Extracellular Vesicles Secreted by Adipose Tissue during Obesity and Type 2 Diabetes Mellitus Influence Reverse Cholesterol Transport-Related Gene Expression in Human Macrophages
Abstract
:1. Introduction
2. Results
2.1. Patient Clinical and Anthropometric Data
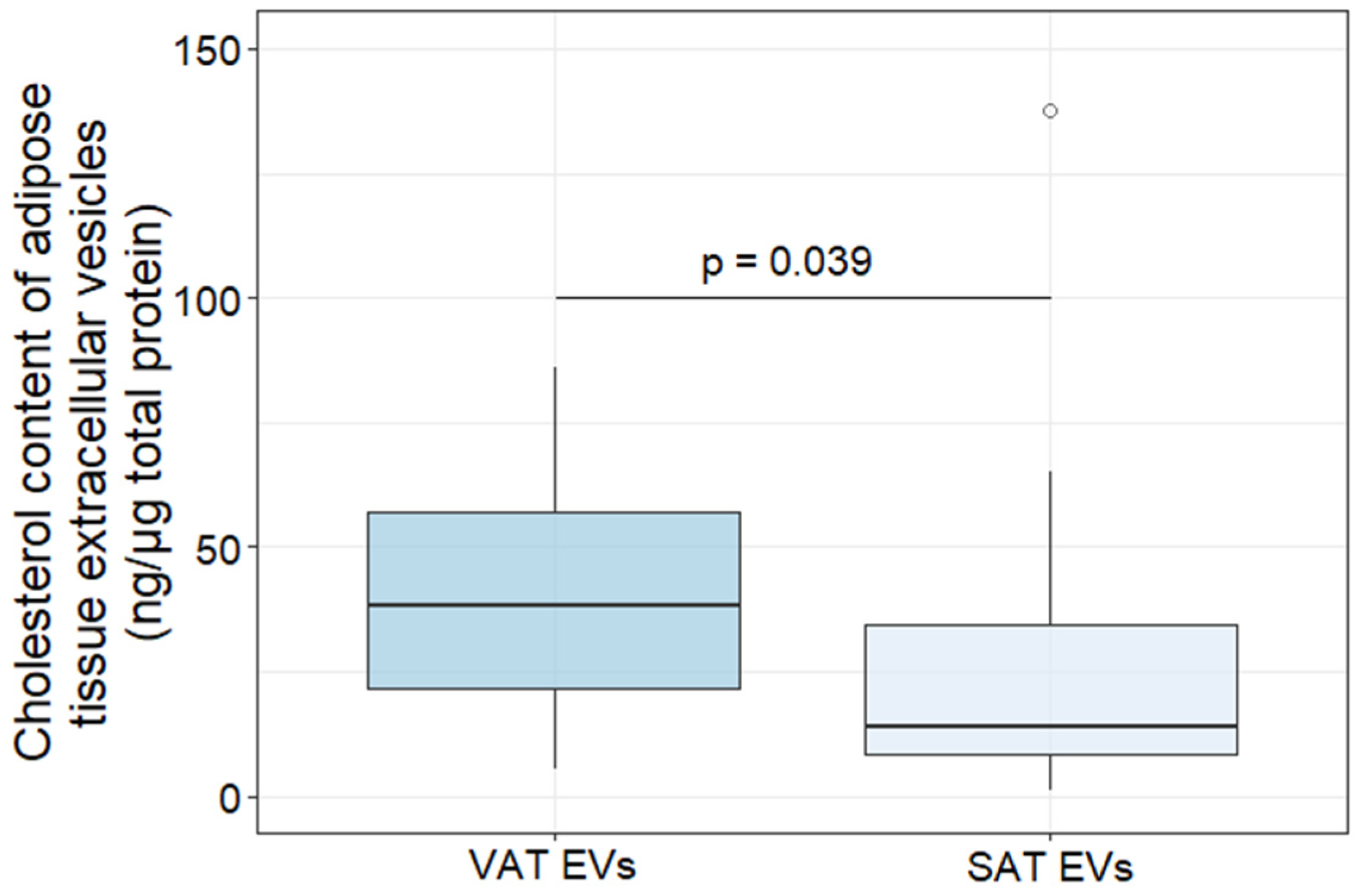
2.2. Characterization of AT EVs
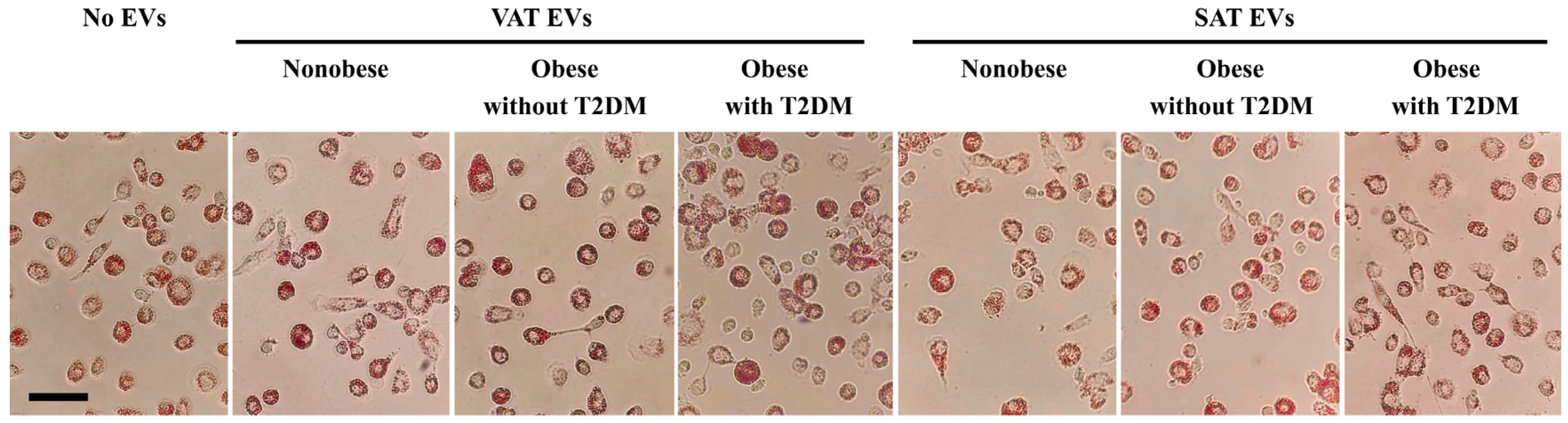
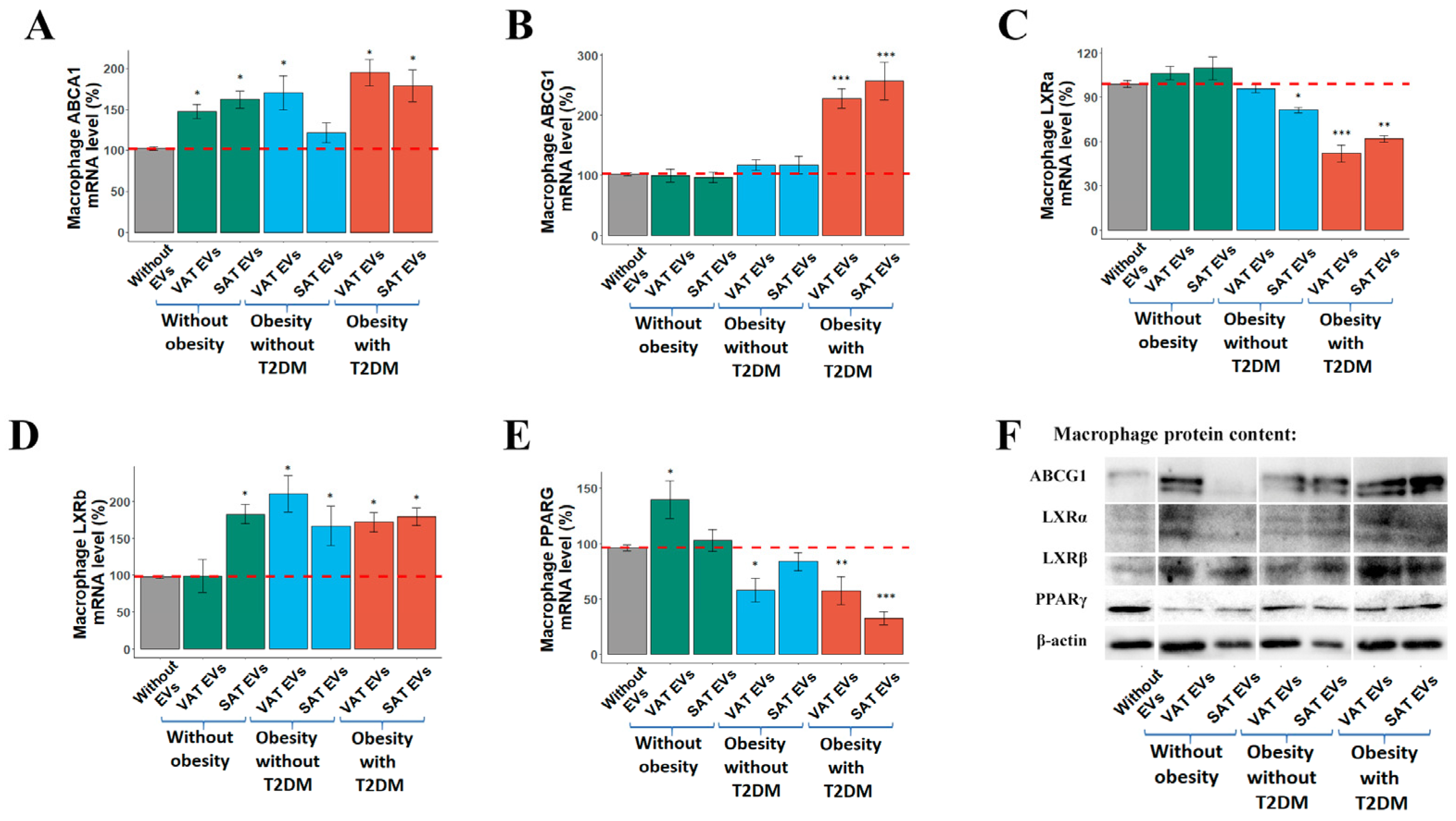
2.3. Effects of AT EVs on RCT Gene Expression in MDMs
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Adipose Tissue Cultivation and Extraction of Extracellular Vesicles
4.3. Characterization Adipose Tissue Extracellular Vesicles
4.4. Human Monocyte Derived Macrophages
4.5. Oil Red Staining
4.6. Extraction of RNA and qRT-PCR
4.7. Western Blotting for MDMs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Yonetsu, T.; Kato, K.; Uemura, S.; Kim, B.K.; Jang, Y.; Kang, S.Y.; Park, S.; Lee, S.; Kim, S.Y.; Jia, H.; et al. Features of coronary plaque in patients with metabolic syndrome and diabetes mellitus assessed by 3-vessel optical coherence tomography. Circ. Cardiovasc. Imaging 2013, 6, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Woodward, M.; Peters, S.A.E. Duration of diabetes and the risk of major cardiovascular events in women and men: A prospective cohort study of UK Biobank participants. Diabetes Res. Clin. Pract. 2022, 188, 109899. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, B.; Yang, Y.; Jia, Q.; Qi, Z.; Zhang, A.; Lv, S.; Zhang, J. Exosomes in ischemic heart disease: Novel carriers for bioinformation. Biomed. Pharmacother. 2019, 120, 109451. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Kuang, G.; Wu, Y.; Ou, C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin. Transl. Med. 2021, 11, e468. [Google Scholar] [CrossRef] [PubMed]
- Camino, T.; Lago-Baameiro, N.; Pardo, M. Extracellular Vesicles as Carriers of Adipokines and Their Role in Obesity. Biomedicines 2023, 11, 422. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Stepanian, A.; Bourguignat, L.; Hennou, S.; Coupaye, M.; Hajage, D.; Salomon, L.; Alessi, M.C.; Msika, S.; de Prost, D. Microparticle increase in severe obesity: Not related to metabolic syndrome and unchanged after massive weight loss. Obesity 2013, 21, 2236–2243. [Google Scholar] [CrossRef]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef]
- Miroshnikova, V.V.; Dracheva, K.V.; Kamyshinsky, R.A.; Yastremsky, E.V.; Garaeva, L.A.; Pobozheva, I.A.; Landa, S.B.; Anisimova, K.A.; Balandov, S.G.; Hamid, Z.M.; et al. Cryo-electron microscopy of adipose tissue extracellular vesicles in obesity and type 2 diabetes mellitus. PLoS ONE 2023, 18, e0279652. [Google Scholar] [CrossRef]
- Connolly, K.D.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterisation of adipocyte-derived extracellular vesicles released pre-and post-adipogenesis. J. Extracell. Vesicles 2015, 4, 29159. [Google Scholar] [CrossRef] [PubMed]
- Dracheva, K.V.; Pobozheva, I.A.; Anisimova, K.A.; Balandov, S.G.; Grunina, M.N.; Hamid, Z.M.; Vasilevsky, D.I.; Pchelina, S.N.; Miroshnikova, V.V. Downregulation of Exosomal hsa-miR-551b-3p in Obesity and Its Link to Type 2 Diabetes Mellitus. Noncoding RNA 2023, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Barberio, M.D.; Kasselman, L.J.; Playford, M.P.; Epstein, S.B.; Renna, H.A.; Goldberg, M.; DeLeon, J.; Voloshyna, I.; Barlev, A.; Salama, M.; et al. Cholesterol efflux alterations in adolescent obesity: Role of adipose-derived extracellular vesical microRNAs. J. Transl. Med. 2019, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef]
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem. Biophys. Res. Commun. 2010, 398, 723–729. [Google Scholar] [CrossRef]
- Flaherty, I.I.I.S.E.; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W., Jr. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef]
- Eguchi, A.; Mulya, A.; Lazic, M.; Radhakrishnan, D.; Berk, M.P.; Povero, D.; Gornicka, A.; Feldstein, A.E. Microparticles Release by Adipocytes Act as “Find-Me” Signals to Promote Macrophage Migration. PLoS ONE 2015, 10, e0123110. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Bastiaan-Net, S.; Garssen, J.; Pellegrini, N.; Willemsen, L.E.M.; Wichers, H.J. Functional differences between primary monocyte-derived and THP-1 macrophages and their response to LCPUFAs. PharmaNutrition 2022, 22, 100322. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce foam cell formation by regulating expression of cholesterol transporters. Front. Cardiovasc. Med. 2021, 8, 697510. [Google Scholar] [CrossRef] [PubMed]
- Shavva, V.S.; Babina, A.V.; Nekrasova, E.V.; Lisunov, A.V.; Dizhe, E.B.; Oleinikova, G.N.; Orlov, S.V. Insulin Downregulates the Expression of ATP-binding Cassette Transporter AI in Human Hepatoma Cell Line HepG2 in a FOXO1 and LXR Dependent Manner. Cell Biochem. Biophys. 2023, 81, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, M.S.; Smith, P.; Portman, J.R.; Jackson-Jones, L.H.; Bain, C.C.; Ramachandran, P.; Michailidou, Z.; Stimson, R.H.; Dweck, M.R.; Denby, L.; et al. Role of Tim4 in the regulation of ABCA1+ adipose tissue macrophages and post-prandial cholesterol levels. Nat. Commun. 2021, 12, 4434. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Tonyan, Z.N.; Barbitoff, Y.A.; Nasykhova, Y.A.; Danilova, M.M.; Kozyulina, P.Y.; Mikhailova, A.A.; Bulgakova, O.L.; Vlasova, M.E.; Golovkin, N.V.; Glotov, A.S. Plasma microRNA Profiling in Type 2 Diabetes Mellitus: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 17406. [Google Scholar] [CrossRef]
- Huang-Doran, I.; Zhang, C.Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication in Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef]
- Hartwig, S.; De Filippo, E.; Göddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 140172. [Google Scholar] [CrossRef]
- Tsubakio-Yamamoto, K.; Matsuura, F.; Koseki, M.; Oku, H.; Sandoval, J.C.; Inagaki, M.; Nakatani, K.; Nakaoka, H.; Kawase, R.; Yuasa-Kawase, M.; et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem. Biophys. Res. Commun. 2008, 375, 390–394. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.A.; González-King, H.; Grueso, E.; Sánchez, R.; Martinez-Romero, A.; Jávega, B.; O’Connor, E.; Simons, P.J.; Handberg, A.; Sepúlveda, P. Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: Possible role of CD36. PLoS ONE 2019, 14, e0217546. [Google Scholar] [CrossRef] [PubMed]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins [S]. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Mauerer, R.; Ebert, S.; Langmann, T. High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages. Exp. Mol. Med. 2009, 41, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Acebes, S.; Palacios, N.; Botella-Carretero, J.I.; Olea, N.; Crespo, L.; Peromingo, R.; Gómez-Coronado, D.; Lasunción, M.A.; Vázquez, C.; Martínez-Botas, J. Gene expression profiling of subcutaneous adipose tissue in morbid obesity using a focused microarray: Distinct expression of cell-cycle- and differentiation-related genes. BMC Med. Genom. 2010, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Panteleeva, A.A.; Razgildina, N.D.; Pobozheva, I.A.; Polyakova, E.A.; Dracheva, K.V.; Belyaeva, O.D.; Berkovich, A.; Baranova, E.I.; Pchelina, S.N.; Miroshnikova, V.V. Expression of Genes Encoding Nuclear Factors PPARγ, LXRβ, and RORα in Epicardial and Subcutaneous Adipose Tissues in Patients with Coronary Heart Disease. Bull. Exp. Biol. Med. 2021, 170, 654–657. [Google Scholar] [CrossRef]
- Yu, L.; Gao, Y.; Aaron, N.; Qiang, L. A glimpse of the connection between PPARγ and macrophage. Front. Pharmacol. 2023, 14, 1254317. [Google Scholar] [CrossRef] [PubMed]
- Szanto, A.; Rőszer, T. Nuclear receptors in macrophages: A link between metabolism and inflammation. FEBS Lett. 2008, 582, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, H.B.; Napimoga, M.H.; Lopes, A.H.; de Macedo Maganin, A.G.; Cunha, T.M.; Van Dyke, T.E.; Napimoga, J.T.C. Activation of PPAR-γ induces macrophage polarization and reduces neutrophil migration mediated by heme oxygenase 1. Int. Immunopharmacol. 2020, 84, 106565. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Yao, Q.; Qian, L.; Liu, J.; Xie, X.; Ma, W.; Nie, X.; Lai, B.; Wang, N. Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages. Front. Immunol. 2019, 10, 1895. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Qin, W.; Zheng, Y.; Wan, Z.; Liu, L. Role of Adipose Tissue Derived Exosomes in Metabolic Disease. Front. Endocrinl. 2022, 13, 873865. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127 (Suppl. S1), S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Breit, S.N. Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV. J. Leukoc. Biol. 1994, 56, 236–240. [Google Scholar] [CrossRef]
- Mogilenko, D.A.; Kudriavtsev, I.V.; Trulioff, A.S.; Shavva, V.S.; Dizhe, E.B.; Missyul, B.V.; Zhakhov, A.V.; Ischenko, A.M.; Perevozchikov, A.P.; Orlov, S.V. Modified Low Density Lipoprotein Stimulates Complement C3 Expression and Secretion via Liver X Receptor and Toll-like Receptor 4 Activation in Human Macrophages. J. Biol. Chem. 2012, 287, 5954–5968. [Google Scholar] [CrossRef] [PubMed]
- Vincent, V.; Thakkar, H.; Aggarwal, S.; Mridha, A.R.; Ramakrishnan, L.; Singh, A. ATP-binding cassette transporter A1 (ABCA1) expression in adipose tissue and its modulation with insulin resistance in obesity. Diabetes Metab Syndr Obes. 2019, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Oram, J.F. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 2005, 280, 30150–30157. [Google Scholar] [CrossRef]
- Miroshnikova, V.V.; Panteleeva, A.A.; Pobozheva, I.A.; Razgildina, N.D.; Polyakova, E.A.; Markov, A.V.; Belyaeva, O.D.; Berkovich, O.A.; Baranova, E.I.; Nazarenko, M.S.; et al. ABCA1 and ABCG1 DNA methylation in epicardial adipose tissue of patients with coronary artery disease. BMC Cardiovasc. Disord. 2021, 21, 566. [Google Scholar] [CrossRef]
- Panteleeva, A.A.; Razgildina, N.D.; Brovin, D.L.; Pobozheva, I.A.; Dracheva, K.V.; Berkovich, O.A.; Polyakova, E.A.; Belyaeva, O.D.; Baranova, E.I.; Pchelina, S.N.; et al. The Expression of Genes Encoding ABCA1 and ABCG1 Transporters and PPARγ, LXRβ, and RORα Transcriptional Factors in Subcutaneous and Visceral Adipose Tissue in Women with Metabolic Syndrome. Mol. Biol. 2021, 55, 56–65. [Google Scholar] [CrossRef]
- Miroshnikova, V.V.; Panteleeva, A.A.; Bazhenova, E.A.; Demina, E.P.; Usenko, T.S.; Nikolaev, M.A.; Semenova, I.A.; Neimark, A.E.; He, J.; Belyaeva, O.D.; et al. Regulation of ABCA1 and ABCG1 gene expression in the intraabdominal adipose tissue. Biomeditsinskaya Khimiya 2016, 62, 283–289. [Google Scholar] [CrossRef]

| Studied Groups | Obesity without Type 2 Diabetes Mellitus N = 27 | Obesity with Type 2 Diabetes Mellitus N = 26 | Control Group N = 15 | p |
|---|---|---|---|---|
| Age | 41.7 ± 11.3 | 44.4 ± 10.8 | 47.0 ± 13.5 | 1 0.476 2 0.721 3 0.974 |
| Sex (male/female) | 6/21 | 7/19 | 4/11 | |
| Body mass index, kg/m2 | 42.9 ± 6.4 | 49.6 ± 6.9 | 25.2 ± 3.2 | 1 0.027 2 0.000 3 0.000 |
| Weight, kg | 121.1 ± 18.0 | 137.5 ± 23.3 | 74.5 ± 11.9 | 1 0.099 2 0.001 3 0.000 |
| Waist circumference, cm | 120.2 ± 14.3 | 139 ± 16.0 | nd | 1 0.006 |
| Hip, cm | 129.4 ± 13.8 | 134.6 ± 16.2 | nd | 1 0.361 |
| Waist-to-hip ratio | 0.9 ± 0.1 | 1.0 ± 0.1 | nd | 1 0.037 |
| Glucose, nmol/L | 5.4 (4.3–8.1) | 7.3 (5.4–14.9) | 5 (4.3–6.6) | 1 0.000 2 0.700 3 0.000 |
| Insulin, µIU/mL | 14.3 (9.4–41.4) | 26.6 (8.7–79.4) | nd | 1 0.035 |
| HOMA-IR index | 3.5 (1.8–10.8) | 8.8 (4.2–23.4) | nd | 1 0.004 |
| C-peptide. ng/mL | 2.7 (1.8–4.6) | 3.9 (1.9–11.9) | nd | 1 0.014 |
| HbA1c, % | 5.5 (5.1–6.0) | 6.8 (5–11.9) | nd | 1 0.000 |
| Total cholesterol, mmol/L | 4.9 ± 1.1 | 5.1 ± 0.9 | nd | 1 0.529 |
| HDL, mmol/L | 1.4 ± 0.3 | 1.2 ± 0.2 | nd | 1 0.383 |
| LDL, mmol/L | 2.8 ± 1.0 | 2.7 ± 0.8 | nd | 1 0.897 |
| Triglycerides, mmol/L | 1.3 (0.6–4.3) | 2.0 (0.9–4.4) | nd | 1 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dracheva, K.V.; Pobozheva, I.A.; Anisimova, K.A.; Panteleeva, A.A.; Garaeva, L.A.; Balandov, S.G.; Hamid, Z.M.; Vasilevsky, D.I.; Pchelina, S.N.; Miroshnikova, V.V. Extracellular Vesicles Secreted by Adipose Tissue during Obesity and Type 2 Diabetes Mellitus Influence Reverse Cholesterol Transport-Related Gene Expression in Human Macrophages. Int. J. Mol. Sci. 2024, 25, 6457. https://doi.org/10.3390/ijms25126457
Dracheva KV, Pobozheva IA, Anisimova KA, Panteleeva AA, Garaeva LA, Balandov SG, Hamid ZM, Vasilevsky DI, Pchelina SN, Miroshnikova VV. Extracellular Vesicles Secreted by Adipose Tissue during Obesity and Type 2 Diabetes Mellitus Influence Reverse Cholesterol Transport-Related Gene Expression in Human Macrophages. International Journal of Molecular Sciences. 2024; 25(12):6457. https://doi.org/10.3390/ijms25126457
Chicago/Turabian StyleDracheva, Kseniia V., Irina A. Pobozheva, Kristina A. Anisimova, Aleksandra A. Panteleeva, Luiza A. Garaeva, Stanislav G. Balandov, Zarina M. Hamid, Dmitriy I. Vasilevsky, Sofya N. Pchelina, and Valentina V. Miroshnikova. 2024. "Extracellular Vesicles Secreted by Adipose Tissue during Obesity and Type 2 Diabetes Mellitus Influence Reverse Cholesterol Transport-Related Gene Expression in Human Macrophages" International Journal of Molecular Sciences 25, no. 12: 6457. https://doi.org/10.3390/ijms25126457
APA StyleDracheva, K. V., Pobozheva, I. A., Anisimova, K. A., Panteleeva, A. A., Garaeva, L. A., Balandov, S. G., Hamid, Z. M., Vasilevsky, D. I., Pchelina, S. N., & Miroshnikova, V. V. (2024). Extracellular Vesicles Secreted by Adipose Tissue during Obesity and Type 2 Diabetes Mellitus Influence Reverse Cholesterol Transport-Related Gene Expression in Human Macrophages. International Journal of Molecular Sciences, 25(12), 6457. https://doi.org/10.3390/ijms25126457





