Comparative Analysis of mRNA, microRNA of Transcriptome, and Proteomics on CIK Cells Responses to GCRV and Aeromonas hydrophila
Abstract
1. Introduction
2. Results
2.1. Viruses and Bacteria Change Effect
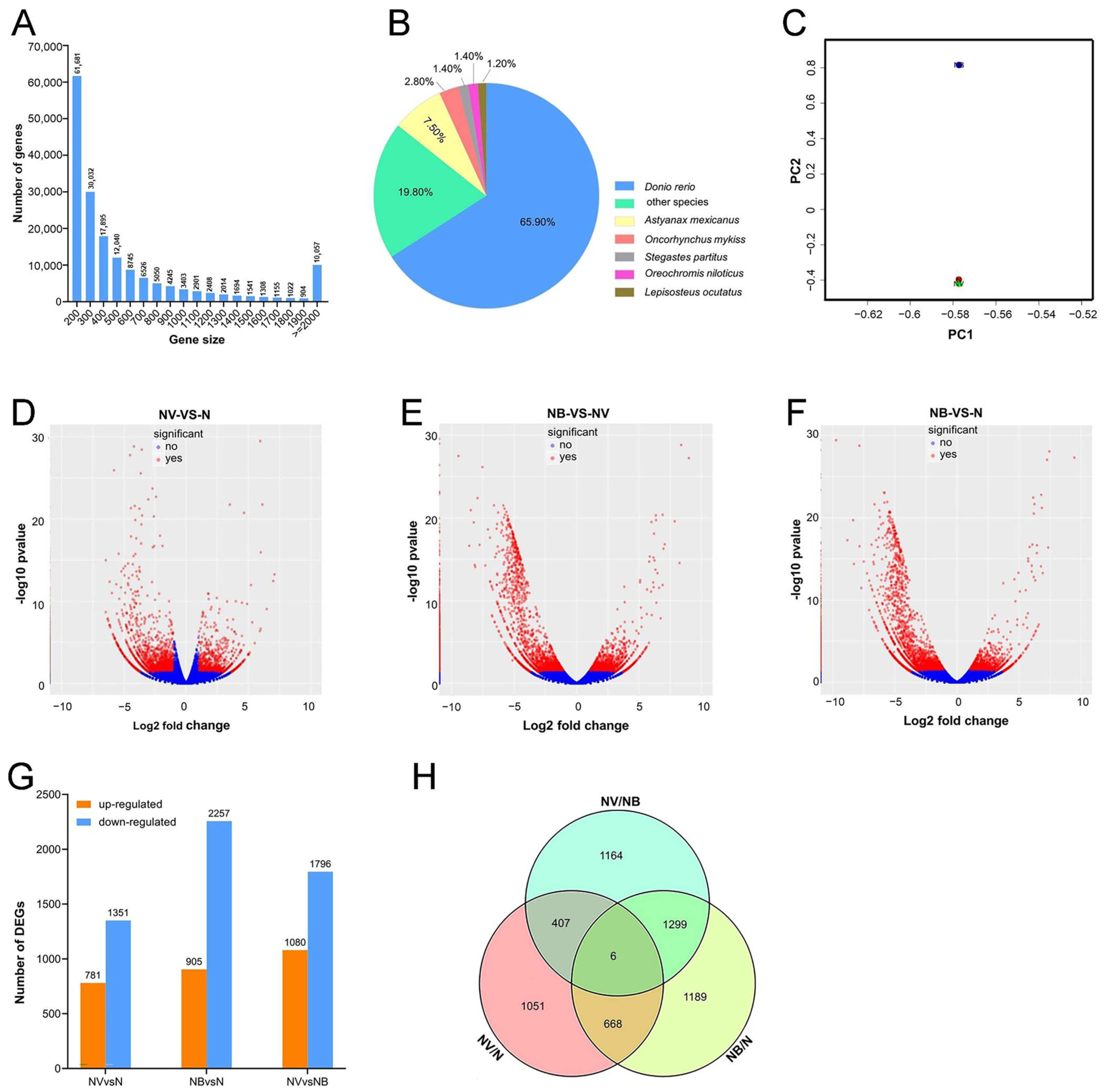
2.2. Preliminary Transcriptome Analysis and Identification of DEGs
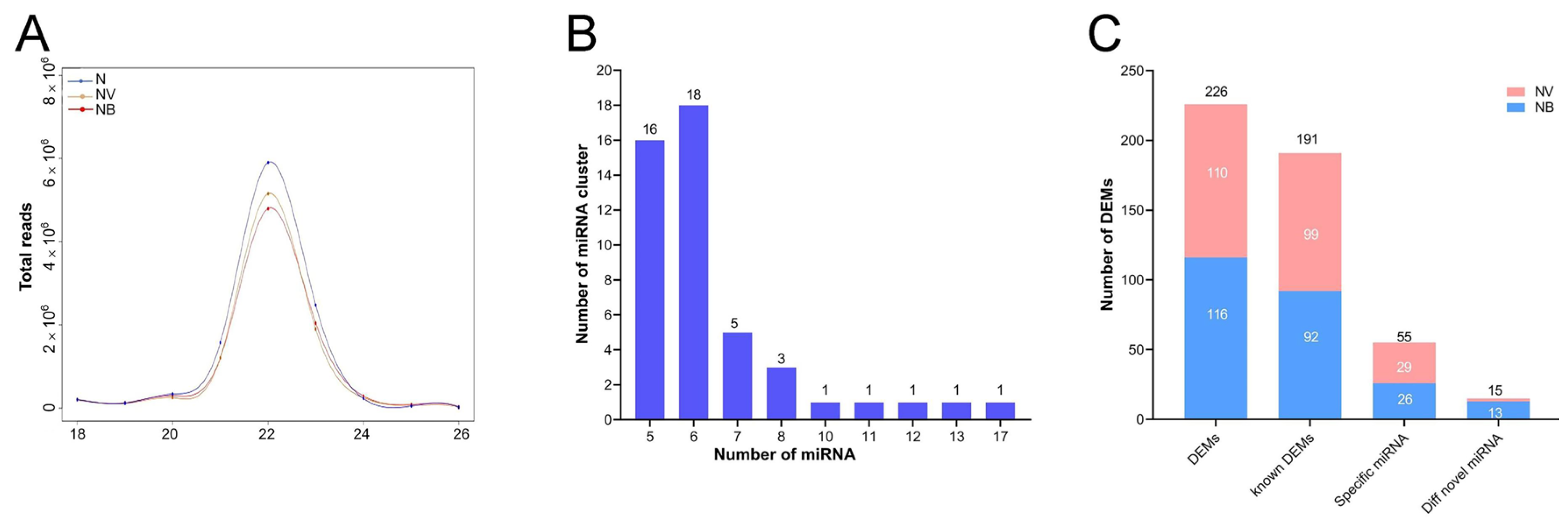
2.3. Analysis of miRNA-seq and Identification of DEMs
2.4. Validation of DEGs, DEMs, and Target Genes
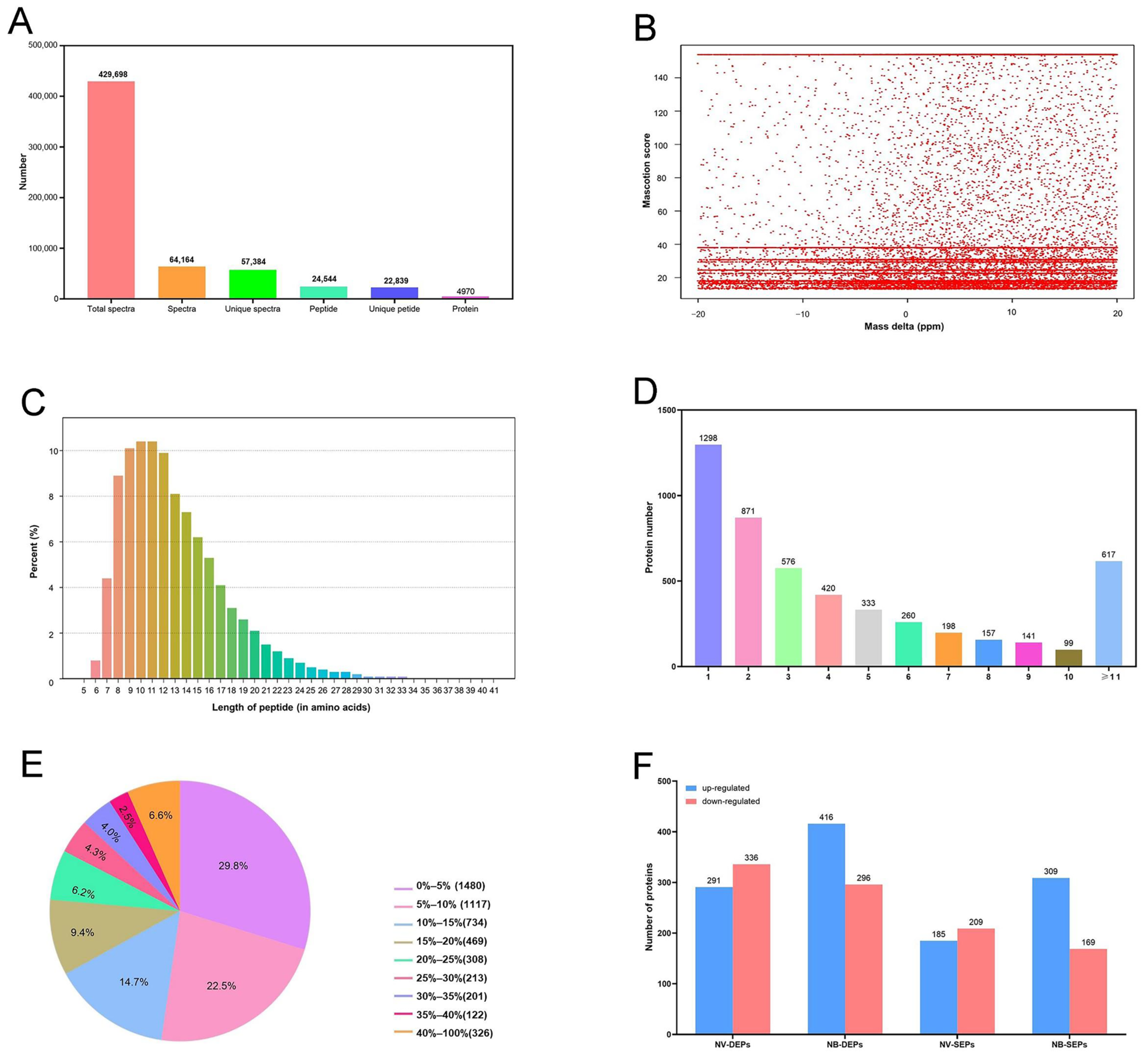
2.5. iTRAQ Quantitative Data Analysis and Identification of DEPs
2.6. Functional Annotation Based on GO and KEGG Analysis
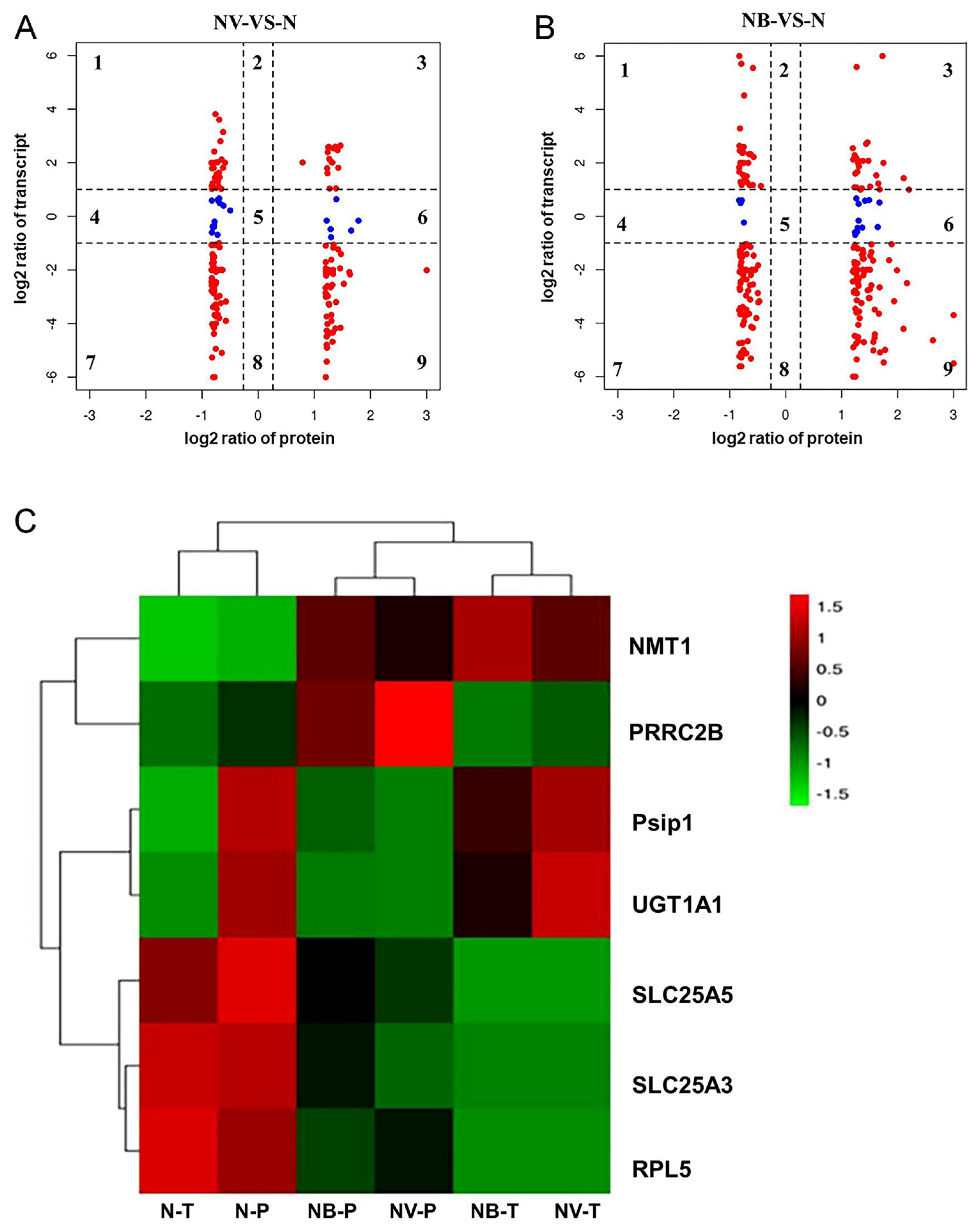
2.7. Correlation Analysis of DEM, DEG, and DEP
3. Discussion
4. Materials and Methods
4.1. Cells’ Infection and Sample Collection
4.2. RNA Isolation, cDNA Library Construction, and Transcriptome Sequencing
4.3. Preliminary Analysis of RNA-seq Data
4.4. Data Analysis and Bioinformatics Analysis of Small RNA
4.5. Validation of mRNA and miRNA by RT-qPCR
4.6. Target Prediction and miRNA Overexpression
4.7. Protein Sample Preparation, iTRAQ Labelling, and LC-MS/MS Analysis
4.8. Bioinformatics Analysis of iTRAQ Data
4.9. Integrated mRNA and microRNA of Transcriptome and Proteome
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, Q.; Yan, X.; Liu, L.; Xu, T. The Inducible microRNA-21 Negatively Modulates the Inflammatory Response in Teleost Fish via Targeting IRAK4. Front. Immunol. 2019, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.P.; Shen, Y.B.; Chen, Y.; Wang, W.J.; Li, J.L. Molecular characterization, expression, and immunological response analysis of the TWEAK and APRIL genes in grass carp, Ctenopharyngodon idella. Genet. Mol. Res. 2014, 13, 10105–10120. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Xu, X.; Shen, Y.; Hu, M.; Zhang, M.; Li, L.; Lv, L.; Li, J. Transcriptome Analysis of the Innate Immunity-Related Complement System in Spleen Tissue of Ctenopharyngodon idella Infected with Aeromonas hydrophila. PLoS ONE 2016, 11, e0157413. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Su, J. Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp. J. Immunol. Res. 2015, 2015, 670437. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, J.; Hu, C.; Wang, F.; Wang, B.; Shi, X.; Hou, Q.; Huang, W.; Lin, G. Transcriptome data analysis of grass carp (Ctenopharyngodon idella) infected by reovirus provides insights into two immune-related genes. Fish Shellfish. Immunol. 2017, 64, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Heng, J.; Huang, T.; Peng, L.; Yang, C.; Li, Q. Identification, mRNA expression and genomic structure of TLR22 and its association with GCRV susceptibility/resistance in grass carp (Ctenopharyngodon idella). Dev. Comp. Immunol. 2012, 36, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, Y.; Fu, J.; Lu, L.; Li, J. De novo assembly of the grass carp Ctenopharyngodon idella transcriptome to identify miRNA targets associated with motile aeromonad septicemia. PLoS ONE 2014, 9, e112722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, G.; He, L.; Luo, L.; Huang, R.; Liao, L.; Li, Y.; Zhu, Z.; Wang, Y. Transcriptomics Sequencing Provides Insights into Understanding the Mechanism of Grass Carp Reovirus Infection. Int. J. Mol. Sci. 2018, 19, 488. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Yang, S.; Cao, Y.; Song, X.; Xiao, J.; Feng, H. Black carp PRMT6 inhibits TBK1-IRF3/7 signaling during the antiviral innate immune activation. Fish Shellfish. Immunol. 2019, 93, 108–115. [Google Scholar] [CrossRef]
- Mu, Y.; Ding, F.; Cui, P.; Ao, J.; Hu, S.; Chen, X. Transcriptome and expression profiling analysis revealed changes of multiple signaling pathways involved in immunity in the large yellow croaker during Aeromonas hydrophila infection. BMC Genom. 2010, 11, 506. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, Q.; Wang, Z.; Ma, T.; Zhou, J.; Holland, J.W.; Gao, Q. Transcriptome analysis of the endangered Chinese giant salamander (Andrias davidianus): Immune modulation in response to Aeromonas hydrophila infection. Vet. Immunol. Immunopathol. 2016, 169, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Fan, Y.; Zhou, Y.; Wang, W.; Ma, J.; Zeng, L. Transcriptome analysis of Aeromonas hydrophila infected hybrid sturgeon (Huso dauricus × Acipenser schrenckii). Sci. Rep. 2018, 8, 17925. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Martens, M.; Anasetti, C.; Carreras, J.; Horowitz, M.; Lee, S.J.; Antin, J.; Cutler, C.; Logan, B. Factors Associated with Successful Discontinuation of Immune Suppression after Allogeneic Hematopoietic Cell Transplantation. JAMA Oncol. 2020, 6, e192974. [Google Scholar] [CrossRef] [PubMed]
- Guérin-El Khourouj, V.; Dalle, J.H.; Pédron, B.; Yakouben, K.; Bensoussan, D.; Cordeiro, D.J.; Peltier, L.; Ouachée-Chardin, M.; Baruchel, A.; Sterkers, G. Quantitative and qualitative CD4 T cell immune responses related to adenovirus DNAemia in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 476–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, I.; Kondo, M.; Yamamori, S.; Kobayashi-Sun, J.; Taniguchi, M.; Kanemaru, K.; Katakura, F.; Traver, D. Enrichment of hematopoietic stem/progenitor cells in the zebrafish kidney. Sci. Rep. 2019, 9, 14205. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Iyer, S.; Lobbardi, R.; Moore, J.C.; Chen, H.; Lareau, C.; Hebert, C.; Shaw, M.L.; Neftel, C.; Suva, M.L.; et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J. Exp. Med. 2017, 214, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wei, X.; Liao, Z.; Gao, Y.; Liu, X.; Su, J.; Yuan, G. Transcriptome Analysis Provides Insights into the Markers of Resting and LPS-Activated Macrophages in Grass Carp (Ctenopharyngodon idella). Int. J. Mol. Sci. 2018, 19, 3562. [Google Scholar] [CrossRef] [PubMed]
- Zwollo, P. The humoral immune system of anadromous fish. Dev. Comp. Immunol. 2018, 80, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ren, M.; Xu, T.; Gao, M.; Liu, H.; Lin, H. Selenium alleviates lead-induced CIK cells pyroptosis and inflammation through IRAK1/TAK1/IKK pathway. Fish Shellfish. Immunol. 2023, 142, 109101. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, Q.; Mao, H.; Liu, Y.; Hu, C. GRP78 from grass carp (Ctenopharyngodon idella) provides cytoplasm protection against thermal and Pb2+ stress. Fish Shellfish. Immunol. 2013, 34, 617–622. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef]
- Shi, W.J.; Jiang, Y.X.; Ma, D.D.; Huang, G.Y.; Xie, L.; Chen, H.X.; Huang, M.Z.; Ying, G.G. Dydrogesterone affects the transcription of genes in visual cycle and circadian rhythm network in the eye of zebrafish. Ecotoxicol. Environ. Saf. 2019, 183, 109556. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cui, X.; Shen, X.; Wang, L.; Jiang, L.; Liu, H.; Liu, Y.; Liu, Q.; Jiang, C. De novo transcriptome analysis and differentially expressed genes in the ovary and testis of the Japanese mantis shrimp Oratosquilla oratoria by RNA-Seq. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 26, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, Y.; Song, C.; Cui, Z. Discovery of sex-related genes from embryonic development stage based on transcriptome analysis in Eriocheir sinensis. Gene 2019, 710, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Tan, X.; Xu, M.; Liu, Q.; Ye, H.; Zou, C.; Ye, C. Liver transcriptome analysis and de novo annotation of the orange-spotted groupers (Epinephelus coioides) under cold stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, X.; Lü, A.; Liu, R.; Sun, J.; Sung, Y.Y.; Song, Y. Transcriptome analysis in the skin of Carassius auratus challenged with Aeromonas hydrophila. Fish Shellfish. Immunol. 2019, 94, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.; Lodish, H.; Sun, L. MicroRNAs in adipogenesis and as therapeutic targets for obesity. Expert Opin. Ther. Targets. 2011, 15, 623–636. [Google Scholar] [CrossRef]
- Xing, K.; Zhao, X.; Ao, H.; Chen, S.; Yang, T.; Tan, Z.; Wang, Y.; Zhang, F.; Liu, Y.; Ni, H.; et al. Transcriptome analysis of miRNA and mRNA in the livers of pigs with highly diverged backfat thickness. Sci. Rep. 2019, 9, 16740. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, R.; Fan, Y.; Huang, X.; Luo, H.; Xiong, F.; Liu, J.; Zhang, R.; Lei, J.; Zhou, H.; et al. Integrated mRNA and small RNA sequencing reveals microRNA regulatory network associated with internode elongation in sugarcane (Saccharum officinarum L.). BMC Genom. 2019, 20, 817. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, Z.; Wu, M.; Zhang, J.; Jia, L.; Chen, X. Identification and differential expression of microRNAs during metamorphosis of the Japanese flounder (Paralichthys olivaceus). PLoS ONE 2011, 6, e22957. [Google Scholar] [CrossRef]
- Herkenhoff, M.E.; Oliveira, A.C.; Nachtigall, P.G.; Costa, J.M.; Campos, V.F.; Hilsdorf, A.W.S.; Pinhal, D. Fishing into the MicroRNA Transcriptome. Front. Genet. 2018, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Y.; Shi, Z.; Su, Y.; Zhang, J. miR-17 is involved in Japanese Flounder (Paralichthys olivaceus) development by targeting the Cdc42 mRNA. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 191, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, S.; Ji, C.; Li, F.; Cong, M.; Shan, X.; Wu, H. iTRAQ-based proteomic analysis on the mitochondrial responses in gill tissues of juvenile olive flounder Paralichthys olivaceus exposed to cadmium. Environ. Pollut. 2020, 257, 113591. [Google Scholar]
- Jin, S.; Sun, D.; Song, D.; Wang, N.; Fu, H.; Ji, F.; Zhang, Y. iTRAQ-based quantitative proteomic analysis of embryonic developmental stages in Amur sturgeon, Acipenser schrenckii. Sci. Rep. 2018, 8, 6255. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Reidegeld, K.A.; Meyer, H.E.; Warscheid, B. Protein labeling by iTRAQ: A new tool for quantitative mass spectrometry in proteome research. Proteomics 2007, 7, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, Y.; Zhao, X.; Xu, P. Recent advance in high accuracy iTRAQ for quantitative proteomics, Chin. J. Biotechnol. 2014, 30, 1073–1082. [Google Scholar]
- Liu, L.; Li, Q.; Lin, L.; Wang, M.; Lu, Y.; Wang, W.; Yuan, J.; Li, L.; Liu, X. Proteomic analysis of epithelioma papulosum cyprini cells infected with spring viremia of carp virus. Fish Shellfish. Immunol. 2013, 35, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Encinas, P.; Rodriguez-Milla, M.A.; Novoa, B.; Estepa, A.; Figueras, A.; Coll, J. Zebrafish fin immune responses during high mortality infections with viral haemorrhagic septicemia rhabdovirus. A proteomic and transcriptomic approach. BMC Genom. 2010, 11, 518. [Google Scholar] [CrossRef]
- Lü, A.; Hu, X.; Wang, Y.; Shen, X.; Li, X.; Zhu, A.; Tian, J.; Ming, Q.; Feng, Z. iTRAQ analysis of gill proteins from the zebrafish (Danio rerio) infected with Aeromonas hydrophila. Fish Shellfish. Immunol. 2014, 36, 229–239. [Google Scholar] [CrossRef]
- Wang, L.; Shao, C.; Xu, W.; Zhou, Q.; Wang, N.; Chen, S. Proteome profiling reveals immune responses in Japanese flounder (Paralichthys olivaceus) infected with Edwardsiella tarda by iTRAQ analysis. Fish Shellfish. Immunol. 2017, 66, 325–333. [Google Scholar] [CrossRef]
- Xu, X.; Shen, Y.; Fu, J.; Lu, L.; Li, J. Next-generation sequencing identified microRNAs that associate with motile aeromonad septicemia in grass carp. Fish Shellfish. Immunol. 2015, 45, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Song, L.; Wang, H.; Xu, X.; Wang, T.; Lu, L. Proteomic analysis of cellular protein expression profiles in response to grass carp reovirus infection. Fish Shellfish. Immunol. 2015, 44, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, H.; Li, H.; Wang, A. Transcriptome profiling of grass carp (Ctenopharyngodon idellus) infected with Aeromonas hydrophila. Fish Shellfish. Immunol. 2016, 51, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Li, W.; Xu, M.; Zhu, Y.; Zhou, Y.; Wang, Q. Transcriptome-wide analysis of immune responses in Eriocheir sinensis hemocytes after challenge with different microbial derivatives. Dev. Comp. Immunol. 2019, 101, 103457. [Google Scholar]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Xu, B.H.; Zhong, L.; Liu, Q.L.; Xiao, T.Y.; Su, J.M.; Chen, K.J.; Wang, H.Q.; Dai, Y.J.; Chen, J. Characterization of grass carp spleen transcriptome during GCRV infection. Genet. Mol. Res. 2016, 15, gmr.15026650. [Google Scholar] [CrossRef]
- da Rosa, V.M.; Ariotti, K.; Bressan, C.A.; da Silva, E.G.; Dallaporta, M.; Júnior, G.B.; da Costa, S.T.; de Vargas, A.C.; Baldisserotto, B.; Finamor, I.A.; et al. Dietary addition of rutin impairs inflammatory response and protects muscle of silver catfish (Rhamdia quelen) from apoptosis and oxidative stress in Aeromonas hydrophila-induced infection. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108611. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Z.; Zhang, J.; Bonami, J.R. Purification and characterization of a new reovirus from the Chinese mitten crab, Eriocheir sinensis. J. Fish Dis. 2004, 27, 687–692. [Google Scholar] [CrossRef]
- Song, X.; Hu, X.; Sun, B.; Bo, Y.; Wu, K.; Xiao, L.; Gong, C. A transcriptome analysis focusing on inflammation-related genes of grass carp intestines following infection with Aeromonas hydrophila. Sci. Rep. 2017, 7, 40777. [Google Scholar] [CrossRef]
- Ba, C.Y.; Du, X.Y.; Zhang, P.J.; Chen, P.; Cai, Y.N.; Li, Y.H. Transcriptome Analysis of Epithelioma Papulosum Cyprini Cells Infected by Reovirus Isolated from Allogynogenetic Silver Crucian Carp. Viruses 2018, 10, 135. [Google Scholar] [CrossRef]
- Ferreira, P.A. The coming-of-age of nucleocytoplasmic transport in motor neuron disease and neurodegeneration. Cell Mol. Life Sci. 2019, 76, 2247–2273. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kao, T.H.; Chang, S.W. The structural changes of the mutated ankyrin repeat domain of the human TRPV4 channel alter its ATP binding ability. J. Mech. Behav. Biomed. Mater. 2020, 101, 103407. [Google Scholar] [CrossRef] [PubMed]
- Irannejad, R.; Tsvetanova, N.G.; Lobingier, B.T.; von Zastrow, M. Effects of endocytosis on receptor-mediated signaling. Curr. Opin. Cell Biol. 2015, 35, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Yang, C.; Wan, Q.; Rao, Y.; Su, J. The destiny of the resistance/susceptibility against GCRV is controlled by epigenetic mechanisms in CIK cells. Sci. Rep. 2017, 7, 4551. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, R.; Høyheim, B. miRNAs associated with immune response in teleost fish. Dev. Comp. Immunol. 2017, 75, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, J.; Luo, Y.; Chen, H.; Zheng, P.; et al. MicroRNA-499-5p regulates porcine myofiber specification by controlling Sox6 expression. Animal 2017, 11, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Gu, T.; Lu, L.; Cao, Z.; Song, Q.; Wang, Z.; Zhang, Y.; Chang, G.; Xu, Q.; Chen, G. Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle. J. Cell Physiol. 2019, 234, 3490–3499. [Google Scholar] [CrossRef]
- Izarra, A.; Moscoso, I.; Cañón, S.; Carreiro, C.; Fondevila, D.; Martín-Caballero, J.; Blanca, V.; Valiente, I.; Díez-Juan, A.; Bernad, A. miRNA-1 and miRNA-133a are involved in early commitment of pluripotent stem cells and demonstrate antagonistic roles in the regulation of cardiac differentiation. J. Tissue Eng. Regen. Med. 2017, 11, 787–799. [Google Scholar] [CrossRef]
- Wang, F.; Song, G.; Liu, M.; Li, X.; Tang, H. miRNA-1 targets fibronectin1 and suppresses the migration and invasion of the HEp2 laryngeal squamous carcinoma cell line. FEBS Lett. 2011, 585, 3263–3269. [Google Scholar] [CrossRef]
- He, L.; Zhang, A.; Chu, P.; Li, Y.; Huang, R.; Liao, L.; Zhu, Z.; Wang, Y. Deep Illumina sequencing reveals conserved and novel microRNAs in grass carp in response to grass carp reovirus infection. BMC Genom. 2017, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Hu, X.; Wang, R.; Lü, A.; Sun, J. MicroRNA expression profile analysis of skin immune response in crucian carp (Carassius auratus) infected by Aeromonas hydrophila. Fish Shellfish. Immunol. 2020, 104, 673–685. [Google Scholar] [CrossRef]
- Cui, L.; Hu, H.; Wei, W.; Wang, W.; Liu, H. Identification and Characterization of MicroRNAs in the Liver of Blunt Snout Bream (Megalobrama amblycephala) Infected by Aeromonas hydrophila. Int. J. Mol. Sci. 2016, 17, 1972. [Google Scholar]
- Lim, K.H.; Staudt, L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013, 5, a011247. [Google Scholar] [CrossRef]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Fiuji, H.; Soleimanpour, S.; Ferns, G.A.; Avan, A.; Khazaei, M. Toll like receptor signaling pathway as a potential therapeutic target in colorectal cancer. J. Cell Physiol. 2018, 233, 5613–5622. [Google Scholar] [CrossRef]
- Liu, P.; Lu, Z.; Liu, L.; Li, R.; Liang, Z.; Shen, M.; Xu, H.; Ren, D.; Ji, M.; Yuan, S.; et al. NOD-like receptor signaling in inflammation-associated cancers: From functions to targeted therapies. Phytomedicine 2019, 64, 152925. [Google Scholar] [CrossRef]
- Hauser, M.A.; Legler, D.F. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 2016, 99, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, R.J.; Zamoyska, R. T cell receptor signaling networks: Branched, diversified and bounded. Nat. Rev. Immunol. 2013, 13, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef]
- McGlincy, N.J.; Ingolia, N.T. Transcriptome-wide measurement of translation by ribosome profiling. Methods 2017, 126, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shem, A.; Garreau de Loubresse, N.; Melnikov, S.; Jenner, L.; Yusupova, G.; Yusupov, M. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 2011, 334, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, P.; Altvater, M.; Gerhardy, S.; Schütz, S.; Fischer, U.; Weirich, C.; Panse, V.G. Eukaryotic Ribosome Assembly and Nuclear Export. Int. Rev. Cell Mol. Biol. 2015, 319, 107–140. [Google Scholar] [PubMed]
- Qu, X.; Hu, M.; Shang, Y.; Pan, L.; Jia, P.; Fu, C.; Liu, Q.; Wang, Y. Liver Transcriptome and miRNA Analysis of Silver Carp (Hypophthalmichthys molitrix) Intraperitoneally Injected with Microcystin-LR. Front. Physiol. 2018, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Raabe, K.; Honys, D.; Michailidis, C. The role of eukaryotic initiation factor 3 in plant translation regulation. Plant Physiol. Biochem. 2019, 145, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, J.; Wang, Y.; Bu, C.; Zhou, Y.; Fang, Q. Comparative Proteomic Analysis of Lysine Acetylation in Fish CIK Cells Infected with Aquareovirus. Int. J. Mol. Sci. 2017, 18, 2419. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, K.; Lohikangas, L.; Gullberg, D.; Pfaff, M.; Johansson, S.; Fässler, R. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J. Cell Biol. 1996, 132, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.E.; Shin, K.C.; Natale, R.B.; Todd, R.F., 3rd. Beta 1 integrin expression on human small cell lung cancer cells. Cancer Res. 1991, 51, 1065–1070. [Google Scholar]
- Li, G.; Zhao, Y.; Guo, S.; Liu, B.; Chen, Y.; Sun, X.; Feng, J. Comparative analysis of spleen transcriptome detects differences in evolutionary adaptation of immune defense functions in bighead carp and silver carp. Fish Shellfish. Immunol. 2019, 84, 148–157. [Google Scholar] [CrossRef]
- Kelly, C.; Salinas, I. Under Pressure: Interactions between Commensal Microbiota and the Teleost Immune System. Front. Immunol. 2017, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Schrama, D.; Ritter, C.; Becker, J.C. T cell receptor repertoire usage in cancer as a surrogate marker for immune responses. Semin. Immunopathol. 2017, 39, 255–268. [Google Scholar] [CrossRef]
- Agorastos, A.; Bozikas, V.P. Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki 2019, 30, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Siggs, O.M.; Berger, M.; Krebs, P.; Arnold, C.N.; Eidenschenk, C.; Huber, C.; Pirie, E.; Smart, N.G.; Khovananth, K.; Xia, Y.; et al. A mutation of Ikbkg causes immune deficiency without impairing degradation of IkappaB alpha. Proc. Natl. Acad. Sci. USA 2010, 107, 3046–3051. [Google Scholar] [CrossRef] [PubMed]
- Meisel, M.; Hermann-Kleiter, N.; Hinterleitner, R.; Gruber, T.; Wachowicz, K.; Pfeifhofer-Obermair, C.; Fresser, F.; Leitges, M.; Soldani, C.; Viola, A.; et al. The kinase PKCα selectively upregulates interleukin-17A during Th17 cell immune responses. Immunity 2013, 38, 41–52. [Google Scholar] [CrossRef]
- Zuo, W.; Qian, H.; Xu, Y.; Du, S.; Yang, X. A Cell Line Derives from the Kidney of Grass Carp (Ctenopharyngodon idella). J. Fish. China 1986, 10, 11–17. [Google Scholar]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y.; Zhang, Y.; Ning, Z.; Li, Y.; Zhao, Q.; Lu, H.; Huang, R.; Xia, X.; Feng, Q.; et al. The draft genome of the grass carp (Ctenopharyngodon idellus) provides insights into its evolution and vegetarian adaptation. Nat. Genet. 2015, 47, 625–631. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Jin, H.; Wan, Y.W.; Liu, Z. Comprehensive evaluation of RNA-seq quantification methods for linearity. BMC Bioinform. 2017, 18 (Suppl. 4), 117. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [PubMed]
- Aedo, J.E.; Fuentes-Valenzuela, M.; Molina, A.; Valdés, J.A. Quantitative proteomics analysis of membrane glucocorticoid receptor activation in rainbow trout skeletal muscle. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100627. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A.; et al. WEGO 2.0: A web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef]
- Jin, W.; Wu, F. Proteome-Wide Identification of Lysine Succinylation in the Proteins of Tomato (Solanum lycopersicum). PLoS ONE 2016, 11, e0147586. [Google Scholar] [CrossRef]
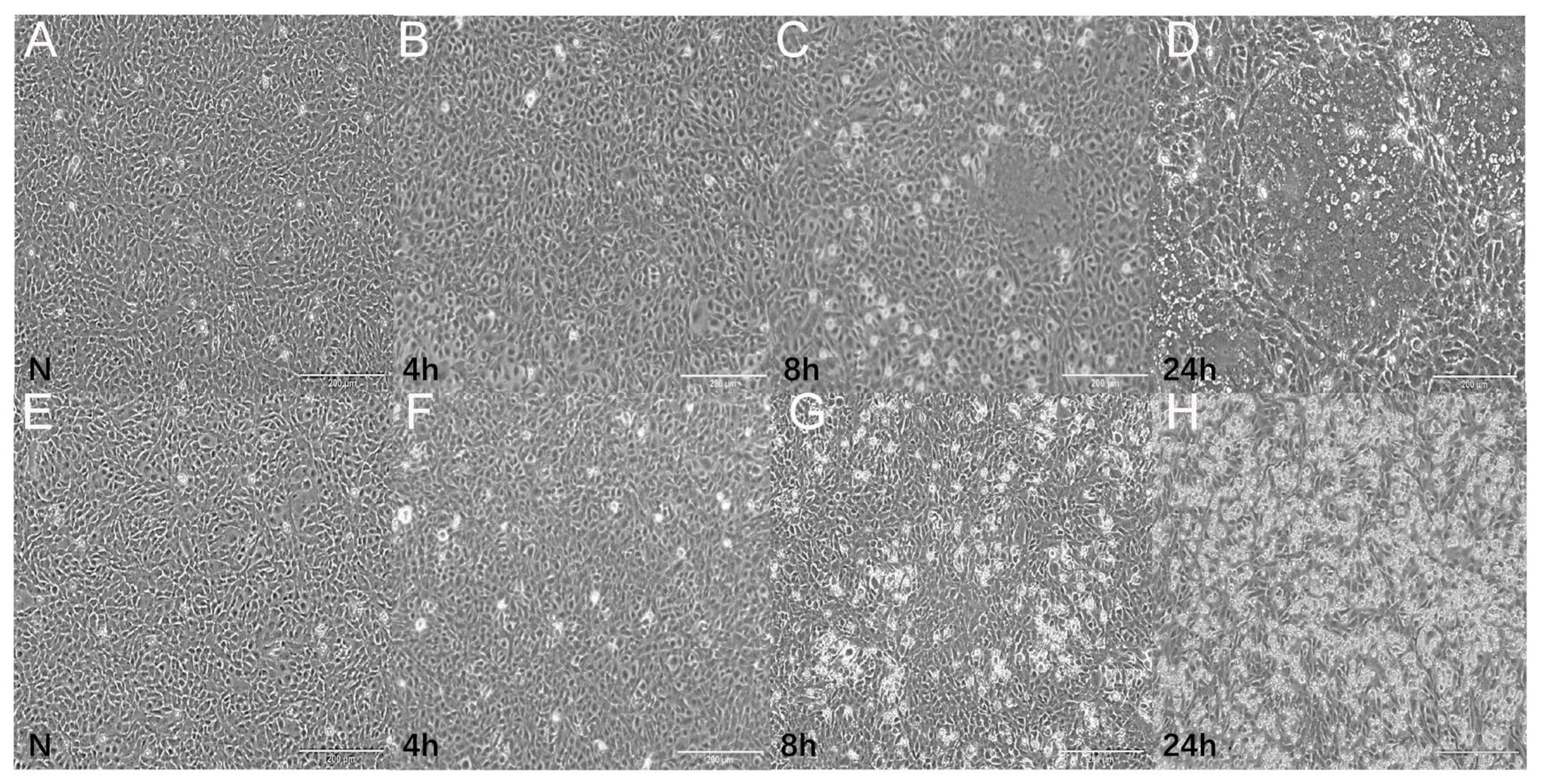



| Sample | N | 4 h | 8 h | 24 h |
|---|---|---|---|---|
| NV | 1.00 × 106 | 0.77 × 106 | 0.43 × 106 | 0.14 × 106 |
| NB | 1.00 × 106 | 0.80 × 106 | 0.47 × 106 | 0.21 × 106 |
| Sample | KEGG Pathway | Pathway_ID | Gene Num |
|---|---|---|---|
| NV | Immune system | ko04620, ko04012, ko04062, ko04621 | 4 |
| Carbohydrate metabolism | ko00010, ko00053, ko00040 | 5 | |
| Translation | ko03010 | 5 | |
| NB | Carbohydrate metabolism | ko00053, ko00010, ko00020, ko00030 ko00040, ko00051 | 14 |
| Membrane transport | ko02010, ko03070 | 9 | |
| Transport and catabolism | ko04145, ko04142, ko04144 | 6 | |
| Immune system | ko04612, ko04621 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lin, Y.; Li, W.; Cheng, Y.; Zhang, J.; Qiu, J.; Fu, Y. Comparative Analysis of mRNA, microRNA of Transcriptome, and Proteomics on CIK Cells Responses to GCRV and Aeromonas hydrophila. Int. J. Mol. Sci. 2024, 25, 6438. https://doi.org/10.3390/ijms25126438
Li X, Lin Y, Li W, Cheng Y, Zhang J, Qiu J, Fu Y. Comparative Analysis of mRNA, microRNA of Transcriptome, and Proteomics on CIK Cells Responses to GCRV and Aeromonas hydrophila. International Journal of Molecular Sciences. 2024; 25(12):6438. https://doi.org/10.3390/ijms25126438
Chicago/Turabian StyleLi, Xike, Yue Lin, Wenjuan Li, Yuejuan Cheng, Junling Zhang, Junqiang Qiu, and Yuanshuai Fu. 2024. "Comparative Analysis of mRNA, microRNA of Transcriptome, and Proteomics on CIK Cells Responses to GCRV and Aeromonas hydrophila" International Journal of Molecular Sciences 25, no. 12: 6438. https://doi.org/10.3390/ijms25126438
APA StyleLi, X., Lin, Y., Li, W., Cheng, Y., Zhang, J., Qiu, J., & Fu, Y. (2024). Comparative Analysis of mRNA, microRNA of Transcriptome, and Proteomics on CIK Cells Responses to GCRV and Aeromonas hydrophila. International Journal of Molecular Sciences, 25(12), 6438. https://doi.org/10.3390/ijms25126438






