JNK Signaling Positively Regulates Acute Ethanol Tolerance in C. elegans
Abstract
:1. Introduction
2. Results
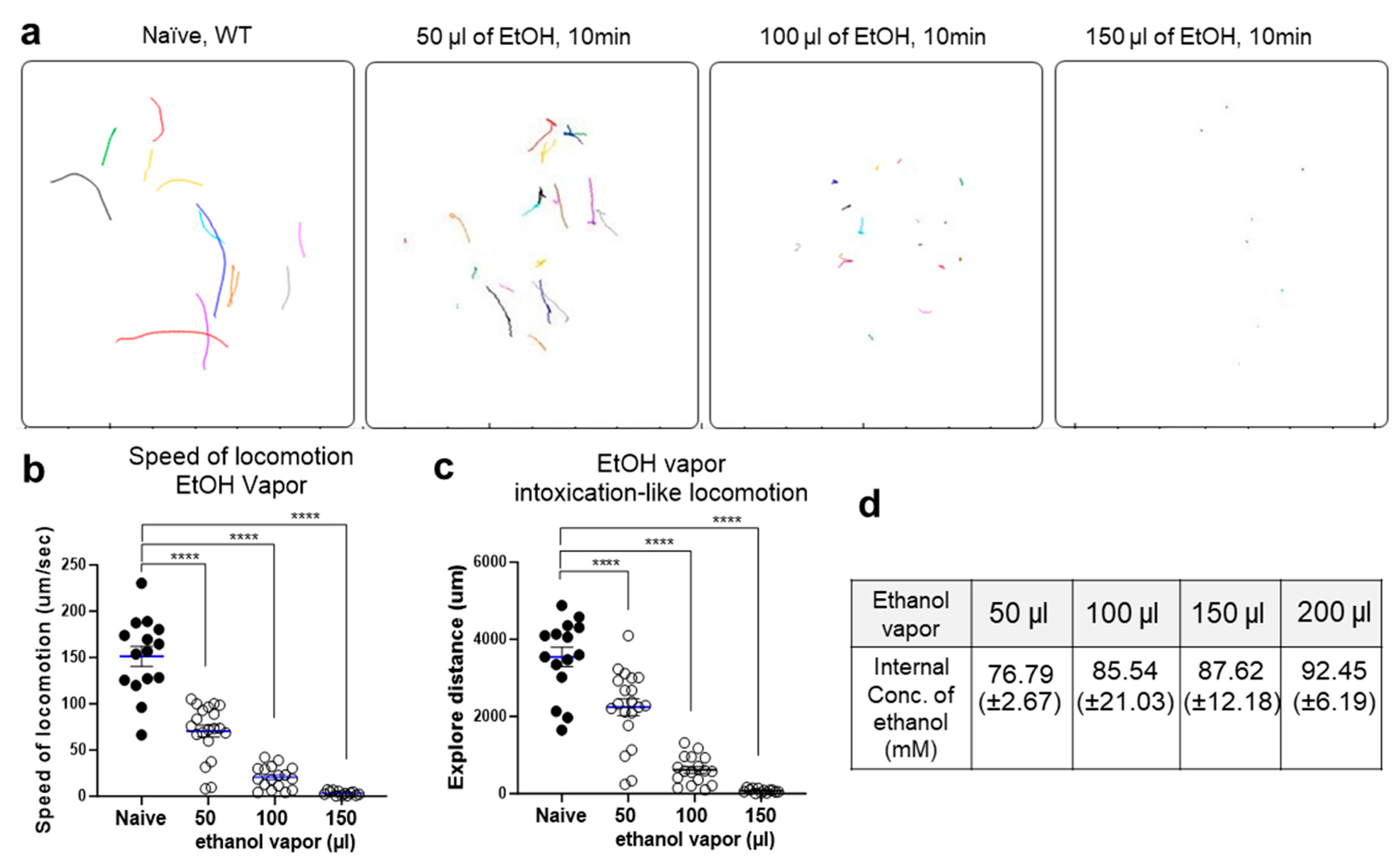
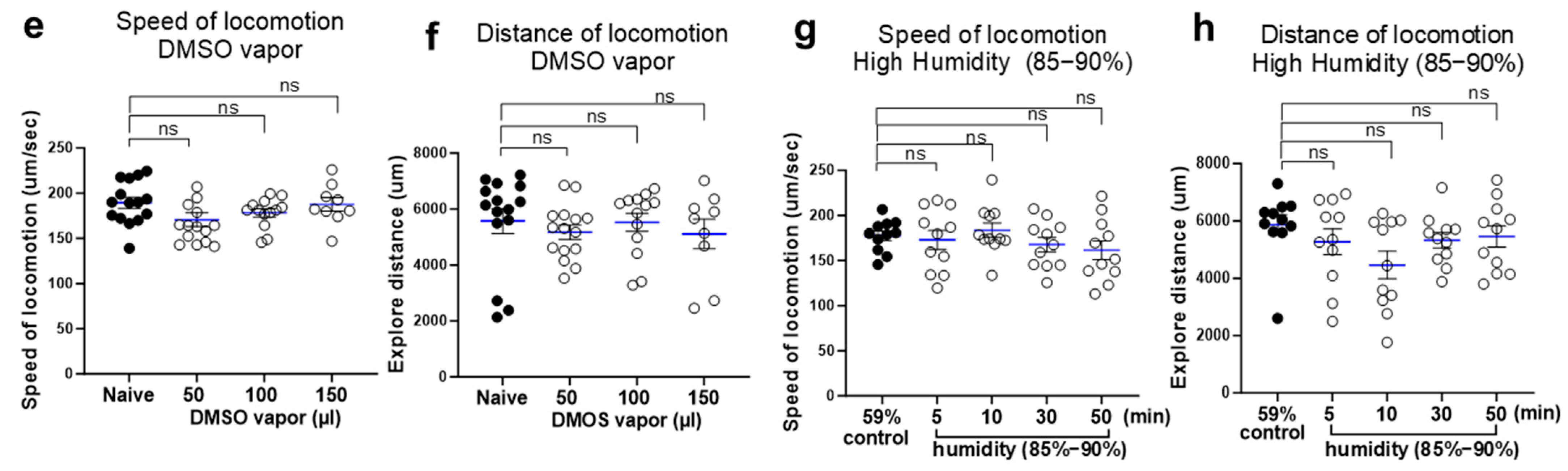
2.1. Ethanol Vapor Exposure Elicits Intoxication-like Behavioral Responses in C. elegans
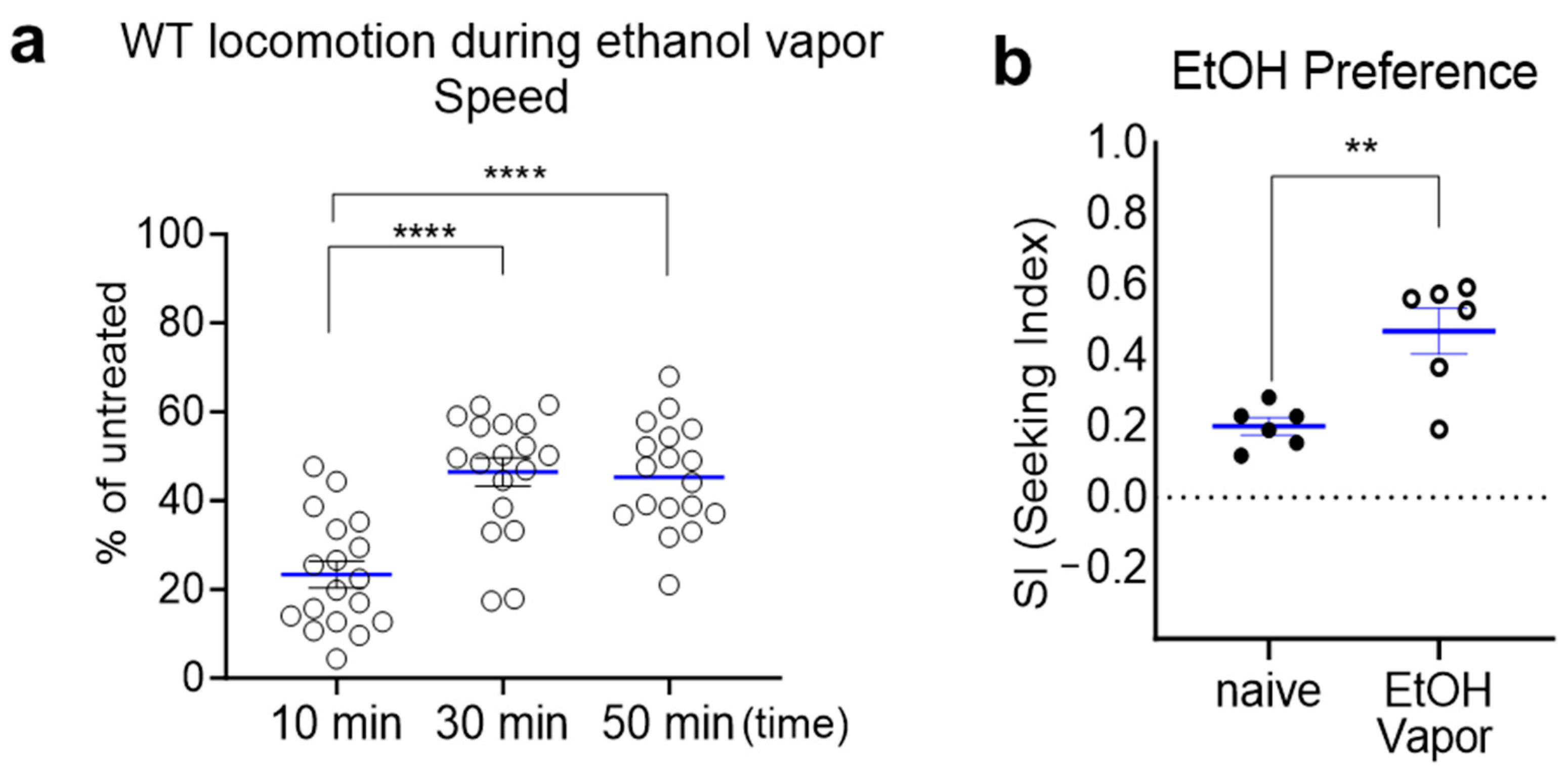
2.2. Prolonged Exposure to Ethanol Vapor Induces Ethanol Tolerance and Ethanol Preference
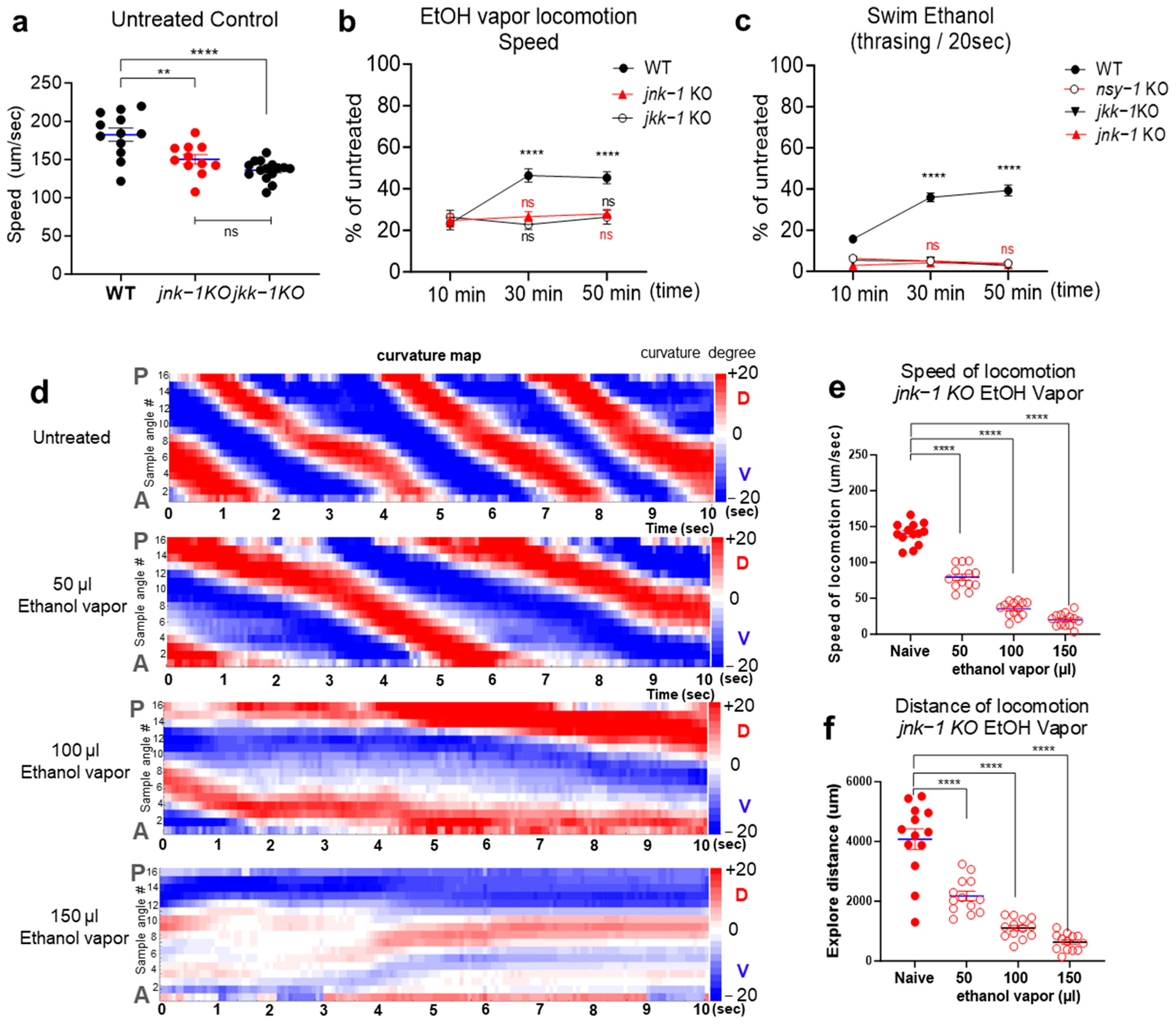
2.3. JNK Signaling Cascades Positively Regulate Acute Ethanol Tolerance
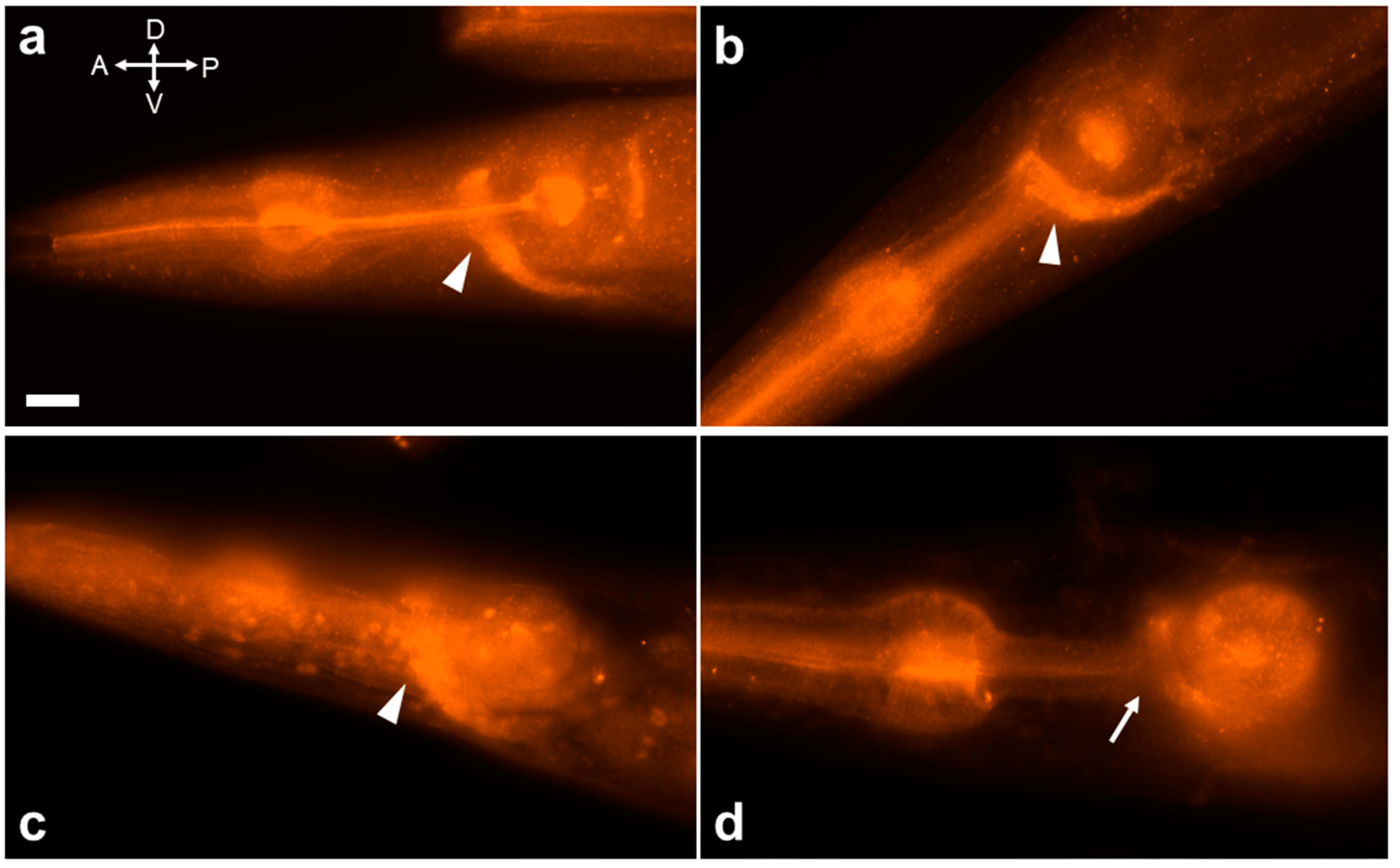
2.4. Activation of JNK-1 upon Exposure to Ethanol
3. Discussion
4. Materials and Methods
4.1. Strains and Maintenance
4.2. Locomotion Analysis and Ethanol Vapor
4.3. Curvature Map Analysis
4.4. Measurement of Internal Ethanol Concentration Using Spectrophotometric Analysis
4.5. Ethanol Tolerance Assay
4.6. Ethanol Preference Assay
4.7. Statistical Analysis
4.8. Immunochemistry for Phosphorylated JNK-1
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollard, M.S.; Tucker, J.S.; Green, H.D., Jr. Changes in Adult Alcohol Use and Consequences During the COVID-19 Pandemic in the US. JAMA Netw. Open 2020, 3, e2022942. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.L.; Sellers, E.M.; Hamilton, C.; Naranjo, C.A.; Dorian, P. Is there acute tolerance to alcohol at steady state? J. Stud. Alcohol. 1985, 46, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Korpi, E.R.; den Hollander, B.; Farooq, U.; Vashchinkina, E.; Rajkumar, R.; Nutt, D.J.; Hyytiä, P.; Dawe, G.S. Mechanisms of Action and Persistent Neuroplasticity by Drugs of Abuse. Pharmacol. Rev. 2015, 67, 872–1004. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Newton, P.M.; Oyasu, M.; McMahon, T.; Chou, W.-H.H.; Connolly, J.; Messing, R.O. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology 2007, 32, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Rohsenow, D.J.; Monti, P.M. Does urge to drink predict relapse after treatment? Alcohol. Res. Health 1999, 23, 225–232. [Google Scholar] [PubMed]
- Koob, G.F. Animal models of craving for ethanol. Addiction 2000, 95 (Suppl. 2), S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.A.; Roberts, A.J.; Cooney, N.; Swift, R.M.; Anton, R.F.; Rohsenow, D.J. The role of craving in alcohol use, dependence, and treatment. Alcohol. Clin. Exp. Res. 2001, 25, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.G.; Pierce-Shimomura, J.T.; Kim, H.; VanHoven, M.K.; Thiele, T.R.; Bonci, A.; Bargmann, C.I.; McIntire, S.L. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 2003, 115, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jee, C.; McIntire, S.L. Ethanol preference in C. elegans. Genes. Brain Behav. 2009, 8, 578–585. [Google Scholar] [CrossRef]
- Jee, C.; Lee, J.; Lim, J.P.; Parry, D.; Messing, R.O.; McIntire, S.L. SEB-3, a CRF receptor-like GPCR, regulates locomotor activity states, stress responses and ethanol tolerance in Caenorhabditis elegans. Genes. Brain Behav. 2013, 12, 250–262. [Google Scholar] [CrossRef]
- Barclay, J.W.; Graham, M.E.; Edwards, M.R.; Johnson, J.R.; Morgan, A.; Burgoyne, R.D. Presynaptic targets for acute ethanol sensitivity. Biochem. Soc. Trans. 2010, 38, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, J.C.; Davies, A.G. The role of the BK channel in ethanol response behaviors: Evidence from model organism and human studies. Front. Physiol. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Cook, J.; Choquet, H.; Jorgenson, E.; Yin, J.; Kinnunen, T.; Barclay, J.; Morris, A.P.; Pirmohamed, M. Functional validity, role, and implications of heavy alcohol consumption genetic loci. Sci. Adv. 2020, 6, eaay5034. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.G.; Bettinger, J.C.; Thiele, T.R.; Judy, M.E.; McIntire, S.L. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron 2004, 42, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.R.; Li, Y.; Rankin, C.H. Effects of developmental exposure to ethanol on Caenorhabditis elegans. Alcohol. Clin. Exp. Res. 2008, 32, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Salim, C.; Kan, A.K.; Batsaikhan, E.; Patterson, E.C.; Jee, C. Neuropeptidergic regulation of compulsive ethanol seeking in C. elegans. Sci. Rep. 2022, 12, 1804. [Google Scholar] [CrossRef] [PubMed]
- Jee, C.; Batsaikhan, E.; Salim, C. Neurobiological Basis of Aversion-Resistant Ethanol Seeking in C. elegans. Metabolites 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.V. Recent Progress in Regulation of Aging by Insulin/IGF-1 Signaling in Caenorhabditis elegans. Mol. Cells 2022, 45, 763–770. [Google Scholar] [CrossRef]
- Kwon, H.C.; Bae, Y.; Lee, S.V. The Role of mRNA Quality Control in the Aging of Caenorhabditis elegans. Mol. Cells 2023, 46, 664–671. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.A.; Willicott, C.W.; Caldwell, G.A. Modeling neurodegeneration in Caenorhabditiselegans. Dis. Model. Mech. 2020, 13, dmm046110. [Google Scholar] [CrossRef] [PubMed]
- Morcos, M.; Hutter, H. The model Caenorhabditis elegans in diabetes mellitus and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.C.; Allouche, L.; Chapot, P.A.; Vranizan, K.; Moore, M.S.; Heberlein, U.; Wolf, F.W. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol. Clin. Exp. Res. 2010, 34, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.G.; Sedensky, M.M. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol. Clin. Exp. Res. 1995, 19, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, J.T.; Davis, S.J.; Song, S.S.; Burnette, C.R.; Grotewiel, M.; Shelton, K.L.; Pierce-Shimomura, J.T.; Davies, A.G.; Bettinger, J.C. Ethanol Metabolism and Osmolarity Modify Behavioral Responses to Ethanol in C. elegans. Alcohol. Clin. Exp. Res. 2012, 36, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Pierce-Shimomura, J.T.; Chen, B.L.; Mun, J.J.; Ho, R.; Sarkis, R.; McIntire, S.L. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc. Natl. Acad. Sci. USA 2008, 105, 20982–20987. [Google Scholar] [CrossRef] [PubMed]
- Salim, C.; Batsaikhan, E.; Kan, A.K.; Chen, H.; Jee, C. Nicotine Motivated Behavior in C. elegans. Int. J. Mol. Sci. 2024, 25, 1634. [Google Scholar] [CrossRef]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef]
- Ronkina, N.; Gaestel, M. MAPK-Activated Protein Kinases: Servant or Partner? Annu. Rev. Biochem. 2022, 91, 505–540. [Google Scholar] [CrossRef]
- Aroor, A.R.; Shukla, S.D. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004, 74, 2339–2364. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; James, T.T.; Jackson, D.E.; Shukla, S.D. Differential changes in MAP kinases, histone modifications, and liver injury in rats acutely treated with ethanol. Alcohol. Clin. Exp. Res. 2010, 34, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Ju, X.; Simpkins, J.W.; Metzger, D.B.; Yan, L.J.; Wen, Y. Ethanol withdrawal acts as an age-specific stressor to activate cerebellar p38 kinase. Neurobiol. Aging 2011, 32, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Kapfhamer, D.; King, I.; Zou, M.E.; Lim, J.P.; Heberlein, U.; Wolf, F.W. JNK pathway activation is controlled by Tao/TAOK3 to modulate ethanol sensitivity. PLoS ONE 2012, 7, e50594. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Lu, L.; Tan, Y.; Pan, X.; Cai, Y.; Wang, X.; Hong, J.; Zhong, C.; Wang, F.; Zhang, X.Y.; et al. Genome-wide association discoveries of alcohol dependence. Am. J. Addict. 2014, 23, 526–539. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Underwood, R.S.; Greenwald, I.; Shaye, D.D. OrthoList 2: A New Comparative Genomic Analysis of Human and Caenorhabditis elegans Genes. Genetics 2018, 210, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Lozano, J.; Morales, A.; Lin, X.; Deng, X.; Hengartner, M.O.; Kolesnick, R.N. jkk-1 and mek-1 regulate body movement coordination and response to heavy metals through jnk-1 in Caenorhabditis elegans. Embo J. 2001, 20, 5114–5128. [Google Scholar] [CrossRef]
- Kawasaki, M.; Hisamoto, N.; Iino, Y.; Yamamoto, M.; Ninomiya-Tsuji, J.; Matsumoto, K. A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. Embo J. 1999, 18, 3604–3615. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Sagasti, A.; Hisamoto, N.; Hyodo, J.; Tanaka-Hino, M.; Matsumoto, K.; Bargmann, C.I. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell 2001, 105, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.A.; McIntire, S.L. A Caenorhabditis elegans p38 MAP kinase pathway mutant protects from dopamine, methamphetamine, and MDMA toxicity. Neurosci. Lett. 2011, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 2002, 12, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef]
- Payne, D.M.; Rossomando, A.J.; Martino, P.; Erickson, A.K.; Her, J.H.; Shabanowitz, J.; Hunt, D.F.; Weber, M.J.; Sturgill, T.W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). Embo J. 1991, 10, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Nunes, F.; Henkel, A.; Heinick, A.; Paul, R.J. The MAP kinase JNK-1 of Caenorhabditis elegans: Location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J. Cell Physiol. 2008, 214, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Larnerd, C.; Adhikari, P.; Valdez, A.; Del Toro, A.; Wolf, F.W. Rapid and Chronic Ethanol Tolerance Are Composed of Distinct Memory-Like States in Drosophila. J. Neurosci. 2023, 43, 2210–2220. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Copeland, R.L., Jr.; Alex, P.K.; Pradhan, S.N. Behavioral effects of ethanol inhalation in rats. Pharmacol. Biochem. Behav. 1991, 38, 699–704. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Richardson, H.N.; Cole, M.; Koob, G.F. Vapor inhalation of alcohol in rats. Curr. Protoc. Neurosci. 2008, 44, 9.29.1–9.29.19. [Google Scholar] [CrossRef] [PubMed]
- Vierkant, V.; Xie, X.; Wang, X.; Wang, J. Experimental Models of Alcohol Use Disorder and Their Application for Pathophysiological Investigations. Curr. Protoc. 2023, 3, e831. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; Koob, G.F. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007, 30, 399–406. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, L.E.; Roberts, A.J.; Smith, R.T.; Koob, G.F. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 2004, 28, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Rimondini, R.; Arlinde, C.; Sommer, W.; Heilig, M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. Faseb J. 2002, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gage, G.A.; Muench, M.A.; Jee, C.; Kearns, D.N.; Chen, H.; Tunstall, B.J. Intermittent-access operant alcohol self-administration promotes binge-like drinking and drinking despite negative consequences in male and female heterogeneous stock rats. Neuropharmacology 2023, 235, 109564. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kwon, A.; Oh, Y.; Rhee, S.; Song, W.K. Microtubule Acetylation-Specific Inhibitors Induce Cell Death and Mitotic Arrest via JNK/AP-1 Activation in Triple-Negative Breast Cancer Cells. Mol. Cells 2023, 46, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ruediger, C.; Shapira, M. Integration of Stress Signaling in Caenorhabditis elegans Through Cell-Nonautonomous Contributions of the JNK Homolog KGB-1. Genetics 2018, 210, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Gerke, P.; Keshet, A.; Mertenskötter, A.; Paul, R.J. The JNK-like MAPK KGB-1 of Caenorhabditis elegans promotes reproduction, lifespan, and gene expressions for protein biosynthesis and germline homeostasis but interferes with hyperosmotic stress tolerance. Cell Physiol. Biochem. 2014, 34, 1951–1973. [Google Scholar] [CrossRef]
- Mizuno, T.; Fujiki, K.; Sasakawa, A.; Hisamoto, N.; Matsumoto, K. Role of the Caenorhabditis elegans Shc adaptor protein in the c-Jun N-terminal kinase signaling pathway. Mol. Cell Biol. 2008, 28, 7041–7049. [Google Scholar] [CrossRef]
- Neumann-Haefelin, E.; Qi, W.; Finkbeiner, E.; Walz, G.; Baumeister, R.; Hertweck, M. SHC-1/p52Shc targets the insulin/IGF-1 and JNK signaling pathways to modulate life span and stress response in C. elegans. Genes. Dev. 2008, 22, 2721–2735. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Hino, M.; Sagasti, A.; Hisamoto, N.; Kawasaki, M.; Nakano, S.; Ninomiya-Tsuji, J.; Bargmann, C.I.; Matsumoto, K. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 2002, 3, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Raber, J.; Yang, D.; Su, B.; Mucke, L. Dynamic regulation of c-Jun N-terminal kinase activity in mouse brain by environmental stimuli. Proc. Natl. Acad. Sci. USA 1997, 94, 12655–12660. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Waudby, C.A.; Alvarez-Teijeiro, S.; Josue Ruiz, E.; Suppinger, S.; Pinotsis, N.; Brown, P.R.; Behrens, A.; Christodoulou, J.; Mylona, A. An intrinsic temporal order of c-JUN N-terminal phosphorylation regulates its activity by orchestrating co-factor recruitment. Nat. Commun. 2022, 13, 6133. [Google Scholar] [CrossRef] [PubMed]
- Kallunki, T.; Deng, T.; Hibi, M.; Karin, M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 1996, 87, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Eferl, R.; Wagner, E.F. AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 2003, 3, 859–868. [Google Scholar] [CrossRef]
- Angel, P.; Allegretto, E.A.; Okino, S.T.; Hattori, K.; Boyle, W.J.; Hunter, T.; Karin, M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature 1988, 332, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Hattori, K.; Smeal, T.; Karin, M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell 1988, 55, 875–885. [Google Scholar] [CrossRef]
- Rauscher, F.J., 3rd; Voulalas, P.J.; Franza, B.R., Jr.; Curran, T. Fos and Jun bind cooperatively to the AP-1 site: Reconstitution in vitro. Genes. Dev. 1988, 2, 1687–1699. [Google Scholar] [CrossRef]
- Hiatt, S.M.; Duren, H.M.; Shyu, Y.J.; Ellis, R.E.; Hisamoto, N.; Matsumoto, K.; Kariya, K.; Kerppola, T.K.; Hu, C.D. Caenorhabditis elegans FOS-1 and JUN-1 regulate plc-1 expression in the spermatheca to control ovulation. Mol. Biol. Cell 2009, 20, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Los, F.C.; Huffman, D.L.; Wachi, S.; Kloft, N.; Husmann, M.; Karabrahimi, V.; Schwartz, J.L.; Bellier, A.; Ha, C.; et al. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 2011, 7, e1001314. [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Zehorai, E.; Hanoch, T.; Blenis, J.; Seger, R. The nuclear translocation of the kinases p38 and JNK promotes inflammation-induced cancer. Sci. Signal 2018, 11, eaao3428. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Kuan, C.Y.; Whitmarsh, A.J.; Rincón, M.; Zheng, T.S.; Davis, R.J.; Rakic, P.; Flavell, R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 1997, 389, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Schauwecker, P.E. Seizure-induced neuronal death is associated with induction of c-Jun N-terminal kinase and is dependent on genetic background. Brain Res. 2000, 884, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Herdegen, T.; Waetzig, V. AP-1 proteins in the adult brain: Facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene 2001, 20, 2424–2437. [Google Scholar] [CrossRef] [PubMed]
- Pahng, A.R.; Paulsen, R.I.; McGinn, M.A.; Farooq, M.A.; Edwards, K.N.; Edwards, S. Dysregulation of c-Jun N-terminal kinase phosphorylation in alcohol dependence. Alcohol 2019, 75, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Jee, C.; Lee, J.; Lee, J.I.; Lee, W.H.; Park, B.-J.J.; Yu, J.-R.R.; Park, E.; Kim, E.; Ahnn, J. SHN-1, a Shank homologue in C. elegans, affects defecation rhythm via the inositol-1,4,5-trisphosphate receptor. FEBS Lett. 2004, 561, 29–36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, C.; Batsaikhan, E. JNK Signaling Positively Regulates Acute Ethanol Tolerance in C. elegans. Int. J. Mol. Sci. 2024, 25, 6398. https://doi.org/10.3390/ijms25126398
Jee C, Batsaikhan E. JNK Signaling Positively Regulates Acute Ethanol Tolerance in C. elegans. International Journal of Molecular Sciences. 2024; 25(12):6398. https://doi.org/10.3390/ijms25126398
Chicago/Turabian StyleJee, Changhoon, and Enkhzul Batsaikhan. 2024. "JNK Signaling Positively Regulates Acute Ethanol Tolerance in C. elegans" International Journal of Molecular Sciences 25, no. 12: 6398. https://doi.org/10.3390/ijms25126398




