IGF-1 and IGFBP-1 as Possible Predictors of Response to Lifestyle Intervention—Results from Randomized Controlled Trials
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Responses to Lifestyle Interventions
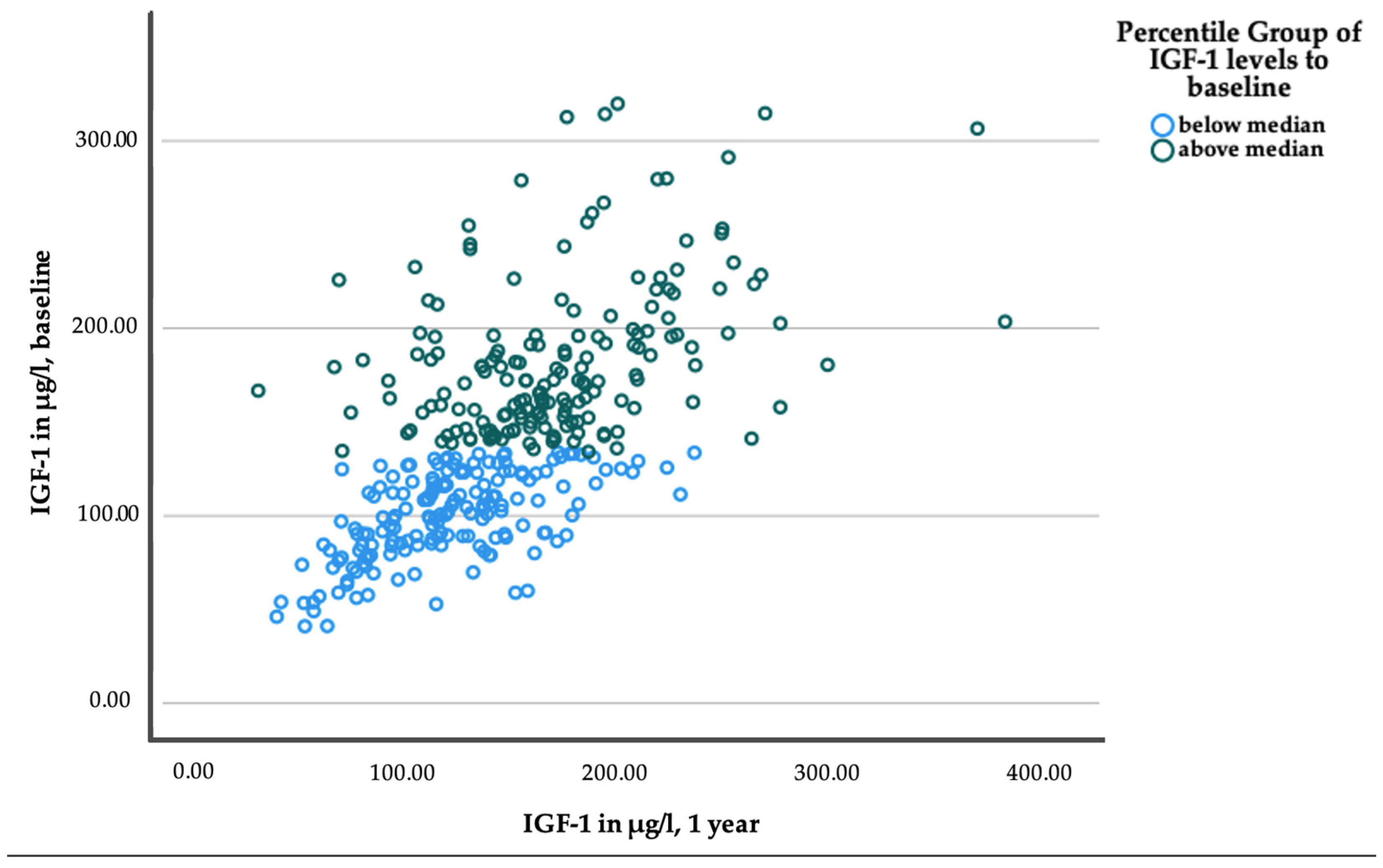
2.3. Responses to Lifestyle Interventions within Median Subgroups of IGF-1 and IGFBP-1 Baseline Levels
2.4. Differential Response to Lifestyle Interventions Depending on Baseline Levels of IGF-1 and IGFBP-1
3. Discussion
4. Materials and Methods
4.1. Project Design and Participants
4.2. Interventions
4.3. Sample Collection and Anthropometric and Metabolic Assessments
4.4. Laboratory Analyses
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvado, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, A.; Wagner, R.; Heni, M.; Kantartzis, K.; Machann, J.; Schick, F.; Lehmann, R.; Peter, A.; Dannecker, C.; Fritsche, L.; et al. Different Effects of Lifestyle Intervention in High- and Low-Risk Prediabetes: Results of the Randomized Controlled Prediabetes Lifestyle Intervention Study (PLIS). Diabetes 2021, 70, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Yakar, S. Mechanisms of disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Alic, N.; Bjedov, I.; Piper, M.D. Ageing in Drosophila: The role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 2011, 46, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Milman, S.; Huffman, D.M.; Barzilai, N. The Somatotropic Axis in Human Aging: Framework for the Current State of Knowledge and Future Research. Cell Metab. 2016, 23, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Colao, A. The GH-IGF-I axis and the cardiovascular system: Clinical implications. Clin. Endocrinol. 2008, 69, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.; Partridge, L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem. J. 2009, 418, 1–12. [Google Scholar] [CrossRef]
- Teppala, S.; Shankar, A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care 2010, 33, 2257–2259. [Google Scholar] [CrossRef]
- Meyer, N.M.T.; Kabisch, S.; Dambeck, U.; Honsek, C.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Schwarz, P.E.H.; Machann, J.; et al. Low IGF-1 and high IGFBP-1 predict diabetes onset in prediabetic patients. Eur. J. Endocrinol. 2022, 187, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.C.; Matheny, A.P., Jr.; Lang, C.A. Insulin-like growth factor-I comparisons in healthy twin children. J. Clin. Endocrinol. Metab. 1994, 78, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, D.R. Role of IGF Binding Proteins in Regulating Metabolism. Trends Endocrinol. Metab. 2016, 27, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef] [PubMed]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Arafat, A.M.; Weickert, M.O.; Frystyk, J.; Spranger, J.; Schofl, C.; Mohlig, M.; Pfeiffer, A.F. The Role of Insulin-Like Growth Factor (IGF) Binding Protein-2 in the Insulin-Mediated Decrease in IGF-I Bioactivity. J. Clin. Endocrinol. Metab. 2009, 94, 5093–5101. [Google Scholar] [CrossRef]
- Petersson, U.; Ostgren, C.J.; Brudin, L.; Brismar, K.; Nilsson, P.M. Low levels of insulin-like growth-factor-binding protein-1 (IGFBP-1) are prospectively associated with the incidence of type 2 diabetes and impaired glucose tolerance (IGT): The Soderakra Cardiovascular Risk Factor Study. Diabetes Metab. 2009, 35, 198–205. [Google Scholar] [CrossRef]
- Kotronen, A.; Lewitt, M.; Hall, K.; Brismar, K.; Yki-Jarvinen, H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J. Clin. Endocrinol. Metab. 2008, 93, 4867–4872. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Hilding, A.; Ostenson, C.G.; Efendic, S.; Brismar, K.; Hall, K. Insulin-like growth factor-binding protein-1 in the prediction and development of type 2 diabetes in middle-aged Swedish men. Diabetologia 2008, 51, 1135–1145. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Hilding, A.; Brismar, K.; Efendic, S.; Ostenson, C.G.; Hall, K. IGF-binding protein 1 and abdominal obesity in the development of type 2 diabetes in women. Eur. J. Endocrinol. 2010, 163, 233–242. [Google Scholar] [CrossRef]
- Lewitt, M.S. The Role of the Growth Hormone/Insulin-Like Growth Factor System in Visceral Adiposity. Biochem. Insights 2017, 10, 1178626417703995. [Google Scholar] [CrossRef] [PubMed]
- Teumer, A.; Qi, Q.; Nethander, M.; Aschard, H.; Bandinelli, S.; Beekman, M.; Berndt, S.I.; Bidlingmaier, M.; Broer, L.; Group, C.L.W.; et al. Genomewide meta-analysis identifies loci associated with IGF-I and IGFBP-3 levels with impact on age-related traits. Aging Cell 2016, 15, 811–824. [Google Scholar] [CrossRef]
- Hong, Y.; Brismar, K.; Hall, K.; Pedersen, N.L.; de Faire, U. Associations between insulin-like growth factor-I (IGF-I), IGF-binding protein-1, insulin and other metabolic measures after controlling for genetic influences: Results from middle-aged and elderly monozygotic twins. J. Endocrinol. 1997, 153, 251–257. [Google Scholar] [CrossRef]
- Hong, Y.; Pedersen, N.L.; Brismar, K.; Hall, K.; de Faire, U. Quantitative genetic analyses of insulin-like growth factor I (IGF-I), IGF-binding protein-1, and insulin levels in middle-aged and elderly twins. J. Clin. Endocrinol. Metab. 1996, 81, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Thissen, J.P.; Ketelslegers, J.M.; Underwood, L.E. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 1994, 15, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.S.; Heald, A.H.; Gibson, J.M.; Cruickshank, J.K.; Dunger, D.B.; Wareham, N.J. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: A prospective observational study. Lancet 2002, 359, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; He, M.; Sun, Q.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Gunter, M.J.; Pollak, M.; Kim, M.; Pessin, J.E.; et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes 2012, 61, 2248–2254. [Google Scholar] [CrossRef]
- Larsson, S.C.; Michaelsson, K.; Burgess, S. IGF-1 and cardiometabolic diseases: A Mendelian randomisation study. Diabetologia 2020, 63, 1775–1782. [Google Scholar] [CrossRef]
- Saito, T.; Watanabe, M.; Nishida, J.; Izumi, T.; Omura, M.; Takagi, T.; Fukunaga, R.; Bandai, Y.; Tajima, N.; Nakamura, Y.; et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: A randomized controlled trial. Arch. Intern. Med. 2011, 171, 1352–1360. [Google Scholar] [CrossRef]
- Kulkarni, R.N.; Holzenberger, M.; Shih, D.Q.; Ozcan, U.; Stoffel, M.; Magnuson, M.A.; Kahn, C.R. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat. Genet. 2002, 31, 111–115. [Google Scholar] [CrossRef]
- Ueki, K.; Okada, T.; Hu, J.; Liew, C.W.; Assmann, A.; Dahlgren, G.M.; Peters, J.L.; Shackman, J.G.; Zhang, M.; Artner, I.; et al. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat. Genet. 2006, 38, 583–588. [Google Scholar] [CrossRef]
- Rajwani, A.; Ezzat, V.; Smith, J.; Yuldasheva, N.Y.; Duncan, E.R.; Gage, M.; Cubbon, R.M.; Kahn, M.B.; Imrie, H.; Abbas, A.; et al. Increasing Circulating IGFBP1 Levels Improves Insulin Sensitivity, Promotes Nitric Oxide Production, Lowers Blood Pressure, and Protects Against Atherosclerosis. Diabetes 2012, 61, 915–924. [Google Scholar] [CrossRef]
- Telgenkamp, I.; Kusters, Y.H.A.M.; Schalkwijk, C.G.; Houben, A.J.H.M.; Kooi, M.E.; Lindeboom, L.; Bons, J.A.P.; Schaper, N.C.; Joris, P.J.; Plat, J.; et al. Contribution of Liver Fat to Weight Loss-Induced Changes in Serum Hepatokines: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2019, 104, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Honsek, C.; Kabisch, S.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Dambeck, U.; Osterhoff, M.A.; Weickert, M.O.; Pfeiffer, A.F.H. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT). Diabetologia 2018, 61, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Kulzer, B.; Hermanns, N.; Gorges, D.; Schwarz, P.; Haak, T. Prevention of diabetes self-management program (PREDIAS): Effects on weight, metabolic risk factors, and behavioral outcomes. Diabetes Care 2009, 32, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Thamer, C.; Schnoedt, B.; Stefan, N.; Haring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Hepatic lipid accumulation in healthy subjects: A comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn. Reson. Med. 2006, 55, 913–917. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Balas, B.; DeFronzo, R.A. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007, 30, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, H.S.; Allen, E.W.; Herron, A.L.; Brennan, M.T. Insulin secretion in response to glycemic stimulus: Relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J. Clin. Investig. 1967, 46, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Shen, S.; Hanley, A.J.; Vuksan, V.; Hamilton, J.K.; Zinman, B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity 2008, 16, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Schüler, R.; Markova, M.; Osterhoff, M.A.; Arafat, A.; Pivovarova, O.; Machann, J.; Hierholzer, J.; Hornemann, S.; Rohn, S.; Pfeiffer, A.F.H. Similar dietary regulation of IGF-1- and IGF-binding proteins by animal and plant protein in subjects with type 2 diabetes. Eur. J. Nutr. 2021, 60, 3499–3504. [Google Scholar] [CrossRef]
- Rasch, D.; Kubinger, K.D.; Moder, K. The two-sample t test: Pre-testing its assumptions does not pay off. Stat. Pap. 2011, 52, 219–231. [Google Scholar] [CrossRef]
| Parameters | Value | No. |
|---|---|---|
| Women (%) | 54.0 | 186 |
| Age (years) | 62.7 ± 8.7 | 345 |
| Study allocation | ||
| PLIS (%) | 39.1 | 135 |
| DiNA-P (%) | 33.6 | 116 |
| OptiFiT (%) | 27.2 | 94 |
| IGF-1 (µg/L) | 141.8 ± 53.7 | 345 |
| IGFBP-1 (µg/L) | 2.1 [1.4; 4.1] | 345 |
| IGFBP-2 (µg/L) | 259.1 [134.2; 422.6] | 345 |
| BMI (kg/m2) | 30.9 ± 5.4 | 345 |
| Present overweight (%) | 38.0 | 132 |
| Present obesity (%) | 50.7 | 175 |
| Grade I (%) | 29.3 | 101 |
| Grade II (%) | 15.1 | 52 |
| Grade III (%) | 6.4 | 22 |
| WHR (cm/cm) | 0.93 ± 0.09 | 341 |
| Body fat content-BIA [%] | 34.7 ± 8.5 | 312 |
| VAT-MRI (l) | 5.5 ± 2.4 | 225 |
| IHL-MRS (%-abs.) | 7.0 [3.0; 14.4] | 231 |
| Present MASLD (%) | 39.4 | 136 |
| Fasting glucose (mmol/L) | 5.7 ± 0.7 | 345 |
| 2 h glucose (mmol/L) | 8.2 ± 1.6 | 345 |
| Fasting insulin (pmol/L) | 73.4 [51.7; 105.5] | 337 |
| Present IFG + NGT (%) | 31.9 | 110 |
| Present NFG + IGT (%) | 31.6 | 109 |
| Present IFG + IGT (%) | 36.5 | 126 |
| HOMA-IR | 2.6 [1.7; 3.8] | 337 |
| Matsuda Index | 2.6 [1.8; 3.5] | 238 |
| HIRI | 37.2 [30.6; 44.4] | 242 |
| IGI | 11.7 [7.5; 21.2] | 242 |
| DI | 30.9 [21.6; 43.6] | 238 |
| Parameters | Baseline | 1 Year | n | p | d/r | Baseline | 1 Year | n | p | d/r |
|---|---|---|---|---|---|---|---|---|---|---|
| (a) | ||||||||||
| IGF-1 < 134.2 µg/L | IGF-1 ≥ 134.2 µg/L | |||||||||
| IGF-1 [µg/L] | 99.9 ± 23.3 | 117.2 ± 38.8 | 172 | <0.001 | −0.56 | 183.5 ± 41.5 | 168.7 ± 51.0 | 173 | <0.001 | 0.29 |
| IGFBP-1 [µg/L] | 2.2 [1.2; 4.4] | 2.5 [1.3; 4.5] | 172 | 0.460 | −0.06 | 2.1 [0.9; 3.7] | 1.9 [1.2; 4.0] | 173 | 0.015 | −0.18 |
| IGFBP-2 [µg/L] | 269.6 [148.1; 453.6] | 271.7 [162.0; 431.9] | 170 | 0.290 | −0.08 | 251.5 [133.9; 385.2] | 250.7 [164.8; 427.7] | 172 | 0.057 | −0.14 |
| Body mass index [kg/m2] | 30.8 ± 5.2 | 29.9 ± 5.1 | 171 | <0.001 | 0.51 | 31.1 ± 5.6 | 29.9 ± 5.4 | 171 | <0.001 | 0.65 |
| Waist-to-hip ratio [cm/cm] | 0.94 ± 0.09 | 0.92 ± 0.09 | 166 | 0.011 | 0.18 | 0.93 ± 0.09 | 0.93 ± 0.09 | 166 | 0.359 | 0.03 |
| Body fat content-BIA [%] | 35.1 ± 8.6 | 34.0 ± 9.0 | 145 | <0.001 | 0.34 | 34.2 ± 8.5 | 33.1 ± 9.1 | 147 | 0.002 | 0.25 |
| Visceral fat volume-MRI [L] | 5.6 ± 2.5 | 5.2 ± 2.4 | 111 | <0.001 | 0.43 | 5.6 ± 2.3 | 5.0 ± 2.1 | 86 | <0.001 | 0.71 |
| Intrahepatic lipid content-MRS [%-abs.] | 7.0 [3.0; 14.7] | 4.4 [2.3; 8.9] | 113 | <0.001 | −0.41 | 7.2 [3.0; 14.2] | 3.1 [1.1; 7.1] | 89 | <0.001 | −0.68 |
| Fasting glucose [mmol/L] | 5.8 ± 0.7 | 5.6 ± 0.8 | 164 | <0.001 | 0.34 | 5.7 ± 0.7 | 5.5 ± 0.7 | 157 | <0.001 | 0.31 |
| 2 h glucose [mmol/L] | 8.3 ± 1.5 | 7.6 ± 1.9 | 164 | <0.001 | 0.36 | 8.1 ± 1.6 | 7.3 ± 2.0 | 157 | <0.001 | 0.46 |
| Fasting insulin [pmol/L] | 79.7 [55.8; 108.2] | 77.8 [54.9; 111.2] | 170 | 0.239 | −0.09 | 66.0 [49.6; 99.7] | 61.5 [44.1;88.3] | 165 | <0.001 | −0.34 |
| HOMA-IR | 3.0 [1.9; 3.9] | 2.7 [1.7; 3.9] | 170 | 0.051 | −0.15 | 2.4 [1.6; 3.7] | 2.0 [1.4; 3.1] | 164 | <0.001 | −0.36 |
| Matsuda index | 2.5 [1.8; 3.3] | 2.9 [2.0; 4.3] | 122 | <0.001 | −0.34 | 2.8 [1.9; 3.6] | 3.6 [2.5; 5.0] | 112 | <0.001 | −0.50 |
| HIRI | 37.5 [30.8; 45.3] | 34.2 [29.8; 42.0] | 127 | 0.003 | −0.26 | 36.7 [30.0; 42.6] | 33.5 [27.2; 39.3] | 114 | <0.001 | −0.41 |
| IGI | 11.7 [7.3; 21.2] | 12.4 [7.8; 19.8] | 127 | 0.273 | −0.10 | 11.6 [7.5; 19.2] | 11.2 [7.0; 17.0] | 114 | 0.232 | −0.11 |
| DI | 28.2 [19.5; 43.6] | 34.3 [21.4; 63.1] | 122 | <0.001 | −0.36 | 33.6 [22.9; 44.5] | 38.7 [25.0; 68.0] | 112 | <0.001 | −0.31 |
| (b) | ||||||||||
| IGFBP-1 < 2.13 µg/L | IGFBP-1 ≥ 2.13 µg/L | |||||||||
| IGF-1 [µg/L] | 141.5 ± 48.5 | 150.5 ± 52.5 | 172 | 0.002 | −0.23 | 142.1 ± 58.5 | 135.6 ± 50.6 | 173 | 0.043 | 0.13 |
| IGFBP-1 [µg/L] | 1.0 [0.7; 1.5] | 1.5 [0.9; 2.2] | 172 | <0.001 | −0.53 | 4.1 [2.8; 6.8] | 3.9 [2.3; 5.6] | 173 | 0.045 | −0.15 |
| IGFBP-2 [µg/L] | 223.6 [119.5; 369.2] | 237.4 [141.2; 352.5] | 172 | 0.080 | −0.13 | 310.2 [175.4; 463.2] | 319.5 [190.2; 515.7] | 170 | 0.179 | −0.10 |
| Body mass index [kg/m2] | 31.8 ± 5.0 | 30.7 ± 4.8 | 171 | <0.001 | 0.68 | 30.0 ± 5.7 | 29.1 ± 5.6 | 171 | <0.001 | 0.49 |
| Waist-to-hip ratio [cm/cm] | 0.94 ± 0.08 | 0.93 ± 0.08 | 165 | 0.035 | 0.14 | 0.93 ± 0.10 | 0.92 ± 0.09 | 167 | 0.096 | 0.10 |
| Body fat content-BIA [%] | 35.5 ± 8.1 | 34.3 ± 8.8 | 149 | <0.001 | 0.35 | 33.6 ± 9.1 | 32.7 ± 9.6 | 143 | 0.003 | 0.24 |
| Visceral fat volume-MRI [L] | 6.0 ± 2.1 | 5.5 ± 2.1 | 106 | <0.001 | 0.64 | 5.1 ± 2.7 | 4.7 ± 2.3 | 91 | <0.001 | 0.46 |
| Intrahepatic lipid content-MRS [%-abs.] | 9.4 [5.1; 17.1] | 5.3 [2.4; 10.5] | 110 | <0.001 | −0.55 | 4.1 [1.5; 9.2] | 2.5 [.7; 6.5] | 92 | <0.001 | −0.50 |
| Fasting glucose [mmol/L] | 5.8 ± 0.6 | 5.6 ± 0.7 | 159 | <0.001 | 0.31 | 5.7 ± 0.7 | 5.5 ± 0.8 | 162 | <0.001 | 0.34 |
| 2 h glucose [mmol/L] | 8.2 ± 1.5 | 7.3 ± 2.0 | 159 | <0.001 | 0.47 | 8.3 ± 1.6 | 7.6 ± 2.0 | 162 | <0.001 | 0.35 |
| Fasting insulin [pmol/L] | 82.0 [59.3; 115.3] | 74.3 [55.5; 111.1] | 165 | 0.002 | −0.24 | 64.2 [43.2 98.0] | 62.8 [44.1; 87.6] | 170 | 0.019 | −0.18 |
| HOMA-IR | 3.0 [2.1; 4.1] | 2.7 [1.8; 3.9] | 165 | <0.001 | −0.28 | 2.3 [1.5; 3.4] | 2.0 [1.3; 3.1] | 169 | 0.003 | −0.23 |
| Matsuda index | 2.4 [1.7; 3.2] | 2.8 [2.0;4.1] | 128 | <0.001 | −0.53 | 2.9 [2.2; 4.6] | 3.7 [2.4; 5.6] | 106 | 0.002 | −0.30 |
| HIRI | 38.3 [32.8; 45.5] | 35.8 [31.3; 42.3] | 133 | <0.001 | −0.37 | 34.9 [27.9; 40.9] | 31.2 [25.8; 38.6] | 108 | 0.003 | −0.29 |
| IGI | 13.7 [8.9; 23.5] | 15.2 [8.8; 19.8] | 133 | 0.560 | −0.05 | 8.5 [5.7; 15.7] | 9.9 [6.0; 15.9] | 108 | 0.434 | −0.08 |
| DI | 32.9 [22.1; 46.1] | 38.2 [22.6; 65.5] | 128 | <0.001 | −0.31 | 28.4 [19.5; 39.2] | 33.8 [23.1; 63.4] | 106 | <0.001 | −0.3 |
| Parameters | Mean Difference | 95% CI | p | d/r |
|---|---|---|---|---|
| (a) | ||||
| Subgroups of IGF-1 baseline levels: above vs. below the median | ||||
| ∆ IGF-1 [µg/L] | −32.09 | [−41.12; 23.05] | <0.001 | −0.75 |
| ∆ IGFBP-1 [µg/L] | 0.06 | [−0.76; 0.88] | 0.396 a | 0.05 |
| ∆ IGFBP-2 [µg/L] | 17.90 | [−22.16; 57.96] | 0.422 a | 0.04 |
| ∆ Body mass index [kg/m2] | −0.31 | [−0.68; 0.07] | 0.053 | −0.18 |
| ∆ Waist-to-hip ratio [cm/cm] | 0.01 | [−0.00; 0.03] | 0.046 | 0.19 |
| ∆ Body fat content-BIA [%] | 0.13 | [−0.74; 0.99] | 0.386 | 0.03 |
| ∆ Visceral fat volume-MRI [L] | −0.24 | [−0.48; 0.00] | 0.027 | −0.28 |
| ∆ Intrahepatic lipid content-MRS [%-abs.] | −1.75 | [−3.44; −0.054] | 0.011 a | −0.18 |
| ∆ Fasting glucose [mmol/L] | 0.03 | [−0.09; 0.15] | 0.321 | 0.05 |
| ∆ 2 h glucose [mmol/L] | −0.06 | [−0.46; 0.35] | 0.394 | −0.03 |
| ∆ Fasting insulin [pmol/L] | −11.31 | [−27.74; 5.12] | 0.031 a | −0.12 |
| ∆ HOMA-IR | −0.36 | [−0.96; 0.25] | 0.086 a | −0.09 |
| ∆ Matsuda index | 0.45 | [−0.09; 0.98] | 0.019 a | 0.15 |
| ∆ HIRI | −1.16 | [−3.35; 1.02] | 0.232 a | −0.08 |
| ∆ IGI | −5.11 | [−10.84; 0.62] | 0.118 a | −0.10 |
| ∆ DI | −7.59 | [−22.13; 6.95] | 0.679 a | −0.03 |
| (b) | ||||
| Subgroups of IGFBP-1 baseline levels: below vs. above the median | ||||
| ∆ IGF-1 [µg/L] | 15.49 | [5.97; 25.00] | <0.001 | 0.34 |
| ∆ IGFBP-1 [µg/L] | 1.79 | [0.99; 2.58] | <0.001 a | −0.22 |
| ∆ IGFBP-2 [µg/L] | −3.60 | [−43.78; 36.58] | 0.430 a | 0.00 |
| ∆ Body mass index [kg/m2] | −0.17 | [−0.54; 0.20] | 0.183 | −0.10 |
| ∆ Waist-to-hip ratio [cm/cm] | 0.00 | [−0.01; 0.02] | 0.465 | 0.01 |
| ∆ Body fat content-BIA [%] | −0.26 | [−1.13; 0.61] | 0.276 | −0.07 |
| ∆ Visceral fat volume-MRI [L] | −0.11 | [−0.36; 0.14] | 0.193 | −0.13 |
| ∆ Intrahepatic lipid content-MRS [%-abs.] | −1.28 | [−2.95; 0.38] | 0.049 a | −0.14 |
| ∆ Fasting glucose [mmol/L] | 0.05 | [−0.08; 0.17] | 0.221 | 0.08 |
| ∆ 2 h glucose [mmol/L] | −0.12 | [−0.53; 0.28] | 0.275 | −0.06 |
| ∆ Fasting insulin [pmol/L] | −6.11 | [−22.58; 10.35] | 0.642 a | −0.03 |
| ∆ HOMA-IR | −0.22 | [−0.82; 0.39] | 0.703 a | −0.02 |
| ∆ Matsuda index | 0.27 | [−0.29; 0.83] | 0.484 a | −0.05 |
| ∆ HIRI | −0.60 | [−2.82; 1.62] | 0.785 a | −0.02 |
| ∆ IGI | 1.72 | [−3.75; 7.19] | 0.375 a | −0.06 |
| ∆ DI | 4.07 | [−9.74; 17.89] | 0.786 a | −0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, N.M.T.; Kabisch, S.; Dambeck, U.; Honsek, C.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Schwarz, P.E.H.; Machann, J.; et al. IGF-1 and IGFBP-1 as Possible Predictors of Response to Lifestyle Intervention—Results from Randomized Controlled Trials. Int. J. Mol. Sci. 2024, 25, 6400. https://doi.org/10.3390/ijms25126400
Meyer NMT, Kabisch S, Dambeck U, Honsek C, Kemper M, Gerbracht C, Arafat AM, Birkenfeld AL, Schwarz PEH, Machann J, et al. IGF-1 and IGFBP-1 as Possible Predictors of Response to Lifestyle Intervention—Results from Randomized Controlled Trials. International Journal of Molecular Sciences. 2024; 25(12):6400. https://doi.org/10.3390/ijms25126400
Chicago/Turabian StyleMeyer, Nina M. T., Stefan Kabisch, Ulrike Dambeck, Caroline Honsek, Margrit Kemper, Christiana Gerbracht, Ayman M. Arafat, Andreas L. Birkenfeld, Peter E. H. Schwarz, Jürgen Machann, and et al. 2024. "IGF-1 and IGFBP-1 as Possible Predictors of Response to Lifestyle Intervention—Results from Randomized Controlled Trials" International Journal of Molecular Sciences 25, no. 12: 6400. https://doi.org/10.3390/ijms25126400







