Donor Sites and Harvesting Techniques Affect miRNA Cargos of Extracellular Vesicles Released by Human Adipose-Derived Mesenchymal Stromal Cells
Abstract
1. Introduction
2. Results
2.1. Phenotype Characterization of ASCs and Secreted EVs
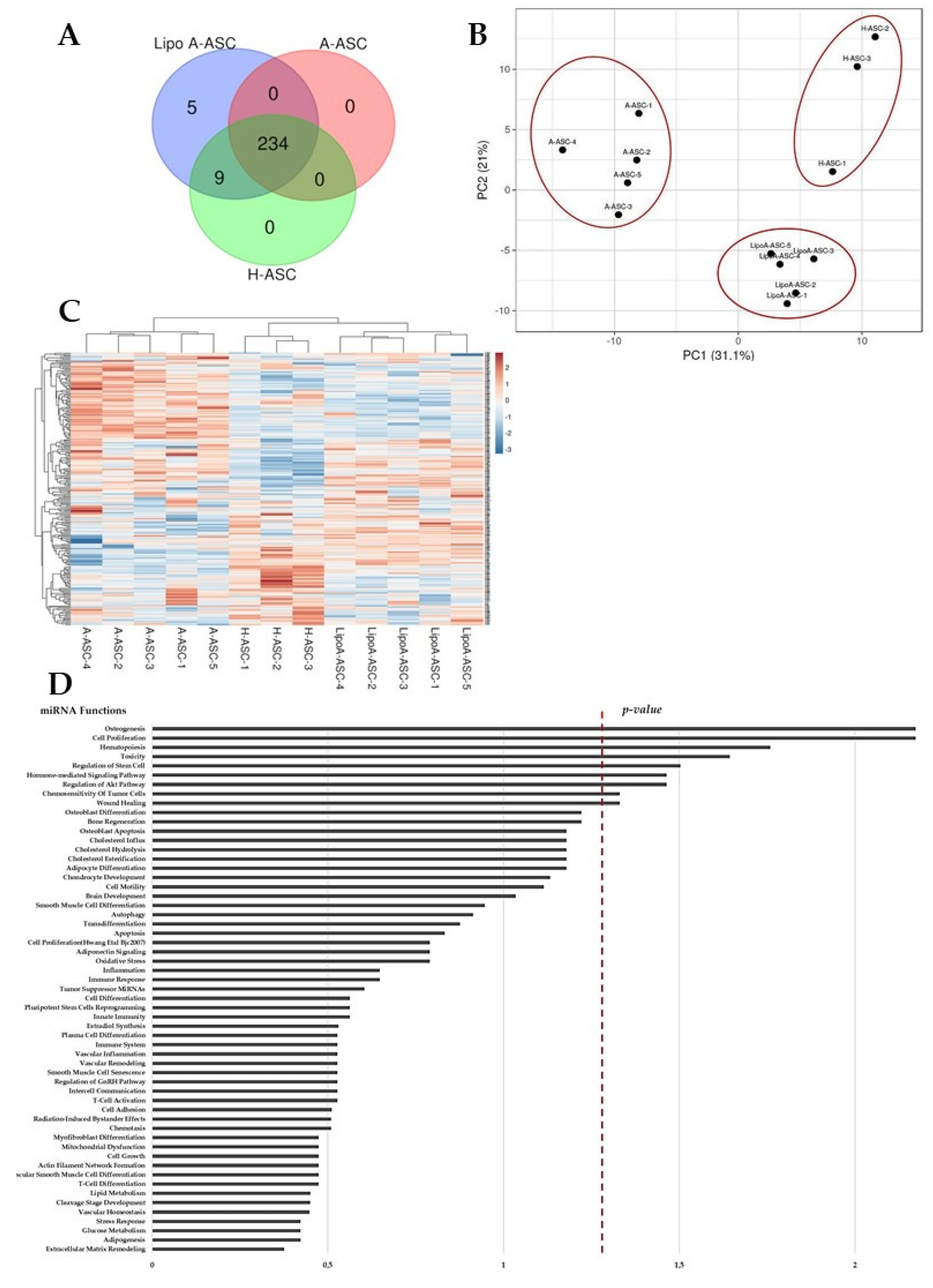
2.2. Comparison of LipoA-ASC-, A-ASC- and H-ASC-Derived Ev-miRNA Profile
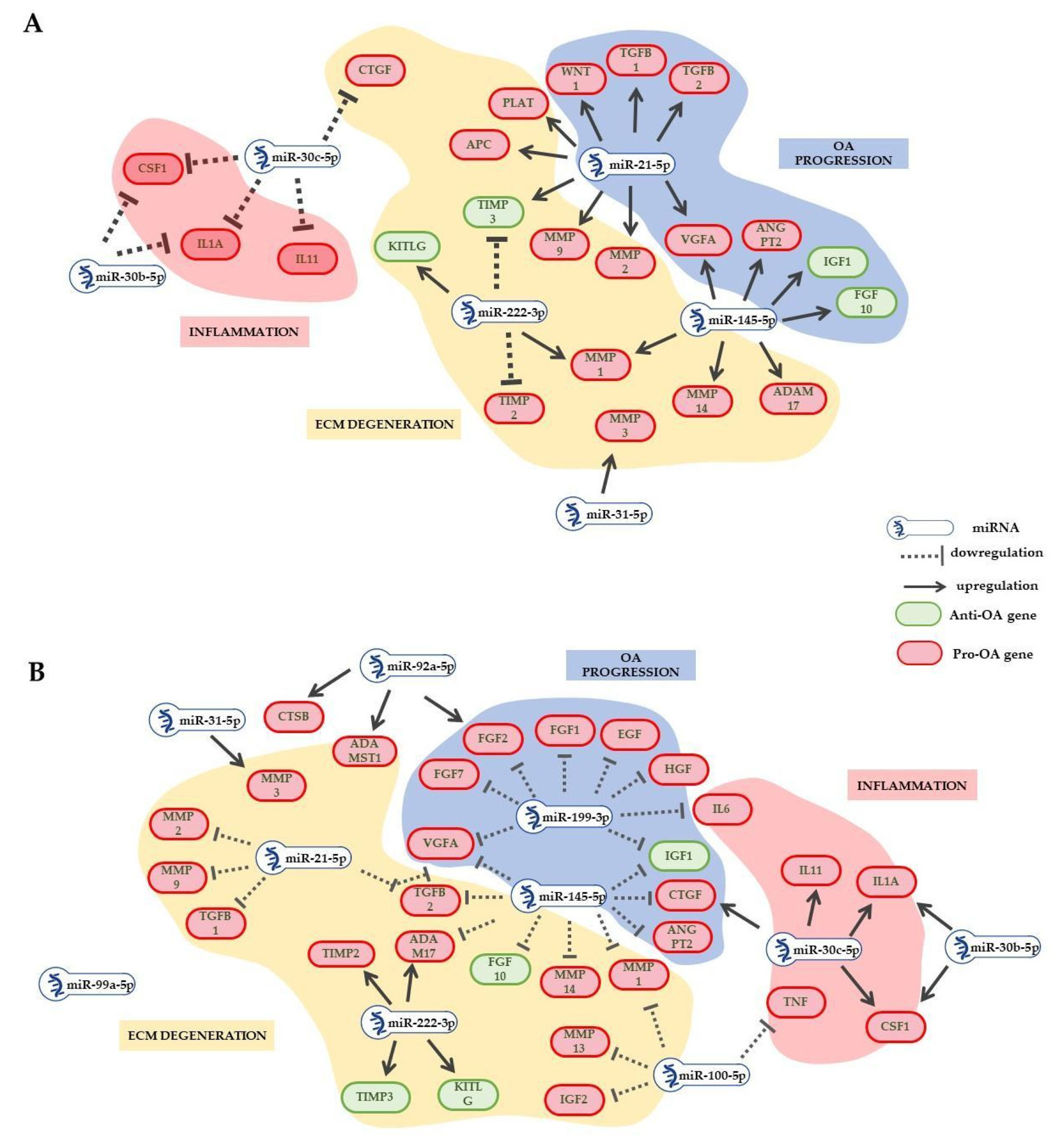
2.3. EV-miRNAs Involved in OA Pathological State
2.4. EV-miRNA Target Identification
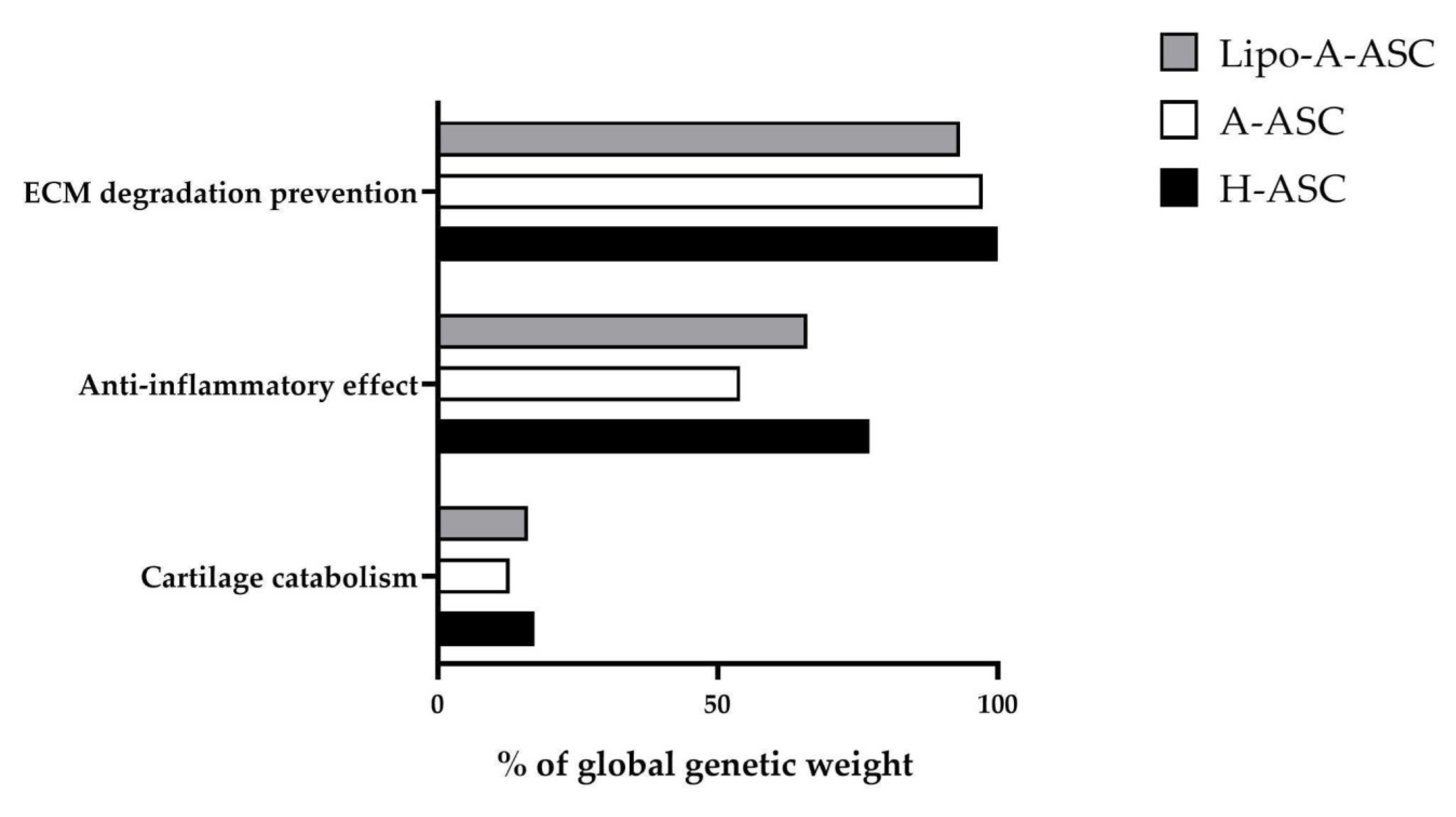
2.5. Target Effect Prediction of EV-miRNAs and Comparisons among ASC Types
3. Discussion
4. Materials and Methods
4.1. hASC Isolation and Culture
4.2. hASC Characterization by Flow Cytometry
4.3. hASC Culture Supernatant Collection and EVs Isolation
4.4. EVs Characterization by Flow Cytometry
4.5. EV-miRNA Cargo Evaluation and Normalization
4.6. EV-miRNA Target Identification
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Pessler, F.; Dai, L.; Diaz-Torne, C.; Gomez-Vaquero, C.; Paessler, M.E.; Zheng, D.-H.; Einhorn, E.; Range, U.; Scanzello, C.; Schumacher, H.R. The Synovitis of “Non-Inflammatory” Orthopaedic Arthropathies: A Quantitative Histological and Immunohistochemical Analysis. Ann. Rheum. Dis. 2008, 67, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Tanaka, H.; Katoh, K.; Nakamura, H.; Nagashima, M.; Yoshino, S. Characterization of Infiltrating T Cells and Th1/Th2-Type Cytokines in the Synovium of Patients with Osteoarthritis. Osteoarthr. Cartil. 2002, 10, 277–281. [Google Scholar] [CrossRef]
- Altman, R.; Barkin, R.L. Topical Therapy for Osteoarthritis: Clinical and Pharmacologic Perspectives. Postgrad. Med. 2009, 121, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Pelletier, J.-P.; Martel-Pelletier, J. Efficacy and Safety of Topical NSAIDs in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S18–S21. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory Mechanisms of Mesenchymal Stem and Stromal Cells in Inflammatory Diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Borakati, A.; Mafi, R.; Mafi, P.; Khan, W.S. A Systematic Review And Meta-Analysis of Clinical Trials of Mesenchymal Stem Cell Therapy for Cartilage Repair. Curr. Stem Cell Res. Ther. 2018, 13, 215–225. [Google Scholar] [CrossRef]
- Kim, S.H.; Djaja, Y.P.; Park, Y.-B.; Park, J.-G.; Ko, Y.-B.; Ha, C.-W. Intra-Articular Injection of Culture-Expanded Mesenchymal Stem Cells Without Adjuvant Surgery in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2020, 48, 2839–2849. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The Function of microRNAs in Cartilage and Osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 40–47. [Google Scholar] [PubMed]
- Sondag, G.R.; Haqqi, T.M. The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-Derived Stem Cells: Sources, Potency, and Implications for Regenerative Therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Orfei, C.P.; Papait, A.; De Girolamo, L. Comparison of miRNA Cargo in Human Adipose-Tissue vs. Amniotic-Membrane Derived Mesenchymal Stromal Cells Extracellular Vesicles for Osteoarthritis Treatment. Extracell Vesicles Circ. Nucleic Acids 2021, 2, 202–221. [Google Scholar] [CrossRef]
- Colombini, A.; Ragni, E.; Mortati, L.; Libonati, F.; Perucca Orfei, C.; Viganò, M.; Brayda-Bruno, M.; de Girolamo, L. Adipose-Derived Mesenchymal Stromal Cells Treated with Interleukin 1 Beta Produced Chondro-Protective Vesicles Able to Fast Penetrate in Cartilage. Cells 2021, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of Human Adipose-Derived Cells: Temporal Changes in Stromal-Associated and Stem Cell-Associated Markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A Reduction in CD90 (THY-1) Expression Results in Increased Differentiation of Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.L.; Sánchez-Abarca, L.I.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC Surface Markers (CD44, CD73, and CD90) Can Identify Human MSC-Derived Extracellular Vesicles by Conventional Flow Cytometry. Cell Commun. Signal 2016, 14, 2. [Google Scholar] [CrossRef]
- Ragni, E.; Colombini, A.; Viganò, M.; Libonati, F.; Perucca Orfei, C.; Zagra, L.; de Girolamo, L. Cartilage Protective and Immunomodulatory Features of Osteoarthritis Synovial Fluid-Treated Adipose-Derived Mesenchymal Stem Cells Secreted Factors and Extracellular Vesicles-Embedded miRNAs. Cells 2021, 10, 1072. [Google Scholar] [CrossRef]
- Mehta, S.; Akhtar, S.; Porter, R.M.; Önnerfjord, P.; Bajpayee, A.G. Interleukin-1 Receptor Antagonist (IL-1Ra) Is More Effective in Suppressing Cytokine-Induced Catabolism in Cartilage-Synovium Co-Culture than in Cartilage Monoculture. Arthritis Res. Ther. 2019, 21, 238. [Google Scholar] [CrossRef]
- Muthu, S.; Patil, S.C.; Jeyaraman, N.; Jeyaraman, M.; Gangadaran, P.; Rajendran, R.L.; Oh, E.J.; Khanna, M.; Chung, H.Y.; Ahn, B.-C. Comparative Effectiveness of Adipose-Derived Mesenchymal Stromal Cells in the Management of Knee Osteoarthritis: A Meta-Analysis. World J. Orthop. 2023, 14, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Bao, R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, Q.; Lu, L.; Cui, S.; Ma, K.; Zhang, W.; Ma, F.; Li, H.; Fu, X.; Zhang, C. Immunomodulatory Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles: Targeting Immune Cells. Front. Immunol. 2023, 14, 1094685. [Google Scholar] [CrossRef]
- D’Arrigo, D.; Roffi, A.; Cucchiarini, M.; Moretti, M.; Candrian, C.; Filardo, G. Secretome and Extracellular Vesicles as New Biological Therapies for Knee Osteoarthritis: A Systematic Review. J. Clin. Med. 2019, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal Stem Cell-Derived Exosomes: A New Therapeutic Approach to Osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.C.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.D.; Gimble, J.M. Yield of Human Adipose-Derived Adult Stem Cells from Liposuction Aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Wang, Q.; Yan, L.; Fu, X.; Xiao, R. Convergent Alteration of the Mesenchymal Stem Cell Heterogeneity in Adipose Tissue during Aging. FASEB J. 2023, 37, e23114. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, R.; Pham, Q.; Fukagawa, N.K.; Wang, T.T.Y. Individual Variabilities in Adipose Stem Cell Proliferation, Gene Expression and Responses to Lipopolysaccharide Stimulation. Int. J. Mol. Sci. 2022, 23, 12534. [Google Scholar] [CrossRef]
- Jurgens, W.J.F.M.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; Zandiehdoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.P.F.; van Milligen, F.J. Effect of Tissue-Harvesting Site on Yield of Stem Cells Derived from Adipose Tissue: Implications for Cell-Based Therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Babilas, P.; Fruth, S.; Orsó, E.; Schmitz, G.; Mueller, M.B.; Nerlich, M.; Prantl, L. Harvesting Human Adipose Tissue-Derived Adult Stem Cells: Resection versus Liposuction. Cytotherapy 2009, 11, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Bajek, A.; Gurtowska, N.; Olkowska, J.; Maj, M.; Kaźmierski, Ł.; Bodnar, M.; Marszałek, A.; Dębski, R.; Drewa, T. Does the Harvesting Technique Affect the Properties of Adipose-Derived Stem Cells?—The Comparative Biological Characterization. J. Cell Biochem. 2017, 118, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Estee, M.M.; Cicuttini, F.M.; Page, M.J.; Wluka, A.E.; Wang, Y. Efficacy of Tumor Necrosis Factor Inhibitors in Hand Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Osteoarthr. Cartil. Open 2023, 5, 100404. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qian, X.; Ding, R. MiR-24-3p Attenuates IL-1β-Induced Chondrocyte Injury Associated with Osteoarthritis by Targeting BCL2L12. J. Orthop. Surg. Res. 2021, 16, 371. [Google Scholar] [CrossRef] [PubMed]
- Philipot, D.; Guérit, D.; Platano, D.; Chuchana, P.; Olivotto, E.; Espinoza, F.; Dorandeu, A.; Pers, Y.-M.; Piette, J.; Borzi, R.M.; et al. p16INK4a and Its Regulator miR-24 Link Senescence and Chondrocyte Terminal Differentiation-Associated Matrix Remodeling in Osteoarthritis. Arthritis Res. Ther. 2014, 16, R58. [Google Scholar] [CrossRef] [PubMed]
- Fordham, J.B.; Naqvi, A.R.; Nares, S. miR-24 Regulates Macrophage Polarization and Plasticity. J. Clin. Cell Immunol. 2015, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-F.; Liu, S.-Y.; Qi, Z.-F.; Lv, Z.-H.; Ding, H.-R.; Zhou, W.-J. MiR-145 Targeting BNIP3 Reduces Apoptosis of Chondrocytes in Osteoarthritis through Notch Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8263–8272. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Lazzarano, S.; Sharma, E.; Lockstone, H.; Murphy, C.L. High-Throughput Identification of MiR-145 Targets in Human Articular Chondrocytes. Life 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, J.; Yang, S.; Liu, X.; Ye, S.; Tian, H. MicroRNA-21 Controls the Development of Osteoarthritis by Targeting GDF-5 in Chondrocytes. Exp. Mol. Med. 2014, 46, e79. [Google Scholar] [CrossRef]
- Francis-West, P.H.; Abdelfattah, A.; Chen, P.; Allen, C.; Parish, J.; Ladher, R.; Allen, S.; MacPherson, S.; Luyten, F.P.; Archer, C.W. Mechanisms of GDF-5 Action during Skeletal Development. Development 1999, 126, 1305–1315. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, F.; Yi, L.; Tang, J.; Zhu, Z.; Pang, Y.; Chen, Y.; Li, D.; Guo, K.; Zheng, X. MicroRNA-21-5p as a Novel Therapeutic Target for Osteoarthritis. Rheumatology 2019, 58, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, C.; Carucci, N.; Colombo, T.; Piconese, S.; Azzalin, G.; Cipolletta, E.; Citarella, F.; Barnaba, V.; Macino, G.; Fulci, V. miR-21 Is a Negative Modulator of T-Cell Activation. Biochimie 2014, 107 Pt B, 319–326. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, L. MiR-320a Upregulation Improves IL-1β-Induced Osteoarthritis via Targeting the DAZAP1 and MAPK Pathways. J. Orthop. Surg. Res. 2023, 18, 541. [Google Scholar] [CrossRef]
- Lineham, B.; Altaie, A.; Harwood, P.; McGonagle, D.; Pandit, H.; Jones, E. A Systematic Review on the Potential Value of Synovial Fluid Biomarkers to Predict Clinical Outcomes in Cartilage Repair Treatments. Osteoarthr. Cartil. 2022, 30, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jin, E.-H.; Kim, D.; Kim, K.Y.; Chun, C.-H.; Jin, E.-J. MicroRNA-222 Regulates MMP-13 via Targeting HDAC-4 during Osteoarthritis Pathogenesis. BBA Clin. 2015, 3, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Tavakkoli Avval, S.; Shoorei, H.; Taheri, M.; Samadian, M. The Impact of Non-Coding RNAs on Macrophage Polarization. Biomed. Pharmacother. 2021, 142, 112112. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.-N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-Abundant Exosomes Derived from Infrapatellar Fat Pad MSCs Protect Articular Cartilage and Ameliorate Gait Abnormalities via Inhibition of mTOR in Osteoarthritis. Biomaterials 2019, 206, 87–100. [Google Scholar] [CrossRef]
- Iyyanki, T.; Hubenak, J.; Liu, J.; Chang, E.I.; Beahm, E.K.; Zhang, Q. Harvesting Technique Affects Adipose-Derived Stem Cell Yield. Aesthet. Surg. J. 2015, 35, 467–476. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. The miRNA–Target Interactions: An Underestimated Intricacy. Nucleic Acids Res. 2023, 52, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, Q.; Yu, J.; Rao, Y.; Kou, Z.; Fang, G.; Shi, X.; Liu, W.; Han, H. A Systematic Way to Infer the Regulation Relations of miRNAs on Target Genes and Critical miRNAs in Cancers. Front. Genet. 2020, 11, 278. [Google Scholar] [CrossRef]
- De Luca, P.; Kouroupis, D.; Viganò, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Contains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xia, J. miRNet-Functional Analysis and Visual Exploration of miRNA-Target Interactions in a Network Context. Methods Mol. Biol. 2018, 1819, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Bleazard, T.; Lamb, J.A.; Griffiths-Jones, S. Bias in microRNA Functional Enrichment Analysis. Bioinformatics 2015, 31, 1592–1598. [Google Scholar] [CrossRef] [PubMed]

| LipoA-ASC-EVs Genetic Weight % | A-ASC-EVs Genetic Weight % | H-ASC-EVs Genetic Weight % | Function | |
|---|---|---|---|---|
| Cartilage protection | ||||
| miR-24-3p | 14.221 | 10.436 | 19.180 | Regulates chondrocyte senescence. |
| miR-125b-5p | 11.061 | 8.598 | 10.468 | Prevents aggrecan loss. |
| miR-193b-3p | 3.533 | 3.782 | 4.000 | Regulates inflammation by repressing TNF-α expression. |
| miR-100-5p | 2.763 | 3.693 | 1.005 | Inhibition of mTOR-autophagy pathway. |
| miR-99a-5p | 2.714 | 3.243 | 1.016 | Suppresses cell apoptosis and extracellular matrix degradation stimulated by IL-6 and |
| miR-222-3p | 4.232 | 2.016 | 5.009 | Reduces cartilage degradation. |
| miR-320a-3p | 1.271 | Chondrocyte viability | ||
| miR-199a-3p | 1.186 | Anti-catabolic. | ||
| miR-92a-3p | 1.261 | 1.892 | Anti-catabolic and increases collagen deposition. | |
| miR-574-3p | 1.044 | Chondrogenesis regulation mediated by SOX9. | ||
| Cartilage destructive | ||||
| miR-21-5p | 4.128 | 9.150 | 2.053 | Negatively regulates chondrogenesis. |
| miR-19b-3p | 1.803 | 1.307 | Induces NF-κB signaling. | |
| miR-214-3p | 1.231 | Negatively regulates NF-κB signaling pathway. | ||
| miR-30b-5p | 1.717 | 2.078 | Pro-apoptotic. ECM degradation. | |
| miR-99b-5p | 1.177 | Activation of NF-κB signaling in chondrocytes. | ||
| Cartilage overlapping | ||||
| miR-145-5p | 1.348 | 4.657 | 1.258 | Chondrocyte proliferation vs. cartilage degradation. |
| miR-221-3p | 4.154 | 2.525 | 6.231 | Prevents ECM degradation vs. pro-inflammatory. |
| miR-30c-5p | 2.260 | 2.399 | Involved in the bone morphogenetic protein (BMP) signaling pathway. | |
| miR-31-5p | 1.112 | 1.262 | Involved in apoptosis and calcification targeting ATF6. | |
| Synovia overlapping | ||||
| miR-191-5p | 1.292 | 1.545 | Hypoxia-induced cell proliferation. | |
| Pro M1 macrophage | ||||
| miR-145-5p | 1.348 | 4.657 | 1.258 | M1-promoting. |
| Pro M2 macrophage | ||||
| miR-24-3p | 14.221 | 10.436 | 19.180 | M2-promoting, M1-suppressing. |
| miR-222-3p | 4.232 | 2.016 | 5.009 | M2-promoting. |
| T cell | ||||
| miR-24-3p | 14.221 | 10.436 | 19.180 | Negative regulation of T cell activation. |
| miR-21-5p | 4.128 | 9.150 | 2.053 | Negative regulation of T cell activation. |
| miR-125b-5p | 11.061 | 8.598 | 10.468 | Negative regulation of T cell activation. |
| miR-145-5p | 1.348 | 4.657 | 1.258 | Negative regulation of T Reg activation. |
| miR-221-3p | 4.154 | 2.525 | 6.231 | T cell activation. |
| miR-19b-3p | 1.803 | 1.307 | T cell activation. | |
| miR-214-3p | 1.231 | T cell activation. | ||
| miR-636 | 13.990 | Unknown. | ||
| miR-520e-3p | 2.253 | 9.266 | Unknown. |
| FC (Lipo A-ASC vs. A-ASC) | FC (Lipo A-ASC vs. H-ASC) | FC (A-ASC vs. H-ASC) | p Value (Lipo A-ASC vs. A-ASC) | p Value (Lipo A-ASC vs. H-ASC) | p Value (A-ASC vs. H-ASC) | |
|---|---|---|---|---|---|---|
| Cartilage protection | ||||||
| miR-193b-3p | 1.013 | 0.959 | 0.946 | ns | ns | ns |
| miR-320a-3p | 0.326 | 0.544 | 1.671 | **** | * | * |
| miR-199a-3p | 0.612 | 1.701 | 2.778 | ** | ** | **** |
| miR-222-3p | 2.27 | 0.91 | 0.040 | * | ns | * |
| miR-24-3p | 1.478 | 0.805 | 0.545 | * | ns | ** |
| miR-92a-3p | 1.52 | 0.72 | 0.47 | ns | ns | * |
| miR-125b-5p | 1.39 | 1.14 | 0.82 | ns | ns | ns |
| miR-574-3p | 0.926 | 0.736 | 0.795 | ns | ns | ns |
| miR-100-5p | 0.811 | 2.986 | 3.681 | ns | *** | *** |
| miR-99a-5p | 0.908 | 2.900 | 3.195 | ns | *** | *** |
| Cartilage destructive | ||||||
| miR-30b-5p | 2.583 | 0.897 | 0.347 | **** | ns | **** |
| miR-21-5p | 0.489 | 2.183 | 4.460 | * | * | *** |
| miR-19b-3p | 0.811 | 2.986 | 3.681 | ** | ns | ns |
| miR-99b-5p | 2.766 | 2.373 | 0.858 | *** | ** | ns |
| miR-214-3p | 0.724 | 1.026 | 1.417 | ns | ns | ns |
| Cartilage overlapping | ||||||
| miR-145-5p | 0.314 | 1.164 | 3.706 | ** | ns | ** |
| miR-221-3p | 1.785 | 0.72 | 0.40 | * | ns | ** |
| miR-30c-5p | 3.803 | 1.023 | 0.269 | **** | ns | **** |
| miR-31-5p | 2.139 | 0.957 | 0.447 | * | ns | * |
| Synovia overlapping | ||||||
| miR-191-5p | 1.519 | 0.908 | 0.598 | ** | ns | ** |
| Pro M1 macrophage | ||||||
| miR-145-5p | 0.314 | 1.164 | 3.706 | ** | ns | ** |
| Pro M2 macrophage | ||||||
| miR-24-3p | 1.478 | 0.805 | 0.545 | * | ns | ** |
| miR-222-3p | 2.27 | 0.91 | 0.040 | * | ns | * |
| T cell | ||||||
| miR-24-3p | 1.478 | 0.805 | 0.545 | * | ns | ** |
| miR-125b-5p | 1.39 | 1.14 | 0.82 | ns | ns | ns |
| miR-214-3p | 0.724 | 1.026 | 1.417 | ns | ns | ns |
| miR-21-5p | 0.489 | 2.183 | 4.460 | * | * | *** |
| miR-221-3p | 1.785 | 0.72 | 0.40 | * | ns | ** |
| miR-19b-3p | 0.811 | 2.986 | 3.681 | ** | ns | ns |
| miR-145-5p | 0.314 | 1.164 | 3.706 | ** | ns | ** |
| miR-520e-3p | 0.264 | 268.914 | 1019.750 | 0.733 | 0.048 | 0.016 |
| miR-636 | 0.018 | 2.000 | 111.430 | 0.056 | >0.9999 | 0.050 |
| Factor | Total Genetic Weight (%) of First-Quartile miRNAs | Expressing Cell Type | |||||
|---|---|---|---|---|---|---|---|
| Lipo-A-ASC | A-ASC | H-ASC | CHO | SYN | HLA-DR * | T CELL | |
| Cytokines | |||||||
| TNF | 28.04 | 22.73 | 30.65 | X | X | ||
| IL6 | 11.73 | 9.78 | 10.90 | X | X | ||
| IL1B | 18.35 | 19.59 | 21.23 | X | X | ||
| IL1A | 5.27 | 2.29 | 6.02 | X | X | ||
| IFNG | 14.22 | 10.44 | 19.18 | ||||
| IL4 | 14.22 | 10.44 | 19.18 | ||||
| CSF1 | 3.98 | 1.37 | 4.48 | X | X | X | |
| CXCL12 | 5.27 | 3.09 | 7.49 | X | X | ||
| WNT1 | 4.13 | 9.15 | 2.05 | ||||
| IL18 | 14.22 | 10.44 | 19.18 | X | X | ||
| EPO | 11.06 | 8.60 | 10.47 | ||||
| LIF | 11.06 | 8.60 | 10.47 | X | X | X | |
| C5 | 3.53 | 3.78 | 4.00 | X | X | ||
| CCL5 | 0.82 | 1.23 | 0.87 | X | X | X | |
| IL11 | 2.26 | 0.64 | 2.40 | X | X | X | |
| Growth Factors | |||||||
| TGFB1 | 19.92 | 22.22 | 23.58 | X | X | X | X |
| EGF | 0.67 | 1.19 | 0.43 | ||||
| IGF1 | 17.93 | 19.31 | 23.04 | X | X | ||
| FGF2 | 1.93 | 2.08 | 2.32 | X | X | ||
| BMP2 | 1.29 | 0.92 | 1.55 | X | X | X | |
| VEGFA | 10.30 | 17.52 | 9.97 | X | X | X | |
| HGF | 0.67 | 1.19 | 0.43 | X | X | ||
| ANGPT2 | 27.45 | 24.92 | 31.78 | X | X | ||
| CTGF | 4.47 | 7.10 | 4.96 | X | X | X | |
| KITLG | 5.10 | 3.82 | 6.32 | X | X | X | |
| TGFB2 | 5.48 | 13.81 | 3.31 | X | X | X | |
| FGF10 | 1.35 | 4.66 | 1.26 | X | X | ||
| FGF7 | 0.67 | 1.19 | 0.43 | X | X | X | |
| IGF2 | 13.82 | 12.29 | 11.47 | X | X | ||
| FGF1 | 0.67 | 1.19 | 0.43 | X | X | ||
| Proteases and Other | |||||||
| ADAM16 | 15.57 | 15.09 | 20.44 | ||||
| ADAM17 | 15.57 | 15.09 | 20.44 | X | X | X | |
| ADAMTS1 | 12.32 | 9.49 | 12.36 | X | X | ||
| MMP1 | 8.34 | 10.37 | 7.27 | X | |||
| MMP2 | 19.34 | 20.27 | 18.75 | X | X | ||
| MMP3 | 1.11 | 0.56 | 1.26 | X | X | ||
| MMP9 | 4.13 | 9.15 | 2.05 | X | X | ||
| MMP13 | 13.82 | 12.29 | 11.47 | ||||
| MMP14 | 15.57 | 15.09 | 20.44 | X | X | ||
| PLAT | 4.13 | 9.15 | 2.05 | X | X | ||
| PLAU | 4.40 | 5.59 | 5.31 | X | X | ||
| CTSB | 1.26 | 0.90 | 1.89 | X | X | X | |
| CTSD | 14.22 | 10.44 | 19.18 | X | X | X | |
| APC | 15.19 | 17.75 | 12.52 | X | X | ||
| TIMP2 | 4.23 | 2.02 | 5.01 | X | X | ||
| TIMP3 | 12.51 | 13.69 | 13.29 | X | X | ||
| IL1RN | 11.06 | 8.60 | 10.47 | X | X | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visconte, C.; Taiana, M.M.; Colombini, A.; De Luca, P.; Ragni, E.; de Girolamo, L. Donor Sites and Harvesting Techniques Affect miRNA Cargos of Extracellular Vesicles Released by Human Adipose-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2024, 25, 6450. https://doi.org/10.3390/ijms25126450
Visconte C, Taiana MM, Colombini A, De Luca P, Ragni E, de Girolamo L. Donor Sites and Harvesting Techniques Affect miRNA Cargos of Extracellular Vesicles Released by Human Adipose-Derived Mesenchymal Stromal Cells. International Journal of Molecular Sciences. 2024; 25(12):6450. https://doi.org/10.3390/ijms25126450
Chicago/Turabian StyleVisconte, Caterina, Michela Maria Taiana, Alessandra Colombini, Paola De Luca, Enrico Ragni, and Laura de Girolamo. 2024. "Donor Sites and Harvesting Techniques Affect miRNA Cargos of Extracellular Vesicles Released by Human Adipose-Derived Mesenchymal Stromal Cells" International Journal of Molecular Sciences 25, no. 12: 6450. https://doi.org/10.3390/ijms25126450
APA StyleVisconte, C., Taiana, M. M., Colombini, A., De Luca, P., Ragni, E., & de Girolamo, L. (2024). Donor Sites and Harvesting Techniques Affect miRNA Cargos of Extracellular Vesicles Released by Human Adipose-Derived Mesenchymal Stromal Cells. International Journal of Molecular Sciences, 25(12), 6450. https://doi.org/10.3390/ijms25126450








