A Study of the Association between Primary Oral Pathologies (Dental Caries and Periodontal Diseases) Using Synchrotron Molecular FTIR Spectroscopy in View of the Patient’s Personalized Clinical Picture (Demographics and Anamnesis)
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR-Spectroscopy
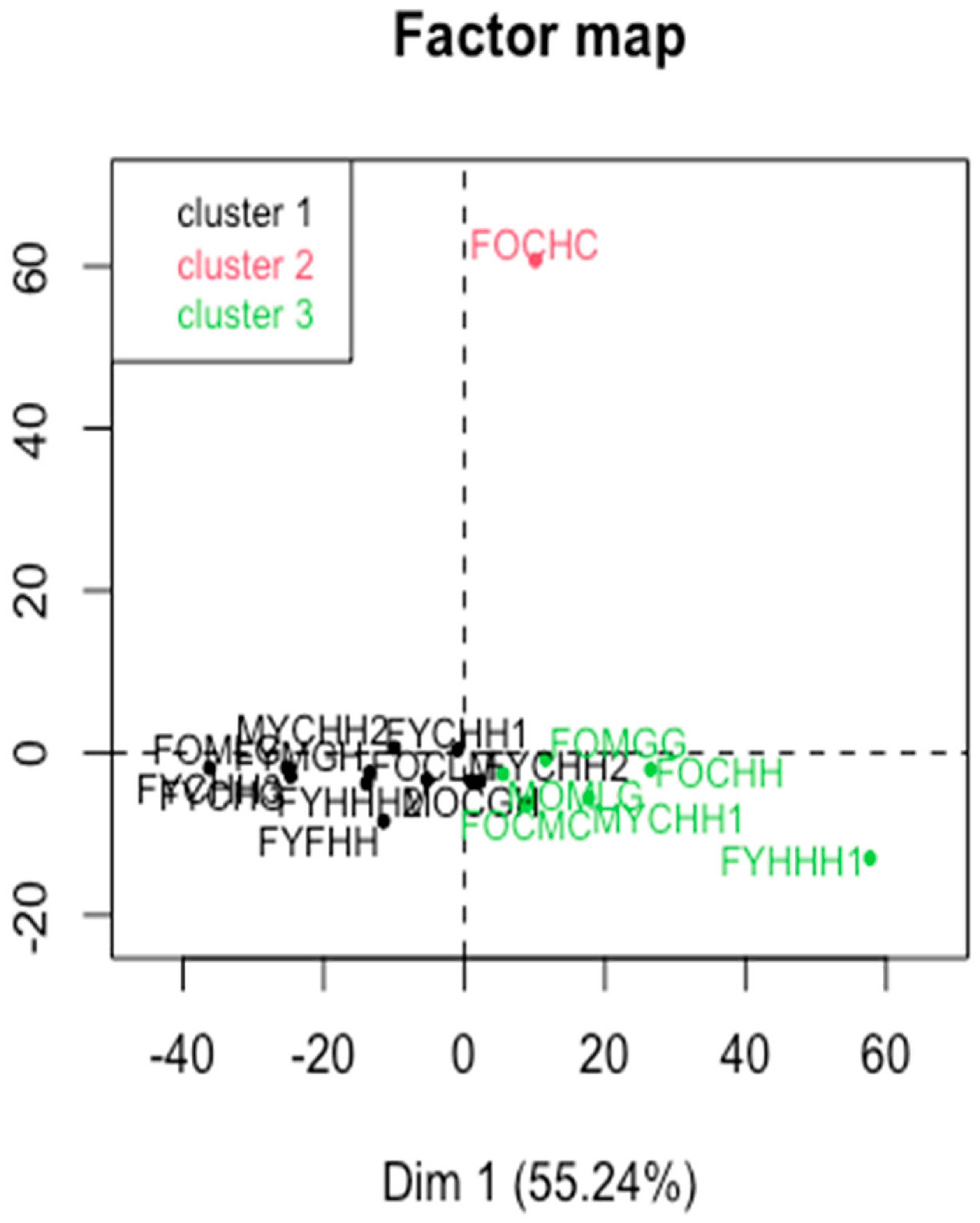
2.2. Multivariate Analysis
3. Methods and Objects of Research
3.1. Experimental Design
3.2. Dataset Description
3.3. Collection of Gingival Fluid
3.4. FTIR-Spectroscopy
3.5. Spectra Processing
3.6. Multivariate Analysis of Spectral Data
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barranca-Enríquez, A.; Romo-González, T. Your Health Is in Your Mouth: A Comprehensive View to Promote General Wellness. Front. Oral Health 2022, 3, 971223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Region Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030: Summary of the WHO European Region. In Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030: Summary of the WHO European Region; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Baima, G.; Shin, H.-S.; Arrica, M.; Laforí, A.; Cordaro, M.; Romandini, M. The Co-Occurrence of the Two Main Oral Diseases: Periodontitis and Dental Caries. Clin. Oral Investig. 2023, 27, 6483–6492. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Wang, X.; Feng, X.P.; Tai, B.J.; Hu, D.Y.; Wang, B.; Wang, C.X.; Zheng, S.G.; Liu, X.N.; Rong, W.S.; et al. The Relationship between Different Types of Caries and Periodontal Disease Severity in Middle-Aged and Elderly People: Findings from the 4th National Oral Health Survey of China. BMC Oral Health 2021, 21, 229. [Google Scholar] [CrossRef] [PubMed]
- Fraihat, N.; Madae’en, S.; Bencze, Z.; Herczeg, A.; Varga, O. Clinical Effectiveness and Cost-Effectiveness of Oral-Health Promotion in Dental Caries Prevention among Children: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2019, 16, 2668. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Nakamura, A.; Hara, T.; Hayakawa, S.; Yoshida, Y.; Van Meerbeek, B. Three-Dimensional Observation and Analysis of Remineralization in Dentinal Caries Lesions. Sci. Rep. 2020, 10, 4387. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, M.; Olianas, A.; Cabras, T.; Manconi, B.; Fanni, D.; Faa, G.; Desiderio, C.; Messana, I.; Castagnola, M. Saliva, a Bodily Fluid with Recognized and Potential Diagnostic Applications. J. Sep. Sci. 2021, 44, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- García-Godoy, F.; Hicks, M.J. Maintaining the Integrity of the Enamel Surface: The Role of Dental Biofilm, Saliva and Preventive Agents in Enamel Demineralization and Remineralization. J. Am. Dent. Assoc. 2008, 139, 25S–34S. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.; Garcia-Godoy, F.; Flaitz, C. Biological Factors in Dental Caries: Role of Saliva and Dental Plaque in the Dynamic Process of Demineralization and Remineralization (Part 1). J. Clin. Pediatr. Dent. 2004, 28, 47–52. [Google Scholar] [CrossRef]
- Aruni, A.W.; Dou, Y.; Mishra, A.; Fletcher, H.M. The Biofilm Community: Rebels with a Cause. Curr. Oral Health Rep. 2015, 2, 48–56. [Google Scholar] [CrossRef]
- Durand, R.; Roufegarinejad, A.; Chandad, F.; Rompré, P.H.; Voyer, R.; Michalowicz, B.S.; Emami, E. Dental Caries Are Positively Associated with Periodontal Disease Severity. Clin. Oral Investig. 2019, 23, 3811–3819. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; Tanner, A.C.R. The Role of Bacterial Biofilms in Dental Caries and Periodontal and Peri-Implant Diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Costa, R.; Barão, V.; Villar, C.C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, M.; d’Apuzzo, F.; Perillo, L.; Grassia, V.; Errico, S.; Lepore, M. Infrared Microspectroscopy Characterization of Gingival Crevicular Fluid during Orthodontic Treatment. J. Mol. Struct. 2019, 1176, 847–854. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, P. Pathology-Specific Molecular Profiles of Saliva in Patients with Multiple Dental Caries—Potential Application for Predictive, Preventive and Personalised Medical Services. EPMA J. 2018, 9, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lips, A.; Antunes, L.S.; Antunes, L.A.; Pintor, A.V.B.; dos Santos, D.A.B.; Bachinski, R.; Küchler, E.C.; Alves, G.G. Salivary Protein Polymorphisms and Risk of Dental Caries: A Systematic Review. Braz. Oral Res. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Kripal, K.; Bhavanam, S.; Anuroopa, P.; Kumar, P.; Chandrasekaran, K.; Paul, P. Comparision of the Microbial Count in Supragingival Plaque, Gingival Crevicular Blood and Saliva Samples Immediately after Diode Laser (970 ± 15 Nm) Application in Chronic Periodontitis Patients: A Randomized Controlled Split Mouth Clinical Trial. Dentistry 2018, 8. [Google Scholar] [CrossRef]
- Delrue, C.; De Bruyne, S.; Speeckaert, M.M. Unlocking the Diagnostic Potential of Saliva: A Comprehensive Review of Infrared Spectroscopy and Its Applications in Salivary Analysis. J. Pers. Med. 2023, 13, 907. [Google Scholar] [CrossRef]
- Saloom, H.F.; Carpenter, G.H. Saliva and Gingival Crevicular Fluid: Contributions to Mucosal Defense. In Oral Mucosa in Health and Disease: A Concise Handbook; Bergmeier, L.A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 91–103. ISBN 978-3-319-56065-6. [Google Scholar]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival Crevicular Fluid as a Source of Biomarkers for Periodontitis. Periodontology 2000 2016, 70, 53–64. [Google Scholar] [CrossRef]
- Bibi, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208. [Google Scholar] [CrossRef]
- Syed, S.; Kankara, V.; Pathakota, K.; Krishnan, P.; Mishra, A. Evaluation of Deoxypyridinoline Levels in Gingival Crevicular Fluid and Serum as Alveolar Bone Loss Biomarker in Patients with Periodontitis. J. Indian Soc. Periodontol. 2020, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- d’Apuzzo, F.; Nucci, L.; Delfino, I.; Portaccio, M.; Minervini, G.; Isola, G.; Serino, I.; Camerlingo, C.; Lepore, M. Application of Vibrational Spectroscopies in the Qualitative Analysis of Gingival Crevicular Fluid and Periodontal Ligament during Orthodontic Tooth Movement. J. Clin. Med. 2021, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Nazar Majeed, Z.; Philip, K.; Alabsi, A.M.; Pushparajan, S.; Swaminathan, D. Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review. Dis. Markers 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Toczewska, J.; Baczyńska, D.; Zalewska, A.; Maciejczyk, M.; Konopka, T. The mRNA Expression of Genes Encoding Selected Antioxidant Enzymes and Thioredoxin, and the Concentrations of Their Protein Products in Gingival Crevicular Fluid and Saliva during Periodontitis. Dent. Med. Probl. 2023, 60, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Khodadadi, H.; Baban, B. Innate Immunity and Oral Microbiome: A Personalized, Predictive, and Preventive Approach to the Management of Oral Diseases. EPMA J. 2019, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ranc, V.; Žižka, R.; Chaloupková, Z.; Ševčík, J.; Zbořil, R. Imaging of Growth Factors on a Human Tooth Root Canal by Surface-Enhanced Raman Spectroscopy. Anal. Bioanal. Chem. 2018, 410, 7113–7120. [Google Scholar] [CrossRef] [PubMed]
- Júnior, C.; Cesar, P.; Strixino, J.F.; Raniero, L.; Júnior, C.; Cesar, P.; Strixino, J.F.; Raniero, L. Analysis of Saliva by Fourier Transform Infrared Spectroscopy for Diagnosis of Physiological Stress in Athletes. Res. Biomed. Eng. 2015, 31, 116–124. [Google Scholar] [CrossRef]
- Lopes, J.; Correia, M.; Martins, I.; Henriques, A.G.; Delgadillo, I.; da Cruz e Silva, O.; Nunes, A. FTIR and Raman Spectroscopy Applied to Dementia Diagnosis Through Analysis of Biological Fluids. J. Alzheimers Dis. 2016, 52, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, U.; Tiriolo, R.; Pullano, S.A.; Dastoli, S.; Amoruso, G.F.; Nisticò, S.P.; Fiorillo, A.S. Infrared Saliva Analysis of Psoriatic and Diabetic Patients: Similarities in Protein Components. IEEE Trans. Biomed. Eng. 2016, 63, 379–384. [Google Scholar] [CrossRef]
- Gupta, G. Gingival Crevicular Fluid as a Periodontal Diagnostic Indicator- II: Inflammatory Mediators, Host-Response Modifiers and Chair Side Diagnostic Aids. J. Med. Life 2013, 6, 7–13. [Google Scholar]
- Mah, J.; Prasad, N. Dentine Phosphoproteins in Gingival Crevicular Fluid during Root Resorption. Eur. J. Orthod. 2004, 26, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, W. Salivary Biomarkers for Caries Risk Assessment. J. Calif. Dent. Assoc. 2013, 41, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.d.A. Salivary Biomarkers of Dental Caries; Universidade do Porto: Porto, Portugal, 2013. [Google Scholar]
- Camerlingo, C.; Portaccio, M.; d’Apuzzo, F.; Nucci, L.; Perillo, L.; Lepore, M. μ-FTIR, μ-Raman, and SERS Analysis of Amide I Spectral Region in Oral Biofluid Samples during Orthodontic Treatment. Sensors 2022, 22, 7874. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Comparative Analysis of Dentine and Gingival Fluid Molecular Composition and Protein Conformations during Development of Dentine Caries: A Pilot Study. Vib. Spectrosc. 2020, 108, 103058. [Google Scholar] [CrossRef]
- Abdelkawi, S.; El Saeid, A.; El-Rashedi, A.; Abd-Eldaiem, M. Photobiomodulation Therapy for Diabetic Macular Edema: Fourier Transform Infrared Spectroscopy Study. J. Arab Soc. Med. Res. 2018, 13, 25. [Google Scholar] [CrossRef]
- Fujii, S.; Sato, S.; Fukuda, K.; Okinaga, T.; Ariyoshi, W.; Usui, M.; Nakashima, K.; Nishihara, T.; Takenaka, S. Diagnosis of Periodontal Disease from Saliva Samples Using Fourier Transform Infrared Microscopy Coupled with Partial Least Squares Discriminant Analysis. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2016, 32, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bellisola, G.; Sorio, C. Infrared Spectroscopy and Microscopy in Cancer Research and Diagnosis. Am. J. Cancer Res. 2012, 2, 1–21. [Google Scholar] [PubMed]
- Titus, J.; Ghimire, H.; Viennois, E.; Merlin, D.; Perera, A.G.U. Protein Secondary Structure Analysis of Dried Blood Serum Using Infrared Spectroscopy to Identify Markers for Colitis Screening. J. Biophotonics 2018, 11, e201700057. [Google Scholar] [CrossRef] [PubMed]
- Babot-Marquillas, C.; Sánchez-Martín, M.-J.; Amigo, J.M.; Yousef, I.H.; Valido, I.; Boada, R.; Valiente, M. Tooth Whitening, Oxidation or Reduction? Study of Physicochemical Alterations in Bovine Enamel Using Synchrotron Based Micro-FTIR. Dent. Mater. 2022, 38, 670–679. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Khydyakov, Y.; Nesterov, D.; Ippolitov, I.; Ippolitov, Y.; Vongsvivut, J. Development of a Hybrid Biomimetic Enamel-Biocomposite Interface and a Study of Its Molecular Features Using Synchrotron Submicron ATR-FTIR Microspectroscopy and Multivariate Analysis Techniques. Int. J. Mol. Sci. 2022, 23, 11699. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics 2021, 11, 1294. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Development of a New Approach to Diagnosis of the Early Fluorosis Forms by Means of FTIR and Raman Microspectroscopy. Sci. Rep. 2020, 10, 20891. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Kashkarov, V.; Lukin, A.; Ippolitov, Y.; Julian, R.; Doyle, S. Local Study of Fissure Caries by Fourier Transform Infrared Microscopy and X-Ray Diffraction Using Synchrotron Radiation. J. Synchrotron Radiat. 2013, 20, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Jersie-Christensen, R.R.; Lyon, D.; Damgaard, C.; Jensen, L.J.; Holmstrup, P.; Olsen, J.V. Metaproteomics of Saliva Identifies Human Protein Markers Specific for Individuals with Periodontitis and Dental Caries Compared to Orally Healthy Controls. PeerJ 2016, 4, e2433. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Age and Gender Characteristics of the Infrared Spectra of Normal Human Saliva. Appl. Spectrosc. 2020, 74, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, L.; Wang, Q.; Wen, Z.; Liu, C.; Ding, Y. Raman Spectroscopy: A Potential Diagnostic Tool for Oral Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 775236. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A. Biochemical Composition and Characteristics of Salivary FTIR Spectra: Correlation Analysis. J. Mol. Liq. 2021, 341, 117380. [Google Scholar] [CrossRef]
- Caixeta, D.C.; Carneiro, M.G.; Rodrigues, R.; Alves, D.C.T.; Goulart, L.R.; Cunha, T.M.; Espindola, F.S.; Vitorino, R.; Sabino-Silva, R. Salivary ATR-FTIR Spectroscopy Coupled with Support Vector Machine Classification for Screening of Type 2 Diabetes Mellitus. Diagnostics 2023, 13, 1396. [Google Scholar] [CrossRef]
- Durlik-Popińska, K.; Żarnowiec, P.; Konieczna-Kwinkowska, I.; Lechowicz, Ł.; Gawęda, J.; Kaca, W. Correlations between Autoantibodies and the ATR-FTIR Spectra of Sera from Rheumatoid Arthritis Patients. Sci. Rep. 2021, 11, 17886. [Google Scholar] [CrossRef]
- Sanchez-Brito, M.; Vazquez-Zapien, G.J.; Luna-Rosas, F.J.; Mendoza-Gonzalez, R.; Martinez-Romo, J.C.; Mata-Miranda, M.M. Attenuated Total Reflection FTIR Dataset for Identification of Type 2 Diabetes Using Saliva. Comput. Struct. Biotechnol. J. 2022, 20, 4542–4548. [Google Scholar] [CrossRef]
- Giorgini, E.; Balercia, P.; Conti, C.; Ferraris, P.; Sabbatini, S.; Rubini, C.; Tosi, G. Insights on Diagnosis of Oral Cavity Pathologies by Infrared Spectroscopy: A Review. J. Mol. Struct. 2013, 1051, 226–232. [Google Scholar] [CrossRef]
- Derruau, S.; Gobinet, C.; Mateu, A.; Untereiner, V.; Lorimier, S.; Piot, O. Shedding Light on Confounding Factors Likely to Affect Salivary Infrared Biosignatures. Anal. Bioanal. Chem. 2019, 411, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25. [Google Scholar] [CrossRef]
- Baker, M.J.; Hussain, S.R.; Lovergne, L.; Untereiner, V.; Hughes, C.; Lukaszewski, R.A.; Thiéfin, G.; Sockalingum, G.D. Developing and Understanding Biofluid Vibrational Spectroscopy: A Critical Review. Chem. Soc. Rev. 2016, 45, 1803–1818. [Google Scholar] [CrossRef]
- Orphanou, C.-M. The Detection and Discrimination of Human Body Fluids Using ATR FT-IR Spectroscopy. Forensic Sci. Int. 2015, 252, e10–e16. [Google Scholar] [CrossRef]
- Matthäus, C.; Bird, B.; Miljković, M.; Chernenko, T.; Romeo, M.; Diem, M. Infrared and Raman Microscopy in Cell Biology. Methods Cell Biol. 2008, 89, 275–308. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Bambery, K. The Investigations of Changes in Mineral–Organic and Carbon–Phosphate Ratios in the Mixed Saliva by Synchrotron Infrared Spectroscopy. Results Phys. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Nesterov, D.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. Effect of Exo/Endogenous Prophylaxis Dentifrice/Drug and Cariogenic Conditions of Patient on Molecular Property of Dental Biofilm: Synchrotron FTIR Spectroscopic Study. Pharmaceutics 2022, 14, 1355. [Google Scholar] [CrossRef]
- Naumann, D. Infrared Spectroscopy of Cells, Tissues, and Biofluids. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 1057–1065. ISBN 978-3-642-16712-6. [Google Scholar]
- Scherdin-Almhöjd, U. Identification of Esters in Carious Dentine Staining and Chemo-Mechanical Excavation. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 2017. [Google Scholar]
- Almhöjd, U.S.; Norén, J.G.; Arvidsson, A.; Nilsson, Å.; Lingström, P. Analysis of Carious Dentine Using FTIR and ToF-SIMS. Oral Health Dent. Manag. 2014, 13, 735–744. [Google Scholar] [CrossRef]
- Larmas, M. A Chromatographic and Histochemical Study of Nonspecific Esterases in Human Carious Dentine. Arch. Oral Biol. 1972, 17, 1121–1132. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein Secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Liao, H.-F.; Wang, S.-L.; Lin, S.-Y. Glycation and Secondary Conformational Changes of Human Serum Albumin: Study of the FTIR Spectroscopic Curve-Fitting Technique. AIMS Biophys. 2016, 3, 247–260. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; Analytical Techniques in the Sciences; J. Wiley: Chichester, UK; Hoboken, NJ, USA, 2004; ISBN 978-0-470-85427-3. [Google Scholar]
- Depciuch, J.; Sowa-Kućma, M.; Nowak, G.; Dudek, D.; Siwek, M.; Styczeń, K.; Parlińska-Wojtan, M. Phospholipid-Protein Balance in Affective Disorders: Analysis of Human Blood Serum Using Raman and FTIR Spectroscopy. A Pilot Study. J. Pharm. Biomed. Anal. 2016, 131, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.V.; Goloshchapov, D.L.; Ippolitov, Y.A.; Kalivradzhiyan, E.S. Does Dentifrice Provide the Necessary Saturation of Ions in Oral Fluids to Favour Remineralisation? Russ. Open Med. J. 2018, 7, e0106. [Google Scholar] [CrossRef]
- Makhnii, T.; Ilchenko, O.; Reynt, A.; Pilgun, Y.; Kutsyk, A.; Krasnenkov, D.; Ivasyuk, M.; Kukharskyy, V. Age-Related Changes in FTIR and Raman Spectra of Human Blood. Ukr. J. Phys. 2016, 61, 853–862. [Google Scholar] [CrossRef]
- Tajmir-Riahi, H.A.; N’soukpoe-kossi, C.N.; Joly, D. Structural Analysis of Protein-DNA and Protein-RNA Interactions by FTIR Spectroscopy. Adv. Biomed. Spectrosc. 2009, 288–311. [Google Scholar] [CrossRef]
- Elkins, K.M. Rapid Presumptive “Fingerprinting” of Body Fluids and Materials by ATR FT-IR Spectroscopy. J. Forensic Sci. 2011, 56, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.M.; Liu, K.Z.; Man, A.; Ghiabi, E.; Cholakis, A.; Scott, D.A. Periodontitis-Specific Molecular Signatures in Gingival Crevicular Fluid. J. Periodontal Res. 2010, 45, 345–352. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Lukin, A.; Peshkov, Y.; Ippolitov, I.; Ippolitov, Y.; Litvinova, T.; Vongsvivut, J.; Chae, B.; et al. Changes in Dental Biofilm Proteins’ Secondary Structure in Groups of People with Different Cariogenic Situations in the Oral Cavity and Using Medications by Means of Synchrotron FTIR-Microspectroscopy. Int. J. Mol. Sci. 2023, 24, 15324. [Google Scholar] [CrossRef]
- Kim, S.; Song, Y.; Kim, S.; Kim, S.; Na, H.; Lee, S.; Chung, J.; Kim, S. Identification of a Biomarker Panel for Diagnosis of Early Childhood Caries Using Salivary Metabolic Profile. Metabolites 2023, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; Angeli, R.; Muniz, A.M.S.; Gonsalves, E.; Santos, R.; Nadal, J.; Almeida, F.C.L.; Valente, A.P.; Souza, I.P.R. Salivary Metabolite Signatures of Children with and without Dental Caries Lesions. Metabolomics 2013, 9, 657–666. [Google Scholar] [CrossRef]
- Cattell, R.B. The Scree Test for The Number of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Lê, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R, 2nd ed.; Computer Science and Data Analysis Series; CRC Press Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; ISBN 978-0-367-65802-1. [Google Scholar]
- Thrun, M.C. Projection-Based Clustering through Self-Organization and Swarm Intelligence; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2018; ISBN 978-3-658-20539-3. [Google Scholar]
- Department of Economic and Social Affairs. World Population Ageing 2019; United Nations: New York, NY, USA, 2020; ISBN 978-92-1-004554-4. [Google Scholar]
- Ahmad, O.B.; Boschi Pinto, C.; Lopez, A.D. Age Standardization of Rates: A New WHO Standard. GPE Discuss. Pap. Ser. No 31 2001, 10–12. [Google Scholar]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y. Jitraporn Vongsvivut Spectroscopic Signature of the Pathological Processes of Carious Dentine Based on FTIR Investigations of the Oral Biological Fluids. Biomed. Opt. Express 2019, 10, 4050–4058. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Deepa, A.; Madhu Babu, D.; Chandra, N.; Subba Reddy, C.; Kumar, A. Estimation of Tissue Inhibitor of Matrix Metalloproteinase-1 Levels in Gingival Crevicular Fluid in Periodontal Health, Disease and after Treatment. J. Indian Soc. Periodontol. 2014, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Martin, F.L.; Dickinson, A.W.; Saba, T.; Bongers, T.; Singh, M.N.; Bury, D. ATR-FTIR Spectroscopy with Chemometrics for Analysis of Saliva Samples Obtained in a Lung-Cancer-Screening Programme: Application of Swabs as a Paradigm for High Throughput in a Clinical Setting. J. Pers. Med. 2023, 13, 1039. [Google Scholar] [CrossRef]
- González-Solís, J.L.; Martínez-Cano, E.; Magaña-López, Y. Early Detection of Dental Fluorosis Using Raman Spectroscopy and Principal Component Analysis. Lasers Med. Sci. 2015, 30, 1675–1681. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A.; Lukitaningsih, E.; Rafi, M.; Betania, K.; Fadzillah, N.A. The Use of FTIR and Raman Spectroscopy in Combination with Chemometrics for Analysis of Biomolecules in Biomedical Fluids: A Review. Biomed. Spectrosc. Imaging 2020, 8, 55–71. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses 2020. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 7 June 2024).
- FactoMineR: Catdes. Available online: http://factominer.free.fr/factomethods/categories-description.html (accessed on 11 April 2024).
- Lebart, L.; Morineau, A.; Piron, M. Statistique Exploratoire Multidimensionnelle, 3rd ed.; Sciences Sup; Dunod: Paris, France, 2000; ISBN 978-2-10-005351-3. [Google Scholar]
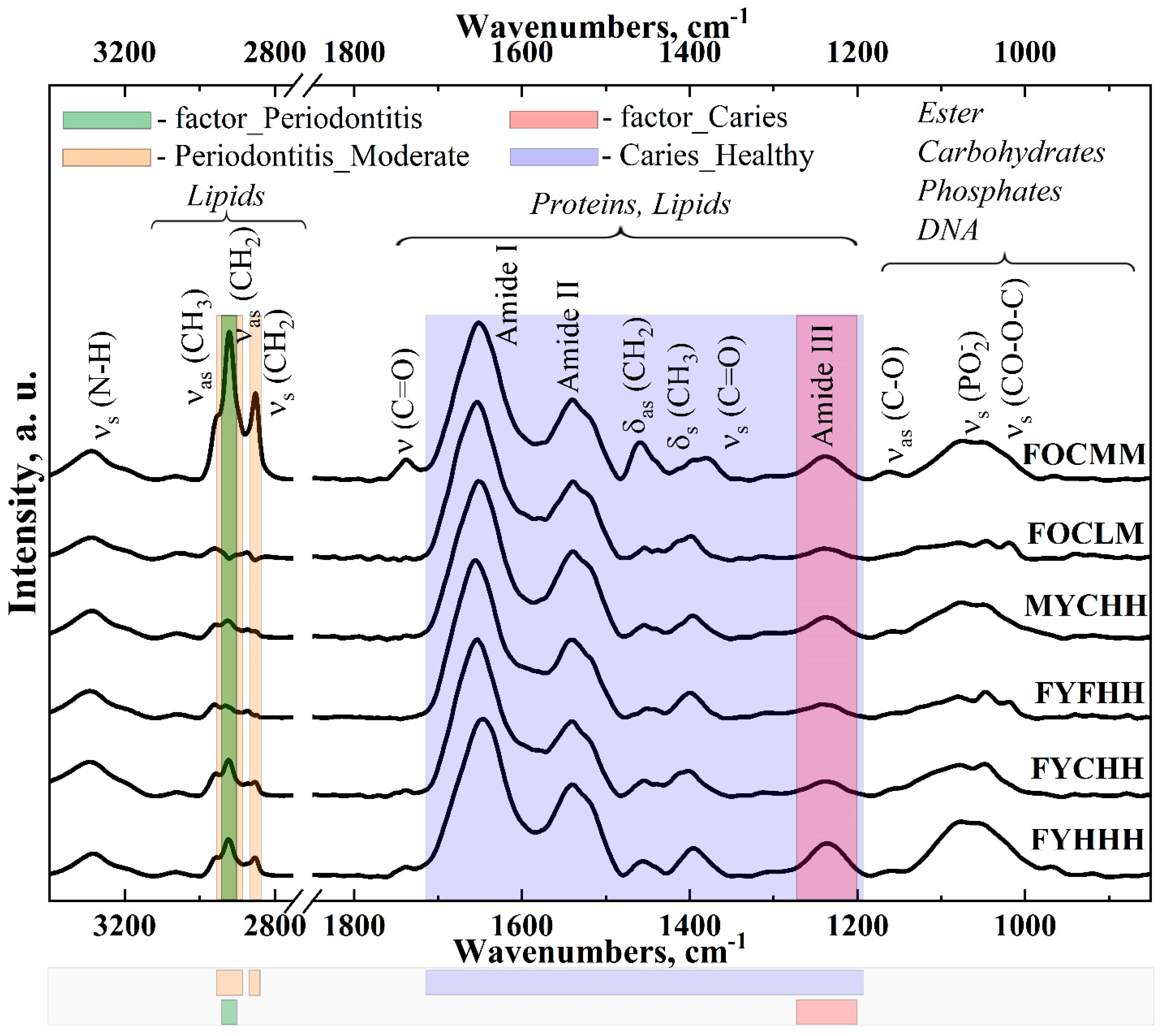



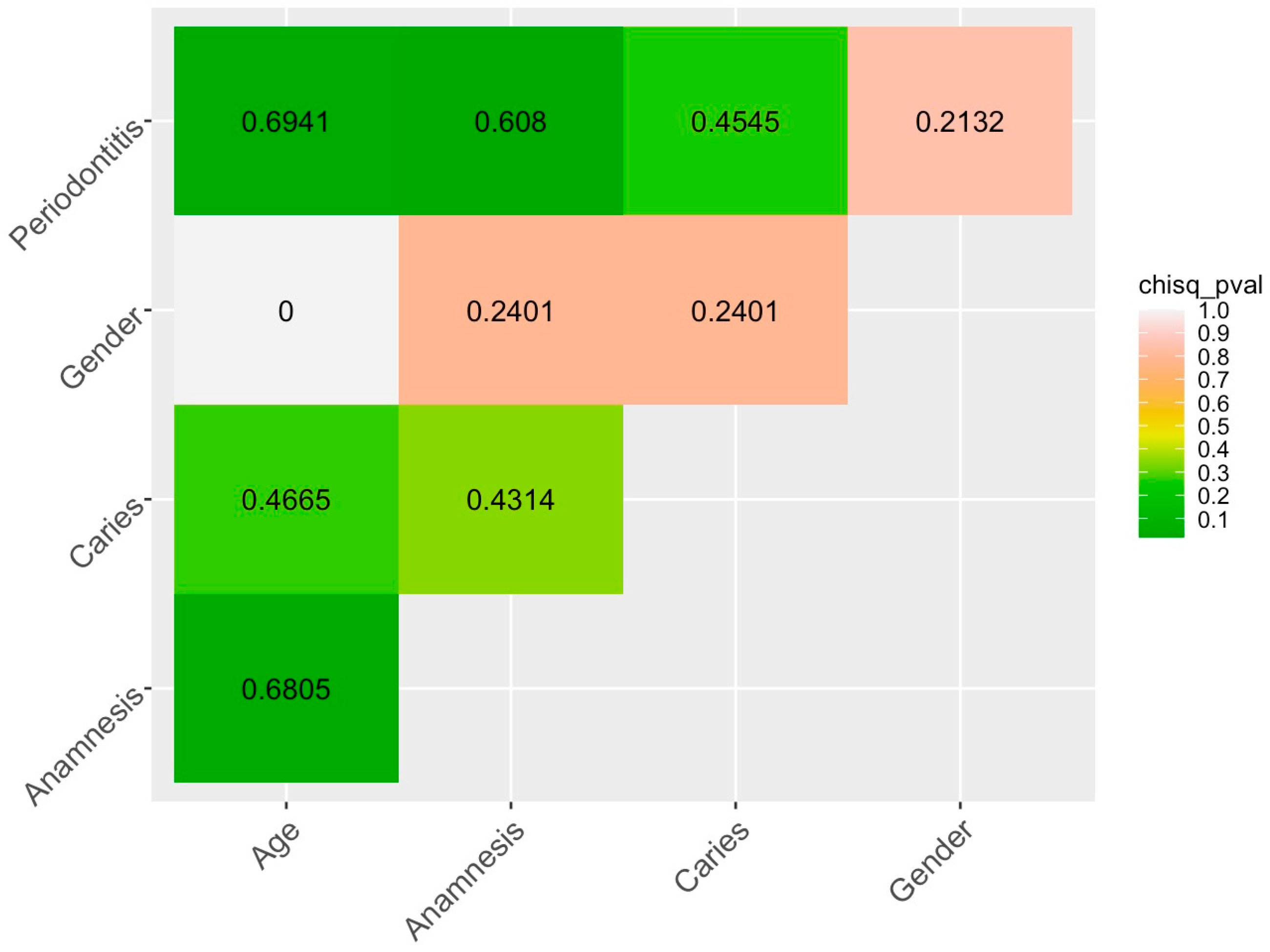

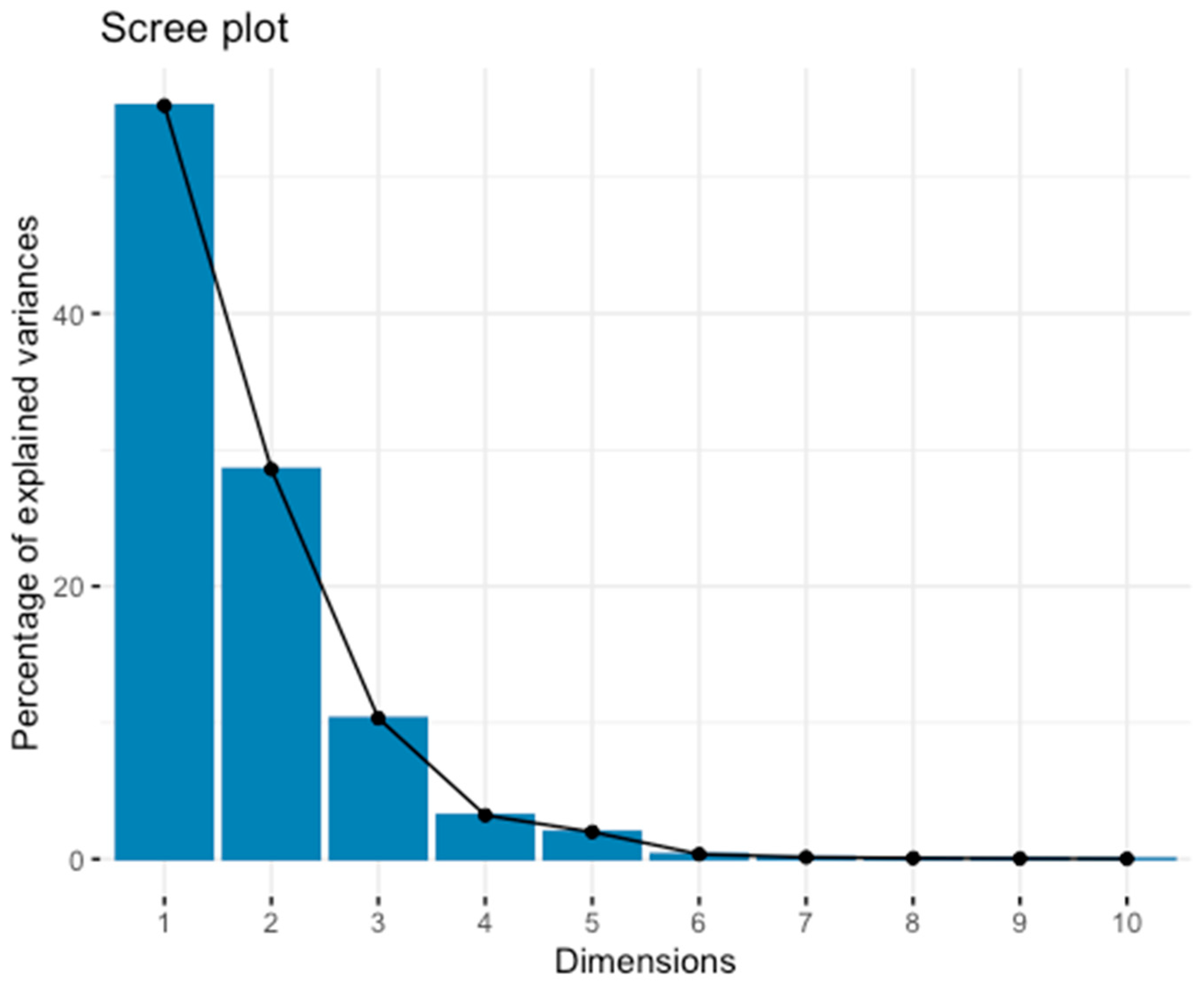


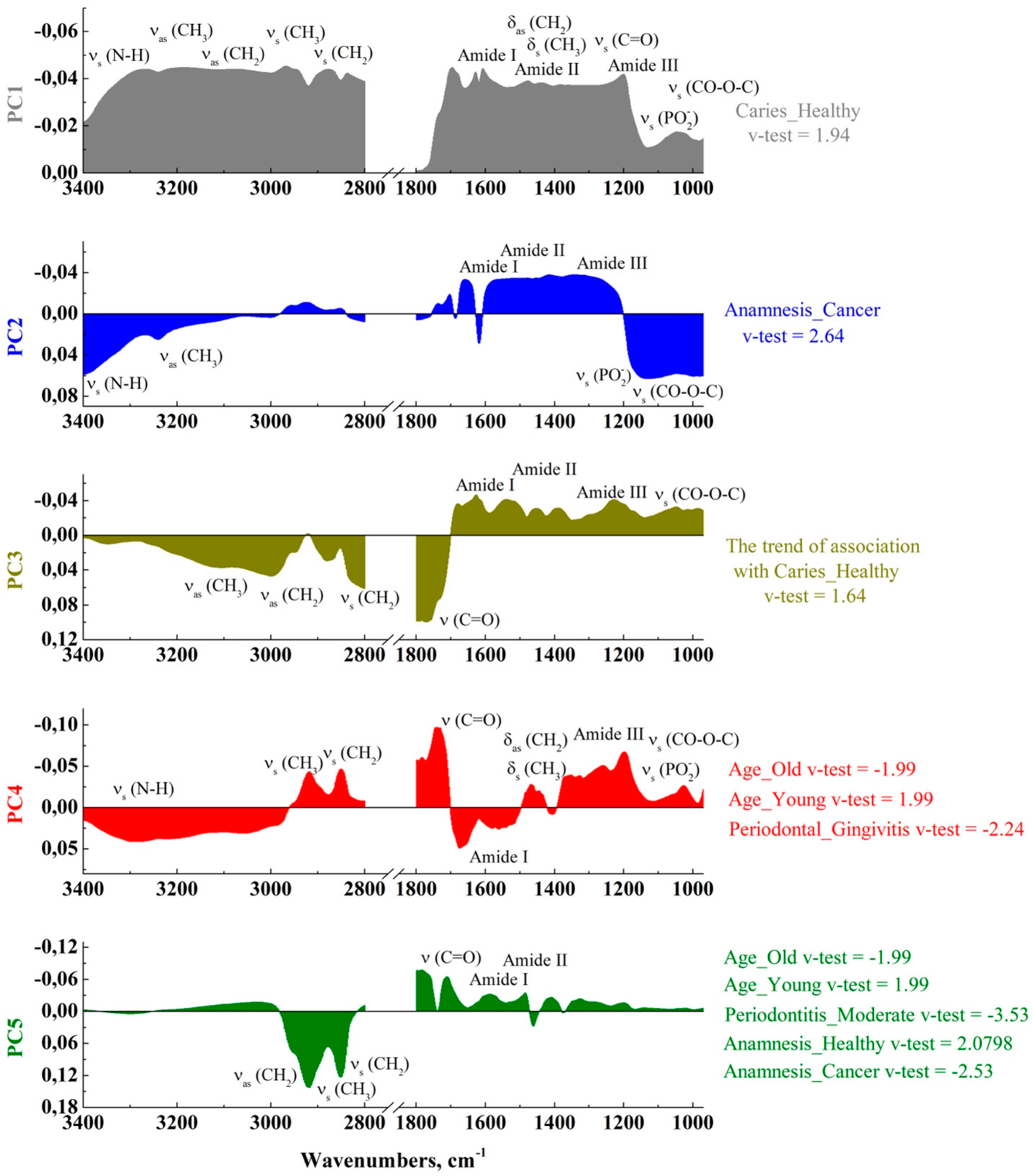
| Factor | Factor Level | Dist |
|---|---|---|
| Gender | Male | 2.09 |
| Female | 7.33 | |
| Age | Young | 5.52 |
| Old | 6.90 | |
| Dental caries | Healthy | 29.19 |
| Initial | 17.43 | |
| Fissure | 7.26 | |
| Multiple | 3.90 | |
| Periodontum | Healthy | 3.728241 |
| Gingivitis | 7.590230 | |
| Light | 11.756492 | |
| Moderate | 20.138446 | |
| Anamnesis | Healthy | 5.064526 |
| Gastrointestinal | 9.235628 | |
| Multiple | 14.845186 | |
| Cancer | 30.593537 |
| Sample | Name of Participants | Gender | Age | Caries Status | Periodontal Status | Anamnesis |
|---|---|---|---|---|---|---|
| 1 | FYHHH | F | Y | H | H | H |
| 2 | FYCHH | F | Y | C | H | H |
| 3 | FYCHH | F | Y | C | H | H |
| 4 | FOCHH | F | O | C | H | H |
| 5 | MYCGG | M | Y | C | G | G |
| 6 | MOCLH | M | O | C | L | H |
| 7 | FOCMC | F | O | C | M | C |
| 8 | FYCHG | F | Y | C | H | G |
| 9 | MYCLH | M | Y | C | L | H |
| 10 | FYCHM | F | Y | C | H | M |
| 11 | FOMLG | F | O | M | L | G |
| 12 | MOMLG | M | O | M | L | G |
| 13 | FOMHC | F | O | M | H | C |
| 14 | FOMLM | F | O | M | L | M |
| 15 | FOMLG | F | O | M | L | G |
| 16 | FYMLM | F | Y | M | L | M |
| 17 | FYFHH | F | Y | F | H | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Litvinova, T.; Ippolitov, Y.; Goloshchapov, D.; Peshkov, Y.; Kashkarov, V.; Ippolitov, I.; Chae, B. A Study of the Association between Primary Oral Pathologies (Dental Caries and Periodontal Diseases) Using Synchrotron Molecular FTIR Spectroscopy in View of the Patient’s Personalized Clinical Picture (Demographics and Anamnesis). Int. J. Mol. Sci. 2024, 25, 6395. https://doi.org/10.3390/ijms25126395
Seredin P, Litvinova T, Ippolitov Y, Goloshchapov D, Peshkov Y, Kashkarov V, Ippolitov I, Chae B. A Study of the Association between Primary Oral Pathologies (Dental Caries and Periodontal Diseases) Using Synchrotron Molecular FTIR Spectroscopy in View of the Patient’s Personalized Clinical Picture (Demographics and Anamnesis). International Journal of Molecular Sciences. 2024; 25(12):6395. https://doi.org/10.3390/ijms25126395
Chicago/Turabian StyleSeredin, Pavel, Tatiana Litvinova, Yuri Ippolitov, Dmitry Goloshchapov, Yaroslav Peshkov, Vladimir Kashkarov, Ivan Ippolitov, and Boknam Chae. 2024. "A Study of the Association between Primary Oral Pathologies (Dental Caries and Periodontal Diseases) Using Synchrotron Molecular FTIR Spectroscopy in View of the Patient’s Personalized Clinical Picture (Demographics and Anamnesis)" International Journal of Molecular Sciences 25, no. 12: 6395. https://doi.org/10.3390/ijms25126395







