
| CRP (mg/dL) | 96 | 150 (90–218) | 72 | 152 (91–220) | 24 | 146 (86–216 | 0.460 |
| PCT (ug/L) | 69 | 0.63 (0.20–2.29) | 52 | 0.585 (0.185–2.125) | 17 | 0.9 (0.32–2.29) | 0.293 |
| Hematology at baseline | |||||||
| Hb (g/dL) | 98 | 8.85 (8.10–10.1) | 74 | 8.85 (7.9–10.1) | 24 | 8.85 (8.4–9.65) | 0.880 |
| WBC (109/L) | 96 | 11.5 (8.29–14.3) | 72 | 11.87 (9.01–14.31) | 24 | 10.43 (5.93–14.31) | 0.405 |
| Thrombocytes (109/L) | 96 | 162 (106–250) | 72 | 166 (114–256) | 24 | 137 (94.5–244.5) | 0.875 |
| INR | 96 | 1.07 (1–1.12) | 72 | 1.055 (1–1.11) | 24 | 1.1 (1–1.19) | 0.589 |
| Vital signs at baseline | |||||||
| Temperature (°C) | 96 | 37.1 (36.8–37.7) | 72 | 37.1 (36.8–37.7) | 24 | 37.1 (36.8–37.6) | 0.993 |
| Resp. rate (1/min) | 98 | 19 (16–22) | 74 | 19 (16–22) | 24 | 20 (16–25) | 0.199 |
| Horovitz (mmHg) | 98 | 280 (220–343) | 74 | 301 (229–347) | 24 | 218 (185–297) | 0.102 |
| Shock index | 98 | 0.70 (0.61–0.88) | 74 | 0.71 (0.60–0.87) | 24 | 0.69 (0.62–0.95) | 0.491 |
| Clinical scores at baseline | |||||||
| RASS | 97 | −3 (−5–0) | 74 | −3 (−5–−1) | 23 | −1 (−5–0) | 0.298 |
| TISS | 98 | 18 (10–22) | 74 | 17.5 (10–22) | 24 | 22 (11.5–25) | 0.092 |
| SAPS II | 98 | 35 (28–43) | 74 | 33 (25–39) | 24 | 42 (36–51) | <0.001 |
| SOFA | 99 | 8 (5–11) | 75 | 8 (5–10) | 24 | 11 (7–13) | 0.013 |
| Hypoxia biomarkers at baseline | |||||||
| Lactate (mmol/L) | 98 | 1 (0.7–1.6) | 74 | 0.9 (0.7–1.5) | 24 | 1.1 (0.9–1.65) | 0.045 |
| SAH (nmol/L) | 99 | 21.7 (13.5–47.8) | 75 | 18.61 (13.86–32.01) | 24 | 47.56 (23.43–91.32) | <0.001 |
| Early Period (24 h Following Study Inclusion, Time Points 1–4) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lactate | LacMax | LacMean | LacNAS | SAH | SAHMax | SAHMean | SAHNAS | SAPS | SOFA | ||
| Total study period (up to 112 h, time points 1–15) | Lactate | 1.00 | 0.85 <0.01 | 0.84 <0.01 | 0.84 <0.01 | 0.44 <0.01 | 0.44 <0.01 | 0.46 <0.01 | 0.45 <0.01 | 0.33 <0.01 | 0.34 <0.01 |
| LacMax | 0.75 <0.01 | 1.00 | 0.90 <0.01 | 0.92 <0.01 | 0.41 <0.01 | 0.43 <0.01 | 0.44 <0.01 | 0.44 <0.01 | 0.25 <0.01 | 0.30 <0.01 | |
| LacMean | 0.73 <0.01 | 0.92 <0.01 | 1.00 | 0.92 <0.01 | 0.43 <0.01 | 0.44 <0.01 | 0.45 <0.01 | 0.45 <0.01 | 0.31 <0.01 | 0.28 0.01 | |
| LacNAS | 0.70 <0.01 | 0.90 <0.01 | 0.99 <0.01 | 1.00 | 0.40 <0.01 | 0.42 <0.01 | 0.43 <0.01 | 0.42 <0.01 | 0.28 0.01 | 0.33 <0.01 | |
| SAH | 0.44 <0.01 | 0.28 0.01 | 0.32 <0.01 | 0.34 <0.01 | 1.00 | 0.93 <0.01 | 0.94 <0.01 | 0.93 <0.01 | 0.51 <0.01 | 0.37 <0.01 | |
| SAHMax | 0.40 <0.01 | 0.40 <0.01 | 0.41 <0.01 | 0.43 <0.01 | 0.82 <0.01 | 1.00 | 0.99 <0.01 | 0.98 <0.01 | 0.50 <0.01 | 0.37 <0.01 | |
| SAHMean | 0.40 <0.01 | 0.34 <0.01 | 0.36 <0.01 | 0.38 <0.01 | 0.91 <0.01 | 0.94 <0.01 | 1.00 | 1.00 <0.01 | 0.51 <0.01 | 0.38 <0.01 | |
| SAHNAS | 0.40 <0.01 | 0.34 <0.01 | 0.36 <0.01 | 0.38 <0.01 | 0.90 <0.01 | 0.93 <0.01 | 1.00 <0.01 | 1.00 | 0.52 <0.01 | 0.38 <0.01 | |
| SAPS | 0.33 <0.01 | 0.25 0.02 | 0.34 <0.01 | 0.34 <0.01 | 0.51 <0.01 | 0.45 <0.01 | 0.49 <0.01 | 0.49 <0.01 | 1.00 | 0.10 0.34 | |
| SOFA | 0.34 <0.01 | 0.38 <0.01 | 0.34 <0.01 | 0.31 <0.01 | 0.37 <0.01 | 0.44 <0.01 | 0.41 <0.01 | 0.41 <0.01 | 0.10 0.34 | 1.00 | |
| All (N = 99) | Survivors (S) (N = 75) | Non-Survivors (NS) (N = 24) | p-Value S vs. NS | ||
|---|---|---|---|---|---|
| Early period (24 h following study inclusion) | Lactate (mmol/L) | ||||
| Initial/n | 1.0 (0.7–1.6)/98 | 0.9 (0.7–1.5)/74 | 1.1 (0.9–1.7)/24 | 0.045 | |
| Maximum/n | 1.3 (1.0–1.8.0)/98 | 1.2 (0.9–1.7)/74 | 1.7 (1.3–2.3)/24 | 0.016 | |
| Mean/n | 1.1 (0.8–1.5)/371 | 1.0 (0.8–1.4)/278 | 1.3 (1.0–1.8)/93 | 0.007 | |
| Normalized area score/n | 1.0 (0.8–1.4)/371 | 0.7 (1.0–1.3)/278 | 1.2 (1.0–1.8)/93 | 0.005 | |
| SAH (nmol/L) | |||||
| Initial/n | 21.7 (13.5–47.8)/99 | 18.6 (13.4–32.0)/75 | 47.6 (22.8–92.6)/24 | <0.001 | |
| Maximum/n | 26.8 (17.5–52.6)/99 | 23.2 (17.4–40.8)/75 | 55.0 (32.6–122.3)/24 | <0.001 | |
| Mean/n | 20.9 (14.2–42.8)/385 | 19.4 (14.1–32.3)/290 | 46.0 (25.1–98.6)/95 | <0.001 | |
| Normalized area score/n | 21.6 (14.0–44.0)/385 | 19.9(13.8–33.1)/290 | 46.7 (26.0–100.1)/95 | <0.001 | |
| Total study period (up to 112 h) | Lactate (mmol/L) | ||||
| Maximum/n | 1.4 (1.1–2.0)/98 | 1.4 (1.0–1.8)/74 | 1.9 (1.4–3.0)/24 | 0.001 | |
| Mean/n | 1.0 (0.8–1.3)/1126 | 0.9 (0.8–1.2)/859 | 1.4 (1.0–1.7)/267 | <0.001 | |
| Normalized area score/n | 1.0 (0.8–1.3)/1126 | 1.0 (0.8–1.2)/859 | 1.4 (1.0–1.6)/267 | <0.001 | |
| SAH (nmol/L) | |||||
| Maximum/n | 33.7 (21.7–65.9)/99 | 30.4 (20.2–46.9)/75 | 88.5 (32.8–176.7)/24 | <0.001 | |
| Mean/n | 23.3 (14.8–51.4)/1248 | 20.6 (14.2–29.2)/947 | 62.4 (24.8–122.6)/301 | <0.001 | |
| Normalized area score/n | 23.9 (15.3–51.6)/1248 | 20.7(14.2–29.4)/947 | 62.9 (25.4–123.74)/301 | <0.001 |
| Coefficient (Means, SE) | Odds Ratio (95% CI) | p-Value | AUROC (SE) | p-Value | ||
|---|---|---|---|---|---|---|
| Early period (24 h following study inclusion) | Lactate | |||||
| Initial | 0.446 (0.243) | 1.563 (0.971–2.514) | 0.066 | 0.636 (0.065) | 0.046 | |
| Maximum | 0.587 (0.294) | 1.816 (1.021–3.232) | 0.042 | 0.663 (0.065) | 0.017 | |
| Mean | 1.000 (0.442) | 2.718 (1.142–6.469) | 0.024 | 0.684 (0.064) | 0.007 | |
| Normalized area score | 1.010 (0.432) | 2.746 (1.177–6.407) | 0.019 | 0.690 (0.062) | 0.005 | |
| SAH | ||||||
| Initial | 0.028 (0.008) | 1.028 (1.013–1.044) | <0.001 | 0.732 (0.067) | 0.001 | |
| Maximum | 0.023 (0.006) | 1.023 (1.011–1.035) | <0.001 | 0.744 (0.068) | <0.001 | |
| Mean | 0.027 (0.007) | 1.027 (1.013–1.042) | <0.001 | 0.738 (0.069) | <0.001 | |
| Normalized area score | 0.026 (0.007) | 1.027 (1.013–1.041) | <0.001 | 0.743 (0.069) | <0.001 | |
| Total study period (up to 112 h) | Lactate | |||||
| Maximum | 0.864 (0.305) | 2.373 (1.306–4.313) | 0.005 | 0.718 (0.061) | 0.001 | |
| Mean | 2.452 (0.751) | 11.607 (2.664–50.569) | 0.001 | 0.742 (0.060) * | 0.001 | |
| Normalized area score | 2.412 (0.753) | 11.160 (2.552–48.800) | 0.001 | 0.740 (0.060) * | <0.001 | |
| SAH | ||||||
| Maximum | 0.017 (0.005) | 1.017 (1.008–1.027) | <0.001 | 0.757 (0.064) | <0.001 | |
| Mean | 0.030 (0.007) | 1.030 (1.015–1.045) | <0.001 | 0.751 (0.067) | <0.001 | |
| Normalized area score | 0.026 (0.007) | 1.027 (1.013–1.041) | <0.001 | 0.751 (0.067) | <0.001 |
| Early Period (24 h Following Study Inclusion) | Total Study Period (Up to 112 h) | |||||||
|---|---|---|---|---|---|---|---|---|
| AUROC (p-Value) | Model Parameters | OR (95% CI) | p-Value of OR | AUROC (p-Value) | Model Parameters | OR (95% CI) | p-Value of OR | |
| Initial lactate | 0.781 (<0.001) | Vascular CM SAPS II | 2.86 (0.93–8.85) 1.11 (1.04–1.17) | 0.068 0.001 | 0.781 (<0.001) | Vascular CM SAPS II | 2.86 (0.93–8.85) 1.11 (1.04–1.17) | 0.068 0.001 |
| Maximum lactate | 0.788 (<0.001) | Vascular CM SAPS II Maximum lactate | 2.81 (0.85–9.28) 1.10 (1.04–1.17) 1.60 (0.85–3.00) | 0.097 0.002 0.111 | 0.830 (<0.001) | Vascular CM SAPS II Maximum lactate | 2.87 (0.86–9.52) 1.08 (1.01–1.15) 2.29 (1.23–4.26) | 0.086 0.003 0.008 |
| Mean lactate | 0.781 (0.001) | SAPS II Mean lactate | 1.10 (1.04–1.17) 2.46 (0.99–6.10) | 0.001 0.053 | 0.838 (<0.001) | Vascular CM SAPS II Creatinine Mean lactate | 2.94 (0.84–10.25) 1.06 (0.995–1.14) 1.71 (0.95–3.08) 8.45 (1.72–41.41) | 0.091 0.072 0.076 0.009 |
| Normalized lactate area score | 0.805 (<0.001) | Vascular CM SAPS II Lactate area score | 2.77 (0.85–8.98) 1.10 (1.04–1.17) 2.61 (1.08–6.34) | 0.090 0.002 0.034 | 0.837 (<0.001) | Vascular CM SAPS II Creatinine Lactate area score | 2.91 (0.84–10.09) 1.06 (0.996–1.14) 1.67 (0.93–3.00) 7.85 (1.59–38.70) | 0.092 0.067 0.085 0.011 |
| Initial SAH | 0.802 (<0.001) | SAPS II SAH | 1.08 (1.01–1.15) 1.02 (1.002–1.04) | 0.023 0.032 | 0.802 (<0.001) | SAPS II SAH | 1.08 (1.01–1.15) 1.02 (1.002–1.037) | 0.023 0.032 |
| Maximum SAH | 0.810 (<0.001) | SAPS II Maximum SAH | 1.08 (1.02–1.15) 1.02 (1.01–1.03) | 0.013 0.005 | 0.836 (<0.001) | Vascular CM SAPS II Maximum SAH | 2.88 (0.84–9.84) 1.09 (1.03–1.17) 1.01 (1.004–1.02) | 0.091 0.005 0.004 |
| Mean SAH | 0.804 (<0.001) | SAPS II Mean SAH | 1.08 (1.01–1.15) 1.02 (1.01–1.04) | 0.018 0.010 | 0.842 (<0.001) | Vascular CM SAPS II Mean SAH | 3.01 (0.86–10.59) 1.07 (1.01–1.14) 1.03 (1.01–1.06) | 0.085 0.034 0.002 |
| Normalized SAH area score | 0.805 (<0.001) | SAPS II SAH area score | 1.08 (1.01–1.15) 1.02 (1.01–1.04) | 0.016 0.009 | 0.840 (<0.001) | Vascular CM SAPS II SAH area score | 3.00 (0.87–10.38) 1.08 (1.02–1.15) 1.02 (1.01–1.04) | 0.083 0.015 0.003 |
| AUROC (p-Value) | Model Parameters | OR (95% CI) | p-Value of OR | |
|---|---|---|---|---|
| Early period (24 h following study inclusion) | 0.840 | SAPS II | 1.10 (1.03–1.17) | 0.007 |
| (<0.001) | Maximum SAH | 1.01 (1.00–1.03) | 0.052 | |
| Lactate | 0.20 (0.04–1.12) | 0.066 | ||
| Lactate area score | 16.70 (1.41–197.78) | 0.026 | ||
| Total study period (up to 112 h) | 0.869 | SAPS II | 1.08 (1.01–1.16) | 0.017 |
| (<0.001) | Mean SAH | 1.02 (1.01–1.04) | 0.007 | |
| Lactate | 0.30 (0.09–0.989) | 0.048 | ||
| Maximum lactate | 5.01 (1.45–17.32) | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoettler, J.J.; Brohm, K.; Mindt, S.; Jäger, E.; Hahn, B.; Fuderer, T.; Lindner, H.A.; Schneider-Lindner, V.; Krebs, J.; Neumaier, M.; et al. Mortality Prediction by Kinetic Parameters of Lactate and S-Adenosylhomocysteine in a Cohort of Critically Ill Patients. Int. J. Mol. Sci. 2024, 25, 6391. https://doi.org/10.3390/ijms25126391
Schoettler JJ, Brohm K, Mindt S, Jäger E, Hahn B, Fuderer T, Lindner HA, Schneider-Lindner V, Krebs J, Neumaier M, et al. Mortality Prediction by Kinetic Parameters of Lactate and S-Adenosylhomocysteine in a Cohort of Critically Ill Patients. International Journal of Molecular Sciences. 2024; 25(12):6391. https://doi.org/10.3390/ijms25126391
Chicago/Turabian StyleSchoettler, Jochen J., Kathrin Brohm, Sonani Mindt, Evelyn Jäger, Bianka Hahn, Tanja Fuderer, Holger A. Lindner, Verena Schneider-Lindner, Joerg Krebs, Michael Neumaier, and et al. 2024. "Mortality Prediction by Kinetic Parameters of Lactate and S-Adenosylhomocysteine in a Cohort of Critically Ill Patients" International Journal of Molecular Sciences 25, no. 12: 6391. https://doi.org/10.3390/ijms25126391
APA StyleSchoettler, J. J., Brohm, K., Mindt, S., Jäger, E., Hahn, B., Fuderer, T., Lindner, H. A., Schneider-Lindner, V., Krebs, J., Neumaier, M., Thiel, M., & Centner, F.-S. (2024). Mortality Prediction by Kinetic Parameters of Lactate and S-Adenosylhomocysteine in a Cohort of Critically Ill Patients. International Journal of Molecular Sciences, 25(12), 6391. https://doi.org/10.3390/ijms25126391





