Highly Efficient Separation of Ethanol Amines and Cyanides via Ionic Magnetic Mesoporous Nanomaterials
Abstract
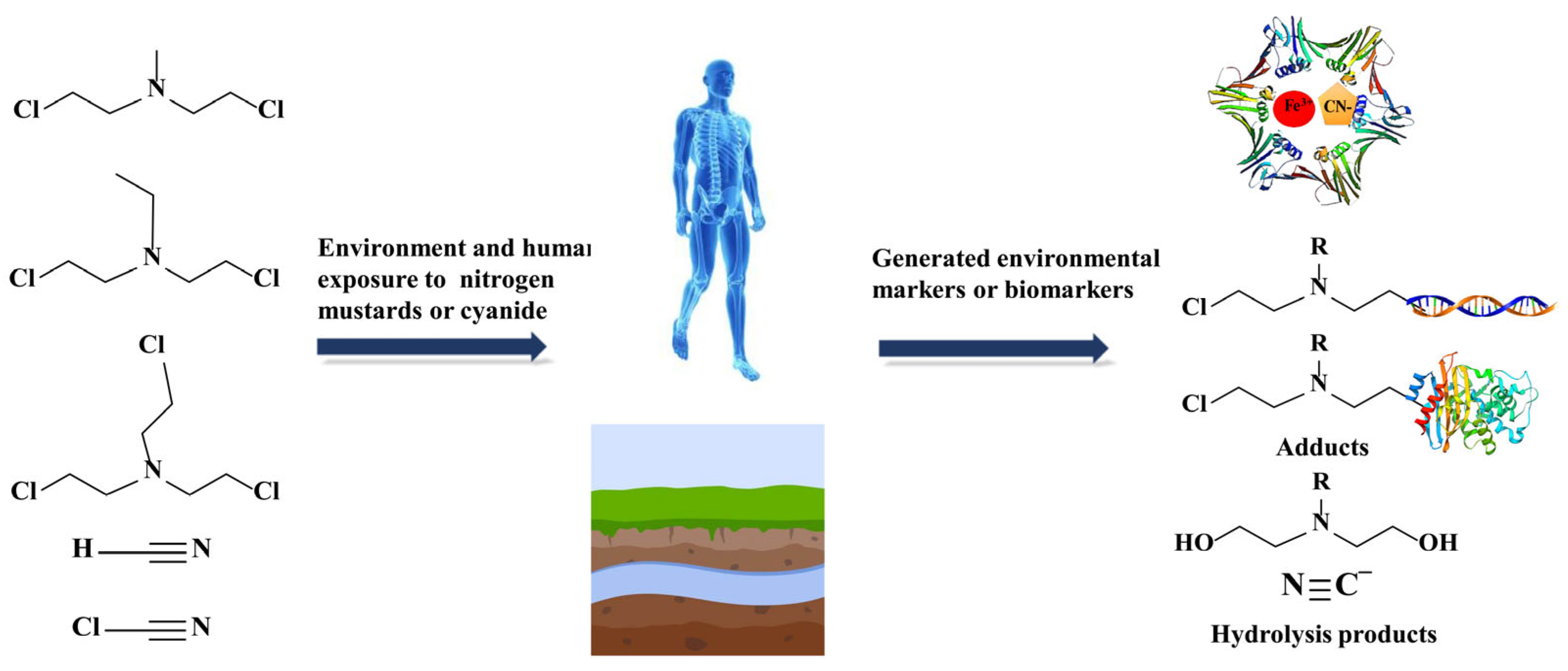
:1. Introduction
2. Results
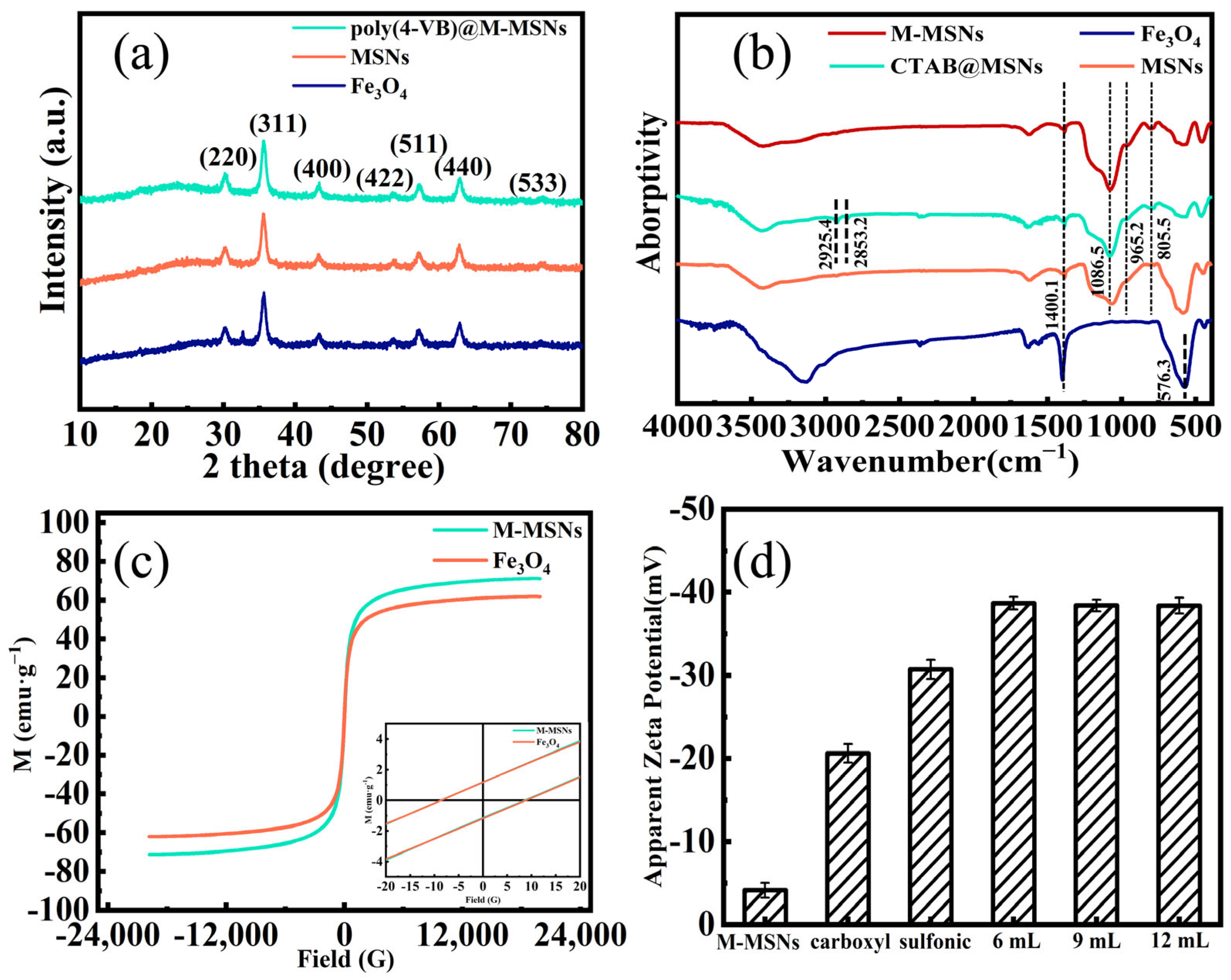
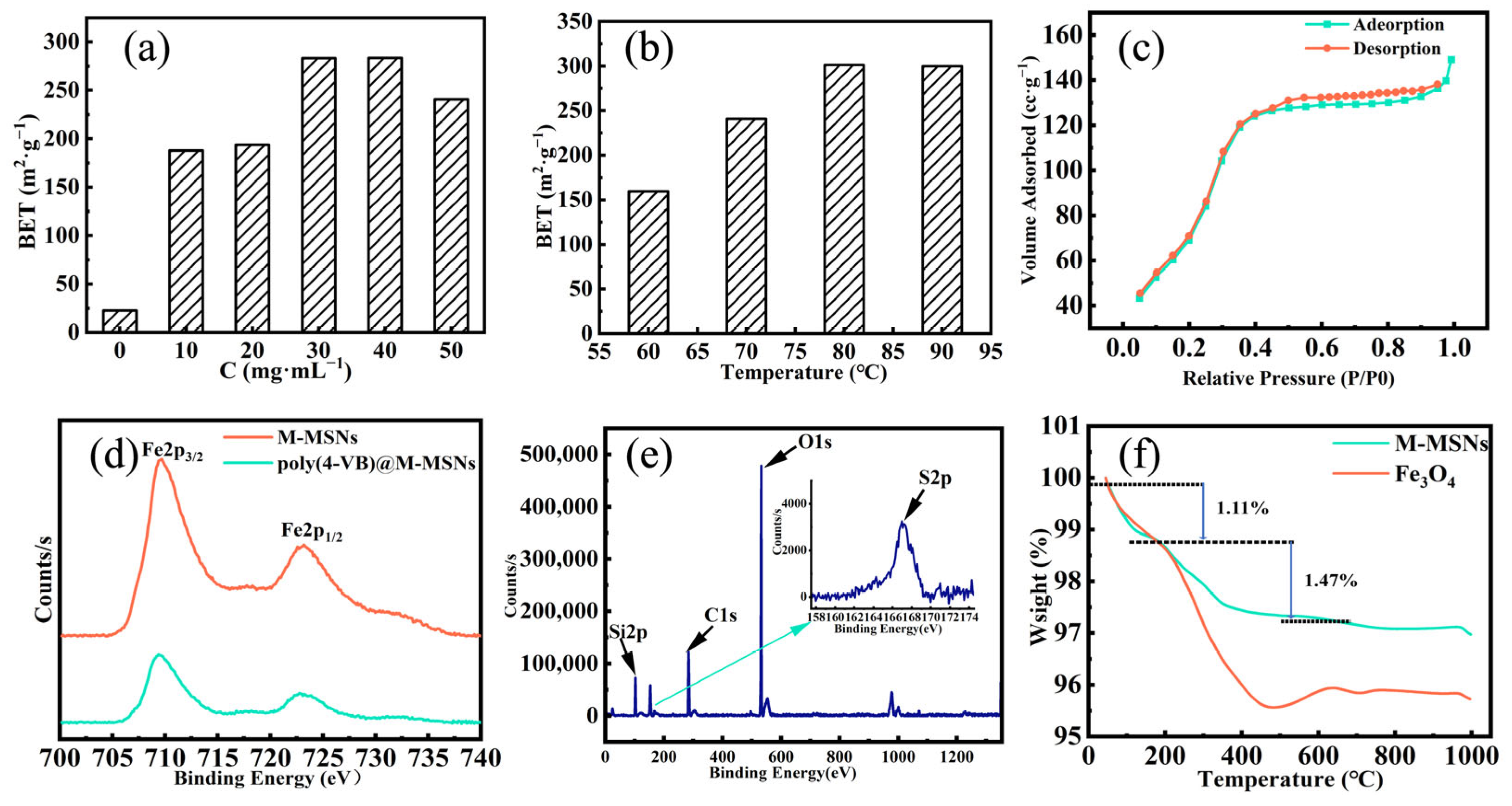
Characterization
3. Discussion
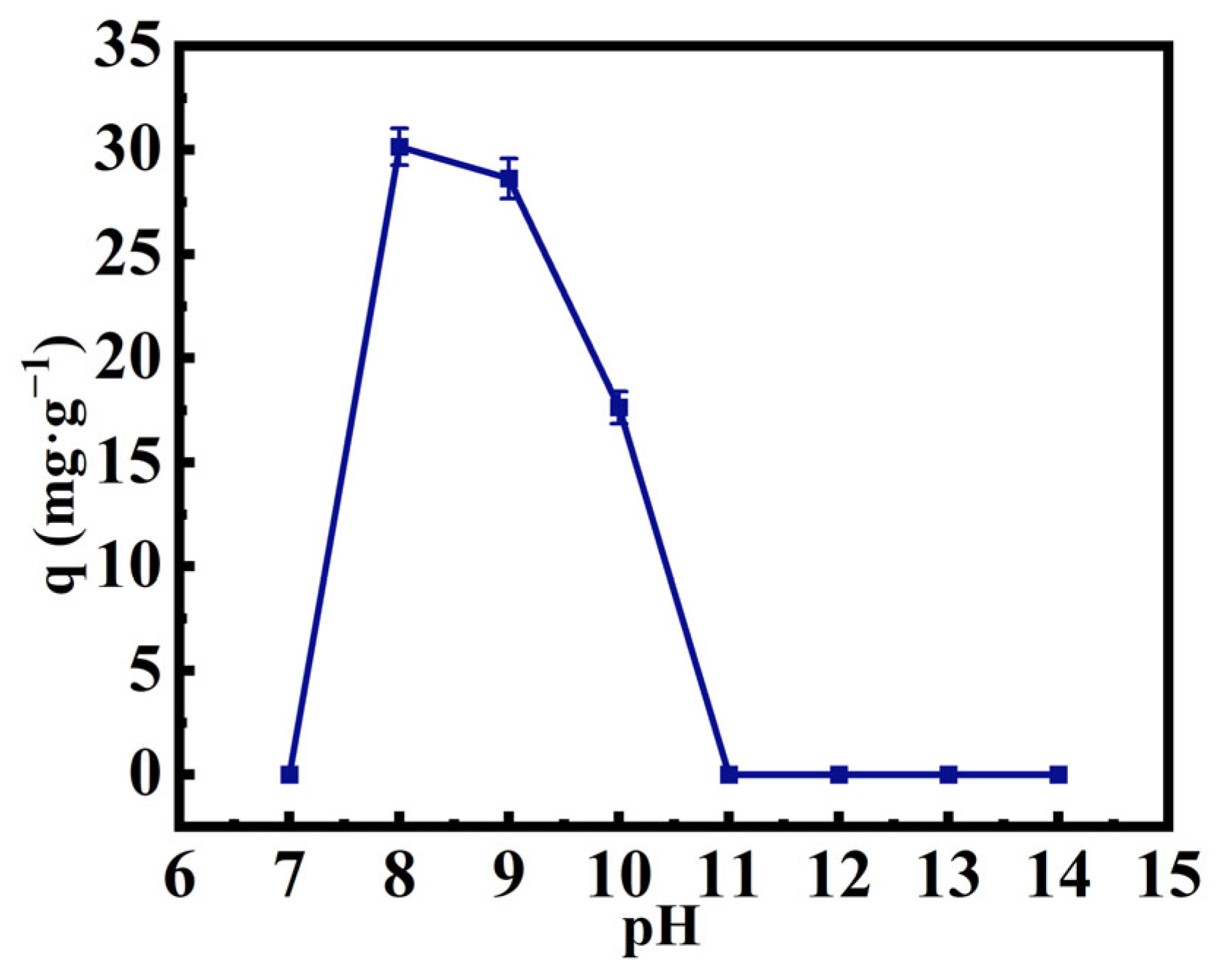
3.1. Optimum pH of Cyanide Adsorption
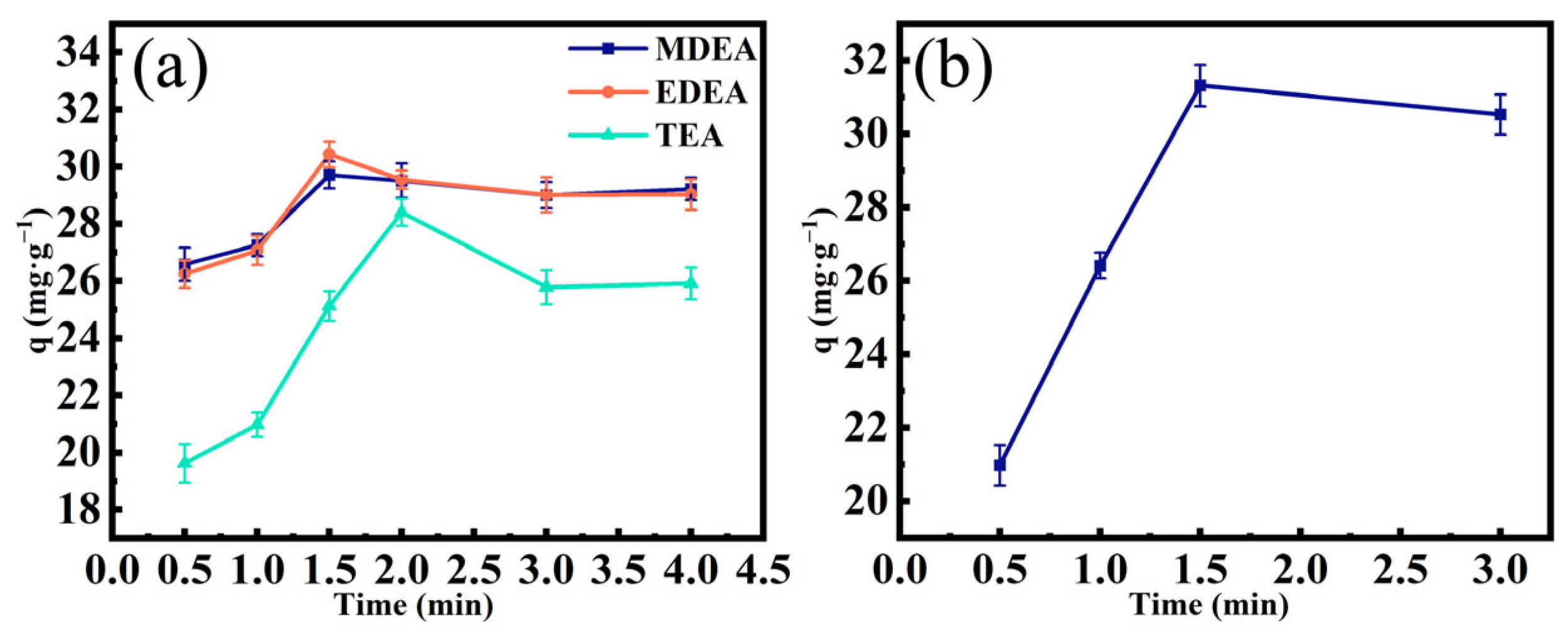
3.2. Adsorption kinetics
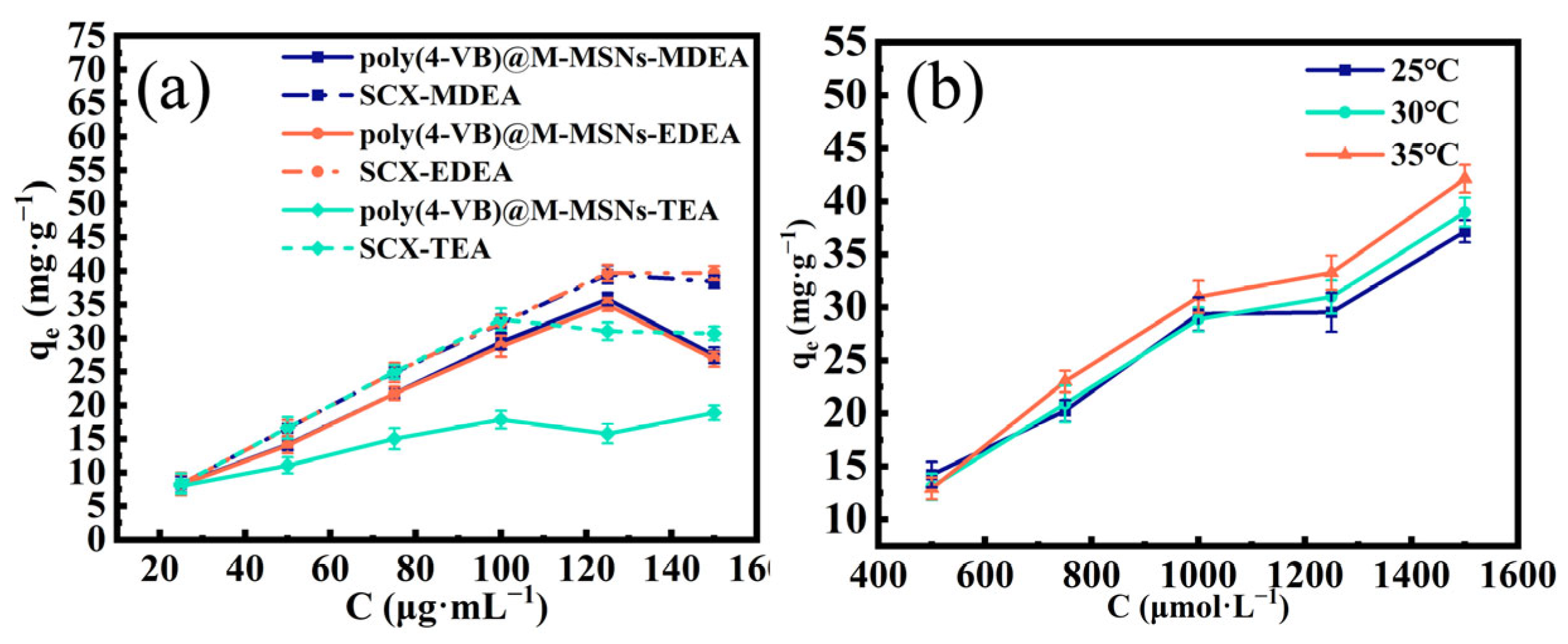
3.3. Adsorption Isotherm Experiment
3.4. DFT
3.5. Matrix Effect
3.6. Repeatability
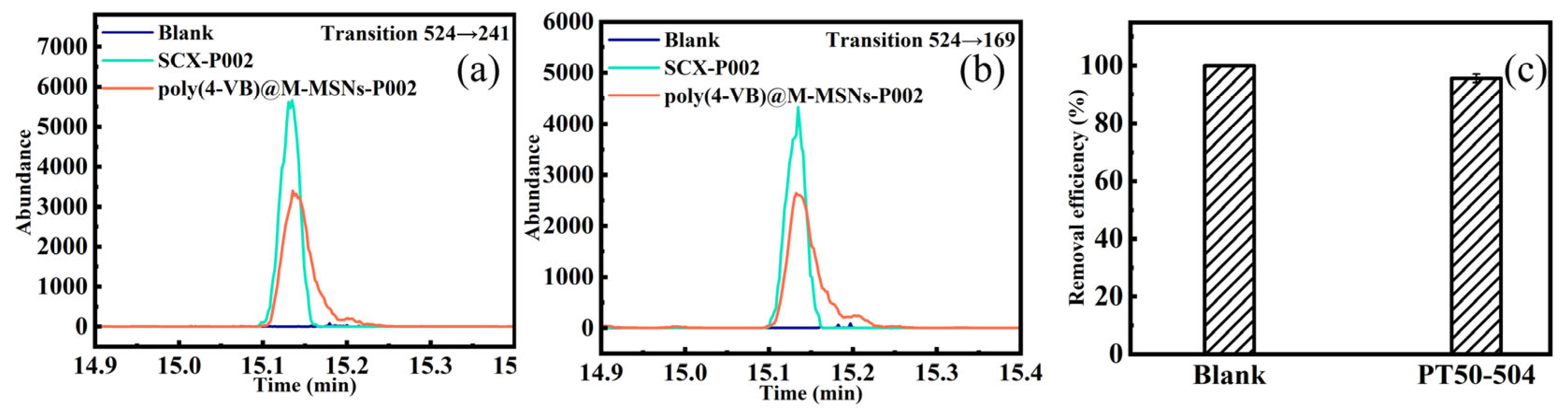
3.7. Application to the Actual Samples
4. Materials and Methods
4.1. Materials and Instruments
4.1.1. Reagents and Instruments
4.1.2. Instrument Conditions
4.2. Preparation of Ionic Magnetic Mesoporous Materials
4.2.1. Synthesis of Magnetic Mesoporous Silicon Nanoparticles (M-MSNs)
4.2.2. Synthesis of Poly(4-VB)@M-MSNs and Co2+@M-MSNs
4.3. Sample Preparation
4.3.1. Preparation of EA Samples
4.3.2. Preparation of Cyanide Samples
4.4. Characterization
4.5. Adsorption Isotherm
4.6. Adsorption Kinetics
4.7. DFT Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, C.; Liu, X.; Chen, C.; Gong, Y.; Xiong, W.; Guo, Y.; Wang, C.; Zhao, J.; Che, Y. Ultrasensitive detection of sulfur mustard via differential noncovalent interactions. Anal. Chem. 2019, 91, 6408–6412. [Google Scholar] [CrossRef] [PubMed]
- Szinicz, L. History of chemical and biological warfare agents. Toxicology 2005, 214, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Sezigen, S.; Kenar, L. Recent sulfur mustard attacks in Middle East and experience of health professionals. Toxicol. Lett. 2020, 320, 52–57. [Google Scholar] [CrossRef]
- Yang, F.C.; Yang, Y.; Yan, L.; Wang, F.Y.; Wu, L.; Xia, M.Z.; Li, X.S. Fluoride derivatization-enabled sensitive and simultaneous detection of biomarkers for nitrogen mustard in human plasma and urine via gas chromatography tandem mass spectrometry. RSC Adv. 2023, 13, 27535–27548. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Yadav, P.; Behl, A.; Sharma, R.K.; Kulshrestha, S.; Butola, B.S.; Sharma, N. Toxic blister agents: Chemistry, mode of their action and effective treatment strategies. Chem-Biol. Interact. 2021, 350, 109654. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V.R.; DeCaprio, A.P. Covalent adduction of nitrogen mustards to model protein nucleophiles. Chem. Res. Toxicol. 2013, 26, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Miyaguchi, H.; Uchiyama, M. Analysis of nitrogen mustard degradation products via post-pentafluorobenzoylation liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1625, 461306. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Purohit, A.; Tak, V.K.; Dubey, D.K. Enhanced detectability of fluorinated derivatives of N, N-dialkylamino alcohols and precursors of nitrogen mustards by gas chromatography coupled to Fourier transform infrared spectroscopy analysis for verification of chemical weapons convention. J. Chromatogr. A 2009, 1216, 7906–7914. [Google Scholar] [CrossRef] [PubMed]
- Prihed, H.; Shifrovich, A.; Yamin, T.S.; Madmon, M.; Smolkin, B.; Chen, R.; Blanca, M.; Weissberg, A. A novel approach for the detection and identification of sulfur mustard using liquid chromatography-electrospray ionization-tandem mass spectrometry based on its selective oxidation to sulfur mustard monoxide with N-iodosuccinimide. J. Mass Spectrom. 2021, 56, e4721. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, C.; Yang, Y.; Liang, L.; Chen, B.; Yu, H.; Xia, J.; Liu, S.; Li, Y. Advances in sulfur mustard-induced DNA adducts: Characterization and detection. Toxicol. Lett. 2021, 344, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Sun, M.; Xu, Q.; Cen, J.; Cao, Y.; Li, Z.; Xiao, K. Development of a series of fluorescent probes for the early diagnostic imaging of sulfur mustard poisoning. Acs. Sens. 2019, 4, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Sperry, M.L.; Skanchy, D.; Marino, M.T. High-performance liquid chromatographic determination of N-2-(hydroxyethyl)-N-(2-(7-guaninyl)ethyl) methylamine, a reaction product between nitrogen mustard and DNA and its application to biological samples. J. Chromatogr. B 1998, 716, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rozsypal, T.; Kobliha, Z. Identification of nitrogen mustard chemical warfare agents in sand by gas chromatography-mass spectrometry (GC-MS) in a military deployable laboratory. Anal. Lett. 2023, 56, 1–13. [Google Scholar] [CrossRef]
- Popiel, S.; Nawala, J.; Dziedzic, D.; Soderstrom, M.; Vanninen, P. Determination of mustard gas hydrolysis products thiodiglycol and thiodiglycol sulfoxide by gas chromatography-tandem mass spectrometry after trifluoroacetylation. Anal. Chem. 2014, 86, 5865–5872. [Google Scholar] [CrossRef] [PubMed]
- Palit, M.; Mallard, G. Dispersive derivatization liquid-liquid extraction of degradation products/precursors of mustards and V-agents from aqueous samples. J. Chromatogr. A 2011, 1218, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Hu, Z.L.; Chen, M.L.; Tu, C.S.; Zhu, Y. Ionic liquid based dispersive liquid-liquid microextraction of aromatic amines in water samples. Chin. Chem. Lett. 2008, 19, 985–987. [Google Scholar] [CrossRef]
- Jiang, P.Y.; Yuan, L.; Liu, S.L.; Lv, Q.; Yu, H.L.; Liang, L.H.; Yang, Y.; Liu, C.C. Simultaneous solvent extraction and quantification of eleven amine compounds related to Chemical Weapon Convention in soils via hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2022, 1671, 462990. [Google Scholar] [CrossRef] [PubMed]
- Studzinska, S.; Nuckowski, L.; Buszewski, B. Oligonucleotides isolation and separation-A review on adsorbent selection. Int. J. Mol. Sci. 2022, 23, 9546. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, I.; Ciarlantini, C.; Francolini, I.; Tomai, P.; Gentili, A.; Dal Bosco, C.; Piozzi, A. Chitosan-graphene oxide composite membranes for solid-phase extraction of pesticides. Int. J. Mol. Sci. 2021, 22, 8374. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lv, B.; Shen, H.; Jing, G.; Zhou, Z. Coupling life cycle assessment with scenario analysis for sustainable management of disperse blue environ. Environ. Sci. Pollut. 2020, 27, 25197–25208. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, B.; Jena, S.; Das, S.; Das, K. A comprehensive review of various non-cyanide electroplating baths for the production of silver and gold coatings. Int. Mater. Rev. 2023, 7, 825–861. [Google Scholar] [CrossRef]
- Mondal, M.; Mukherjee, R.; Sinha, A.; Sarkar, S.; De, S. Removal of cyanide from steel plant effluent using coke breeze, a waste product of steel industry. J. Water. Process. Eng. 2019, 28, 135–143. [Google Scholar] [CrossRef]
- Kuyucak, N.; Akcil, A. Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner. Eng. 2013, 50–51, 13–29. [Google Scholar] [CrossRef]
- Suzuki, Y.; Taguchi, K.; Kure, T.; Enoki, Y.; Otagiri, M.; Sakai, H.; Matsumoto, K. Long-term pharmaceutical stability of liposome-encapsulated methemoglobin as an antidote for cyanide poisoning. Int. J. Pharmaceut. 2021, 610, 121260. [Google Scholar] [CrossRef] [PubMed]
- Harikandei, K.B.; Salehi, P.; Ebrahimi, S.N.; Bararjanian, M.; Kaiser, M.; Khavasi, H.R.; Al-Harrasi, A. N-substituted noscapine derivatives as new antiprotozoal agents: Synthesis, antiparasitic activity and molecular docking study. Bioorg. Chem. 2019, 91, 103116. [Google Scholar]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Sener, A. Hydrogen sulfide metabolite, sodium thiosulfate: Clinical applications and underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 6452. [Google Scholar] [CrossRef] [PubMed]
- Benaissa, M.L.; Hantson, P.; Bismuth, C.; Baud, F.J. Mercury oxycyanide and mercuric cyanide poisoning: Two cases. Intens. Care. Med. 1995, 21, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, I.; Boston, D.J.; Higgins, R.F.; Klug, C.M.; Shores, M.P.; Gupta, T. Naked eye detection of cyanide in water with CoII bis(terpyridine) complexes. Sensor. Actuat. B-Chem. 2016, 235, 325–329. [Google Scholar] [CrossRef]
- Biradar, A.A.; Biradar, A.V.; Sun, T.; Chan, Y.; Huang, X.; Asefa, T. Bicinchoninic acid-based colorimetric chemosensor for detection of low concentrations of cyanide. Sensor. Actuat. B-Chem. 2016, 222, 112–119. [Google Scholar] [CrossRef]
- Virbickas, P.; Valiuniene, A.; Baryseva, D.; Popkirov, G.; Ramanavicius, A. Determination of cyanide concentration by chronoamperometry, cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy. J. Electroanal. Chem. 2021, 895, 115449. [Google Scholar] [CrossRef]
- Takekawa, K.; Oya, M.; Kido, A.; Suzuki, O. Analysis of cyanide in blood by headspace solid-phase microextraction (SPME) and capillary gas chromatography. Chromatographia 1998, 47, 209–214. [Google Scholar] [CrossRef]
- Marton, D.; Tapparo, A.; Di Marco, V.B.; Repice, C.; Giorio, C.; Bogialli, S. Ultratrace determination of total and available cyanides in industrial wastewaters through a rapid headspace-based sample preparation and gas chromatography with nitrogen phosphorous detection analysis. J. Chromatogr. A 2013, 1300, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Ya, V.; Naddeo, V.; Choo, K.H.; Li, C.W. Cyanide removal and recovery by electrochemical crystallization process. Water 2021, 13, 2704. [Google Scholar] [CrossRef]
- Han, W.W.; Yang, H.Y.; Tong, L.L. Cyanide removal for ultrafine gold cyanide residues by chemical oxidation methods. T. Nonferr. Metal. Soc. 2022, 32, 4129–4138. [Google Scholar] [CrossRef]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Application of the anion-exchange resin as a complementary technique to remove residual cyanide complexes in industrial plating wastewater after conventional treatment. Environ. Sci. Pollut. Res. 2020, 27, 41688–41701. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Jin, C.; Shao, M.; Huang, Z.; Xie, X. Removal of metal-cyanide complexes and recovery of Pt(II) and Pd(II) from wastewater using an alkali-tolerant metal-organic resin. J. Hazard. Mater. 2021, 406, 124315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, H.; He, Y. Application and mechanism of sludge-based activated carbon for phenol and cyanide removal from bio-treated effluent of coking wastewater. Processes 2020, 8, 82. [Google Scholar] [CrossRef]
- Tyagi, M.; Rana, A.; Kumari, S.; Jagadevan, S. Adsorptive removal of cyanide from coke oven wastewater onto zero-valent iron: Optimization through response surface methodology, isotherm and kinetic studies. J. Clean. Prod. 2018, 178, 398–407. [Google Scholar] [CrossRef]
- Aliprandini, P.; Veiga, M.M.; Marshall, B.G.; Scarazzato, T.; Espinosa, D.C.R. Investigation of mercury cyanide adsorption from synthetic wastewater aqueous solution on granular activated carbon. J. Water. Process. Eng. 2020, 34, 101154. [Google Scholar] [CrossRef]
- Hamedi, A.; Trotta, F.; Borhani Zarandi, M.; Zanetti, M.; Caldera, F.; Anceschi, A.; Nateghi, M.R. In situ synthesis of MIL-100(Fe) at the surface of Fe3O4@AC as highly efficient dye adsorbing nanocomposite. Int. J. Mol. Sci. 2019, 20, 5612. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, Q.; Li, H.; Wang, J.; Tai, G.; Wang, F.; Han, J.; Zhu, Y.; Wu, G. Waste-to-Resource strategy to fabricate functionalized MOFs composite material based on durian shell biomass carbon fiber and Fe3O4 for highly efficient and recyclable dye adsorption. Int. J. Mol. Sci. 2022, 23, 5900. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sathish, C.I.; Guan, X.; Wang, S.; Palanisami, T.; Vinu, A.; Yi, J. Advances in magnetic materials for microplastic separation and degradation. J. Hazard. Mater. 2024, 461, 132537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Song, X.; Liu, M.; Chen, M.; Li, J.; Han, J. Review on the use of magnetic nanoparticles in the detection of environmental pollutants. Water 2023, 15, 17. [Google Scholar] [CrossRef]
- Melo, A.F.; Singh, s.; Chinnamuthu, P.; Crespilho, F.; Rydzek, G. Magnetically stimulated bio-and electrochemical systems: State-of-the-art, applications, and future directions. Chem. Nano. Mat. 2023, 9, e202300192. [Google Scholar] [CrossRef]
- Qu, G.; Chen, B.; Liu, S.; Zhang, Q.; Yang, Y.; Fu, Q. A modified QuEChERS-DART-MS/MS technique for high-throughput detection of organophosphate nerve agent hydrolysis products in environmental samples. J. Anal. Test. 2022, 7, 163–171. [Google Scholar] [CrossRef]
- Erdem, B.; Erdem, S.; Tekin, N. Cationic surfactant templated synthesis of magnetic mesoporous nanocomposites for efficient removal of light green. Korean J. Chem. Eng. 2021, 38, 1425–1437. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Qin, L.; Li, H.; Liang, W. Magnetic coagulation and flocculation of a kaolin suspension using Fe3O4 coated with SiO2. J. Environ. Chem. Eng. 2021, 9, 105980. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Zhu, L. Hydrothermal growth and characterization of magnetite (Fe3O4) thin films. Surf. Coat. Technol. 2007, 201, 5870–5874. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Schroeder, G. Modification of magnetite nanoparticles with triazine-based dendrons and their application as drug-transporting systems. Int. J. Mol. Sci. 2021, 22, 11353. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef]



| PFO | PSO | |||||
|---|---|---|---|---|---|---|
| R2 | k1 (min−1) | qe (mg·g−1) | R2 | k2 (g·mg−1·min−1) | qe (mg·g−1) | |
| MDEA | 2.590 × 10−3 | 0.1652 | 0.1270 | 0.9992 | 0.9967 | 29.41 |
| EDEA | 1.105 × 10−2 | 0.4028 | 0.1118 | 0.9985 | 1.089 | 29.43 |
| TEA | 0.7807 | 0.3622 | 8.770 | 0.9904 | 0.2124 | 27.49 |
| KCN | 0.9932 | −0.9044 | 14.64 | 0.9879 | 1.086 × 10−2 | 66.05 |
| Langmuir Equation | Freundlich Equation | |||||
|---|---|---|---|---|---|---|
| R2 | KL (L·mg−1) | qm (mg·g−1) | R2 | KF | 1·n−1 | |
| MDEA | 0.9768 | 0.6226 | 28.47 | 0.7399 | 1.068 × 10−3 | 2.950 |
| EDEA | 0.9761 | 0.5917 | 27.99 | 0.7549 | 3.088 × 10−3 | 2.666 |
| TEA | 0.9705 | 0.1591 | 18.84 | 0.8943 | 7.976 × 10−5 | 4.789 |
| Temperature/°C | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| R2 | KL (L·mg−1) | qm (mg·g−1) | R2 | KF | 1·n−1 | |
| 25 | 0.2404 | 1.251 × 10−2 | 85.91 | 0.6940 | 2.168 | 0.8273 |
| 30 | 3.334 × 10−2 | 4.668 × 10−3 | 154.80 | 0.8726 | 2.122 | 0.6724 |
| 35 | 3.000 × 10−3 | 1.180 × 10−3 | 613.50 | 0.8231 | 4.383 | 0.7289 |
| Binding Product | Ev (kJ·mol−1) |
|---|---|
| MDEA-COOH | −592.10 |
| MDEA-SO3H | −1028.68 |
| EDEA-COOH | −632.22 |
| EDEA-SO3H | −1067.96 |
| TEA-COOH | −707.60 |
| TEA-SO3H | −1143.28 |
| Metal Ions | Ev (kJ·mol−1) |
|---|---|
| Cu | 28.49 |
| Ca | −18.20 |
| Fe | −25.63 |
| Mg | −22.46 |
| Co | −27.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yang, F.; Wu, J.; Qu, G.; Yang, Y.; Yang, Y.; Li, X. Highly Efficient Separation of Ethanol Amines and Cyanides via Ionic Magnetic Mesoporous Nanomaterials. Int. J. Mol. Sci. 2024, 25, 6470. https://doi.org/10.3390/ijms25126470
Zhao Y, Yang F, Wu J, Qu G, Yang Y, Yang Y, Li X. Highly Efficient Separation of Ethanol Amines and Cyanides via Ionic Magnetic Mesoporous Nanomaterials. International Journal of Molecular Sciences. 2024; 25(12):6470. https://doi.org/10.3390/ijms25126470
Chicago/Turabian StyleZhao, Yuxin, Fangchao Yang, Jina Wu, Gang Qu, Yuntao Yang, Yang Yang, and Xiaosen Li. 2024. "Highly Efficient Separation of Ethanol Amines and Cyanides via Ionic Magnetic Mesoporous Nanomaterials" International Journal of Molecular Sciences 25, no. 12: 6470. https://doi.org/10.3390/ijms25126470
APA StyleZhao, Y., Yang, F., Wu, J., Qu, G., Yang, Y., Yang, Y., & Li, X. (2024). Highly Efficient Separation of Ethanol Amines and Cyanides via Ionic Magnetic Mesoporous Nanomaterials. International Journal of Molecular Sciences, 25(12), 6470. https://doi.org/10.3390/ijms25126470




