Enhancing Bone Infection Diagnosis with Raman Handheld Spectroscopy: Pathogen Discrimination and Diagnostic Potential
Abstract
1. Introduction
2. Results
2.1. Spectroscopy Data Evaluation
2.2. Diagnostic Performance PCA
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Development of Biofilm on Bone Allografts
4.3. MIRA Raman Handheld
4.4. Data Processing
4.5. Principal Component Analyses (PCA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone Jt. Surg. Br. Vol. 2007, 89, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Zwitser, E.W.; Jiya, T.U.; George Licher, H.; van Royen, B.J. Design and management of an orthopaedic bone bank in The Netherlands. Cell Tissue Bank. 2012, 13, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Nodzo, S.R.; Boyle, K.K.; Pavlesen, S.; Rachala, S. Bone morphogenic protein-2 use in revision total hip arthroplasty with acetabular defects. Int. Orthop. 2018, 42, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Coraca-Huber, D.C.; Hausdorfer, J.; Fille, M.; Nogler, M. Effect of storage temperature on gentamicin release from antibiotic-coated bone chips. Cell Tissue Bank. 2013, 14, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, M.; Muylle, L.; Eastlund, T.; Fehily, D.; Noel, L.; Strong, D.M. Adverse reactions and events related to musculoskeletal allografts: Reviewed by the World Health Organisation Project NOTIFY. Int. Orthop. 2012, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.S.; Katz, J.; Baker, M.I.; Supronowicz, P.R.; Gill, E.; Cobb, R.R. Local antibiotic delivery with bovine cancellous chips. J. Biomater. Appl. 2011, 26, 491–506. [Google Scholar] [CrossRef]
- Sommerfeldt, D.W.; Linhart, W.; Schmandra, T.C.; Konold, P.; Rueger, J.M. Die Knochenbank Richtlinien—Probleme—Anwendung. Unfallchirurgie 1998, 24, 236–244. [Google Scholar] [CrossRef]
- Slooff, T.J.; Buma, P.; Schreurs, B.W.; Schimmel, J.W.; Huiskes, R.; Gardeniers, J. Acetabular and femoral reconstruction with impacted graft and cement. Clin. Orthop. Relat. Res. 1996, 324, 108–115. [Google Scholar] [CrossRef]
- Brewster, N.T.; Gillespie, W.J.; Howie, C.R.; Madabhushi, S.P.; Usmani, A.S.; Fairbairn, D.R. Mechanical considerations in impaction bone grafting. J. Bone Jt. Surg. Br. Vol. 1999, 81, 118–124. [Google Scholar] [CrossRef]
- Malkani, A.L.; Voor, M.J.; Fee, K.A.; Bates, C.S. Femoral component revision using impacted morsellised cancellous graft. A biomechanical study of implant stability. J. Bone Jt. Surg. Br. Vol. 1996, 78, 973–978. [Google Scholar] [CrossRef]
- Putzer, D.; Mayr, E.; Haid, C.; Reinthaler, A.; Nogler, M. Impaction bone grafting: A laboratory comparison of two methods. J. Bone Jt. Surg. Br. Vol. 2011, 93, 1049–1053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Putzer, D.; Coraca-Huber, D.; Wurm, A.; Schmoelz, W.; Nogler, M. Optimizing the grain size distribution of allografts in bone impaction grafting. J. Orthop. Res. 2014, 32, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.G.; Dos Santos Kotake, B.G.; de Figueiredo, F.A.T.; Feldman, S.; Ervolino, E.; Dos Santos, M.C.G.; Issa, J.P.M. Effectiveness of rhBMP-2 association to autogenous, allogeneic, and heterologous bone grafts. Microsc. Res. Tech. 2019, 82, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Coraca-Huber, D.C.; Hausdorfer, J.; Fille, M.; Steidl, M.; Nogler, M. Effect of two cleaning processes for bone allografts on gentamicin impregnation and in vitro antibiotic release. Cell Tissue Bank. 2013, 14, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Nogler, M.; Ammann, C.G.; Coraca-Huber, D.C. Effect of storage temperature and antibiotic impregnation on the quantity of bone morphogenetic protein seven in human bone grafts. Int. Orthop. 2014, 38, 1513–1517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef]
- Steinmetz, S.; Wernly, D.; Moerenhout, K.; Trampuz, A.; Borens, O. Infection after fracture fixation. EFORT Open Rev. 2019, 4, 468–475. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Grieb, T.A.; Forng, R.Y.; Stafford, R.E.; Lin, J.; Almeida, J.; Bogdansky, S.; Ronholdt, C.; Drohan, W.N.; Burgess, W.H. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials 2005, 26, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- DePaula, C.A.; Truncale, K.G.; Gertzman, A.A.; Sunwoo, M.H.; Dunn, M.G. Effects of hydrogen peroxide cleaning procedures on bone graft osteoinductivity and mechanical properties. Cell Tissue Bank. 2005, 6, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Vastel, L.; Meunier, A.; Siney, H.; Sedel, L.; Courpied, J.P. Effect of different sterilization processing methods on the mechanical properties of human cancellous bone allografts. Biomaterials 2004, 25, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Uchida, K.; Naruse, K.; Suto, M.; Urabe, K.; Uchiyama, K.; Suto, K.; Moriya, M.; Itoman, M.; Takaso, M. Quality assessment for processed and sterilized bone using Raman spectroscopy. Cell Tissue Bank. 2012, 13, 409–414. [Google Scholar] [CrossRef]
- McCreadie, B.R.; Morris, M.D.; Chen, T.C.; Sudhaker Rao, D.; Finney, W.F.; Widjaja, E.; Goldstein, S.A. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006, 39, 1190–1195. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Buchinger, B.; Zoehrer, R.; Phipps, R.; Klaushofer, K.; Paschalis, E.P. Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 2011, 49, 1160–1165. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Esmonde-White, F.W.; Holmes, C.M.; Morris, M.D.; Roessler, B.J. Alterations to bone mineral composition as an early indication of osteomyelitis in the diabetic foot. Diabetes Care 2013, 36, 3652–3654. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Hofstetter, B.; Zwettler, E.; Recker, R.; Gasser, J.A.; Eriksen, E.F.; Klaushofer, K.; Paschalis, E.P. Effects of 3 years treatment with once-yearly zoledronic acid on the kinetics of bone matrix maturation in osteoporotic patients. Osteoporos Int. 2013, 24, 339–347. [Google Scholar] [CrossRef]
- Busse, B.; Bale, H.A.; Zimmermann, E.A.; Panganiban, B.; Barth, H.D.; Carriero, A.; Vettorazzi, E.; Zustin, J.; Hahn, M.; Ager, J.W., 3rd; et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci. Transl. Med. 2013, 5, 193ra188. [Google Scholar] [CrossRef]
- Olejnik, C.; Falgayrac, G.; During, A.; Vieillard, M.H.; Maes, J.M.; Cortet, B.; Penel, G. Molecular alterations of bone quality in sequesters of bisphosphonates-related osteonecrosis of the jaws. Osteoporos Int. 2014, 25, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Imbert, L.; Auregan, J.C.; Pernelle, K.; Hoc, T. Mechanical and mineral properties of osteogenesis imperfecta human bones at the tissue level. Bone 2014, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Bora, T.; Ghaithi, A.A.; Thukral, S.; Dutta, J. Raman Spectroscopy detects changes in Bone Mineral Quality and Collagen Cross-linkage in Staphylococcus Infected Human Bone. Sci. Rep. 2018, 8, 9417. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Kuhn, J.; Kugel, K.; Putzer, D.; Arora, R.; Coraca-Huber, D.C.; Zelger, P.; Badzoka, J.; Kappacher, C.; Huck, C.W.; et al. Raman microscopic spectroscopy as a diagnostic tool to detect Staphylococcus epidermidis in bone grafts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121570. [Google Scholar] [CrossRef] [PubMed]
- Wurm, A.; Steiger, R.; Ammann, C.G.; Putzer, D.; Liebensteiner, M.C.; Nogler, M.; Coraca-Huber, D.C. Changes in the Chemical Quality of Bone Grafts During Clinical Preparation Detected by Raman Spectroscopy. Biopreserv. Biobank. 2016, 14, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Lasch, P.; Beekes, M.; Schmitt, J.; Naumann, D. Biomedical Vibrational Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Salzer, R.; Siesler, H.W. Infrared and Raman Spectroscopic Imaging; Vch Pub: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hutengs, C.; Ludwig, B.; Jung, A.; Eisele, A.; Vohland, M. Comparison of Portable and Bench-Top Spectrometers for Mid-Infrared Diffuse Reflectance Measurements of Soils. Sensors 2018, 18, 993. [Google Scholar] [CrossRef] [PubMed]
- Bec, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chemistry 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Hedegaard, M.; Krafft, C.; Ditzel, H.J.; Johansen, L.E.; Hassing, S.; Popp, J. Discriminating isogenic cancer cells and identifying altered unsaturated fatty acid content as associated with metastasis status, using k-means clustering and partial least squares-discriminant analysis of Raman maps. Anal. Chem. 2010, 82, 2797–2802. [Google Scholar] [CrossRef]
- Swain, R.J.; Stevens, M.M. Raman microspectroscopy for non-invasive biochemical analysis of single cells. Biochem. Soc. Trans. 2007, 35, 544–549. [Google Scholar] [CrossRef]
- Morris, M.D.; Mandair, G.S. Raman assessment of bone quality. Clin. Orthop. Relat. Res. 2011, 469, 2160–2169. [Google Scholar] [CrossRef]
- Gasior-Glogowska, M.; Komorowska, M.; Hanuza, J.; Ptak, M.; Kobielarz, M. Structural Alteration of Collagen Fibres—Spectroscopic and Mechanical Studies. Acta Bioeng. Biomech. 2010, 12, 55–62. [Google Scholar] [PubMed]
- Maiti, N.C.; Apetri, M.M.; Zagorski, M.G.; Carey, P.R.; Anderson, V.E. Raman spectroscopic characterization of secondary structure in natively unfolded proteins: Alpha-synuclein. J. Am. Chem. Soc. 2004, 126, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Happillon, T.; Feru, J.; Brassart-Passco, S.; Angiboust, J.-F.; Manfait, M.; Piot, O. Raman comparison of skin dermis of different ages: Focus on spectral markers of collagen hydration. J. Raman Spectrosc. 2013, 44, 1230–1237. [Google Scholar] [CrossRef]
- Prieto, J.L.; Magaña, C.; Ubelaker, D.H. Interpretation of postmortem change in cadavers in Spain. J. Forensic Sci. 2004, 49, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Liendl, L.; Grillari, J.; Schosserer, M. Raman fingerprints as promising markers of cellular senescence and aging. GeroScience 2020, 42, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, T.; Niciejewski, K.; Kozielski, M.; Szybowicz, M.; Siatkowski, M.; Krauss, H. Identifying compositional and structural changes in spongy and subchondral bone from the hip joints of patients with osteoarthritis using Raman spectroscopy. J. Biomed. Opt. 2012, 17, 017007. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of bone and apatitic biomaterials as revealed by intravital Raman microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, M.; Buchwald, T.; Szybowicz, M.; Błaszczak, Z.; Piotrowski, A.; Ciesielczyk, B. Determination of composition and structure of spongy bone tissue in human head of femur by Raman spectral mapping. J. Mater. Sci. Mater. Med. 2011, 22, 1653–1661. [Google Scholar] [CrossRef]
- Kazanci, M.; Wagner, H.D.; Manjubala, N.I.; Gupta, H.S.; Paschalis, E.; Roschger, P.; Fratzl, P. Raman imaging of two orthogonal planes within cortical bone. Bone 2007, 41, 456–461. [Google Scholar] [CrossRef]
- Goodyear, S.R.; Gibson, I.R.; Skakle, J.M.; Wells, R.P.; Aspden, R.M. A comparison of cortical and trabecular bone from C57 Black 6 mice using Raman spectroscopy. Bone 2009, 44, 899–907. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Body, D.R. The lipid composition of adipose tissue. Prog. Lipid Res. 1988, 27, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Paschou, A.M.; Katsikini, M.; Christofilos, D.; Arvanitidis, J.; Ves, S. High pressure Raman study of type-I collagen. FEBS J. 2018, 285, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Redman, S.; Milz, S.; Büttner, A.; Amin, A.; Moriggl, B.; Brenner, E.; Emery, P.; McGonagle, D.; Bydder, G. Adipose tissue at entheses: The rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann. Rheum. Dis. 2004, 63, 1549–1555. [Google Scholar] [CrossRef]
- Shaw, H.M.; Santer, R.M.; Watson, A.H.; Benjamin, M. Adipose tissue at entheses: The innervation and cell composition of the retromalleolar fat pad associated with the rat Achilles tendon. J. Anat. 2007, 211, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta 1992, 1120, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Orilisi, G.; Monterubbianesi, R.; Notarstefano, V.; Tosco, V.; Vitiello, F.; Giuliani, G.; Putignano, A.; Orsini, G. New insights from Raman MicroSpectroscopy and Scanning Electron Microscopy on the microstructure and chemical composition of vestibular and lingual surfaces in permanent and deciduous human teeth. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 260, 119966. [Google Scholar] [CrossRef]
- Orsini, G.; Orilisi, G.; Notarstefano, V.; Monterubbianesi, R.; Vitiello, F.; Tosco, V.; Belloni, A.; Putignano, A.; Giorgini, E. Vibrational Imaging Techniques for the Characterization of Hard Dental Tissues: From Bench-Top to Chair-Side. Appl. Sci. 2021, 11, 11953. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; Al-Umran, K.; Al-Habdan, I.; Al-Mulhim, F. Ultrasonography: Can it differentiate between vasoocclusive crisis and acute osteomyelitis in sickle cell disease? J. Pediatr. Orthop. 1998, 18, 552–554. [Google Scholar] [CrossRef]
- Berger, E.; Saunders, N.; Wang, L.; Friedman, J.N. Sickle cell disease in children: Differentiating osteomyelitis from vaso-occlusive crisis. Arch. Pediatr. Adolesc. Med. 2009, 163, 251–255. [Google Scholar] [CrossRef]
- Goswami, K.; Parvizi, J.; Maxwell Courtney, P. Current Recommendations for the Diagnosis of Acute and Chronic PJI for Hip and Knee-Cell Counts, Alpha-Defensin, Leukocyte Esterase, Next-generation Sequencing. Curr. Rev. Musculoskelet. Med. 2018, 11, 428–438. [Google Scholar] [CrossRef]
- Talsma, D.T.; Ploegmakers, J.J.W.; Jutte, P.C.; Kampinga, G.; Wouthuyzen-Bakker, M. Time to positivity of acute and chronic periprosthetic joint infection cultures. Diagn. Microbiol. Infect. Dis. 2021, 99, 115178. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Carvalho, A.; Santos, A.C.; Abreu, M.A. Optimal microbiological sampling for the diagnosis of osteoarticular infection. EFORT Open Rev. 2021, 6, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.T.; Della Valle, C.J. Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J. Arthroplast. 2017, 32, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Bürger, J.; Palmowski, Y.; Strube, P.; Perka, C.; Putzier, M.; Pumberger, M. Low sensitivity of histopathological examination of peri-implant tissue samples in diagnosing postoperative spinal implant infection. Bone Jt. J. 2020, 102-b, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Ahmed, R.; Mahadevan-Jansen, A.; Nyman, J.S. Compositional assessment of bone by Raman spectroscopy. Analyst 2021, 146, 7464–7490. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.D.C.A.; Limirio, P.H.J.O.; Novais, V.R.; Dechichi, P. Fourier transform infrared spectroscopy (FTIR) application chemical characterization of enamel, dentin and bone. Appl. Spectrosc. Rev. 2018, 53, 747–769. [Google Scholar] [CrossRef]
- Rubio, L.; Vargas, A.; Rivera, P.; López-Gambero, A.J.; Tovar, R.; Christians, J.K.; Martín-de-las-Heras, S.; Rodríguez de Fonseca, F.; Chowen, J.A.; Argente, J.; et al. Recombinant IGF-1 Induces Sex-Specific Changes in Bone Composition and Remodeling in Adult Mice with Pappa2 Deficiency. Int. J. Mol. Sci. 2021, 22, 4048. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared Assessment of Bone Quality: A Review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef]
- Paschalis, E.P. Fourier Transform Infrared Imaging of Bone. In Bone Research Protocols; Idris, A.I., Ed.; Springer: New York, NY, USA, 2019; pp. 641–649. [Google Scholar]
- Taylor, E.A.; Lloyd, A.A.; Salazar-Lara, C.; Donnelly, E. Raman and Fourier Transform Infrared (FT-IR) Mineral to Matrix Ratios Correlate with Physical Chemical Properties of Model Compounds and Native Bone Tissue. Appl. Spectrosc. 2017, 71, 2404–2410. [Google Scholar] [CrossRef]
- Rubio, L.; Suárez, J.; Martin-de-las-Heras, S.; Zapico, S.C. Partners in Postmortem Interval Estimation: X-ray Diffraction and Fourier Transform Spectroscopy. Int. J. Mol. Sci. 2023, 24, 6793. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.L.; Nespeca, M.G.; Pavini, W.D.; Ferreira, E.C.; Gomes Neto, J.A. A user-friendly excel spreadsheet for dealing with spectroscopic and chromatographic data. Chemom. Intell. Lab. Syst. 2019, 194, 103816. [Google Scholar] [CrossRef]
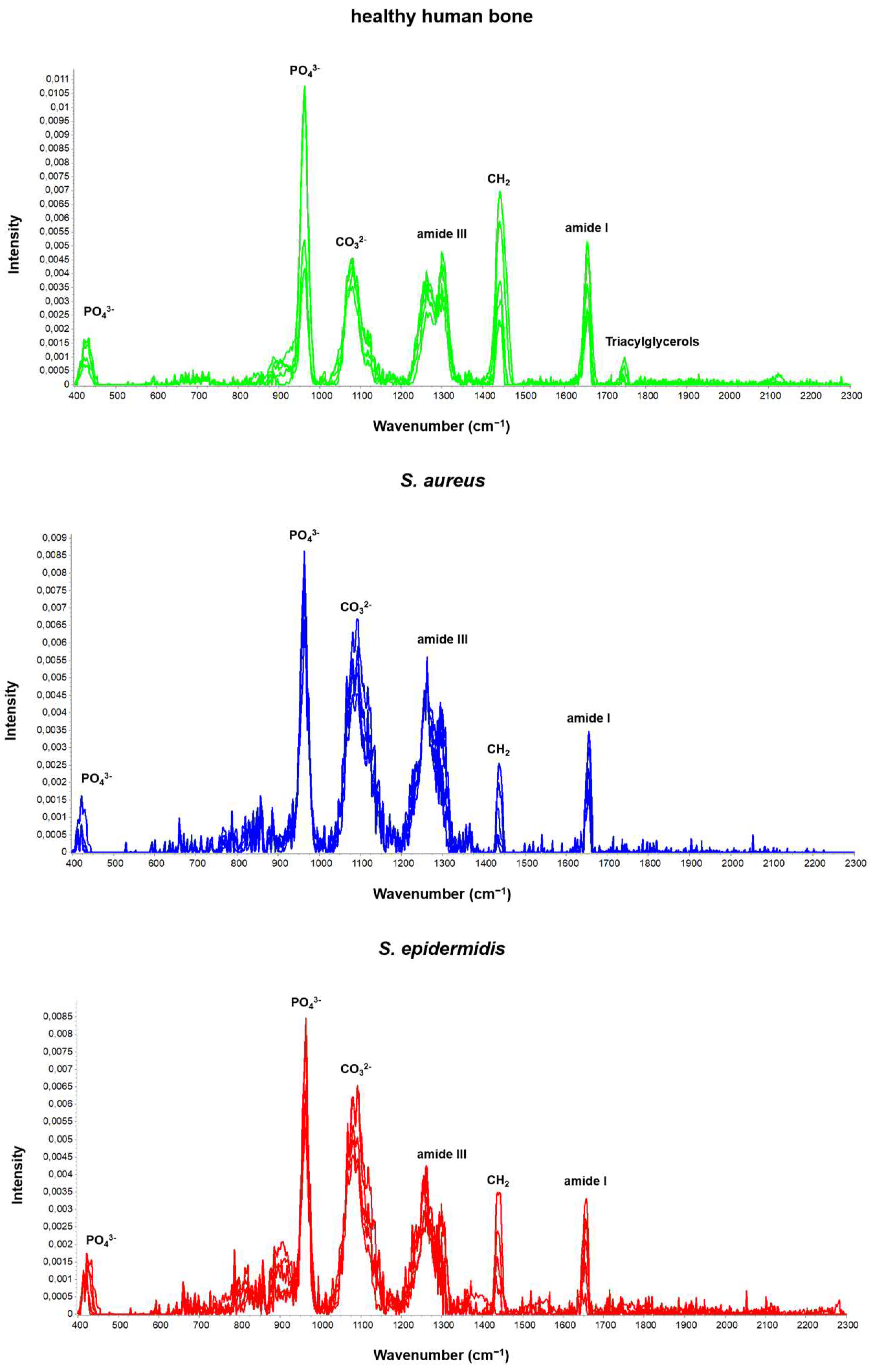
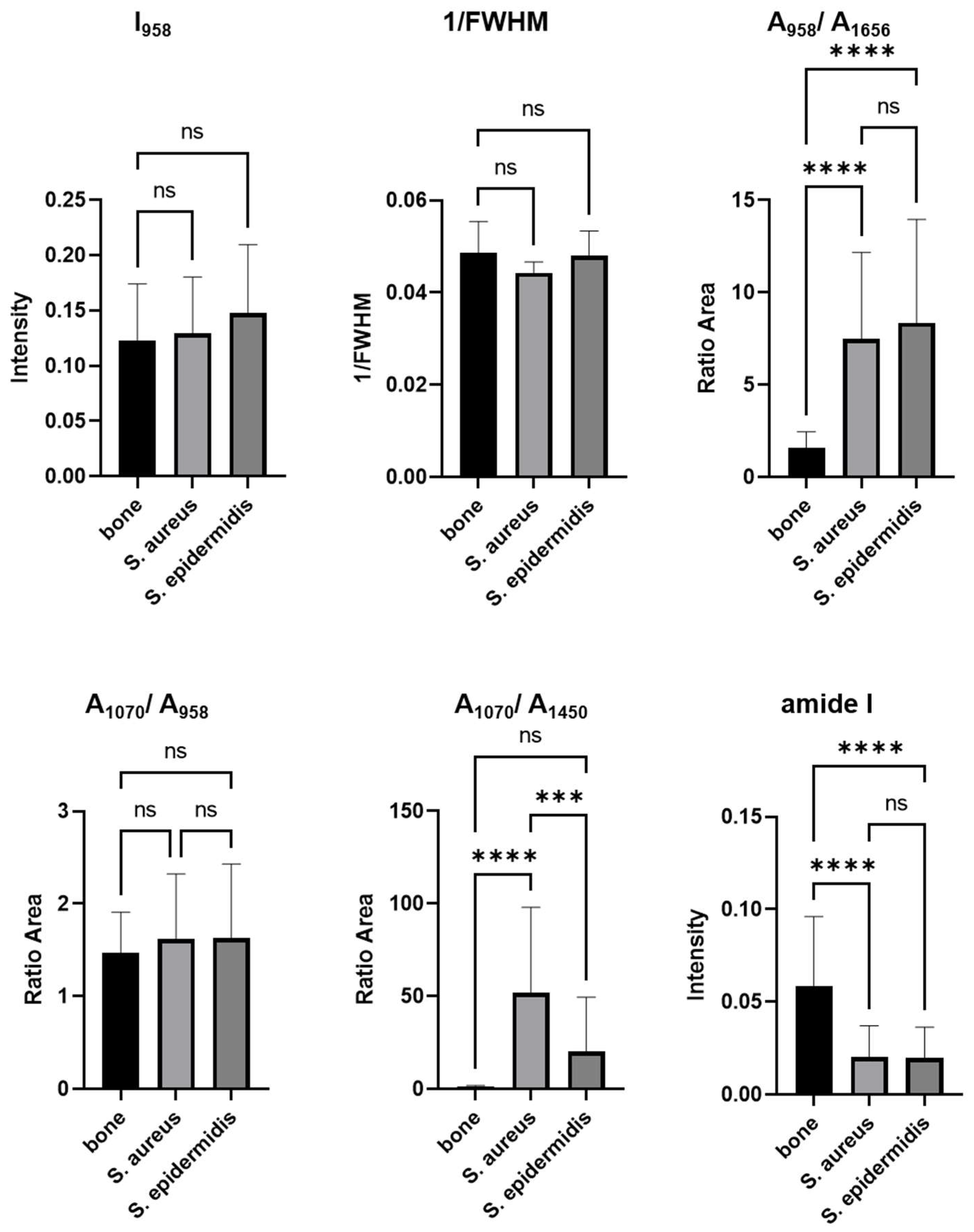

| Name | Description | Determination | p-Values Based on a One-Factor ANOVA | ||
|---|---|---|---|---|---|
| Healthy Human Bone | Staphylococcus epidermidis | Staphylococcus aureus | |||
| Phosphate | ν3PO43− Amount of phosphate | (I958) | <0.0001 **** | <0.0001 **** | 0.9946 ns |
| Crystallinity | 1/FWHM958 | 0.1572 ns | 0.9431 ns | 0.2728 ns | |
| Mineral/matrix (MMR) phosphate/amide I | ν3PO43−/amide I Mineral component amount to the organic one | (A958/A1656) | <0.0001 **** | <0.0001 **** | 0.7055 ns |
| Mineral quality and crystallinity carbonate/phosphate | ν1CO32−/ν1PO43− Carbonate incorporation extent in the hydroxyapatite lattice | (A1070/A958) | 0.6208 ns | 0.5697 ns | 0.9968 ns |
| Mineral carbonate content (MinCarb) | ν1CO32−/(C-H) bend; CH2 wag | (A1070/A1450) | <0.0001 **** | 0.0943 ns | 0.0007 *** |
| Amide I | Amide I of α-helical structures Arrangement and quantity of collagen | (I1656) | <0.0001 **** | <0.0001 **** | 0.9946 ns |
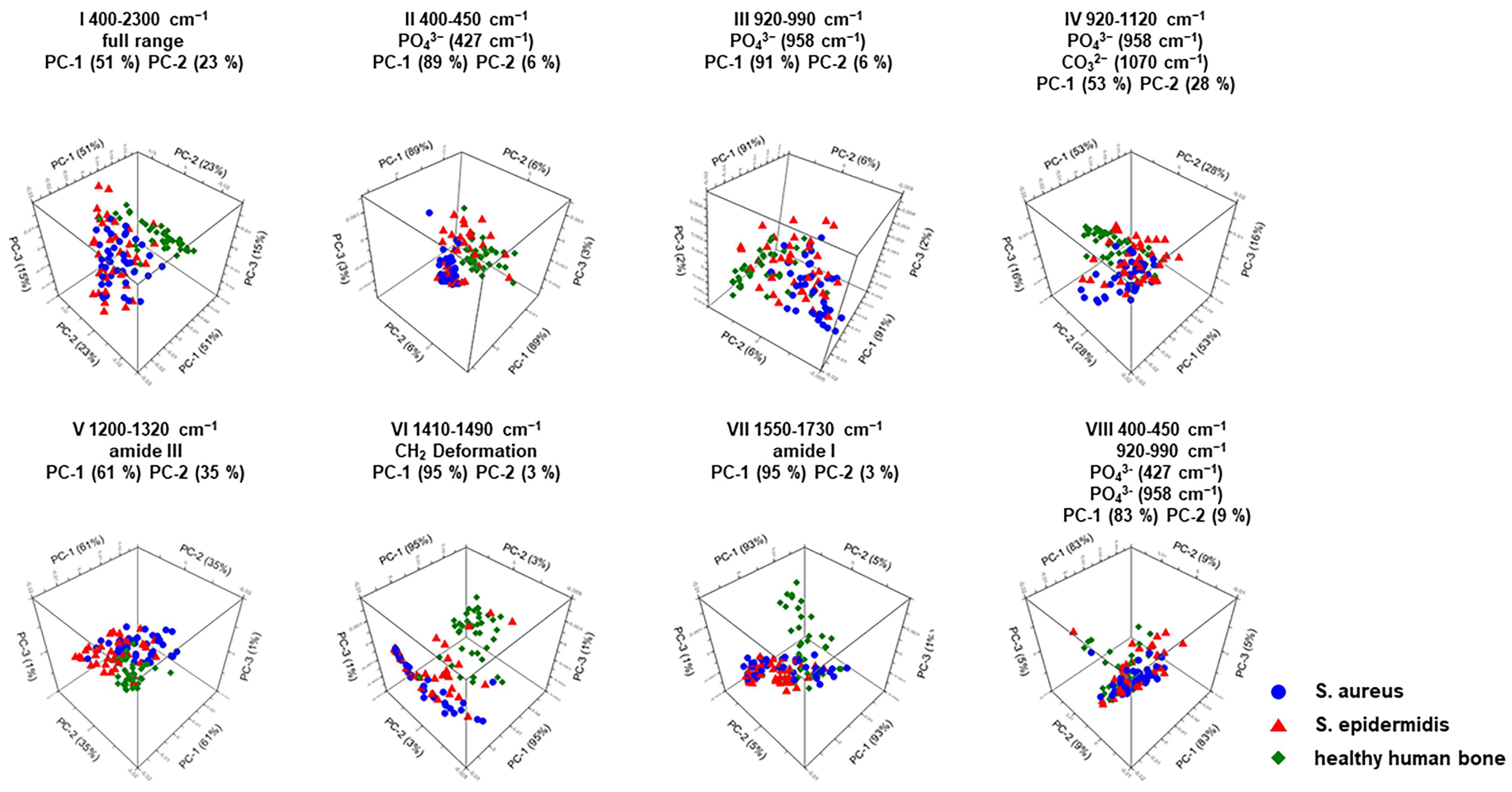
| Wavenumber Range Number | PCA | Assignment | Spectral Region |
|---|---|---|---|
| I | PC-1 (51%) PC-2 (23%) | Full wavenumber range | 400 cm−1 to 2300 cm−1 |
| II | PC-1 (89%) PC-2 (6%) | PO43− (427 cm−1) | 400 cm−1 to 450 cm−1 |
| III | PC-1 (91%) PC-2 (6%) | PO43− (958 cm−1) | 920 cm−1 to 990 cm−1 |
| IV | PC-1 (53%) PC-2 (28%) | PO43− (958 cm−1) CO32− (1070 cm−1) | 920 cm−1 to 1120 cm−1 |
| V | PC-1 (61%) PC-2 (35%) | Amide III (1246 cm−1) | 1200 cm−1 to 1320 cm−1 |
| VI | PC-1 (95%) PC-2 (3%) | CH2 deformation (1450 cm−1) | 1410 cm−1 to 1490 cm−1 |
| VII | PC-1 (93%) PC-2 (5%) | Amide I (1656 cm−1) | 1550 cm−1 to 1730 cm−1 |
| VIII | PC-1 (83%) PC-2 (9%) | PO43− (427 cm−1) PO43− (958 cm−1) | 400 cm−1 to 450 cm−1 920 cm−1 to 990 cm−1 |
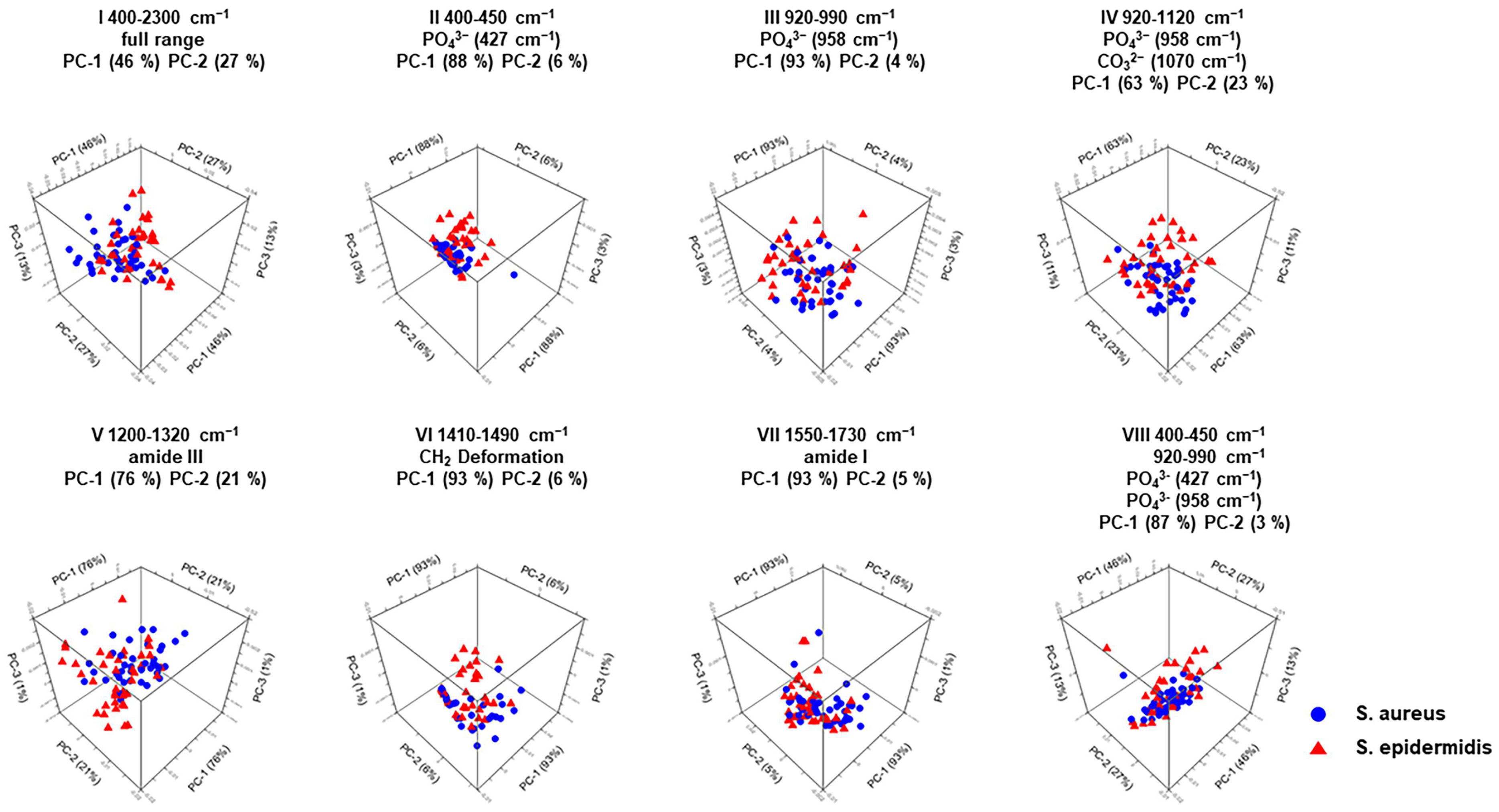
| Wavenumber Range Number | PCA | Assignment | Spectral Region |
|---|---|---|---|
| I | PC-1 (46%) PC-2 (27%) | Full wavenumber range | 400 cm−1 to 2300 cm−1 |
| II | PC-1 (88%) PC-2 (6%) | PO43− (427 cm−1) | 400 cm−1 to 450 cm−1 |
| III | PC-1 (93%) PC-2 (4%) | PO43− (958 cm−1) | 920 cm−1 to 990 cm−1 |
| IV | PC-1 (63%) PC-2 (23%) | PO43− (958 cm−1) CO32− (1070 cm−1) | 920 cm−1 to 1120 cm−1 |
| V | PC-1 (76%) PC-2 (21%) | Amide III (1246 cm−1) | 1200 cm−1 to 1320 cm−1 |
| VI | PC-1 (93%) PC-2 (6%) | CH2 deformation (1450 cm−1) | 1410 cm−1 to 1490 cm−1 |
| VII | PC-1 (93%) PC-2 (5%) | Amide I (1656 cm−1) | 1550 cm−1 to 1730 cm−1 |
| VIII | PC-1 (87%) PC-2 (3%) | PO43− (427 cm−1) PO43− (958 cm−1) | 400 cm−1 to 450 cm−1 920 cm−1 to 990 cm−1 |
| Age | Female | Male |
|---|---|---|
| <50 | 2 | 3 |
| 50–60 | 6 | 2 |
| 60–70 | 4 | 5 |
| 70–80 | 9 | 7 |
| >80 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindtner, R.A.; Wurm, A.; Pirchner, E.; Putzer, D.; Arora, R.; Coraça-Huber, D.C.; Schirmer, M.; Badzoka, J.; Kappacher, C.; Huck, C.W.; et al. Enhancing Bone Infection Diagnosis with Raman Handheld Spectroscopy: Pathogen Discrimination and Diagnostic Potential. Int. J. Mol. Sci. 2024, 25, 541. https://doi.org/10.3390/ijms25010541
Lindtner RA, Wurm A, Pirchner E, Putzer D, Arora R, Coraça-Huber DC, Schirmer M, Badzoka J, Kappacher C, Huck CW, et al. Enhancing Bone Infection Diagnosis with Raman Handheld Spectroscopy: Pathogen Discrimination and Diagnostic Potential. International Journal of Molecular Sciences. 2024; 25(1):541. https://doi.org/10.3390/ijms25010541
Chicago/Turabian StyleLindtner, Richard Andreas, Alexander Wurm, Elena Pirchner, David Putzer, Rohit Arora, Débora Cristina Coraça-Huber, Michael Schirmer, Jovan Badzoka, Christoph Kappacher, Christian Wolfgang Huck, and et al. 2024. "Enhancing Bone Infection Diagnosis with Raman Handheld Spectroscopy: Pathogen Discrimination and Diagnostic Potential" International Journal of Molecular Sciences 25, no. 1: 541. https://doi.org/10.3390/ijms25010541
APA StyleLindtner, R. A., Wurm, A., Pirchner, E., Putzer, D., Arora, R., Coraça-Huber, D. C., Schirmer, M., Badzoka, J., Kappacher, C., Huck, C. W., & Pallua, J. D. (2024). Enhancing Bone Infection Diagnosis with Raman Handheld Spectroscopy: Pathogen Discrimination and Diagnostic Potential. International Journal of Molecular Sciences, 25(1), 541. https://doi.org/10.3390/ijms25010541










