Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential
Abstract
:1. Introduction
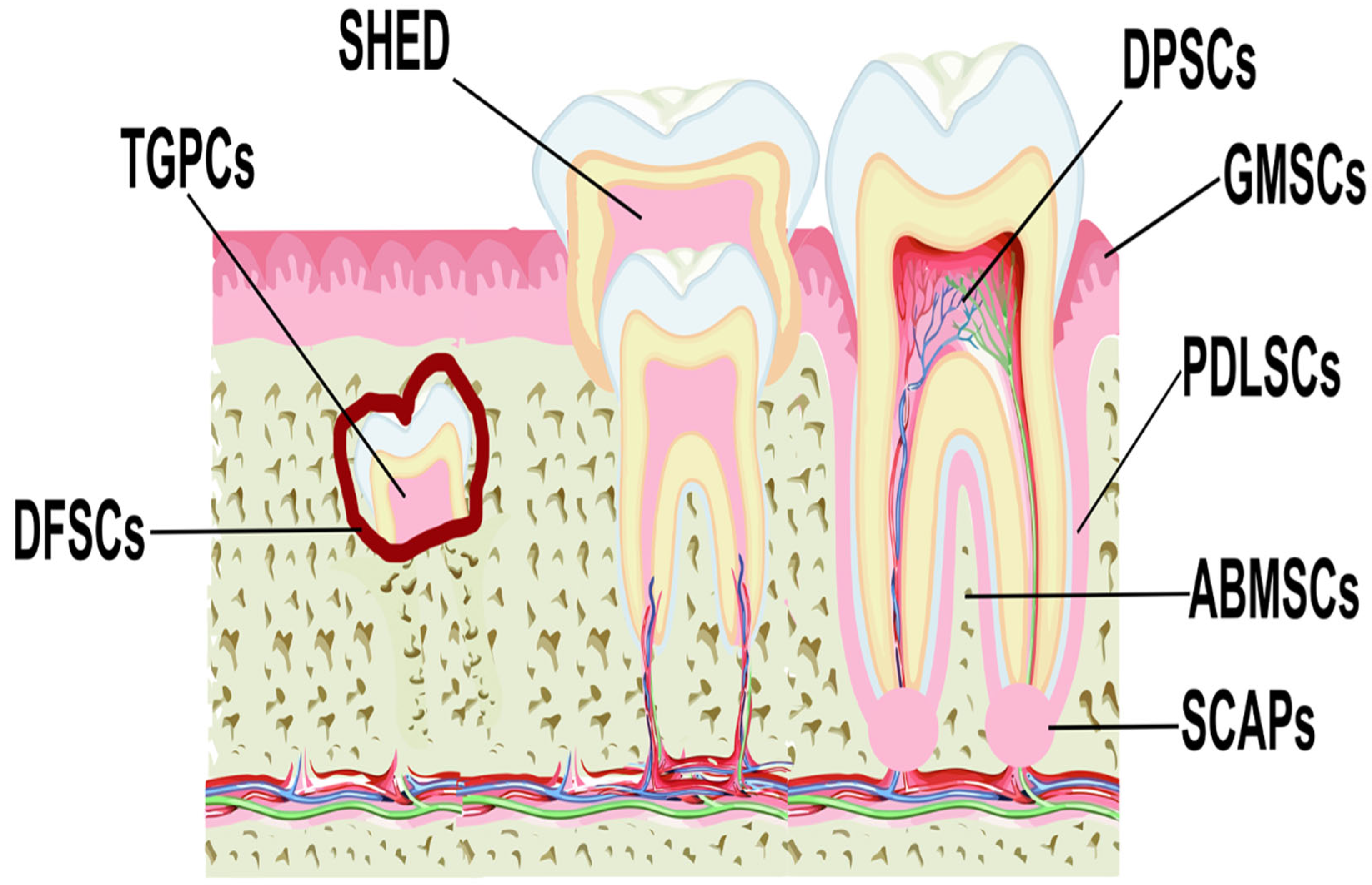
2. MSCs from Dental and Periodontal Tissues
3. Immunomodulatory Properties of MSCs
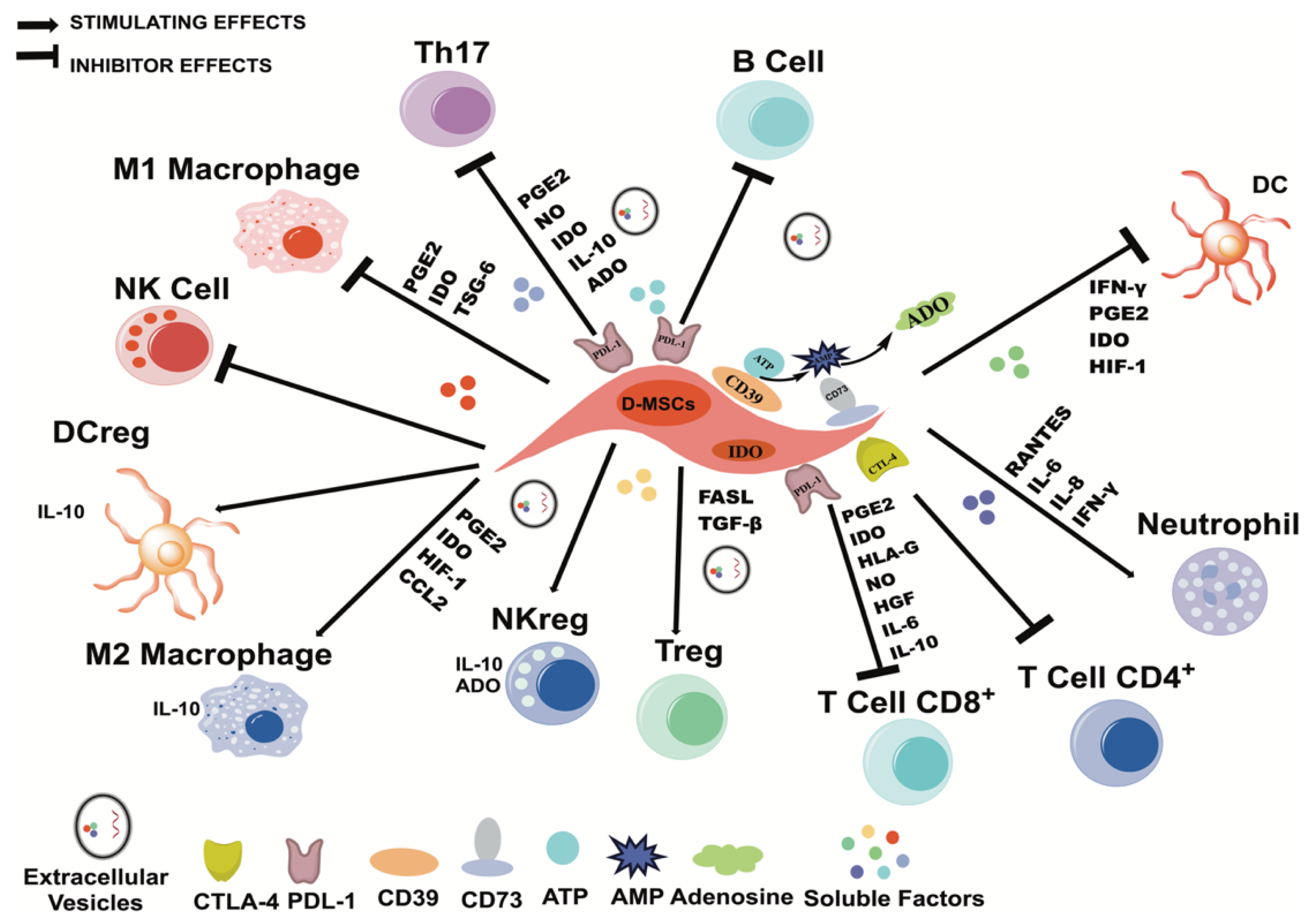
4. Immunomodulatory Properties of D-MSCs
4.1. Dental Pulp MSCs
4.2. Periodontal Ligament MSCs
4.3. Gingival Tissue MSCs
4.4. MSCs of the Apical Papilla
4.5. Dental Follicle MSCs
4.6. MSCs from Human Exfoliated Deciduous Teeth
4.7. MSCs from Alveolar Bone
5. The Induction of the Immunoregulatory Capacity of D-MSCs and the Release of Extracellular Vesicles
6. D-MSCs in Reported Clinical Trials
7. D-MSCs in Registered Clinical Trials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Fan, C.; Li, W.; Liang, R.; Wei, C.; Chen, X.; Yang, Y.; Zhong, Y.; Shao, Y.; Kong, Y.; et al. Mesenchymal Stem Cells: Ideal Seeds for Treating Diseases. Hum. Cell 2021, 34, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A. Successes and Failures of Stem Cell Transplantation in Autoimmune Diseases. Hematol. Am. Soc. Hematol. Educ. Program. 2011, 2011, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-Cell Immunosuppression by Mesenchymal Stromal Cells: What Do We Know So Far? Biomed. Res. Int. 2014, 2014, 216806. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, Donor Age and Gender Affect Function of Human Bone Marrow-Derived Mesenchymal Stromal Cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.J.; Burska, A.N.; Ponchel, F.; Giannoudis, P.V.; Jones, E.A. The Analysis of In Vivo Aging in Human Bone Marrow Mesenchymal Stromal Cells Using Colony-Forming Unit-Fibroblast Assay and the CD45(low) CD271(+) Phenotype. Stem Cells Int. 2019, 2019, 5197983. [Google Scholar] [CrossRef]
- Tokalov, S.V.; Gruner, S.; Schindler, S.; Wolf, G.; Baumann, M.; Abolmaali, N. Age-Related Changes in the Frequency of Mesenchymal Stem Cells in the Bone Marrow of Rats. Stem Cells Dev. 2007, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wong, W.H.; Chan, S.; Chim, J.C.; Cheung, K.M.; Lee, T.L.; Au, W.Y.; Ha, S.Y.; Lie, A.K.; Lau, Y.L.; et al. Factors Affecting Mesenchymal Stromal Cells Yield from Bone Marrow Aspiration. Chin. J. Cancer Res. 2011, 23, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Selle, M.; Koch, J.D.; Ongsiek, A.; Ulbrich, L.; Ye, W.; Jiang, Z.; Krettek, C.; Neunaber, C.; Noack, S. Influence of Age on Stem cells Depends on the Sex of the Bone Marrow Donor. J. Cell. Mol. Med. 2022, 26, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Katsara, O.; Mahaira, L.G.; Iliopoulou, E.G.; Moustaki, A.; Antsaklis, A.; Loutradis, D.; Stefanidis, K.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. Effects of Donor Age, Gender, and In Vitro Cellular Aging on the Phenotypic, Functional, and Molecular Characteristics of Mouse Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2011, 20, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of Mesenchymal Stem Cell In Vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging In Vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Lamas, J.R.; Fernandez-Gutierrez, B.; Mucientes, A.; Marco, F.; Lopiz, Y.; Jover, J.A.; Abasolo, L.; Rodriguez-Rodriguez, L. RNA Sequencing of Mesenchymal Stem Cells Reveals a Blocking of Differentiation and Immunomodulatory Activities Under Inflammatory Conditions in Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2019, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Sibov, T.T.; Severino, P.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; Gamarra, L.F.; et al. Mesenchymal Stem Cells from Umbilical Cord Blood: Parameters for Isolation, Characterization and Adipogenic Differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef]
- Araújo, A.B.; Furlan, J.M.; Salton, G.D.; Schmalfuss, T.; Röhsig, L.M.; Silla, L.M.R.; Passos, E.P.; Paz, A.H. Isolation of Human Mesenchymal Stem Cells from Amnion, Chorion, Placental Decidua and Umbilical Cord: Comparison of Four Enzymatic Protocols. Biotechnol. Lett. 2018, 40, 989–998. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; Ugarte, D.A.D.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Mizuno, M.; Katano, H.; Mabuchi, Y.; Ogata, Y.; Ichinose, S.; Fujii, S.; Otabe, K.; Komori, K.; Ozeki, N.; Koga, H.; et al. Specific Markers and Properties of Synovial Mesenchymal Stem Cells in the Surface, Stromal, and Perivascular Regions. Stem Cell Res. Ther. 2018, 9, 123. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Bonifaz, L.; Castro-Escamilla, O.; Monroy-Garcia, A.; Cortes-Morales, A.; Hernandez-Estevez, E.; Hernandez-Cristino, J.; Mayani, H.; Montesinos, J.J. Mesenchymal Stromal Cells from the Epidermis and Dermis of Psoriasis Patients: Morphology, Immunophenotype, Differentiation Patterns, and Regulation of T Cell Proliferation. Stem Cells Int. 2019, 2019, 4541797. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, F.; Petecchia, L.; Tavian, M.; Jodon de Villeroche, V.; Rossi, G.A.; Brouty-Boye, D. Human Bronchial Fibroblasts Exhibit a Mesenchymal Stem Cell Phenotype and Multilineage Differentiating Potentialities. Lab. Investig. 2005, 85, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Najimi, M.; Khuu, D.N.; Lysy, P.A.; Jazouli, N.; Abarca, J.; Sempoux, C.; Sokal, E.M. Adult-Derived Human Liver Mesenchymal-Like Cells as a Potential Progenitor Reservoir of Hepatocytes? Cell Transplant. 2007, 16, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Ouryazdanpanah, N.; Dabiri, S.; Derakhshani, A.; Vahidi, R.; Farsinejad, A. Peripheral Blood-Derived Mesenchymal Stem Cells: Growth Factor-Free Isolation, Molecular Characterization and Differentiation. Iran. J. Pathol. 2018, 13, 461–466. [Google Scholar] [PubMed]
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Lee, W.Y.W.; Li, G. Characterisation of Multipotent Stem Cells from Human Peripheral Blood Using an Improved Protocol. J. Orthop. Transl. 2019, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Yan, X.; Yuan, F.Z.; Ye, J.; Xu, B.B.; Zhou, Z.X.; Mao, Z.M.; Guan, J.; Song, Y.F.; Sun, Z.W.; et al. The Use of Peripheral Blood-Derived Stem Cells for Cartilage Repair and Regeneration In Vivo: A Review. Front. Pharmacol. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Human Peripheral Blood Derived Mesenchymal Stem Cells Demonstrate Similar Characteristics and Chondrogenic Differentiation Potential to Bone Marrow Derived Mesenchymal Ctem Cells. J. Orthop. Res. 2012, 30, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ouchi, T.; Cao, Y.; Zhao, Z.; Men, Y. Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 2021, 9, 654559. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.L.; Tadi, P. Anatomy, Head and Neck, Teeth. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hovorakova, M.; Lesot, H.; Peterka, M.; Peterkova, R. Early Development of the Human Dentition Revisited. J. Anat. 2018, 233, 135–145. [Google Scholar] [CrossRef]
- Bartlett, J.D. Dental Enamel Development: Proteinases and Their Enamel Matrix Substrates. ISRN Dent. 2013, 2013, 684607. [Google Scholar] [CrossRef]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of Collagen and Mesenchymal Stem Cells in Regenerative Dentistry. Curr. Stem Cell Res. Ther. 2022, 17, 606–620. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front. Biosci. 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Ghannam, M.; Alameddine, H.; Bordoni, B. Anatomy, Head and Neck, Pulp (Tooth). In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Olaru, M.; Sachelarie, L.; Calin, G. Hard Dental Tissues Regeneration-Approaches and Challenges. Materials 2021, 14, 2558. [Google Scholar] [CrossRef]
- Cabana-Munoz, M.E.; Pelaz Fernandez, M.J.; Parmigiani-Cabana, J.M.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications. Pharmaceutics 2023, 15, 2109. [Google Scholar] [CrossRef]
- Torabi, S.; Soni, A. Histology, Periodontium. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Koller, A.; Sapra, A. Anatomy, Head and Neck, Oral Gingiva. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Agha-Hosseini, F.; Jahani, M.A.; Jahani, M.; Mirzaii-Dizgah, I.; Ali-Moghaddam, K. In Vitro Isolation of Stem Cells Derived from Human Dental Pulp. Clin. Transplant. 2010, 24, E23–E28. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) In Vitro and In Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Navabazam, A.R.; Sadeghian Nodoshan, F.; Sheikhha, M.H.; Miresmaeili, S.M.; Soleimani, M.; Fesahat, F. Characterization of Mesenchymal Stem Cells from Human Dental Pulp, Preapical Follicle and Periodontal Ligament. Iran. J. Reprod. Med. 2013, 11, 235–242. [Google Scholar]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Mark Bartold, P.; Batouli, S.; Brahim, J.; Young, M.; Gehron Robey, P.; Wang, C.Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Mitrano, T.I.; Grob, M.S.; Carrion, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and Characterization of Mesenchymal Stem Cells from Human Gingival Tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the Apical papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peca, J.; Santos, J.M. Regeneration of Pulp-Dentin Complex Using Human Stem Cells of the Apical Papilla: In Vivo Interaction with Two Bioactive Materials. Clin. Oral Investig. 2021, 25, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Handa, K.; Saito, M.; Tsunoda, A.; Yamauchi, M.; Hattori, S.; Sato, S.; Toyoda, M.; Teranaka, T.; Narayanan, A.S. Progenitor Cells from Dental Follicle Are Able to Form Cementum Matrix In Vivo. Connect. Tissue Res. 2002, 43, 406–408. [Google Scholar] [CrossRef]
- Qu, G.; Li, Y.; Chen, L.; Chen, Q.; Zou, D.; Yang, C.; Zhou, Q. Comparison of Osteogenic Differentiation Potential of Human Dental-Derived Stem Cells Isolated from Dental Pulp, Periodontal Ligament, Dental Follicle, and Alveolar Bone. Stem Cells Int. 2021, 2021, 6631905. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the Bone Regeneration Ability Between Stem Cells from Human Exfoliated Deciduous Teeth, Human Dental Pulp Stem Cells and Human Bone Marrow Mesenchymal Stem Cells. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Sharpe, P.T. Dental Mesenchymal Stem Cells. Development 2016, 143, 2273. [Google Scholar] [CrossRef]
- Banavar, S.R.; Rawal, S.Y.; Paterson, I.C.; Singh, G.; Davamani, F.; Khoo, S.P.; Tan, E.L. Establishing a Technique for Isolation and Characterization of Human Periodontal Ligament Derived Mesenchymal Stem Cells. Saudi Dent. J. 2021, 33, 693–701. [Google Scholar] [CrossRef]
- Naz, S.; Khan, F.R.; Zohra, R.R.; Lakhundi, S.S.; Khan, M.S.; Mohammed, N.; Ahmad, T. Isolation and Culture of Dental Pulp Stem Cells from Permanent and Deciduous teeth. Pak. J. Med. Sci. 2019, 35, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Raoof, M.; Yaghoobi, M.M.; Derakhshani, A.; Kamal-Abadi, A.M.; Ebrahimi, B.; Abbasnejad, M.; Shokouhinejad, N. A Modified Efficient Method for Dental Pulp Stem Cell Isolation. Dent. Res. J. 2014, 11, 244–250. [Google Scholar]
- Zhang, X.; Zeng, D.; Huang, F.; Wang, J. A Protocol for Isolation and Culture of Mesenchymal stem Cells from human Gingival Tissue. Am. J. Clin. Exp. Immunol. 2019, 8, 21–26. [Google Scholar]
- Remy, M.; Ferraro, F.; Le Salver, P.; Rey, S.; Genot, E.; Djavaheri-Mergny, M.; Thebaud, N.; Boiziau, C.; Boeuf, H. Isolation and Culture of Human Stem Cells from Apical Papilla under Low Oxygen Concentration Highlight Original Properties. Cells 2019, 8, 1485. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C. Dental Follicle Stem Cells. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier: Amsterdam, The Netherlands, 2015; pp. 271–277. [Google Scholar]
- Hernandez-Monjaraz, B.; Santiago-Osorio, E.; Monroy-Garcia, A.; Ledesma-Martinez, E.; Mendoza-Nunez, V.M. Mesenchymal Stem Cells of Dental Origin for Inducing Tissue Regeneration in Periodontitis: A Mini-Review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef]
- Ledesma-Martinez, E.; Mendoza-Nunez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.R.; Tomar, G.B. Dental Tissue–Derived Mesenchymal Stem Cells: Applications in Tissue Engineering. Crit. Rev. Biomed. Eng. 2018, 46, 429–468. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Xia, Y.; Zhou, S.; Peng, H.; Wu, X.; Lu, H.; Wang, H.; Liu, R.; Blazar, B.R.; Gu, J.; et al. Reduction in Murine Acute GVHD Severity by Human Gingival Tissue-Derived Mesenchymal Stem Cells Via the CD39 Pathways. Cell Death Dis. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa-Ruiz, M.d.P.; Álvarez-Pérez, M.A.; Cortés-Morales, V.A.; Monroy-García, A.; Mayani, H.; Fragoso-González, G.; Caballero-Chacón, S.; Diaz, D.; Candanedo-González, F.; Montesinos, J.J. Mesenchymal Stem/Stromal Cells Derived from Dental Tissues: A Comparative In Vitro Evaluation of Their Immunoregulatory Properties Against T cells. Cells 2019, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Fracaro, L.; Senegaglia, A.C.; Herai, R.H.; Leitolis, A.; Boldrini-Leite, L.M.; Rebelatto, C.L.K.; Travers, P.J.; Brofman, P.R.S.; Correa, A. The Expression Profile of Dental Pulp-Derived Stromal Cells Supports Their Limited Capacity to Differentiate into Adipogenic Cells. Int. J. Mol. Sci. 2020, 21, 2753. [Google Scholar] [CrossRef]
- Deng, C.; Sun, Y.; Liu, H.; Wang, W.; Wang, J.; Zhang, F. Selective Adipogenic Differentiation of Human Periodontal Ligament Stem Cells Stimulated with High Doses of Glucose. PLoS ONE 2018, 13, e0199603. [Google Scholar] [CrossRef]
- Du, L.; Yang, P.; Ge, S. Isolation and Characterization of Human Gingiva-Derived Mesenchymal Stem Cells Using Limiting Dilution Method. J. Dent. Sci. 2016, 11, 304–314. [Google Scholar] [CrossRef]
- Dong, R.; Yao, R.; Du, J.; Wang, S.; Fan, Z. Depletion of Histone Demethylase KDM2A Enhanced the Adipogenic and Chondrogenic Differentiation Potentials of Stem Cells from Apical Papilla. Exp. Cell Res. 2013, 319, 2874–2882. [Google Scholar] [CrossRef]
- Koutsoumparis, A.E.; Patsiarika, A.; Tsingotjidou, A.; Pappas, I.; Tsiftsoglou, A.S. Neural Differentiation of Human Dental Mesenchymal Stem Cells Induced by ATRA and UDP-4: A Comparative Study. Biomolecules 2022, 12, 218. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, Z.; Liu, Q.; Wang, T.; Ban, L.K.; Lee, H.H.; Umezawa, A.; Almansour, A.I.; Arumugam, N.; Kumar, R.S.; et al. Neuronal Cell Differentiation of Human Dental Pulp Stem Cells on Synthetic Polymeric Surfaces Coated with ECM Proteins. Front. Cell Dev. Biol. 2022, 10, 893241. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Subbarayan, R.; Dinesh, M.G.; Arumugam, G.; Raja, S.T. Differentiation of Human Gingival Mesenchymal Stem Cells into Neuronal Lineages in 3D Bioconjugated Injectable Protein Hydrogel Construct for the Management of Neuronal Disorder. Exp. Mol. Med. 2016, 48, e209. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, W.; Chen, H.; Li, S.; Dou, R.; Shen, H.; Liu, X.; Liu, X.; Hong, Y.; He, J. Comparison of the Differentiation of Dental Pulp Stem Cells and Periodontal Ligament Stem Cells into Neuron-Like Cells and Their Effects on Focal Cerebral Ischemia. Acta Biochim. Biophys. Sin. 2020, 52, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Dai, X.; Yang, F.; Sun, Y.; Wu, X.; Zhou, Q.; Chen, K.; Sun, J.; Bi, W.; Shi, L.; et al. Spontaneous Spheroids from Alveolar Bone-Derived Mesenchymal Stromal Cells Maintain Pluripotency of Stem Cells by Regulating Hypoxia-Inducible Factors. Biol. Res. 2023, 56, 17. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yan, F.; Mohammed, H.A.G.; Liu, O. Maxillofacial-Derived Mesenchymal Stem Cells: Characteristics and Progress in Tissue Regeneration. Stem Cells Int. 2021, 2021, 5516521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, Y.H.; Zhao, Y.Q.; Gao, Z.R.; Ouyang, Z.Y.; Ye, Q.; Liu, Q.; Chen, Y.; Tan, L.; Zhang, S.H.; et al. Oral Cavity-Derived Stem Cells and Preclinical Models of Jaw-Bone Defects for Bone Tissue Engineering. Stem Cell Res. Ther. 2023, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Rasoolzade, B.; Namanloo, R.A.; Azarpira, N.; Dortaj, H. Stem Cells and Common Biomaterials in Dentistry: A Review Study. J. Mater. Sci. Mater. Med. 2022, 33, 55. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental Stem Cell and Dental Tissue Regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2015, 33, 627–638. [Google Scholar] [CrossRef]
- Aydin, S.; Sahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef]
- Santilli, F.; Fabrizi, J.; Santacroce, C.; Caissutti, D.; Spinello, Z.; Candelise, N.; Lancia, L.; Pulcini, F.; Delle Monache, S.; Mattei, V. Analogies and Differences between Dental Stem Cells: Focus on Secretome in Combination with Scaffolds in Neurological Disorders. Stem Cell Rev. Rep. 2024, 20, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Smojver, I.; Katalinic, I.; Bjelica, R.; Gabric, D.; Matisic, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.E.; Yoo, D.; LeRoux, M.A.; Danilkovitch-Miagkova, A. Treatment of Inflammatory Diseases with Mesenchymal Stem Cells. Inflamm. Allergy Drug Targets 2009, 8, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, J. Regulatory Factors of Mesenchymal Stem Cell Migration into Injured Tissues and Their Signal Transduction Mechanisms. Front. Med. 2011, 5, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Maeda, A. Recruitment of Mesenchymal Stem Cells to Damaged Sites by Plant-Derived Components. Front. Cell Dev. Biol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Sohni, A.; Verfaillie, C.M. Mesenchymal Stem Cells Migration Homing and Tracking. Stem Cells Int. 2013, 2013, 130763. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Atem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Commun. Signal 2011, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, H.; Yuan, X.; Ma, H.; Shi, X.; Ding, Y. Interleukin-10 Secreted by Mesenchymal Stem Cells Attenuates Acute Liver Failure through Inhibiting Pyroptosis. Hepatol. Res. 2018, 48, E194–E202. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Nito, C.; Sowa, K.; Suda, S.; Nishiyama, Y.; Nakamura-Takahashi, A.; Nitahara-Kasahara, Y.; Imagawa, K.; Hirato, T.; Ueda, M.; et al. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol. Ther. Methods Clin. Dev. 2017, 6, 102–111. [Google Scholar] [CrossRef]
- Zhang, C.; Delawary, M.; Huang, P.; Korchak, J.A.; Suda, K.; Zubair, A.C. IL-10 mRNA Engineered MSCs Demonstrate Enhanced Anti-Inflammation in an Acute GvHD Model. Cells 2021, 10, 3101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Huang, G.; Wei, Z.; Nie, K.; Liu, Z.; Deng, C.; Wang, D. IL-10 Gene-Modified Human Amniotic Mesenchymal Stem Cells Augment Regenerative Wound Healing by Multiple Synergistic Effects. Stem Cells Int. 2019, 2019, 9158016. [Google Scholar] [CrossRef] [PubMed]
- Dorronsoro, A.; Lang, V.; Ferrin, I.; Fernandez-Rueda, J.; Zabaleta, L.; Perez-Ruiz, E.; Sepulveda, P.; Trigueros, C. Intracellular Role of IL-6 in Mesenchymal Stromal Cell Immunosuppression and Proliferation. Sci. Rep. 2020, 10, 21853. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, C.; Delawary, M.; Korchak, J.A.; Suda, K.; Zubair, A.C. Development and Evaluation of IL-6 Overexpressing Mesenchymal Stem Cells (MSCs). J. Tissue Eng. Regen. Med. 2022, 16, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yue, W.; Le-Le, Z.; Bin, L.; Hu, X. Mesenchymal Stem Cells Inhibit T Cell Activation by Releasing TGF-β1 from TGF-β1/GARP Complex. Oncotarget 2017, 8, 99784–99800. [Google Scholar] [CrossRef]
- Lynch, K.; Treacy, O.; Chen, X.; Murphy, N.; Lohan, P.; Islam, M.N.; Donohoe, E.; Griffin, M.D.; Watson, L.; McLoughlin, S.; et al. TGF-beta1-Licensed Murine MSCs Show Superior Therapeutic Efficacy in Modulating Corneal Allograft Immune Rejection In Vivo. Mol. Ther. 2020, 28, 2023–2043. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-Secreted TGF-beta Regulates Lipopolysaccharide-Stimulated Macrophage M2-Like Polarization Via the Akt/FoxO1 Pathway. Stem Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef]
- Yanez, R.; Oviedo, A.; Aldea, M.; Bueren, J.A.; Lamana, M.L. Prostaglandin E2 Plays a Key Role in the Immunosuppressive Properties of Adipose and Bone Marrow Tissue-Derived Mesenchymal Stromal Cells. Exp. Cell Res. 2010, 316, 3109–3123. [Google Scholar] [CrossRef]
- Qi, J.; Tang, X.; Li, W.; Chen, W.; Yao, G.; Sun, L. Mesenchymal Stem Cells Inhibited the Differentiation of MDSCs Via COX2/PGE2 in Experimental Sialadenitis. Stem Cell Res. Ther. 2020, 11, 325. [Google Scholar] [CrossRef]
- Sun, X.; Su, Y.; Liu, X.; Liu, F.; Zhang, G.; Chen, Q.; Wang, C.; Fu, H.; Zhu, X.; Liu, K.; et al. PGE2 Dependent Inhibition of Macrophage Pyroptosis by MSCs Contributes to Alleviating aGVHD. Blood 2020, 136, 15. [Google Scholar] [CrossRef]
- Li, Q.; Song, W.J.; Ryu, M.O.; Nam, A.; An, J.H.; Ahn, J.O.; Bhang, D.H.; Jung, Y.C.; Youn, H.Y. TSG-6 Secreted by Human Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorates Severe Acute Pancreatitis Via ER Stress Downregulation in Mice. Stem Cell Res. Ther. 2018, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.Y.; Song, T.; Zhang, L.; Eirin, A.; Conley, S.; Tang, H.; Saadiq, I.; Jordan, K.; Lerman, A.; et al. Mesenchymal Stem Cells protect Renal Tubular Cells Via TSG-6 Regulating Macrophage Sunction and Phenotype Switching. Am. J. Physiol. Ren. Physiol. 2021, 320, F454–F463. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human Leukocyte Antigen-G5 Secretion by Human Mesenchymal Stem Cells is Required to Suppress T Lymphocyte and Natural Killer Function and to Induce CD4+CD25highFOXP3+ Tegulatory T Cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Ding, D.C.; Chou, H.L.; Chang, Y.H.; Hung, W.T.; Liu, H.W.; Chu, T.Y. Characterization of HLA-G and Related Immunosuppressive Effects in Human Umbilical Cord Stroma-Derived Stem Cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal Stem Cells: A Double-Edged Sword in Regulating Immune Responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.T.J.; Rozier, P.; Fonteneau, G.; Sutra, T.; Maumus, M.; Toupet, K.; Cristol, J.P.; Jorgensen, C.; Guilpain, P.; Noel, D. iNOS Activity Is Required for the Therapeutic Effect of Mesenchymal Stem Cells in Experimental Systemic Sclerosis. Front. Immunol. 2018, 9, 3056. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Anti-Inflammatory Mechanisms in the Vascular Wall. Circ. Res. 2001, 88, 877–887. [Google Scholar] [CrossRef]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef]
- Meesuk, L.; Tantrawatpan, C.; Kheolamai, P.; Manochantr, S. The Immunosuppressive Capacity of Human Mesenchymal Stromal Cells Derived from Amnion and Bone Marrow. Biochem. Biophys. Rep. 2016, 8, 34–40. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Uhlig, S.; Elvers-Hornung, S.; Kluter, H.; Bieback, K. Human Adipose Tissue-Derived Stromal Cells Suppress Human, but Not Murine Lymphocyte Proliferation, via Indoleamine 2,3-Dioxygenase Activity. Cells 2020, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Laitinen, A.; Rabina, J.; Valkonen, S.; Takatalo, M.; Larjo, A.; Veijola, J.; Lampinen, M.; Siljander, P.; Lehenkari, P.; et al. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T Cells. Stem Cells 2016, 34, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Saldanha-Araujo, F.; Ferreira, F.I.; Palma, P.V.; Araujo, A.G.; Queiroz, R.H.; Covas, D.T.; Zago, M.A.; Panepucci, R.A. Mesenchymal Stromal Cells Up-Regulate CD39 and Increase Adenosine Production to Suppress Activated T-Lymphocytes. Stem Cell Res. 2011, 7, 66–74. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, F.; Li, W.; Dang, J.L.; Yuan, J.; Wang, J.; Zeng, D.L.; Sun, C.X.; Liu, Y.Y.; Ao, Q.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front. Immunol. 2018, 9, 878. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, W.; Gu, J.; Zhang, X.; Dang, J.; Wang, J.; Zheng, Y.; Huang, F.; Yuan, J.; Xue, Y.; et al. Human Gingival Tissue-Derived MSC Suppress Osteoclastogenesis and Bone Erosion Via CD39-Adenosine Signal Pathway in Autoimmune Arthritis. EBioMedicine 2019, 43, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Haskó, G. Adenosine Regulation of the Immune System. In The Adenosine Receptors; Borea, P.A., Varani, K., Gessi, S., Merighi, S., Vincenzi, F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 499–514. [Google Scholar]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Di Tinco, R.; Bertani, G.; Pisciotta, A.; Bertoni, L.; Pignatti, E.; Maccaferri, M.; Bertacchini, J.; Sena, P.; Vallarola, A.; Tupler, R.; et al. Role of PD-L1 in Licensing Immunoregulatory Function of Dental Pulp Mesenchymal Stem Cells. Stem Cell Res. Ther. 2021, 12, 598. [Google Scholar] [CrossRef]
- Gaber, T.; Schonbeck, K.; Hoff, H.; Tran, C.L.; Strehl, C.; Lang, A.; Ohrndorf, S.; Pfeiffenberger, M.; Rohner, E.; Matziolis, G.; et al. CTLA-4 Mediates Inhibitory Function of Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2018, 19, 2312. [Google Scholar] [CrossRef]
- Montesinos, J.J.; López-García, L.; Cortés-Morales, V.A.; Arriaga-Pizano, L.; Valle-Ríos, R.; Fajardo-Orduña, G.R.; Castro-Manrreza, M.E. Human Bone Marrow Mesenchymal Stem/Stromal Cells Exposed to an Inflammatory Environment Increase the Expression of ICAM-1 and Release Microvesicles Enriched in This Adhesive Molecule: Analysis of the Participation of TNF-α and IFN-γ. J. Immunol. Res. 2020, 2020, 8839625. [Google Scholar] [CrossRef]
- Lopez-Garcia, L.; Castro-Manrreza, M.E. TNF-alpha and IFN-gamma Participate in Improving the Immunoregulatory Capacity of Mesenchymal Stem/Stromal Cells: Importance of Cell-Cell Contact and Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 9531. [Google Scholar] [CrossRef]
- Mattar, P.; Bieback, K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front. Immunol. 2015, 6, 560. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory Properties of Dental Tissue-Derived Mesenchymal Stem Cells: Implication in Disease and Tissue Regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma Does not Break, but Promotes the Immunosuppressive Capacity of Adult Human Mesenchymal Stem Cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Lee, R.H.; Yu, J.M.; Foskett, A.M.; Peltier, G.; Reneau, J.C.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. TSG-6 as a Biomarker to Predict Efficacy of Human Mesenchymal Stem/Progenitor Cells (hMSCs) in Modulating Sterile Inflammation In Vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16766–16771. [Google Scholar] [CrossRef]
- Kim, S.N.; Lee, H.J.; Jeon, M.S.; Yi, T.; Song, S.U. Galectin-9 is Involved in Immunosuppression Mediated by Human Bone Marrow-derived Clonal Mesenchymal Stem Cells. Immune Netw. 2015, 15, 241–251. [Google Scholar] [CrossRef]
- Sioud, M.; Mobergslien, A.; Boudabous, A.; Floisand, Y. Mesenchymal Stem Cell-Mediated T Cell Suppression Occurs Through Secreted Galectins. Int. J. Oncol. 2011, 38, 385–390. [Google Scholar] [CrossRef]
- Giri, J.; Das, R.; Nylen, E.; Chinnadurai, R.; Galipeau, J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020, 30, 1923–1934. [Google Scholar] [CrossRef]
- Yagura, K.; Ohtaki, H.; Tsumuraya, T.; Sato, A.; Miyamoto, K.; Kawada, N.; Suzuki, K.; Nakamura, M.; Kanzaki, K.; Dohi, K.; et al. The Enhancement of CCL2 and CCL5 by Human Bone Marrow-Derived Mesenchymal Stem/Stromal Cells Might Contribute to Inflammatory Suppression and Axonal Extension After Spinal Cord Injury. PLoS ONE 2020, 15, e0230080. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Tang, J.; Chen, Z.; Wei, L.; Chen, J.; Liu, Q. Human Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduce Inflammation and Pyroptosis in Acute Kidney Injury Via miR-223-3p/HDAC2/SNRK. Inflamm. Res. 2023, 72, 553–576. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Strzemecki, D.; Muraca, M.; Janowski, M.; Lukomska, B. Human Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Neuroinflammation Evoked by Focal Brain Injury in Rats. J. Neuroinflamm. 2019, 16, 216. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Mayani, H.; Monroy-Garcia, A.; Flores-Figueroa, E.; Chavez-Rueda, K.; Legorreta-Haquet, V.; Santiago-Osorio, E.; Montesinos, J.J. Human Mesenchymal Stromal Cells from Adult and Neonatal Sources: A Comparative In Vitro Analysis of Their Immunosuppressive Properties Against T Cells. Stem Cells Dev. 2014, 23, 1217–1232. [Google Scholar] [CrossRef]
- Stubbendorff, M.; Deuse, T.; Hua, X.; Phan, T.T.; Bieback, K.; Atkinson, K.; Eiermann, T.H.; Velden, J.; Schroder, C.; Reichenspurner, H.; et al. Immunological Properties of Extraembryonic Human Mesenchymal Stromal Cells Derived from Gestational Tissue. Stem Cells Dev. 2013, 22, 2619–2629. [Google Scholar] [CrossRef]
- Avanzini, M.A.; Bernardo, M.E.; Cometa, A.M.; Perotti, C.; Zaffaroni, N.; Novara, F.; Visai, L.; Moretta, A.; Del Fante, C.; Villa, R.; et al. Generation of Mesenchymal Stromal Cells in the Presence of Platelet Lysate: A Phenotypic and Functional Comparison of Umbilical Cord Blood- and Bone Marrow-Derived Progenitors. Haematologica 2009, 94, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ahn, J.S.; Shin, Y.Y.; Oh, S.J.; Song, M.H.; Kang, M.J.; Oh, J.M.; Lee, D.; Kim, Y.H.; Lee, B.C.; et al. Mesenchymal Stem Cells Target Microglia Via Galectin-1 Production to Rescue Aged Mice from Olfactory Dysfunction. Biomed. Pharmacother. 2022, 153, 113347. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kang, K.S.; Koo, H.C.; Park, J.R.; Choi, E.W.; Park, Y.H. Soluble Factors-Mediated Immunomodulatory Effects of Canine Adipose Tissue-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Roemeling-van Rhijn, M.; Mensah, F.K.; Korevaar, S.S.; Leijs, M.J.; van Osch, G.J.; Ijzermans, J.N.; Betjes, M.G.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Effects of Hypoxia on the Immunomodulatory Properties of Adipose Tissue-Derived Mesenchymal Stem Cells. Front. Immunol. 2013, 4, 203. [Google Scholar] [CrossRef] [PubMed]
- Sineh Sepehr, K.; Razavi, A.; Hassan, Z.M.; Fazel, A.; Abdollahpour-Alitappeh, M.; Mossahebi-Mohammadi, M.; Yekaninejad, M.S.; Farhadihosseinabadi, B.; Hashemi, S.M. Comparative Immunomodulatory Properties of Mesenchymal Stem Cells Derived from Human Breast Tumor and Normal Breast Adipose Tissue. Cancer Immunol. Immunother. 2020, 69, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human Adipose Tissue Mesenchymal Stromal Cells and Their Extracellular Vesicles Act Differentially on Lung Mechanics and Inflammation in Experimental Allergic Asthma. Stem Cell Res. Ther. 2017, 8, 151. [Google Scholar] [CrossRef]
- Heo, J.S.; Kim, S. Human Adipose Mesenchymal Stem Cells Modulate Inflammation and Angiogenesis Through Exosomes. Sci. Rep. 2022, 12, 2776. [Google Scholar] [CrossRef]
- Tomic, S.; Djokic, J.; Vasilijic, S.; Vucevic, D.; Todorovic, V.; Supic, G.; Colic, M. Immunomodulatory Properties of Mesenchymal Stem Cells Derived from Dental Pulp and Dental Follicle are Susceptible to Activation by Toll-Like Receptor Agonists. Stem Cells Dev. 2011, 20, 695–708. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Hashemi, S.M.; Namaki, S.; Ghanbarian, H.; Sattari, M.; Khojasteh, A. Study of the Immunomodulatory Effects of Osteogenic Differentiated Human Dental Pulp Stem Cells. Life Sci. 2019, 216, 111–118. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of Immunomodulatory Properties of Exosomes Derived from Bone Marrow Mesenchymal Stem Cells and Dental Pulp Stem Cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; Ontoria-Oviedo, I.; Ricardo, C.P.; Harding, S.E.; Sacedon, R.; Varas, A.; Zapata, A.; Sepulveda, P.; Vicente, A. Overexpression of Hypoxia-Inducible Factor 1 Alpha Improves Immunomodulation by Dental Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Kong, Y.; Hu, X.; Li, Z.; Li, Y.; Zhong, Y.; Wei, X.; Ling, J. MicroRNA-Enriched Small Extracellular Vesicles Possess Odonto-Immunomodulatory Properties for Modulating the Immune Response of Macrophages and Promoting Odontogenesis. Stem Cell Res. Ther. 2020, 11, 517. [Google Scholar] [CrossRef]
- Kanji, S.; Sarkar, R.; Pramanik, A.; Kshirsagar, S.; Greene, C.J.; Das, H. Dental Pulp-Derived Stem Cells Inhibit Osteoclast Differentiation by Secreting Osteoprotegerin and Deactivating AKT Signalling in Myeloid Cells. J. Cell Mol. Med. 2021, 25, 2390–2403. [Google Scholar] [CrossRef]
- Ahmadi, P.; Yan, M.; Bauche, A.; Smeets, R.; Muller, C.E.; Koch-Nolte, F.; Haag, F.; Fliegert, R.; Kluwe, L.; Schulze Zur Wiesch, J.; et al. Human Dental Pulp Cells Modulate CD8(+) T Cell Proliferation and Efficiently Degrade Extracellular ATP to Adenosine In Vitro. Cell Immunol. 2022, 380, 104589. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory Properties of Human Periodontal Ligament Stem Cells. J. Cell Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic Periodontal Ligament Stem Cell Therapy for Periodontitis in Swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef]
- Arora, P.; Li, W.; Huang, X.; Yu, W.; Huang, R.; Jiang, Q.; Chen, C. Metabolic Reconfiguration Activates Stemness and Immunomodulation of PDLSCs. Int. J. Mol. Sci. 2022, 23, 4038. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jo, C.H.; Kim, H.R.; Hwang, Y.I. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.Y.O.; Silverio Ruiz, K.G.; Nociti, F.H., Jr.; Albiero, M.L.; Saito, M.T.; Nobrega Stipp, R.; Condino-Neto, A.; Holzhausen, M.; Palombo, H.; Villar, C.C. Periodontal Ligament-Derived Mesenchymal Stem Cells Modulate Neutrophil Responses Via Paracrine Mechanisms. J. Periodontol. 2019, 90, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Su, W.R.; Zhang, Q.Z.; Shi, S.H.; Nguyen, A.L.; Le, A.D. Human Gingiva-Derived Mesenchymal Stromal Cells Attenuate Contact Hypersensitivity Via Prostaglandin E2-Dependent Mechanisms. Stem Cells 2011, 29, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Z.; Liu, P.; Hu, Y.; Li, T.; Yang, J.; Gao, P.; Xu, Q. Exosomes Derived from Human Gingival Mesenchymal Stem Cells Attenuate the Inflammatory Response in Periodontal Ligament Stem Cells. Front. Chem. 2022, 10, 863364. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, W.; Lin, X.; Guo, Z.; Wang, J.; Zhang, Q.; Brand, D.; Ryffel, B.; Huang, J.; Liu, Z.; et al. Adoptive Transfer of Human Gingiva-Derived Mesenchymal Stem Cells Ameliorates Collagen-Induced Arthritis via Suppression of Th1 and Th17 Cells and Enhancement of Regulatory T Cell Differentiation. Arthritis Rheum. 2013, 65, 1181–1193. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Dang, J.; Zhang, X.; Wang, J.; Chen, Y.; Liang, J.; Li, D.; Ma, J.; Yuan, J.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Ameliorate Streptozoticin-induced T1DM in Mice via Suppression of T effector cells and Up-regulating Treg Subsets. Sci. Rep. 2017, 7, 15249. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, M.; Chen, W.; Gu, J.; Yuan, J.; Xue, Y.; Dang, J.; Su, W.; Wang, J.; Zadeh, H.H.; et al. Human Gingiva-Derived Mesenchymal Stem Cells Inhibit Xeno-Graft-Versus-Host Disease Via CD39–CD73–Adenosine and IDO Signals. Front. Immunol. 2017, 8, 68. [Google Scholar] [CrossRef]
- Dang, J.; Xu, Z.; Xu, A.; Liu, Y.; Fu, Q.; Wang, J.; Huang, F.; Zheng, Y.; Qi, G.; Sun, B.; et al. Human Gingiva-Derived Mesenchymal Stem Cells are Therapeutic in Lupus Nephritis Through Targeting of CD39(−) CD73 Signaling Pathway. J. Autoimmun. 2020, 113, 102491. [Google Scholar] [CrossRef]
- Jiang, C.M.; Liu, J.; Zhao, J.Y.; Xiao, L.; An, S.; Gou, Y.C.; Quan, H.X.; Cheng, Q.; Zhang, Y.L.; He, W.; et al. Effects of Hypoxia on the Immunomodulatory Properties of Human Gingiva-Derived Mesenchymal Stem Cells. J. Dent. Res. 2015, 94, 69–77. [Google Scholar] [CrossRef]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 Complex Regulates IL-1RA Secretion in Mesenchymal Stem Cells to Accelerate Wound Healing. Sci. Transl. Med. 2018, 10, eaai8524. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-Treated Human Gingiva-Derived MSCs Enhance M2 Macrophage Polarization and Inhibit Periodontal Bone Loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human Gingiva-Derived Mesenchymal Stem Cells Elicit Polarization of M2 Macrophages and Enhance Cutaneous Wound Healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, Y.; Ma, Y.; Ge, L. Profiling the Secretome of Human Stem Cells from Dental Apical Papilla. Stem Cells Dev. 2016, 25, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, J.; Zhao, Y.; Li, X.; Ge, L. Comparative Secretome Analysis of Mesenchymal Stem Cells from Dental Apical Papilla and Bone Marrow During Early Odonto/Osteogenic Differentiation: Potential Role of Transforming Growth Factor-beta2. Front. Physiol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, X.; Chen, P.; Li, Q.; Du, H. Proteomic Profile of Human Dental Follicle Stem Cells and Apical Papilla Stem Cells. J. Proteom. 2021, 231, 103928. [Google Scholar] [CrossRef]
- Meneses, C.C.B.; Pizzatto, L.N.; Andrade, F.F.F.; Sipert, C.R. Prostaglandin E(2) Affects Interleukin 6 and Monocyte Chemoattractant Protein 1/CCL2 Production by Cultured Stem Cells of Apical Papilla. J. Endod. 2020, 46, 413–418. [Google Scholar] [CrossRef]
- Genc, D.; Zibandeh, N.; Nain, E.; Gokalp, M.; Ozen, A.O.; Goker, M.K.; Akkoc, T. Dental Follicle Mesenchymal Stem Cells Down-Regulate Th2-Mediated Immune Response in Asthmatic Patients Mononuclear Cells. Clin. Exp. Allergy 2018, 48, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, B.; Tian, J.; Hong, H.; Du, Y.; Li, K.; Li, X.; Wang, N.; Yu, X.; Wei, X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-beta3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol. Biochem. 2018, 51, 2290–2308. [Google Scholar] [CrossRef]
- Hong, H.; Chen, X.; Li, K.; Wang, N.; Li, M.; Yang, B.; Yu, X.; Wei, X. Dental Follicle Stem Cells Rescue the Regenerative Capacity of Inflamed Rat Dental Pulp Through a Paracrine Pathway. Stem Cell Res. Ther. 2020, 11, 333. [Google Scholar] [CrossRef]
- Silva Fde, S.; Ramos, R.N.; de Almeida, D.C.; Bassi, E.J.; Gonzales, R.P.; Miyagi, S.P.; Maranduba, C.P.; Sant’Anna, O.A.; Marques, M.M.; Barbuto, J.A.; et al. Mesenchymal Stem Cells Derived from Human Exfoliated Deciduous Teeth (SHEDs) Induce Immune Modulatory Profile in Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e98050. [Google Scholar] [CrossRef]
- Luo, P.; Jiang, C.; Ji, P.; Wang, M.; Xu, J. Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth as an Anti-Inflammatory Agent in Temporomandibular Joint Chondrocytes Via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, X.; Chen, X.; Yu, S.; Yang, W.; Liu, Y. Stem Cells from Exfoliated Deciduous Teeth Transplantation Ameliorates Sjögren’s Syndrome by Secreting Soluble PD-L1. J. Leukoc. Biol. 2022, 111, 1043–1055. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, S.; Liu, D.; Chen, C.; Xu, X.; Chen, X.; Shi, S. Transplantation of SHED Prevents Bone Loss in the Early Phase of Ovariectomy-Induced Osteoporosis. J. Dent. Res. 2014, 93, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tarle, S.; Kaigler, D. Characterization of the Immunomodulatory Properties of Alveolar Bone-Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Maska, B.; Malik, M.A.; Tagett, R.; Kaigler, D. Immunomodulatory Differences Between Mesenchymal Stem Cells from Different Oral Tissues. Heliyon 2024, 10, e23317. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent Mesenchymal Stem Cells with Immunosuppressive Activity can be Easily Isolated from Dental Pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Shankar, S.R.; Prasad, P.; Sowmya Jahnavi, V.; Bhonde, R.R.; Jyothi Prasanna, S. Functional Differences in Mesenchymal Stromal Cells from Human Dental Pulp and Periodontal Ligament. J. Cell Mol. Med. 2014, 18, 344–354. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertani, G.; Bertoni, L.; Di Tinco, R.; De Biasi, S.; Vallarola, A.; Pignatti, E.; Tupler, R.; Salvarani, C.; de Pol, A.; et al. Modulation of Cell Death and Promotion of Chondrogenic Differentiation by Fas/FasL in Human Dental Pulp Stem Cells (hDPSCs). Front. Cell Dev. Biol. 2020, 8, 279. [Google Scholar] [CrossRef]
- Yan, F.; Liu, O.; Zhang, H.; Zhou, Y.; Zhou, D.; Zhou, Z.; He, Y.; Tang, Z.; Wang, S. Human Dental Pulp Stem Cells Regulate Allogeneic NK Cells’ Function Via Induction of Anti-Inflammatory Purinergic Signalling in Activated NK Cells. Cell Prolif. 2019, 52, e12595. [Google Scholar] [CrossRef]
- Croci, S.; Bonacini, M.; Dolci, G.; Massari, M.; Facciolongo, N.; Pignatti, E.; Pisciotta, A.; Carnevale, G.; Negro, A.; Cassone, G.; et al. Human Dental Pulp Stem Cells Modulate Cytokine Production In Vitro by Peripheral Blood Mononuclear Cells from Coronavirus Disease 2019 Patients. Front. Cell. Dev. Biol. 2020, 8, 609204. [Google Scholar] [CrossRef]
- Cui, S.J.; Zhang, T.; Fu, Y.; Liu, Y.; Gan, Y.H.; Zhou, Y.H.; Yang, R.L.; Wang, X.D. DPSCs Attenuate Experimental Progressive TMJ Arthritis by Inhibiting the STAT1 Pathway. J. Dent. Res. 2020, 99, 446–455. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, J.; Dong, C.; Gu, Z. miR-21 in Exosomes Drived from Dental Pulp Stem Cells Ameliorate the Tregs/Th17 Immune Response Via Targeting Stat3 in Collagen-Induced Arthritis Mice. Ann. Rheum. Dis. 2020, 79, 250. [Google Scholar] [CrossRef]
- Dong, X.; Kong, F.; Liu, C.; Dai, S.; Zhang, Y.; Xiao, F.; Zhang, H.; Wu, C.T.; Wang, H. Pulp Stem Cells with Hepatocyte Growth Factor Overexpression Exhibit Dual Effects in Rheumatoid Arthritis. Stem Cell Res. Ther. 2020, 11, 229. [Google Scholar] [CrossRef]
- Albashari, A.; He, Y.; Zhang, Y.; Ali, J.; Lin, F.; Zheng, Z.; Zhang, K.; Cao, Y.; Xu, C.; Luo, L.; et al. Thermosensitive bFGF-Modified Hydrogel with Dental Pulp Stem Cells on Neuroinflammation of Spinal Cord Injury. ACS Omega 2020, 5, 16064–16075. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Pulp Stem Cells Derived from Human Permanent and Deciduous Teeth: Biological Characteristics and Therapeutic Applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O. Toll-Like Receptors and Dental Mesenchymal Stromal Cells. Front. Oral Health 2021, 2, 648901. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes Derived from Lipopolysaccharide-Preconditioned Human Dental Pulp Stem Cells Regulate Schwann Cell Migration and Differentiation. Connect. Tissue Res. 2021, 62, 277–286. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Gendviliene, I.; Barasa, P.; Rinkunaite, I.; Kaupinis, A.; Seinin, D.; Rutkunas, V.; Bukelskiene, V. Dental Pulp Stem Cell-Derived Extracellular Matrix: Autologous Tool Boosting Bone Regeneration. Cytotherapy 2022, 24, 597–607. [Google Scholar] [CrossRef]
- Zheng, Y.; Dong, C.; Yang, J.; Jin, Y.; Zheng, W.; Zhou, Q.; Liang, Y.; Bao, L.; Feng, G.; Ji, J.; et al. Exosomal microRNA-155-5p from PDLSCs Regulated Th17/Treg Balance by Targeting Sirtuin-1 in Phronic Periodontitis. J. Cell Physiol. 2019, 234, 20662–20674. [Google Scholar] [CrossRef]
- Shin, C.; Kim, M.; Han, J.A.; Choi, B.; Hwang, D.; Do, Y.; Yun, J.H. Human Periodontal Ligament Stem Cells Suppress T-Cell Proliferation Via Down-Regulation of non-Classical Major Histocompatibility Complex-Like Glycoprotein CD1b on Dendritic Cells. J. Periodontal Res. 2017, 52, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, O.; Xu, J.; Ding, G.; Liu, D.; Fan, Z.; Zhang, C.; Chen, W.; Ding, Y.; Tang, Z.; Wang, S. Periodontal Ligament Stem Cells Regulate B Lymphocyte Function via Programmed Cell Death Protein 1. Stem Cells 2013, 31, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, B.; Bao, J.; Zhang, Y.; Lei, L.; Yan, F. Macrophage Polarization in Periodontal Ligament Stem Cells Enhanced Periodontal Regeneration. Stem Cell Res. Ther. 2019, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, X.; Yu, W.; Xu, Y.; Huang, R.; Park, J.; Moshaverinia, A.; Arora, P.; Chen, C. Activation of Functional Somatic Stem Cells Promotes Endogenous Tissue Regeneration. J. Dent. Res. 2022, 101, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Zhang, L.; Li, X.; Ding, X.; Ding, G.; Wei, F. Periodontal Ligament Stem Cells Promote Polarization of M2 Macrophages. J. Leukoc. Biol. 2022, 111, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, X.; Zhou, H.; Zhang, C.; Wang, Y.; Huang, J.; Liu, M.; Yang, P.; Song, A. Enhancement of Periodontal Tissue Regeneration by Conditioned Media from Gingiva-Derived or Periodontal Ligament-Derived Mesenchymal Stem Cells: A Comparative Study in Rats. Stem Cell Res. Ther. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Iwasaki, K.; Akazawa, K.; Komaki, M.; Yokoyama, N.; Izumi, Y.; Morita, I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng. Part. A 2017, 23, 367–377. [Google Scholar] [CrossRef]
- Yu, B.; Li, Q.; Zhou, M. LPS-Induced Upregulation of the TLR4 Signaling Pathway Inhibits Osteogenic Differentiation of Human Periodontal Ligament Stem Cells Under Inflammatory Conditions. Int. J. Mol. Med. 2019, 43, 2341–2351. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, C.; Geng, T.; Liu, Y.; Zhu, S.; Zhang, C.; Liu, Z.; Wang, P. Lipopolysaccharide Inhibits Osteogenic Differentiation of Periodontal Ligament Stem Cells Partially Through Toll-Like Receptor 4-Mediated EphrinB2 Downregulation. Clin. Oral Investig. 2020, 24, 3407–3416. [Google Scholar] [CrossRef]
- Duan, Y.; An, W.; Wu, H.; Wu, Y. Salvianolic Acid C Attenuates LPS-Induced Inflammation and Apoptosis in Human Periodontal Ligament Stem Cells via Toll-Like Receptors 4 (TLR4)/Nuclear Factor kappa B (NF-kappaB) Pathway. Med. Sci. Monit. 2019, 25, 9499–9508. [Google Scholar] [CrossRef]
- Chen, M.; Lin, X.; Zhang, L.; Hu, X. Effects of Nuclear Factor-KappaB Signaling Pathway on Periodontal Ligament Stem Cells Under Lipopolysaccharide-Induced Inflammation. Bioengineered 2022, 13, 7951–7961. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Trubiani, O.; Diomede, F.; Pisciotta, A.; Paganelli, R. Immunomodulating Profile of Dental Mesenchymal Stromal Cells: A Comprehensive Overview. Front. Oral Health 2021, 2, 635055. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed. Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Liu, Y.; An, Y.; Zhang, C.; Shi, S.; Wang, W.; Wang, S. Suppression of T Cell Proliferation by Root Apical Papilla Stem Cells In Vitro. Cells Tissues Organs 2010, 191, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Galler, K.M.; Woelflick, M.; Rosendahl, A.; Moehle, C.; Lenhardt, B.; Buchalla, W.; Widbiller, M. Molecular Biological Comparison of Dental Pulp- and Apical Papilla-Derived Stem Cells. Int. J. Mol. Sci. 2022, 23, 2615. [Google Scholar] [CrossRef]
- Ding, G.; Wang, W.; Liu, Y.; An, Y.; Zhang, C.; Shi, S.; Wang, S. Effect of Cryopreservation on Biological and Immunological Properties of Stem Cells from Apical Papilla. J. Cell Physiol. 2010, 223, 415–422. [Google Scholar] [CrossRef]
- Liu, X.M.; Liu, Y.; Yu, S.; Jiang, L.M.; Song, B.; Chen, X. Potential Immunomodulatory Effects of Stem Cells from the Apical Papilla on Treg Conversion in Tissue Regeneration for Regenerative Endodontic Treatment. Int. Endod. J. 2019, 52, 1758–1767. [Google Scholar] [CrossRef]
- Tatic, N.; Rose, F.; des Rieux, A.; White, L.J. Stem Cells from the Dental Apical Papilla in Extracellular Matrix Hydrogels Mitigate Inflammation of Microglial Cells. Sci. Rep. 2019, 9, 14015. [Google Scholar] [CrossRef]
- De Berdt, P.; Bottemanne, P.; Bianco, J.; Alhouayek, M.; Diogenes, A.; Lloyd, A.; Llyod, A.; Gerardo-Nava, J.; Brook, G.A.; Miron, V.; et al. Stem Cells from Human Apical Papilla Decrease Neuro-Inflammation and Stimulate Oligodendrocyte Progenitor Differentiation Via Activin-A Secretion. Cell Mol. Life Sci. 2018, 75, 2843–2856. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Zhu, Q.; Zhang, Y.; Zhou, P.; Gu, Y. Transplantation of Human Stem Cells from the Apical Papilla for Treating Dextran Sulfate Sodium-Induced Experimental Colitis. Chin. J. Tissue Eng. Res. 2020, 24, 1069–1075. [Google Scholar] [CrossRef]
- Wang, A.; Liu, J.; Zhuang, X.; Yu, S.; Zhu, S.; Liu, Y.; Chen, X. Identification and Comparison of piRNA Expression Profiles of Exosomes Derived from Human Stem Cells from the Apical Papilla and Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2020, 29, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, X.; Liu, Y.; Zhuang, X.Y.; Wang, A.C.; Liu, X.M.; Zhu, S. Exosomes Derived from Stem Cells from the Apical Papilla Alleviate Inflammation in Rat Pulpitis by Upregulating Regulatory T Cells. Int. Endod. J. 2022, 55, 517–530. [Google Scholar] [CrossRef]
- Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules 2021, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Genc, D.; Zibandeh, N.; Nain, E.; Arig, U.; Goker, K.; Aydiner, E.K.; Akkoc, T. IFN-gamma Stimulation of Dental Follicle Mesenchymal Stem Cells Modulates Immune Response of CD4(+) T Lymphocytes in Der p1(+) Asthmatic Patients In Vitro. Allergol. Immunopathol. 2019, 47, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, C.; Zibandeh, N.; Yildirim, S.; Trakas, N.; Zisimopoulou, P.; Kucukerden, M.; Tasli, H.; Tzartos, S.; Goker, K.; Tuzun, E.; et al. Dental Follicle Mesenchymal Stem Cell Administration Ameliorates Muscle Weakness in MuSK-Immunized Mice. J. Neuroinflamm. 2015, 12, 231. [Google Scholar] [CrossRef]
- Guo, R.; Yu, J. Multipotency and Immunomodulatory Benefits of Stem Cells from Human Exfoliated Deciduous Teeth. Front. Dent. Med. 2022, 3, 805875. [Google Scholar] [CrossRef]
- Yamaza, T.; Kentaro, A.; Chen, C.; Liu, Y.; Shi, Y.; Gronthos, S.; Wang, S.; Shi, S. Immunomodulatory Properties of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cell Res. Ther. 2010, 1, 5. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part. A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Rossato, C.; Brandao, W.N.; Castro, S.B.R.; de Almeida, D.C.; Maranduba, C.M.C.; Camara, N.O.S.; Peron, J.P.S.; Silva, F.S. Stem Cells from Human-Exfoliated Deciduous Teeth Reduce Tissue-Infiltrating Inflammatory Cells Improving Clinical Signs in Experimental Autoimmune Encephalomyelitis. Biologicals 2017, 49, 62–68. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Zhu, J.; Guo, H.; Zhai, Q.; Li, B.; Jin, Y.; He, X.; Jin, F. Comparison of Therapeutic Effects of Different Mesenchymal Stem Cells on Rheumatoid Arthritis in Mice. PeerJ 2019, 7, e7023. [Google Scholar] [CrossRef]
- Shimojima, C.; Takeuchi, H.; Jin, S.; Parajuli, B.; Hattori, H.; Suzumura, A.; Hibi, H.; Ueda, M.; Yamamoto, A. Conditioned Medium from the Stem Cells of Human Exfoliated Deciduous Teeth Ameliorates Experimental Autoimmune Encephalomyelitis. J. Immunol. 2016, 196, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Muto, H.; Ito, T.; Tanaka, T.; Yokoyama, S.; Yamamoto, K.; Imai, N.; Ishizu, Y.; Maeda, K.; Honda, T.; Ishikawa, T.; et al. Conditioned Medium from Stem Cells Derived from Human Exfoliated Deciduous Teeth Ameliorates NASH Via the Gut-Liver Axis. Sci. Rep. 2021, 11, 18778. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Abu Kasim, N.H.; Fakurazi, S. Protective Effects of Stem Cells from Human Exfoliated Deciduous Teeth Derived Conditioned Medium on Osteoarthritic Chondrocytes. PLoS ONE 2020, 15, e0238449. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Murata, S.; Kato, H.; Zakaria, F.; Kyumoto-Nakamura, Y.; Uehara, N.; Yamaza, H.; Kukita, T.; Yamaza, T. Targeting of Deciduous Tooth Pulp Stem Cell-Derived Extracellular Vesicles on Telomerase-Mediated Stem Cell Niche and Immune Regulation in Systemic Lupus Erythematosus. J. Immunol. 2021, 206, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Song, Y.; Du, Z.; Yu, F.; Zhang, Y.; Jiang, N.; Ge, X. Exosomes Derived from Human Exfoliated Deciduous Teeth Ameliorate Adult Bone Loss in Mice Through Promoting Osteogenesis. J. Mol. Histol. 2020, 51, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Suardita, K.; Ishii, M.; Sugiyama, M.; Igarashi, A.; Oda, R.; Nishimura, M.; Saito, M.; Nakagawa, K.; Yamanaka, K.; et al. Alveolar Bone Marrow as a Cell Source for Regenerative Medicine: Differences Between Alveolar and Iliac Bone Marrow Stromal Cells. J. Bone Miner. Res. 2005, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Tarle, S.A.; Osibin, W.; Kinfu, Y.; Kaigler, D. Standardization and Safety of Alveolar Bone-Derived Stem Cell Isolation. J. Dent. Res. 2014, 93, 55–61. [Google Scholar] [CrossRef]
- Wang, X.; Xing, H.; Zhang, G.; Wu, X.; Zou, X.; Feng, L.; Wang, D.; Li, M.; Zhao, J.; Du, J.; et al. Restoration of a Critical Mandibular Bone Defect Using Human Alveolar Bone-Derived Stem Cells and Porous Nano-HA/Collagen/PLA Scaffold. Stem Cells Int. 2016, 2016, 8741641. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, Y.; Zhou, J.; Xu, M.; Zheng, W.; Huang, W.; Zhou, W.; Shen, Y.; Zhao, K.; Wu, Y.; et al. Comparison of Intraoral Bone Regeneration with Iliac and Alveolar BMSCs. J. Dent. Res. 2018, 97, 1229–1235. [Google Scholar] [CrossRef]
- Behm, C.; Blufstein, A.; Gahn, J.; Nemec, M.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Cytokines Differently Define the Immunomodulation of Mesenchymal Stem Cells from the Periodontal Ligament. Cells 2020, 9, 1222. [Google Scholar] [CrossRef]
- Behm, C.; Blufstein, A.; Gahn, J.; Kubin, B.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Continuing Effect of Cytokines and Toll-Like Receptor Agonists on Indoleamine-2,3-Dioxygenase-1 in Human Periodontal Ligament Stem/Stromal Cells. Cells 2020, 9, 2696. [Google Scholar] [CrossRef]
- Andrukhov, O.; Hong, J.S.; Andrukhova, O.; Blufstein, A.; Moritz, A.; Rausch-Fan, X. Response of Human Periodontal Ligament Stem Cells to IFN-gamma and TLR-Agonists. Sci. Rep. 2017, 7, 12856. [Google Scholar] [CrossRef]
- Behm, C.; Blufstein, A.; Gahn, J.; Kubin, B.; Nemec, M.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. 1,25(OH)(2)D(3) Differently Affects Immunomodulatory Activities of Mesenchymal Stem Cells Depending on the Presence of TNF-alpha, IL-1beta and IFN-gamma. J. Clin. Med. 2019, 8, 2211. [Google Scholar] [CrossRef]
- Watanabe, Y.; Fukuda, T.; Hayashi, C.; Nakao, Y.; Toyoda, M.; Kawakami, K.; Shinjo, T.; Iwashita, M.; Yamato, H.; Yotsumoto, K.; et al. Extracellular Vesicles Derived from GMSCs Stimulated with TNF-alpha and IFN-alpha Promote M2 Macrophage Polarization Via Enhanced CD73 and CD5L Expression. Sci. Rep. 2022, 12, 13344. [Google Scholar] [CrossRef]
- Tian, J.; Chen, W.; Xiong, Y.; Li, Q.; Kong, S.; Li, M.; Pang, C.; Qiu, Y.; Xu, Z.; Gong, Q.; et al. Small Extracellular Vesicles Derived from Hypoxic Preconditioned Dental Pulp Stem Cells Ameliorate Inflammatory Osteolysis by Modulating Macrophage Polarization and Osteoclastogenesis. Bioact. Mater. 2023, 22, 326–342. [Google Scholar] [CrossRef]
- Cocce, V.; Franze, S.; Brini, A.T.; Gianni, A.B.; Pascucci, L.; Ciusani, E.; Alessandri, G.; Farronato, G.; Cavicchini, L.; Sordi, V.; et al. In Vitro Anticancer Activity of Extracellular Vesicles (EVs) Secreted by Gingival Mesenchymal Stromal Cells Primed with Paclitaxel. Pharmaceutics 2019, 11, 61. [Google Scholar] [CrossRef]
- Klimova, D.; Jakubechova, J.; Altanerova, U.; Nicodemou, A.; Styk, J.; Szemes, T.; Repiska, V.; Altaner, C. Extracellular Vesicles Derived from Dental Mesenchymal Stem/Stromal Cells with Gemcitabine as a Cargo have an Inhibitory Effect on the Growth of Pancreatic Carcinoma Cell Lines In Vitro. Mol. Cell Probes 2023, 67, 101894. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone Defect Pepair by the Grafting of Dental Pulp Stem/Progenitor Cells and Collagen Sponge Biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Akiyama, K.; Liu, Y.; Yamaza, T.; Wang, T.M.; Chen, J.H.; Wang, B.B.; Huang, G.T.; Wang, S.; Shi, S. Utility of PDL Progenitors for In Vivo Tissue Regeneration: A Report of 3 Cases. Oral Dis. 2010, 16, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, G.; Motroni, A.; Graziano, A.; D’Aquino, R.; Zollino, I.; Carinci, F. Sinus Lift Tissue Engineering Using Autologous Pulp Micro-Grafts: A Case Report of Bone Density Evaluation. J. Indian Soc. Periodontol. 2013, 17, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Cricenti, L.; Mariani, G.M.; Romano, F. Autologous Dental Pulp Stem Cells in Periodontal Regeneration: A Case Report. Int. J. Periodontics Restor. Dent. 2014, 34 (Suppl. S3), s27–s33. [Google Scholar] [CrossRef]
- Manimaran, K.; Sankaranarayanan, S.; Ravi, V.R.; Elangovan, S.; Chandramohan, M.; Perumal, S.M. Treatment of Osteoradionecrosis of Mandible with Bone Marrow Concentrate and with Dental Pulp Stem Cells. Ann. Maxillofac. Surg. 2014, 4, 189–192. [Google Scholar] [CrossRef]
- Shiehzadeh, V.; Aghmasheh, F.; Shiehzadeh, F.; Joulae, M.; Kosarieh, E.; Shiehzadeh, F. Healing of Large Periapical Lesions Following Delivery of Dental Stem Cells With an Injectable Scaffold: New Method and Three Case Reports. Indian J. Dent. Res. 2014, 25, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.M.; Cricenti, L.; Romano, F. Use of Dental Pulp Stem Cells/Collagen Sponge Biocomplex in the Treatment of Non-Contained Intrabony Defects: A Case Series. Clin. Adv. Periodontics 2015, 5, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of Periodontal Intrabony Defects Using Autologous Periodontal Ligament Stem Cells: A Randomized Clinical Trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Manimaran, K.; Sharma, R.; Sankaranarayanan, S.; Perumal, S.M. Regeneration of Mandibular Ameloblastoma Defect with the Help of Autologous Dental Pulp Stem Cells and Buccal Pad of Fat Stromal Vascular Fraction. Ann. Maxillofac. Surg. 2016, 6, 97–100. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp Regeneration by Transplantation of Dental Pulp Stem Cells in Pulpitis: A Pilot Clinical Study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Prasad, M.G.S.; Ramakrishna, J.; Babu, D.N. Allogeneic Stem Cells Derived from Human Exfoliated Deciduous Teeth (SHED) for the Management of Periapical Lesions in Permanent Teeth: Two Case Reports of a Novel Biologic Alternative Treatment. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 117–122. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Gamba, M.N.; Giraudi, M.; Romano, F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int. J. Periodontics Restor. Dent. 2018, 38, 51–58. [Google Scholar] [CrossRef]
- Barbier, L.; Ramos, E.; Mendiola, J.; Rodriguez, O.; Santamaria, G.; Santamaria, J.; Arteagoitia, I. Autologous Dental Pulp Mesenchymal Stem Cells for Inferior Third Molar Post-Extraction Socket Healing: A Split-Mouth Randomised Clinical Trial. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e469–e477. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Hernandez-Monjaraz, B.; Santiago-Osorio, E.; Ledesma-Martinez, E.; Alcauter-Zavala, A.; Mendoza-Nunez, V.M. Retrieval of a Periodontally Compromised Tooth by Allogeneic Grafting of Mesenchymal Stem Cells from Dental Pulp: A Case Report. J. Int. Med. Res. 2018, 46, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous Autologous Tooth Stem Cells Regenerate Dental Pulp After Implantation into Injured Teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal Regeneration with Autologous Periodontal Ligament-Derived Cell Sheets—A Safety and Efficacy Study in Ten Patients. Regen. Ther. 2018, 9, 38–44. [Google Scholar] [CrossRef]
- Meza, G.; Urrejola, D.; Saint Jean, N.; Inostroza, C.; Lopez, V.; Khoury, M.; Brizuela, C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J. Endod. 2019, 45, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ghana Shyam Prasad, M.; Juvva, R.; Babu Duvvi, N. Towards a New Era in the Management of Large Periapical Lesion in Permanent Tooth Using Stemcells: A 2-Year Clinical Application Report. J. Dent. 2019, 20, 137–140. [Google Scholar] [CrossRef]
- Sanchez, N.; Fierravanti, L.; Nunez, J.; Vignoletti, F.; Gonzalez-Zamora, M.; Santamaria, S.; Suarez-Sancho, S.; Fernandez-Santos, M.E.; Figuero, E.; Herrera, D.; et al. Periodontal Regeneration Using a Xenogeneic Bone Substitute Seeded with Autologous Periodontal Ligament-Derived Mesenchymal Stem Cells: A 12-Month Quasi-Randomized Controlled Pilot Clinical Trial. J. Clin. Periodontol. 2020, 47, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, D.Y.S.; Pinheiro, C.C.G.; Almeida, M.C.A.; Oliveira, C.; Coudry, R.A.; Rocha, D.L.; Bueno, D.F. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients. Stem Cells Int. 2020, 2020, 6234167. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Horiguchi, Y. Efficacy of a Cultured Conditioned Medium of Exfoliated Deciduous Dental Pulp Stem Cells in Erectile Dysfunction Patients. J. Cell. Mol. Med. 2021, 26, 195–201. [Google Scholar] [CrossRef]
- Li, W.; Jiao, X.; Song, J.; Sui, B.; Guo, Z.; Zhao, Y.; Li, J.; Shi, S.; Huang, Q. Therapeutic Potential of Stem Cells from Human Exfoliated Deciduous Teeth Infusion into Patients with Type 2 Diabetes Pepends on Basal Lipid Levels and Islet Function. Stem Cells Transl. Med. 2021, 10, 956–967. [Google Scholar] [CrossRef]
- da Silva, J.M.; Araldi, R.P.; Colozza-Gama, G.A.; Pagani, E.; Sid, A.; Valverde, C.W.; Kerkis, I. Human Immature Dental Pulp Stem Cells Did Not Graft into a Preexisting Human Lung Adenocarcinoma. Case Rep. Oncol. 2022, 15, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Fukuyama, F.; Iohara, K. Pulp Regenerative Cell Therapy for Mature Molars: A Report of 2 Cases. J. Endod. 2022, 48, 1334.e1331–1340.e1331. [Google Scholar] [CrossRef] [PubMed]
- Cubuk, S.; Oduncuoglu, B.F.; Alaaddinoglu, E.E. The Effect of Dental Pulp Stem Cells and L-PRF when Placed into the Extraction Sockets of Impacted Mandibular Third Molars on the Periodontal Status of Adjacent Second Molars: A Split-Mouth, Randomized, Controlled Clinical Trial. Oral Maxillofac. Surg. 2023, 27, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, H.; Xia, X.; Zhou, C.; Liu, Z.; Xia, Z.E.; Zhang, Z.; Zhao, Y.; Yehenala, J.; Wang, S.; et al. Safety and Efficacy Assessment of Allogeneic Human Dental Pulp Stem Cells to Treat Patients with Severe COVID-19: Structured Summary of a Study Protocol for a Randomized Controlled Trial (Phase I/II). Trials 2020, 21, 520. [Google Scholar] [CrossRef]
- Nagpal, A.; Kremer, K.L.; Hamilton-Bruce, M.A.; Kaidonis, X.; Milton, A.G.; Levi, C.; Shi, S.; Carey, L.; Hillier, S.; Rose, M.; et al. TOOTH (The Open study of dental pulp stem cell Therapy in Humans): Study Protocol for Evaluating Safety and Feasibility of Autologous Human Adult Dental Pulp Stem Cell Therapy in Patients with Chronic Disability After Stroke. Int. J. Stroke 2016, 11, 575–585. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Ihara, M.; Iguchi, Y.; Urabe, T.; Matsumaru, Y.; Sakai, N.; Kimura, K. Randomised Placebo-Controlled Multicentre Trial to Evaluate the Efficacy and Safety of JTR-161, Allogeneic Human Dental Pulp Stem Cells, in Patients with Acute Ischaemic stRoke (J-REPAIR). BMJ Open 2022, 12, e054269. [Google Scholar] [CrossRef]
- Massaro, F.; Corrillon, F.; Stamatopoulos, B.; Dubois, N.; Ruer, A.; Meuleman, N.; Bron, D.; Lagneaux, L. Age-Related Changes in Human Bone Marrow Mesenchymal Stromal Cells: Morphology, Gene Expression Profile, Immunomodulatory Activity and miRNA Expression. Front. Immunol. 2023, 14, 1267550. [Google Scholar] [CrossRef]
- Zhang, Y.; Ravikumar, M.; Ling, L.; Nurcombe, V.; Cool, S.M. Age-Related Changes in the Inflammatory Status of Human Mesenchymal Stem Cells: Implications for Cell Therapy. Stem Cell Rep. 2021, 16, 694–707. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdel-Rasheed, M.; Galal, E.R.; El-Awady, R.R. Factors Defining Human Adipose Stem/Stromal Cell Immunomodulation in Vitro. Stem Cell Rev. Rep. 2023, 20, 175–205. [Google Scholar] [CrossRef]
- Ozgul Ozdemir, R.B.; Ozdemir, A.T.; Kirmaz, C.; Eker Sariboyaci, A.; Karaoz, E.; Erman, G.; Vatansever, H.S.; Mete Gokmen, N. Age-Related Changes in the Immunomodulatory Effects of Human Dental Pulp Derived Mesenchymal Stem Cells on the CD4(+) T Cell Subsets. Cytokine 2021, 138, 155367. [Google Scholar] [CrossRef]
- Dave, J.R.; Chandekar, S.S.; Behera, S.; Desai, K.U.; Salve, P.M.; Sapkal, N.B.; Mhaske, S.T.; Dewle, A.M.; Pokare, P.S.; Page, M.; et al. Human Gingival Mesenchymal Stem Cells Retain their Growth and Immunomodulatory Characteristics Independent of Donor Age. Sci. Adv. 2022, 8, eabm6504. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Wang, H.; Zhao, X.; Zhang, Z.; Ding, G.; Wei, F. The Effect of Aging on the Biological and Immunological Characteristics of Periodontal Ligament Stem Cells. Stem Cell Res. Ther. 2020, 11, 326. [Google Scholar] [CrossRef]
- Morsczeck, C. Effects of Cellular Senescence on Dental Follicle Cells. Pharmacology 2021, 106, 137–142. [Google Scholar] [CrossRef] [PubMed]
- de Mello Palma, V.; Danesi, C.C.; Arend, C.F.; Venturini, A.B.; Blaya, D.S.; Neto, M.M.; Flores, J.A.; Ferrazzo, K.L. Study of Pathological Changes in the Dental Follicle of Disease-Free Impacted Third Molars. J. Maxillofac. Oral Surg. 2018, 17, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.C.; Vitorio, J.G.; Martins-Chaves, R.R.; Leite-Lima, F.; Lebron, Y.A.R.; Moreira, V.R.; Duarte-Andrade, F.F.; Pereira, T.; Santos, L.V.S.; Lange, L.C.; et al. Age-Related Metabolic Pathways Changes in Dental Follicles: A Pilot Study. Front. Oral Health 2021, 2, 677731. [Google Scholar] [CrossRef] [PubMed]
| Source | Efficiency of Isolation | Surface Markers | Embryonic Markers | Neural Markers | Differentiation Potential |
|---|---|---|---|---|---|
| DPSCs | ++++ | CD13, CD29, CD44, CD59, CD73, CD90, CD105, CD146 | STRO-1, OCT-4, Nanog, SSEA-1, SEEA-4, SOX-2 | β3-tubulin, NFM, Nestin, CNPase, S100, CD271 | Adipogenic, osteogenic, odontoblast, angiogenic, and neuronal cells |
| PDLSCs | ++++ | CD10, CD29, CD44, CD73, CD105 | SSEA-1, SSEA-3, SSEA-4, TRA-1–60, TRA-1–81, OCT-4, Nanog, SOX-2, REX1, ALP | Nestin, OCT-4, SSEA-4, CD271, SOX-10 | Adipogenic, chondrogenic, osteogenic, and neuronal cells |
| GMSCs | ++++ | CD73, CD90, CD105 | SSEA-4, OCT-4, Nanog | Nestin, SOX10, β3-tubulin, NFM, CNPase | Adipogenic, chondrogenic, osteogenic, angiogenic, and neuronal cells |
| SCAPs | +++ | CD24, CD44, CD90, CD146, STRO-1 | OCT-4, Nanog, NOTCH-1, SOX-2 | OCT-4, SOX2, Nestin | Adipogenic, chondrogenic osteogenic, odontogenic, and neuronal cells |
| DFSCs | ++ | CD13, CD29, CD44, CD56, CD59, CD90, CD105, CD106, CD166, STRO-1 | OCT-4, Nanog, NOTCH-1, SOX-2 | OCT-4, SOX2, Nestin | Osteogenic, odontogenic, and cementogenic |
| SHED | +++ | CD29, CD73, CD90, CD146, CD166 | OCT-4, Nanog, SSEA-3, SSEA-4, NOTCH-1, SOX-2 | β3-tubulin, NFM, Nestin, CNPase, GAD, NeuN, GFAP, CD271, Vimentin, OCT-4, PAX-6, NSE, MAP-2, PSA- NCAM | Adipogenic, chondrogenic, osteogenic, odontogenic, angiogenic, and neuronal cells |
| ABMSCs | ++++ | CD73, CD90, CD105, STRO-1 | Oct4, KLF4, Sox2, cMyc | NF-M, NeuN, GFAP | Adipogenic, chondrogenic, and osteogenic |
| TGPCs | + | CD29, CD73, CD90, CD105, CD166 | Nanog, OCT-4, SOX-2, Klf4, c-Myc | Nestin | Adipogenic, chondrogenic, osteogenic, and neuronal cells |
| Source | Molecules and Mechanisms Related to Immunomodulatory Effects | References |
|---|---|---|
| BM-MSCs | IDO, TGF-β1, HGF, IL-6, IL-10, HLA-G, PGE2, NO, ICAM-1, PDL-1 and 2, TSG-6, galectins, EVs | [86,89,99,113,116,121,122,123,124,125,126,127,128] |
| UCB-MSCs | IDO, TGF-β, HGF, HLA-G, PDL-1, PGE2, galectins | [129,130,131,132] |
| AT-MSCs | TGF-β, HGF, PGE2, IDO, PDL-1, IL-10, TSG-6, EVs | [97,98,133,134,135,136,137] |
| DPSCs | IL-6, TGF-β, PGE2, IDO, HLAG, HGF, HIF-1, PDL-1, Fas-FasL pathway, osteoprotegerin, EVs, ADO | [60,114,138,139,140,141,142,143,144] |
| PDLSCs | TGF-β, IDO, HGF, PGE2, RANTES, eotaxina, IFN-γ, induced protein 10, MCP-1, IL-6, IL-8, IL-1ra EVs, PD-1, PDL-1 and 2 | [145,146,147,148,149] |
| GMSCs | Fas-FasL pathway, PGE2, IDO, iNOS, IL-10, adenosinergic pathway (CD39, CD73, ADO), EVs | [42,59,110,111,150,151,152,153,154,155,156,157,158,159] |
| SCAPs | IL-6, IL-10, PD-1, PDL-1 y 2 TGF-β 1 y 2, galectin 1, PGE2, EVs | [160,161,162,163] |
| DFSCs | TGF-β, IDO, TGF-β3, thrombospondin 1, IL-4 | [164,165,166] |
| SHED | TGF-β, IL-10, PDL-1, EVs, Fas-FasL pathway | [167,168,169,170] |
| ABMSCs | IL-6, MCP-1, PGE2, TIMP-1 and 2, osteoprotegerin | [171,172] |
| TGPCs | Not described | - |
| Disorder | MSCs Source | Administration Route | Outcomes | Reference |
|---|---|---|---|---|
| Mandibular bone defects after the extraction of third molars | Autologous DPSCs | Implanted at the extraction sites in a collagen sponge | Increased mineralization rate, cortical bone levels, BMP-2, and VEGF | [234] |
| Periodontal bone defects | Autologous periodontal ligament progenitors | Surgery, CALCITITE 4060-2 complex bone graft material | Improves gingival recession and decreases probing depth | [235] |
| Sinus lifting | Autologous DPSCs | Micrografts in the nasal sinus on a collagen sponge | Bone density of the nasal sinus increased | [236] |
| Periodontal bone defects | Autologous DPSCs | Surgery, on a collagen sponge | Bone tissue regeneration | [237] |
| Osteoradionecrosis | Autologous DPSCs | Grafted into the affected area, cells soaked in tricalcium phosphate | Regeneration of mandibular bone tissue | [238] |
| Periradicular periodontitis | Autologous SHED and SCAPs | Implanted in a polyethylene glycol polylactic–polyglycolic acid scaffold | Closure of the apex and bone regeneration | [239] |
| Intrabony defects | Autologous DPSCs | Surgery, on a collagen sponge | Probing depth decreased and bone tissue increased | [240] |
| Periodontal bone defects | Autologous PDLSCs | Surgery, on bovine bone materials | Regeneration of alveolar bone tissue | [241] |
| Mandibular defect due to ameloblastoma | Autologous DPSCs | Packed inside a mesh and placed over the mandible | Mandibular bone regeneration | [242] |
| Irreversible pulpitis | Autologous mobilized DPSCs | Injected with atelocollagen and G-CSF | They regenerated the pulp and formed dentin | [243] |
| Periapical lesions | Allogeneic SHED | Administered in suspension in the root canal | Closure of the apex, healing of the periapical tissue, and regeneration of the pulp | [244] |
| Chronic periodontitis | Autologous DPSCs | Grafted on a collagen sponge | Probing depth decreased and bone tissue increased | [245] |
| Mandibular bone defects after extraction of third molars | Autologous DPSCs | Implanted at extraction sites on collagen scaffolds | No effect was observed | [246] |
| Chronic periodontitis | Autologous DPSCs | Micrografts in a collagen sponge | Reduction in probing depth and bone regeneration | [247] |
| Periodontal disease | Allogeneic DPSCs | Surgery, implantation in a lyophilized collagen–polyvinylpyrrolidone sponge | Probing depth was reduced and bone tissue increased | [248] |
| Pulp necrosis | Autologous SHED | Implantation of the cells in the affected teeth | Pulp tissue regeneration | [249] |
| Periodontitis | Autologous PDLSCs | Cellular sheets | Probing depth was reduced and bone tissue was increased | [250] |
| Irreversible pulpitis | Autologous DPSCs | Administered at the affected site | Regeneration of functional pulp tissue | [251] |
| Apical lesions | Autologous SHED | Administration in root canals | Healing of the periapical tissue and the formation of functional pulp tissue | [252] |
| Intrabony lesions | Autologous PDLSCs | Surgery, implantation in the affected sites | Reduction in probing depth | [253] |
| Cleft lip and palate | Autologous SHED | Surgery, implantation in a hydroxyapatite–collagen sponge | Generation of bone tissue | [254] |
| Erectile dysfunction | CMs SHED | Injections into the corpora cavernosa of the penis | Improvement of erections | [255] |
| Diabetes mellitus 2 | Allogeneic SHED | Intravenous | Decreased glycosylated hemoglobin and glycosylated serum albumin levels and caused fever, fatigue, and rash | [256] |
| Huntington’s disease associated with primary lung adenocarcinoma | Allogeneic SHED | Intravenous | Cells did not graft (homing) in the pre-existing tumor | [257] |
| Irreversible pulpitis | Autologous DPSCs | Administration of the cells in the root canals combined with G-CSF in atelocollagen | Regeneration of functional pulp tissue | [258] |
| Mandibular bone defects after extraction of third molars | Autologous DPSCs | Implanted in the affected areas | Probing depth was reduced | [259] |
| ID | Study Status | Conditions | Interventions | Phases | Study Type |
|---|---|---|---|---|---|
| NCT04302519 | Unknown | COVID-19 | DPSCs | Early Phase 1 | Interventional |
| NCT04983225 | Active not recruiting | Periodontitis | DPSCs | Phase 1 | Interventional |
| NCT02731586 | Unknown | Edentulous alveolar ridge | DPSCs | Early Phase 1 | Interventional |
| NCT05924373 | Not recruiting | Periodontitis | DPSCs | Phase 2 | Interventional |
| NCT05127369 | Not recruiting | Depression | DPSCs | NA | Interventional |
| NCT02728115 | Active not recruiting | Huntington’s disease | DPSCs | Phase 1 | Interventional |
| NCT04130100 | Unknown | Knee osteoarthritis | DPSCs | Early Phase 1 | Interventional |
| NCT03570333 | Active not recruiting | Gingival disorders | GMSCs | Na | Interventional |
| NCT02728115 | Active not recruiting | Huntington’s disease | SHED (Cellavita HD) | Phase 1 | Interventional |
| NCT04219241 | Active not recruiting | Huntington’s disease | SHED (Cellavita HD) | Phase 2|Phase 3 | Interventional |
| NCT03252535 | Completed | Huntington’s disease | SHED (Cellavita HD) | Phase 2 | Interventional |
| NCT01082822 | Unknown | Periodontitis | PDLSCs | Phase 1|Phase 2 | Interventional |
| NCT04336254 | Unknown | COVID-19 | DPSCs | Phase 1|Phase 2 | Interventional |
| NCT02209311 | Unknown | Alveolar bone atrophy | MSCs from oral mucosa | Phase 1|Phase 2 | Interventional |
| NCT06043453 | Recruiting | Apical periodontitis | DPSCs and SCAPs | NA | Observational |
| NCT03957655 | Unknown | Liver cirrhosis | SHED | Early Phase 1 | Interventional |
| NCT01814436 | Unknown | Dental pulp necrosis | SHED | NA | Interventional |
| NCT05728346 | Not recruiting | Dental pulp necrosis | SHED | NA | Interventional |
| NCT03638154 | Completed | Periodontal defects | GMSCs | NA | Interventional |
| NCT03137979 | Unknown | Periodontitis | GMSCs | Phase 1|Phase 2 | Interventional |
| NCT04434794 | Completed | Gingival recession | GMSCs | Phase 1|Phase 2 | Interventional |
| NCT02464202 | Completed | Tooth transplantation | PDLSCs | NA | Interventional |
| NCT02523651 | Unknown | Periodontal diseases | DPSCs | Phase 1|Phase 2 | Interventional |
| ChiCTR2300073144 | Not recruiting | Periodontitis | DPSCs | Phase 2 | Interventional |
| RBR-65trt53 | Not recruiting | COVID-19 | DPSCs | Phase 1|Phase 2 | Interventional |
| IRCT20140911019125N8 | Recruiting | COVID-19 | DPSCs | Phase 2|Phase 3 | Interventional |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poblano-Pérez, L.I.; Castro-Manrreza, M.E.; González-Alva, P.; Fajardo-Orduña, G.R.; Montesinos, J.J. Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential. Int. J. Mol. Sci. 2024, 25, 1986. https://doi.org/10.3390/ijms25041986
Poblano-Pérez LI, Castro-Manrreza ME, González-Alva P, Fajardo-Orduña GR, Montesinos JJ. Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential. International Journal of Molecular Sciences. 2024; 25(4):1986. https://doi.org/10.3390/ijms25041986
Chicago/Turabian StylePoblano-Pérez, Luis Ignacio, Marta Elena Castro-Manrreza, Patricia González-Alva, Guadalupe R. Fajardo-Orduña, and Juan José Montesinos. 2024. "Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential" International Journal of Molecular Sciences 25, no. 4: 1986. https://doi.org/10.3390/ijms25041986
APA StylePoblano-Pérez, L. I., Castro-Manrreza, M. E., González-Alva, P., Fajardo-Orduña, G. R., & Montesinos, J. J. (2024). Mesenchymal Stromal Cells Derived from Dental Tissues: Immunomodulatory Properties and Clinical Potential. International Journal of Molecular Sciences, 25(4), 1986. https://doi.org/10.3390/ijms25041986





