Mechanisms Underlying the Rarity of Skeletal Muscle Cancers
Abstract
1. Introduction
| Cancer Type | Incidence Rate per 100,000 Individuals [Reference] | Incidence Rate Relative to Breast Cancer a | Mean Organ Mass (g) [Reference] | Incidence Rate per Tissue Mass (per 100,000 per g Tissue) b | Incidence Rate per Tissue Mass Relative to Breast Cancer a | Number of Times More Common Than SKM Cancer Relative to Tissue Mass c | Notes |
|---|---|---|---|---|---|---|---|
| Urinary bladder | 5.6 [20] | 0.120 | 37 [21,22] | 0.151 | 1.56 | 30,545 | Mean organ mass was obtained by averaging the values for men and women. |
| Bone | 0.9 [23] | 0.019 | 3465 [24,25] | 2.6 × 10−4 | 2.7 × 10−3 | 53 | Reported bone mass was multiplied by 0.33, the organic mass fraction. |
| Brain | 3.5 [20] | 0.075 | 1294 [26,27,28] | 2.7 × 10−3 | 0.028 | 548 | Mean organ mass was obtained by averaging the values for men and women from two reports each. |
| Breast | 46.8 [20] | 1 | 484 [29] | 0.097 | 1 | 19,581 | Organ mass indicates mean values for women only, although a high variation is present. |
| Colorectal | 17.8 [20] | 0.380 | 1818 [30] | 9.8 × 10−3 | 0.101 | 1982 | Incidence rate was derived by adding the rates of colon and rectal cancers, which were reported separately. |
| Gallbladder | 1.2 [20] | 0.026 | 16.4 [31,32] | 0.073 | 0.757 | 14,817 | |
| Heart | 0.034 [33] | 7.3 × 10−4 | 288 [34,35] | 1.2 × 10−4 | 1.2 × 10−3 | 24 | Mean organ mass was obtained by averaging the values for men and women. |
| Kidney | 4.4 [20] | 0.094 | 287 [27,28] | 0.015 | 0.159 | 3110 | Mean organ mass was obtained by averaging the values for men and women. |
| Leukocyte | 13.7 [20] | 0.292 | 1200 [36] | 0.011 | 0.118 | 2303 | Incidence rate was derived by adding the rates of Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma, and leukemia, which were reported separately. Organ mass includes the total mass of all immune cells. |
| Liver | 8.6 [20] | 0.184 | 1425 [27,28] | 6.0 × 10−3 | 0.062 | 1223 | Mean organ mass was obtained by averaging the values for men and women. |
| Lung | 23.6 [20] | 0.504 | 370 [27,28] | 0.064 | 0.660 | 12,925 | Mean organ mass was obtained by averaging the values for men and women. |
| Ovary | 6.7 [20] | 0.143 | 6.3 [37] | 1.06 | 11.0 | 215,357 | Mean organ mass was derived by multiplying the reported mean ovarian volume (6.3 mL) by the ovarian tissue density (1.00 g/mL). |
| Pancreas | 4.7 [20] | 0.100 | 91.8 [38] | 0.051 | 0.529 | 10,368 | |
| Prostate | 29.4 [20] | 0.628 | 11 [39] | 2.67 | 27.6 | 541,227 | |
| SKM | 0.096 | 0.002 | 19,440 [40] | 4.9 × 10−6 | 5.1 × 10−5 | 1 | Incidence rate was calculated as explained in Section 1. Mean organ mass was derived by multiplying 0.44 (the approximate lower end of SKM mass as a fraction of total body mass [1]) by mean body mass (women and men averaged) [40]. |
| Skin | 13.6 [20] | 0.291 | 3250 [41] | 4.2 × 10−3 | 0.043 | 847 | Incidence rate for skin cancer was derived by adding the rates of melanoma and non-melanoma, which were reported separately. |
| Stomach | 9.2 [20] | 0.197 | 142 [42] | 0.065 | 0.671 | 13,129 | Mean organ mass was obtained by averaging the values for men and women. |
| Testes | 1.7 [20] | 0.036 | 36.6 [43] | 0.046 | 0.480 | 9401 | Organ mass data were obtained in a population of men aged 41–50 years from northwest India. |
| Thyroid | 9.1 [20] | 0.194 | 16 [44] | 0.553 | 5.72 | 112,021 | Mean organ mass was obtained by averaging the values for women aged 20–69 years and men aged 30–69 years. |
| Uterus | 22.5 [20] | 0.481 | 515 [45] | 0.044 | 0.452 | 8849 | Incidence rate for uterine cancers was derived by adding the rates of cervix uteri and corpus uteri cancers, which were reported separately. |
2. Epidemiological and Mechanistic Studies
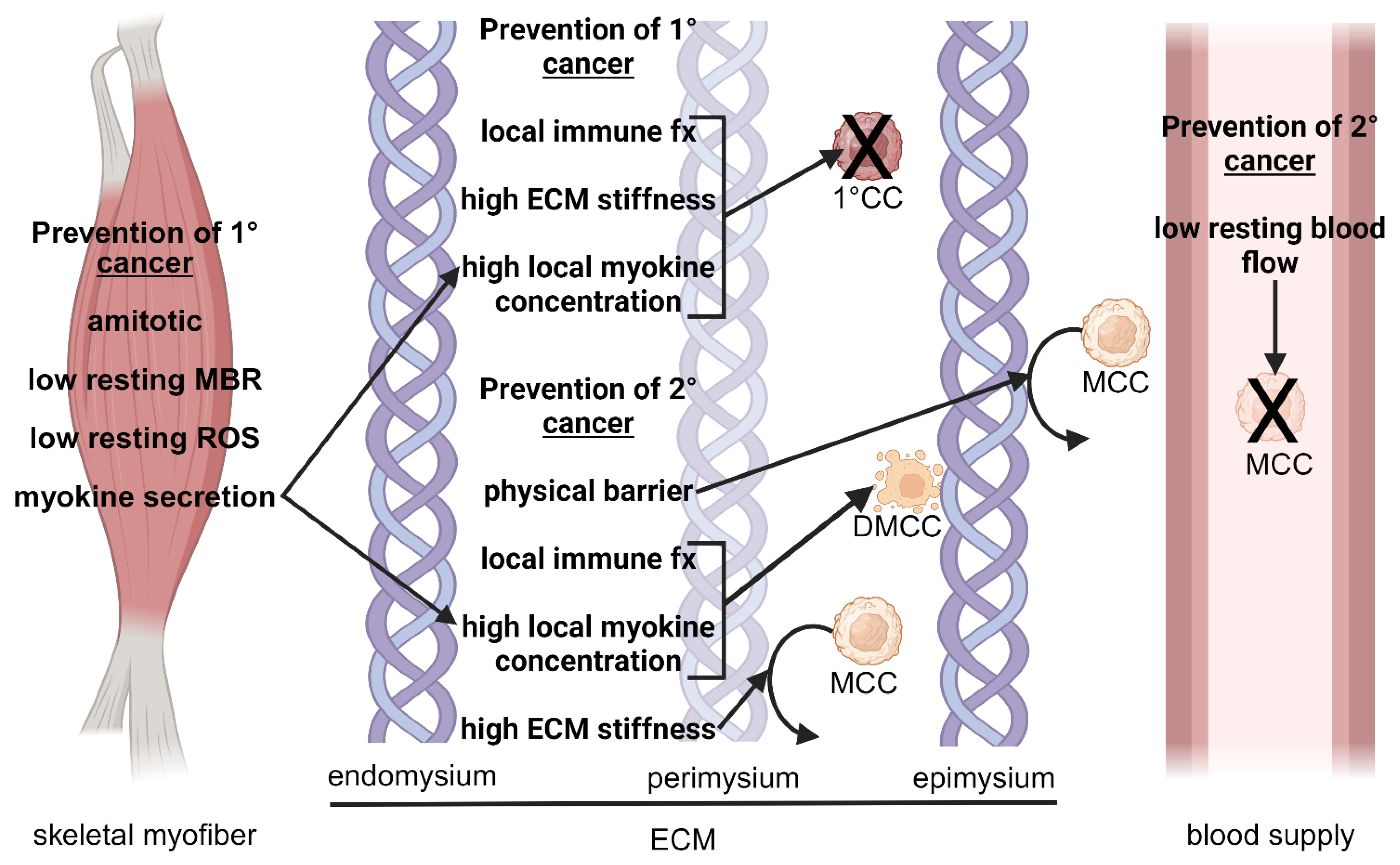
3. Local Mechanisms Alleviating Cancer in Skeletal Muscle
3.1. Skeletal Muscle Characteristics and Architecture
3.1.1. Amitotic Nature of Skeletal Myofibers
3.1.2. Physical Barrier
3.1.3. Mechanical Forces
3.1.4. Low Resting Metabolic Rate
3.1.5. Rich Blood Supply
3.2. Immune-Related Factors
3.2.1. Localized Immune Response
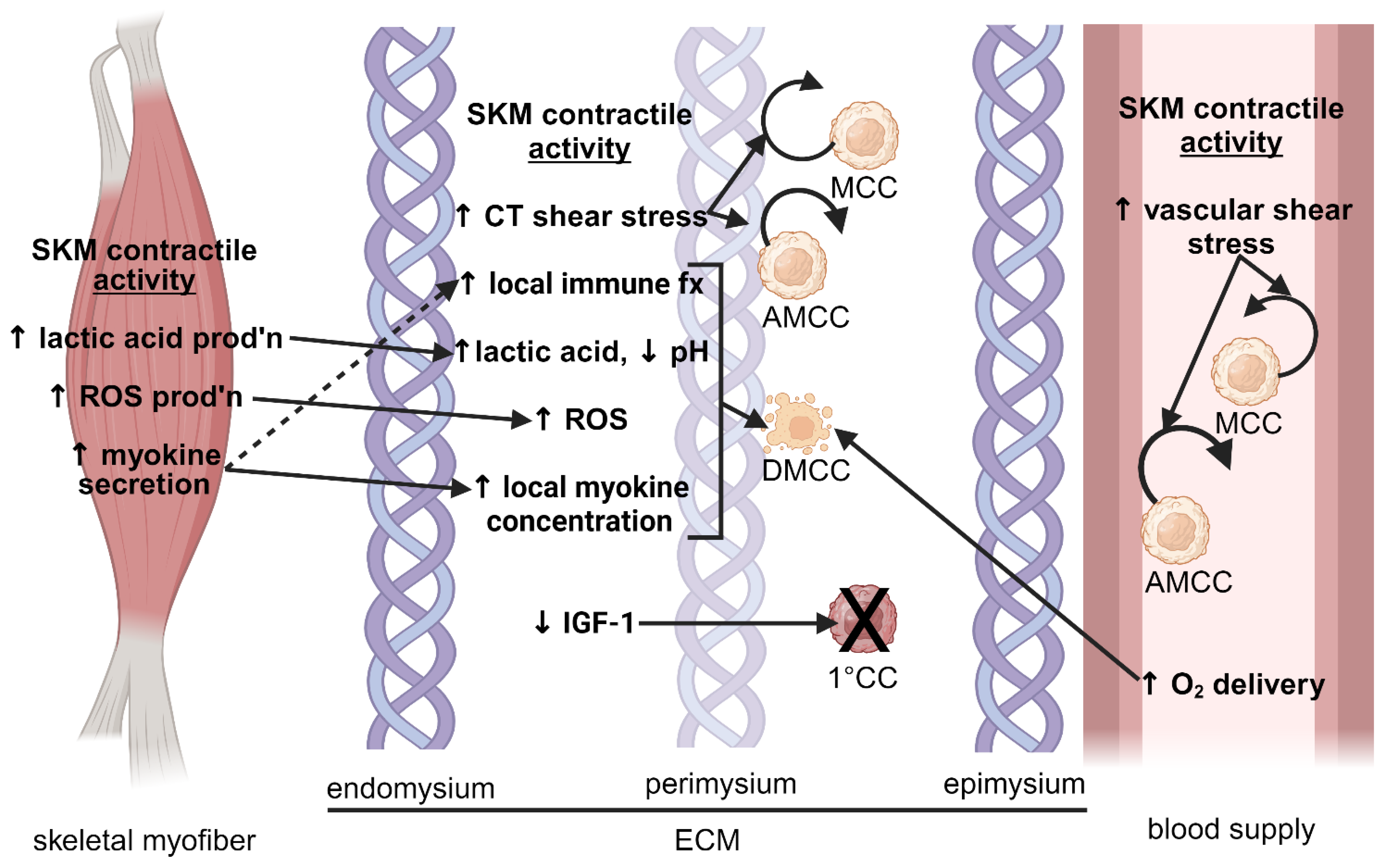
3.2.2. Effect of Contractile Activity
3.3. Skeletal Muscle Microenvironment
3.3.1. Extracellular Matrix Stiffness
3.3.2. Lactic Acid and pH
3.3.3. Oxygen Tension
3.3.4. Oxidative Stress
3.3.5. Lipophilic Ligands of the Lipocalin Protein Family
3.4. Myokines
3.4.1. Oncostatin-M
3.4.2. Irisin
3.4.3. Secreted Protein: Acidic and Rich in Cysteine
3.4.4. Decorin
3.4.5. Brain-Derived Neurotrophic Factor
3.4.6. Interleukin-6
3.4.7. Interleukin-7
3.4.8. Interleukin-10
3.4.9. Interleukin-15
3.5. Insulin-like Growth Factor-1 and Associated Binding Proteins
3.5.1. Role of Insulin-like Growth Factor-1 and Binding Proteins in Cancer
3.5.2. Association of Insulin-like Growth Factor-1 and Binding Proteins with Cancer
3.5.3. Derivation of Insulin-like Growth Factor-1 and Binding Proteins from Skeletal Muscle
3.6. Studies Using Exercise-Conditioned Serum
3.6.1. Animal Studies
3.6.2. Human Studies
3.7. Potential Fiber Type-Specific Effects
3.7.1. Effect of Type I Myofibers
3.7.2. Effect of Type II Myofibers
4. Conclusions
Funding
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Military Nutrition Research. 6, Regulation of Muscle Mass and Function: Effects of Aging and Hormones. Available online: https://www.ncbi.nlm.nih.gov/books/NBK224631/ (accessed on 1 May 2024).
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Nocuń, A.; Chrapko, B. Multiple and Solitary Skeletal Muscle Metastases on 18F-FDG PET/CT Imaging. Nucl. Med. Commun. 2015, 36, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Haygood, T.M.; Wong, J.; Lin, J.C.; Li, S.; Matamoros, A.; Costelloe, C.M.; Yeung, H.; Sandler, C.M.; Nunez, R.F.; Kumar, R.; et al. Skeletal Muscle Metastases: A Three-Part Study of a Not-so-Rare Entity. Skelet. Radiol. 2012, 41, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Pretell-Mazzini, J.; Younis, M.H.; Subhawong, T. Skeletal Muscle Metastases from Carcinomas. JBJS Rev. 2020, 8, e1900114-8. [Google Scholar] [CrossRef] [PubMed]
- Salemis, N.S. Skeletal Muscle Metastasis from Breast Cancer: Management and Literature Review. Breast Dis. 2015, 35, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Kinoshita, I.; Oda, Y. Soft Tissue Special Issue: Skeletal Muscle Tumors: A Clinicopathological Review. Head Neck Pathol. 2020, 14, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, E.R.; Anderson, J.R.; Hawkins, D.S.; Skapek, S.X.; Parham, D.M.; Teot, L.A. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma: A Report from the Children’s Oncology Group. Arch. Pathol. Lab. Med. 2015, 139, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Qualman, S.J.; Coffin, C.M.; Newton, W.A.; Hojo, H.; Triche, T.J.; Parham, D.M.; Crist, W.M. Intergroup Rhabdomyosarcoma Study: Update for Pathologists. Pediatr. Dev. Pathol. 1998, 1, 550–561. [Google Scholar] [CrossRef]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef]
- Hebestreit, H.; Bar-Or, O.; IOC Medical Commission; International Federation of Sports Medicine. The Young Athlete; Blackwell Pub: New York, NY, USA, 2008; ISBN 9781405156479. [Google Scholar]
- Cancer Statistics Branch Cancer Surveillance Research Program Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Available online: https://seer.cancer.gov/archive/publications/childhood/childhood-monograph.pdf (accessed on 29 April 2024).
- Lychou, S.E.; Gustafsson, G.G.; Ljungman, G.E. Higher Rates of Metastatic Disease May Explain the Declining Trend in Swedish Paediatric Rhabdomyosarcoma Survival Rates. Acta Paediatr. 2016, 105, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Parkint, D.M. International Variations in the Incidence of Childhood Soft-tissue Sarcomas. Paediatr. Perinat. Epidemiol. 1994, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.S.; Spunt, S.L.; Skapek, S.X. Children’s Oncology Group’s 2013 Blueprint for Research: Soft Tissue Sarcomas. Pediatr. Blood Cancer 2013, 60, 1001–1008. [Google Scholar] [CrossRef]
- National Cancer Institute Surveillance, Epidemiology and End Results. Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/ (accessed on 29 April 2024).
- Ferrari, A.; Dileo, P.; Casanova, M.; Bertulli, R.; Meazza, C.; Gandola, L.; Navarria, P.; Collini, P.; Gronchi, A.; Olmi, P.; et al. Rhabdomyosarcoma in Adults. Cancer 2003, 98, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Abramson Cancer Center Rhabdomyosarcoma (RMS). Available online: https://www.pennmedicine.org/cancer/types-of-cancer/sarcoma/types-of-sarcoma/soft-tissue-sarcoma/rhabdomyosarcoma#:~:text=But%20they%20are%20still%20rare,soft%20tissue%20sarcomas%20found%20annually (accessed on 6 May 2024).
- International Agency for Research on Cancer-World Health Organization Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 29 April 2024).
- Al-Shaikh, G.; Al-Mandeel, H. Ultrasound Estimated Bladder Weight in Asymptomatic Adult Females. Urol. J. 2012, 9, 586–591. [Google Scholar] [PubMed]
- Chalana, V.; Dudycha, S.; Yuk, J.-T.; McMorrow, G. Automatic Measurement of Ultrasound-Estimated Bladder Weight (UEBW) from Three-Dimensional Ultrasound. Rev. Urol. 2005, 7 (Suppl. 6), S22–S28. [Google Scholar] [PubMed]
- Franchi, A. Epidemiology and Classification of Bone Tumors. Clin. Cases Miner. Bone Metab. 2012, 9, 92–95. [Google Scholar]
- Avtandilashvili, M.; Tolmachev, S.Y. Modeling the Skeleton Weight of an Adult Caucasian Man. Health Phys. 2019, 117, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Capela e Silva, F.; Queiroga, M.C.; Lucena, S.; Potes, J. Bone Mechanotransduction: A Review. J. Biomed. Bioeng. 2011, 2, 37–44. [Google Scholar]
- Hartmann, P.; Ramseier, A.; Gudat, F.; Mihatsch, M.J.; Polasek, W.; Geisenhoff, C. Das Normgewicht Des Gehirns Beim Erwachsenen in Abhängigkeit von Alter, Geschlecht, Körpergröße Und Gewicht. Pathologe 1994, 15, 165–170. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J.M. Normal Organ Weights in Men: Part II-the Brain, Lungs, Liver, Spleen, and Kidneys. Am. J. Forens. Med. Pathol. 2012, 33, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.K.; DiMaio, V.J.M. Normal Organ Weights in Women: Part II-The Brain, Lungs, Liver, Spleen, and Kidneys. Am. J. Forens. Med. Pathol. 2015, 36, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Katch, V.L.; Campaigne, B.; Freedson, P.; Sady, S.; Katch, F.I.; Behnke, A.R. Contribution of Breast Volume and Weight to Body Fat Distribution in Females. Am. J. Phys. Anthropol. 1980, 53, 93–100. [Google Scholar] [CrossRef]
- Drake, R.L.; Vogl, W.; Mitchell, A.W.M. Gray’s Anatomy for Students; Churchill Livingstone: Philadelphia, PA, USA, 2010. [Google Scholar]
- Veghari, G.; Sedaghat, M.; Maghsodlo, S.; Banihashem, S.; Moharloei, P.; Angizeh, A.; Tazik, E.; Moghaddami, A.; Joshaghani, H. The Association between Abdominal Obesity and Serum Cholesterol Level. Int. J. Appl. Basic Med. Res. 2015, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Kubica, K.; Balbus, J. A Computer Study of the Risk of Cholesterol Gallstone Associated with Obesity and Normal Weight. Sci. Rep. 2021, 11, 8868. [Google Scholar] [CrossRef]
- Oliveira, G.H.; Al-Kindi, S.G.; Hoimes, C.; Park, S.J. Characteristics and Survival of Malignant Cardiac Tumors. Circulation 2015, 132, 2395–2402. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J.M. Normal Organ Weights in Men: Part I-the Heart. Am. J. Forens. Med. Pathol. 2012, 33, 362–367. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J.M. Normal Organ Weights in Women: Part I-The Heart. Am. J. Forens. Med. Pathol. 2015, 36, 176–181. [Google Scholar] [CrossRef]
- Sender, R.; Weiss, Y.; Navon, Y.; Milo, I.; Azulay, N.; Keren, L.; Fuchs, S.; Ben-Zvi, D.; Noor, E.; Milo, R. The Total Mass, Number, and Distribution of Immune Cells in the Human Body. Proc. Natl Acad. Sci. USA 2023, 120, e2308511120. [Google Scholar] [CrossRef]
- Rosendahl, M.; Ernst, E.; Rasmussen, P.E.; Andersen, C.Y. True Ovarian Volume Is Underestimated by Two-Dimensional Transvaginal Ultrasound Measurement. Fertil. Steril. 2010, 93, 995–998. [Google Scholar] [CrossRef]
- Innes, J.T.; Carey, L.C. Normal Pancreatic Dimensions in the Adult Human. Am. J. Surg. 1994, 167, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Leissner, K.-H.; Tisell, L.-E. The Weight of the Human Prostate. Scand. J. Urol. Nephrol. 1979, 13, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Fryar, C.D.; Kruszon-Moran, D.; Gu, Q.; Ogden, C.L. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999–2000 through 2015–2016. Natl. Health Stat. Rep. 2018, 122, 1–15. [Google Scholar]
- Leider, M. On the Weight of the Skin. J. Investig. Dermatol. 1949, 12, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Baraki, Y.M.; Traverso, P.; Elariny, H.A.; Fang, Y. Preoperative Prediction of Stomach Weight to Be Removed in Laparoscopic Sleeve Gastrectomy Procedure. Surg. Technol. Int. 2010, 20, 167–171. [Google Scholar]
- Jit, I. Sanjeev Weight of the Testes in Northwest Indian Adults. Am. J. Human Bio. 1991, 3, 671–676. [Google Scholar] [CrossRef]
- Pankow, B.G.; Michalak, J.; McGee, M.K. Adult Human Thyroid Weight. Health Phys. 1985, 49, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, N.W.; Chauhan, S.P.; Carroll, C.S.; Magann, E.F.; Morrison, J.C. Clinical and Sonographic Estimations of Uterine Weight. J. Miss. State Med. Assoc. 2003, 44, 67–73. [Google Scholar] [PubMed]
- Surov, A.; Hainz, M.; Holzhausen, H.-J.; Arnold, D.; Katzer, M.; Schmidt, J.; Spielmann, R.P.; Behrmann, C. Skeletal Muscle Metastases: Primary Tumours, Prevalence, and Radiological Features. Eur. Radiol. 2010, 20, 649–658. [Google Scholar] [CrossRef]
- Caunter, G.; Faeez Md Noh, M.S.; Safri, L.S.; Kumar, K.; Md Idris, M.A.; Harunarashid, H.; Yahaya, A. Delayed Presentation of Metastatic Renal Cell Carcinoma as an Arteriovenous Malformation Mimicking Vascular Tumour of the Forearm. EJVES Short Rep. 2019, 44, 19–22. [Google Scholar] [CrossRef]
- Pop, D.; Nadeemy, A.S.; Venissac, N.; Guiraudet, P.; Otto, J.; Poudenx, M.; Mouroux, J. Skeletal Muscle Metastasis from Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 1236–1241. [Google Scholar] [CrossRef]
- Molina-Garrido, M.J.; Guillén-Ponce, C. Muscle Metastasis of Carcinoma. Clin. Translat. Oncol. 2011, 13, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Acinas García, O.; Fernández, F.A.; Satué, E.G.; Buelta, L.; Val-Bernal, J.F. Metastasis of Malignant Neoplasms to Skeletal Muscle. Rev. Esp. Oncol. 1984, 31, 57–67. [Google Scholar]
- LaBan, M.M.; Nagarajan, R.; Riutta, J.C. Paucity of Muscle Metastasis in Otherwise Widely Disseminated Cancer: A Conundrum. Am. J. Phys. Med. Rehabil. 2010, 89, 931–935. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.B. Sarcopenia in Pancreatic Cancer: Effect on Patient Outcomes. World J. Gastrointest. Oncol. 2022, 14, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Zhou, J.; Xiao, X.; Yang, Y.; Luan, S.; Liang, Z.; Li, X.; Zhang, H.; Shang, Q.; Zeng, X.; et al. The Prognostic Value of Sarcopenia in Oesophageal Cancer: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 3–16. [Google Scholar] [CrossRef]
- Omori, A.; Kawakubo, N.; Takemoto, J.; Souzaki, R.; Obata, S.; Nagata, K.; Matsuura, T.; Tajiri, T.; Taguchi, T. Effects of Changes in Skeletal Muscle Mass on the Prognosis of Pediatric Malignant Solid Tumors. Pediatr. Surg. Int. 2022, 38, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Aduse-Poku, L.; Karanth, S.D.; Wheeler, M.; Yang, D.; Washington, C.; Hong, Y.-R.; Manini, T.M.; Fabregas, J.C.; Cheng, T.-Y.D.; Braithwaite, D. Associations of Total Body Fat Mass and Skeletal Muscle Index with All-Cause and Cancer-Specific Mortality in Cancer Survivors. Cancers 2023, 15, 1081. [Google Scholar] [CrossRef]
- Cortellini, A.; Bozzetti, F.; Palumbo, P.; Brocco, D.; Di Marino, P.; Tinari, N.; De Tursi, M.; Agostinelli, V.; Patruno, L.; Valdesi, C.; et al. Weighing the Role of Skeletal Muscle Mass and Muscle Density in Cancer Patients Receiving PD-1/PD-L1 Checkpoint Inhibitors: A Multicenter Real-Life Study. Sci. Rep. 2020, 10, 1456. [Google Scholar] [CrossRef]
- Hong, S.; Kim, K.W.; Park, H.J.; Ko, Y.; Yoo, C.; Park, S.Y.; Khang, S.; Jeong, H.; Lee, J. Impact of Baseline Muscle Mass and Myosteatosis on the Development of Early Toxicity During First-Line Chemotherapy in Patients with Initially Metastatic Pancreatic Cancer. Front. Oncol. 2022, 12, 878472. [Google Scholar] [CrossRef] [PubMed]
- Limpawattana, P.; Theerakulpisut, D.; Wirasorn, K.; Sookprasert, A.; Khuntikeo, N.; Chindaprasirt, J. The Impact of Skeletal Muscle Mass on Survival Outcome in Biliary Tract Cancer Patients. PLoS ONE 2018, 13, e0204985. [Google Scholar] [CrossRef] [PubMed]
- Protani, M.; Coory, M.; Martin, J.H. Effect of Obesity on Survival of Women with Breast Cancer: Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Cespedes Feliciano, E.M.; Kroenke, C.H. The Importance of Body Composition in Explaining the Overweight Paradox in Cancer—Counterpoint. Cancer Res. 2018, 78, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity with Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, W. Roles and Molecular Mechanisms of Physical Exercise in Cancer Prevention and Treatment. J. Sport Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ma, H.; He, A.; Li, Y.; He, C.; Xia, Y. Exercise in Cancer Prevention and Anticancer Therapy: Efficacy, Molecular Mechanisms and Clinical Information. Cancer Lett. 2022, 544, 215814. [Google Scholar] [CrossRef]
- Goh, J.; Niksirat, N.; Campbell, K.L. Exercise Training and Immune Crosstalk in Breast Cancer Microenvironment: Exploring the Paradigms of Exercise-Induced Immune Modulation and Exercise-Induced Myokines. Am. J. Transl. Res. 2014, 6, 422–438. [Google Scholar]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the Exercise-inducible Myokine Irisin on Malignant and Non-malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-Induced Muscle-Derived Cytokines Inhibit Mammary Cancer Cell Growth. Am. J. Physiol. Endocrin. Metab. 2011, 301, E504–E510. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A Novel Myokine, Secreted Protein Acidic and Rich in Cysteine (SPARC), Suppresses Colon Tumorigenesis via Regular Exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef]
- Roy, P.; Chowdhury, S.; Roy, H.K. Exercise-Induced Myokines as Emerging Therapeutic Agents in Colorectal Cancer Prevention and Treatment. Future Oncol. 2018, 14, 309–312. [Google Scholar] [CrossRef]
- Huang, C.; Chang, Y.; Lee, H.; Wu, J.; Huang, J.; Chung, Y.; Hsu, S.; Chow, L.; Wei, K.; Huang, F. Irisin, an Exercise Myokine, Potently Suppresses Tumor Proliferation, Invasion, and Growth in Glioma. FASEB J. 2020, 34, 9678–9693. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-ΚB Pathway. Med. Sci. Monit. 2019, 25, 6085–6096. [Google Scholar] [CrossRef]
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin Stimulates Cell Proliferation and Invasion by Targeting the PI3K/AKT Pathway in Human Hepatocellular Carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zheng, B.; Lu, Y.; Huang, D.; Liu, J.; Song, J.; Zheng, S. FNDC4 Acts as an Extracellular Factor to Promote the Invasiveness of Hepatocellular Carcinoma Partly via the PI3K/Akt Signalling Pathway. Cancer Med. 2021, 10, 7242–7252. [Google Scholar] [CrossRef]
- Dong, H.; Lv, X.; Gao, P.; Hao, Y. Potential Role of Irisin in Lung Diseases and Advances in Research. Front. Pharmacol. 2023, 14, 1307651. [Google Scholar] [CrossRef]
- Alizadeh Zarei, M.; Seyed Hosseini, E.; Haddad Kashani, H.; Ahmad, E.; Nikzad, H. Effects of the Exercise-Inducible Myokine Irisin on Proliferation and Malignant Properties of Ovarian Cancer Cells through the HIF-1 α Signaling Pathway. Sci. Rep. 2023, 13, 170. [Google Scholar] [CrossRef]
- Kim, J.-S.; Galvão, D.A.; Newton, R.U.; Gray, E.; Taaffe, D.R. Exercise-Induced Myokines and Their Effect on Prostate Cancer. Nat. Rev. Urol. 2021, 18, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Taaffe, D.R.; Galvão, D.A.; Hart, N.H.; Gray, E.; Ryan, C.J.; Kenfield, S.A.; Saad, F.; Newton, R.U. Exercise in Advanced Prostate Cancer Elevates Myokine Levels and Suppresses In-Vitro Cell Growth. Prost. Cancer Prost. Dis. 2022, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Wilson, R.L.; Taaffe, D.R.; Galvão, D.A.; Gray, E.; Newton, R.U. Myokine Expression and Tumor-Suppressive Effect of Serum after 12 Wk of Exercise in Prostate Cancer Patients on ADT. Med. Sci. Sports Exerc. 2022, 54, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.D.; Brady, L.; Pollak, M.; Finn, S.P. Exercise and Prostate Cancer: Evidence and Proposed Mechanisms for Disease Modification. Cancer Epidem. Biomark. Prevent. 2016, 25, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Taaffe, D.R.; Galvão, D.A.; Clay, T.D.; Redfern, A.D.; Hart, N.H.; Gray, E.S.; Ryan, C.J.; Kenfield, S.A.; Saad, F.; et al. Acute Effect of High-Intensity Interval Aerobic Exercise on Serum Myokine Levels and Resulting Tumour-Suppressive Effect in Trained Patients with Advanced Prostate Cancer. Prost. Cancer Prost. Dis. 2023, 26, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Sadr, A.; Ehteram, H.; Seyed Hosseini, E.; Alizadeh Zarei, M.; Hassani Bafrani, H.; Haddad Kashani, H. The Effect of Irisin on Proliferation, Apoptosis, and Expression of Metastasis Markers in Prostate Cancer Cell Lines. Oncol. Ther. 2022, 10, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Dieterich, W.; Reljic, D.; Pilarsky, C.; Mukhopadhyay, D.; Chang, D.K.; Biankin, A.V.; Siebler, J.; Herrmann, H.J.; Neurath, M.F.; et al. Muscle-Derived Cytokines Reduce Growth, Viability and Migratory Activity of Pancreatic Cancer Cells. Cancers 2021, 13, 3820. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin Inhibits Pancreatic Cancer Cell Growth via the AMPK-MTOR Pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef]
- Yang, B.C.; Leung, P.S. Irisin Is a Positive Regulator for Ferroptosis in Pancreatic Cancer. Mol. Ther. Oncolytics 2020, 18, 457–466. [Google Scholar] [CrossRef]
- Holtzer, H.; Marshall, J.M.; Finck, H. An Analysis of Myogenesis by the Use of Fluorescent Antimyosin. J. Cell Biol. 1957, 3, 705–724. [Google Scholar] [CrossRef]
- Borowik, A.K.; Davidyan, A.; Peelor, F.F.; Voloviceva, E.; Doidge, S.M.; Bubak, M.P.; Mobley, C.B.; McCarthy, J.J.; Dupont-Versteegden, E.E.; Miller, B.F. Skeletal Muscle Nuclei in Mice Are Not Post-Mitotic. Function 2022, 4, zqac059. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-X.; Wang, G.; Chen, K.; Ma, X.; Liu, S.-Q.; Miao, W. Amitosis as a Strategy of Cell Division—Insight from the Proliferation of Tetrahymena Thermophila Macronuclei. Theor. Popul. Biol. 2022, 145, 52–62. [Google Scholar] [CrossRef] [PubMed]
- White-Gilbertson, S.; Lu, P.; Esobi, I.; Echesabal-Chen, J.; Mulholland, P.J.; Gooz, M.; Ogretmen, B.; Stamatikos, A.; Voelkel-Johnson, C. Polyploid Giant Cancer Cells Are Dependent on Cholesterol for Progeny Formation through Amitotic Division. Sci. Rep. 2022, 12, 8971. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Lieber, R.L. Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.; Vance, C.; Budnick, S.; Muller, S. Muscle Invasion in Oral Tongue Squamous Cell Carcinoma as a Predictor of Nodal Status and Local Recurrence: Just as Effective as Depth of Invasion? Head Neck Pathol. 2011, 5, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Holzhausen, H.-J.; Arnold, D.; Schmidt, J.; Spielmann, R.-P.; Behrmann, C. Intramuscular Manifestation of Non-Hodgkin Lymphoma and Myeloma: Prevalence, Clinical Signs, and Computed Tomography Features. Acta Radiol. 2010, 51, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Mehta, S.; Sumathi, N.; Dhiwakar, M. Occult Invasion of Sternothyroid Muscle by Differentiated Thyroid Cancer. European Arch. Oto Rhino Laryngol. 2018, 275, 233–238. [Google Scholar] [CrossRef]
- Beunk, L.; Brown, K.; Nagtegaal, I.; Friedl, P.; Wolf, K. Cancer Invasion into Musculature: Mechanics, Molecules and Implications. Semin. Cell Dev. Biol. 2019, 93, 36–45. [Google Scholar] [CrossRef]
- Patel, T.J.; Lieber, R.L. Force Transmission in Skeletal Muscle: From Actomyosin to External Tendons. Exerc. Sport Sci. Rev. 1997, 25, 321–363. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanical Signal Transduction in Skeletal Muscle Growth and Adaptation. J. Appl. Physiol. 2005, 98, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, T.J. Mechanotransduction in Skeletal Muscle. Front. Biosci. 2007, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidis, I.A.; Michalakeas, C.A.; Papadopoulos, C.H.; Anastasiou-Nana, M. Cardiac Tumors. ISRN Oncol. 2011, 2011, 208929. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T. The Role of Skeletal Muscle Contractile Duration throughout the Whole Day: Reducing Sedentary Time and Promoting Universal Physical Activity in All People. J. Physiol. 2018, 596, 1331–1340. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Schautz, B.; Later, W.; Heymsfield, S.B.; Müller, M.J. Specific Metabolic Rates of Major Organs and Tissues across Adulthood: Evaluation by Mechanistic Model of Resting Energy Expenditure. Am. J. Clin. Nutr. 2010, 92, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.; Zhang, L.; Wang, D. Metabolic Characterization and Metabolism-Score of Tumor to Predict the Prognosis in Prostate Cancer. Sci. Rep. 2021, 11, 22486. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.B. Local Control of Blood Flow. In Cunningham’s Textbook of Veterinary Physiology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 253–261. [Google Scholar]
- Dang, C.V. Links between Metabolism and Cancer. Genes Dev. 2012, 26, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Lee, S.; Ju, M.; Jeon, H.; Lee, Y.; Kim, C.; Park, H.; Han, S.; Kang, H. Reactive Oxygen Species Induce Epithelial-mesenchymal Transition, Glycolytic Switch, and Mitochondrial Repression through the Dlx-2/Snail Signaling Pathways in MCF-7 Cells. Mol. Med. Rep. 2019, 20, 2339–2346. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox Regulation in Tumor Cell Epithelial–Mesenchymal Transition: Molecular Basis and Therapeutic Strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Hudlická, O. Development and Adaptability of Microvasculature in Skeletal Muscle. J. Exp. Biol. 1985, 115, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Poole, D.C.; Knight, D.R.; Kurdak, S.S.; Hogan, M.C.; Grassi, B.; Johnson, E.C.; Kendrick, K.F.; Erickson, B.K.; Wagner, P.D. High Muscle Blood Flow in Man: Is Maximal O2 Extraction Compromised? J. Appl. Physiol. 1993, 75, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Egginton, S. Invited Review: Activity-Induced Angiogenesis. Pflugers Arch. 2009, 457, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Li, Y.E.; Fink, L.N.; Brozinick, J.T.; Nikolayev, A.; Kuo, M.-S.; Bilan, P.J.; Klip, A. Nucleotides Released from Palmitate-Challenged Muscle Cells Through Pannexin-3 Attract Monocytes. Diabetes 2014, 63, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology 2015, 29, 908–920+922. [Google Scholar] [PubMed]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S.; et al. Exercise-Induced Engagement of the IL-15/IL-15Rα Axis Promotes Anti-Tumor Immunity in Pancreatic Cancer. Cancer Cell 2022, 40, 720–737.e5. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-Cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef]
- Idorn, M.; Hojman, P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Dinas, P.C.; Tsitoglou, K.; Patramani, I.; Koutedakis, Y.; Kenny, G.P. Non-Invasive Measurement of Tibialis Anterior Muscle Temperature during Rest, Cycling Exercise and Post-Exercise Recovery. Physiol. Meas. 2015, 36, N103–N113. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Webb, P.; Kenny, G.P. Noninvasive Assessment of Muscle Temperature during Rest, Exercise, and Postexercise Recovery in Different Environments. J. Appl. Physiol. 2015, 118, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.T.; Chen, Q.; Skitzki, J.J.; Muhitch, J.B.; Zhou, L.; Appenheimer, M.M.; Vardam, T.D.; Weis, E.L.; Passanese, J.; Wang, W.-C.; et al. IL-6 Trans-Signaling Licenses Mouse and Human Tumor Microvascular Gateways for Trafficking of Cytotoxic T Cells. J. Clin. Investig. 2011, 121, 3846–3859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fisher, D.T.; Clancy, K.A.; Gauguet, J.-M.M.; Wang, W.-C.; Unger, E.; Rose-John, S.; von Andrian, U.H.; Baumann, H.; Evans, S.S. Fever-Range Thermal Stress Promotes Lymphocyte Trafficking across High Endothelial Venules via an Interleukin 6 Trans-Signaling Mechanism. Nat. Immunol. 2006, 7, 1299–1308. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the Thermal Regulation of Immunity: The Immune System Feels the Heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Özdemir, B.H.; Özdemir, A.A. How Exercise Affects the Development and Progression of Hepatocellular Carcinoma by Changing the Biomolecular Status of the Tumor Microenvironment. Exp. Clin. Transplant. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Ashcraft, K.A.; Betof Warner, A.; Nair, S.K.; Dewhirst, M.W. Can Exercise-Induced Modulation of the Tumor Physiologic Microenvironment Improve Antitumor Immunity? Cancer Res. 2019, 79, 2447–2456. [Google Scholar] [CrossRef]
- Wiggins, J.M.; Opoku-Acheampong, A.B.; Baumfalk, D.R.; Siemann, D.W.; Behnke, B.J. Exercise and the Tumor Microenvironment: Potential Therapeutic Implications. Exerc. Sport Sci. Rev. 2018, 46, 56–64. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, M.; Wu, X.; Zhang, Y.; Xia, Y. Muscle-to-Tumor Crosstalk: The Effect of Exercise-Induced Myokine on Cancer Progression. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188761. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, K.; Huang, M.; Liang, C.; Siemann, D.; Wu, L.; Tan, Y.; Tang, X. Biophysics in Tumor Growth and Progression: From Single Mechano-Sensitive Molecules to Mechanomedicine. Oncogene 2023, 42, 3457–3490. [Google Scholar] [CrossRef]
- Lampi, M.C.; Reinhart-King, C.A. Targeting Extracellular Matrix Stiffness to Attenuate Disease: From Molecular Mechanisms to Clinical Trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Liang, C.; Huang, M.; Li, T.; Li, L.; Sussman, H.; Dai, Y.; Siemann, D.W.; Xie, M.; Tang, X. Towards an Integrative Understanding of Cancer Mechanobiology: Calcium, YAP, and MicroRNA under Biophysical Forces. Soft Matter 2022, 18, 1112–1148. [Google Scholar] [CrossRef]
- Tan, Y.; Tajik, A.; Chen, J.; Jia, Q.; Chowdhury, F.; Wang, L.; Chen, J.; Zhang, S.; Hong, Y.; Yi, H.; et al. Matrix Softness Regulates Plasticity of Tumour-Repopulating Cells via H3K9 Demethylation and Sox2 Expression. Nat. Commun. 2014, 5, 4619. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Zhang, H.; Zhang, Y.; Xu, P.; Chen, J.; Poh, Y.-C.; Tang, K.; Wang, N.; Huang, B. Soft Fibrin Gels Promote Selection and Growth of Tumorigenic Cells. Nat. Mater. 2012, 11, 734–741. [Google Scholar] [CrossRef]
- McGrail, D.J.; Kieu, Q.M.N.; Dawson, M.R. Metastatic Ovarian Cancer Cell Malignancy Is Increased on Soft Matrices through a Mechanosensitive Rho/ROCK Pathway. J. Cell Sci. 2014, 127, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.R.; Fabry, B. Cell and Tissue Mechanics in Cell Migration. Exp. Cell Res. 2013, 319, 2418–2423. [Google Scholar] [CrossRef]
- Nijsten, M.W.N.; van Dam, G.M. Hypothesis: Using the Warburg Effect against Cancer by Reducing Glucose and Providing Lactate. Med. Hypotheses 2009, 73, 48–51. [Google Scholar] [CrossRef]
- Aveseh, M.; Nikooie, R.; Aminaie, M. Exercise-induced Changes in Tumour LDH-B and MCT1 Expression Are Modulated by Oestrogen-related Receptor Alpha in Breast Cancer-bearing BALB/c Mice. J. Physiol. 2015, 593, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of Exercise-Induced Metabolic Acidosis. Am. J. Physiol. Reg. Integr. Compar. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K. Mechanism and Its Regulation of Tumor-Induced Angiogenesis. World J. Gastroenterol. 2003, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems Analysis of Intracellular PH Vulnerabilities for Cancer Therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, L.; Li, X.-F. Hypoxia and the Tumor Microenvironment. Technol. Cancer Res. Treat. 2021, 20, 153303382110363. [Google Scholar] [CrossRef]
- McCullough, D.J.; Stabley, J.N.; Siemann, D.W.; Behnke, B.J. Modulation of Blood Flow, Hypoxia, and Vascular Function in Orthotopic Prostate Tumors During Exercise. JNCI J. Natl. Cancer Inst. 2014, 106, dju036. [Google Scholar] [CrossRef]
- Crist, S.B.; Nemkov, T.; Dumpit, R.F.; Dai, J.; Tapscott, S.J.; True, L.D.; Swarbrick, A.; Sullivan, L.B.; Nelson, P.S.; Hansen, K.C.; et al. Unchecked Oxidative Stress in Skeletal Muscle Prevents Outgrowth of Disseminated Tumour Cells. Nat. Cell Biol. 2022, 24, 538–553. [Google Scholar] [CrossRef]
- Al Jaberi, S.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, Function, Distribution and Role in Metabolic Disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Yeh, T.-S.; Wu, R.-C.; Pang, J.-H.S.; Cheng, C.-T.; Wang, S.-Y.; Juang, H.-H.; Yeh, C.-N. Lipocalin 2 (LCN2) Is a Promising Target for Cholangiocarcinoma Treatment and Bile LCN2 Level Is a Potential Cholangiocarcinoma Diagnostic Marker. Sci. Rep. 2016, 6, 36138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, P.; Guo, C.; Zhang, C.; Zhao, Y.; Tan, D.; An, J.; Shi, C. Lipocalin 2 May Be a Key Factor Regulating the Chemosensitivity of Pancreatic Cancer to Gemcitabine. Biochem. Biophys. Rep. 2022, 31, 101291. [Google Scholar] [CrossRef]
- Bao, G.-H.; Ho, C.-T.; Barasch, J. The Ligands of Neutrophil Gelatinase-Associated Lipocalin. RSC Adv. 2015, 5, 104363–104374. [Google Scholar] [CrossRef]
- Kidron, M.; Dudai, M.; Nachshon, I.; Mayer, M. Protease Inhibitor Activity in Human Skeletal Muscle. Biochem. Med. Metab. Biol. 1986, 36, 136–140. [Google Scholar] [CrossRef]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal Muscle Is an Endocrine Organ. J. Pharmacol. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Hwang, B.-O.; Song, N.-Y. The Role of Myokines in Cancer: Crosstalk between Skeletal Muscle and Tumor. BMB Rep. 2023, 56, 365–373. [Google Scholar] [CrossRef]
- Henningsen, J.; Rigbolt, K.T.G.; Blagoev, B.; Pedersen, B.K.; Kratchmarova, I. Dynamics of the Skeletal Muscle Secretome during Myoblast Differentiation. Molec. Cell. Proteom. 2010, 9, 2482–2496. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Marçais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The Metabolic Checkpoint Kinase MTOR Is Essential for IL-15 Signaling during the Development and Activation of NK Cells. Nat. Immunol. 2014, 15, 749–757. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Lesjean-Pottier, S.; Vieira, P.; Sawa, S.; Eberl, G.; Vosshenrich, C.A.J.; Di Santo, J.P. IL-7 and IL-15 Independently Program the Differentiation of Intestinal CD3−NKp46+ Cell Subsets from Id2-Dependent Precursors. J. Exp. Med. 2010, 207, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Exercise-Induced Myokines and Their Role in Chronic Diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Thurmond, D.C. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.; Görgens, S.W.; Raschke, S.; Eckel, J. Myokines in Insulin Resistance and Type 2 Diabetes. Diabetologia 2014, 57, 1087–1099. [Google Scholar] [CrossRef]
- Eckel, J. Myokines in Metabolic Homeostasis and Diabetes. Diabetologia 2019, 62, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Goodyear, L.J. Exercise and Type 2 Diabetes: Molecular Mechanisms Regulating Glucose Uptake in Skeletal Muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef]
- Masjedi, A.; Hajizadeh, F.; Beigi Dargani, F.; Beyzai, B.; Aksoun, M.; Hojjat-Farsangi, M.; Zekiy, A.; Jadidi-Niaragh, F. Oncostatin M: A Mysterious Cytokine in Cancers. Int. Immunopharmacol. 2021, 90, 107158. [Google Scholar] [CrossRef]
- Richards, C.D. The Enigmatic Cytokine Oncostatin M and Roles in Disease. ISRN Inflamm. 2013, 2013, 512103. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.L.; Hammacher, A.; Douglas, A.M.; Goss, G.A.; Mansfield, R.K.; Heath, J.K.; Begley, C.G. An Unexpected Biochemical and Functional Interaction between Gp130 and the EGF Receptor Family in Breast Cancer Cells. Oncogene 2002, 21, 460–474. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-M.; Wang, M.-L.; Chiou, S.-H.; Chen, H.-Y.; Wu, C.-W. Oncostatin M Suppresses Metastasis of Lung Adenocarcinoma by Inhibiting SLUG Expression through Coordination of STATs and PIASs Signalings. Oncotarget 2016, 7, 60395–60406. [Google Scholar] [CrossRef] [PubMed]
- Kourtidis, A.; Lu, R.; Pence, L.J.; Anastasiadis, P.Z. A Central Role for Cadherin Signaling in Cancer. Exp. Cell Res. 2017, 358, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Singhai, R.; Patil, V.; Jaiswal, S.; Patil, S.; Patil, M. E-Cadherin as a Diagnostic Biomarker in Breast Cancer. N. Am. J. Med. Sci. 2011, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Halfter, H.; Friedrich, M.; Resch, A.; Kullmann, M.; Stögbauer, F.; Ringelstein, E.B.; Hengst, L. Oncostatin M Induces Growth Arrest by Inhibition of Skp2, Cks1, and Cyclin A Expression and Induced P21 Expression. Cancer Res. 2006, 66, 6530–6539. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Morikawa, Y. Essential Roles of the Cytokine Oncostatin M in Crosstalk between Muscle Fibers and Immune Cells in Skeletal Muscle after Aerobic Exercise. J. Biol. Chem. 2022, 298, 102686. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Xu, L.; Ye, Y.; Sun, Y.; Zhong, W.; Chi, L.; Lin, Y.; Liu, H.; Li, S.; Chen, H.; Li, C.; et al. Low FNDC5/Irisin Expression Is Associated with Aggressive Phenotypes in Gastric Cancer. Front. Pharmacol. 2022, 13, 981201. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.K.; Chan, W.Y.; Ng, S.-W.; Chan, P.S.; Cheung, K.K.; Berkowitz, R.S.; Mok, S.C. SPARC (Secreted Protein Acidic and Rich in Cysteine) Induces Apoptosis in Ovarian Cancer Cells. Am. J. Pathol. 2001, 159, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Frierson, H.F.; Chernauskas, D.; Conaway, M.; Motamed, K.; Theodorescu, D. The Role of SPARC in the TRAMP Model of Prostate Carcinogenesis and Progression. Oncogene 2009, 28, 3487–3498. [Google Scholar] [CrossRef] [PubMed]
- Brekken, R.A.; Puolakkainen, P.; Graves, D.C.; Workman, G.; Lubkin, S.R.; Sage, E.H. Enhanced Growth of Tumors in SPARC Null Mice Is Associated with Changes in the ECM. J. Clin. Investig. 2003, 111, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Lemke, N.; Ge, S.; Golembieski, W.A.; Rempel, S.A. Secreted Protein Acidic and Rich in Cysteine Promotes Glioma Invasion and Delays Tumor Growth in Vivo. Cancer Res. 2002, 62, 6270–6277. [Google Scholar] [PubMed]
- Ma, J.; Ma, Y.; Chen, S.; Guo, S.; Hu, J.; Yue, T.; Zhang, J.; Zhu, J.; Wang, P.; Chen, G.; et al. SPARC Enhances 5-FU Chemosensitivity in Gastric Cancer by Modulating Epithelial-Mesenchymal Transition and Apoptosis. Biochem. Biophys. Res. Commun. 2021, 558, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Chen, G.-W.; Liu, Y.-C.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Zhu, J.; Gao, H.-Q.; Yin, J.; Wang, W.; et al. Secreted Protein Acidic and Rich in Cysteine (SPARC) Suppresses Angiogenesis by Down-Regulating the Expression of VEGF and MMP-7 in Gastric Cancer. PLoS ONE 2012, 7, e44618. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L. Aberrant Crypt Foci as Microscopic Precursors of Colorectal Cancer. World J. Gastroenterol. 2003, 9, 2642. [Google Scholar] [CrossRef] [PubMed]
- Chern, Y.-J.; Wong, J.C.T.; Cheng, G.S.W.; Yu, A.; Yin, Y.; Schaeffer, D.F.; Kennecke, H.F.; Morin, G.; Tai, I.T. The Interaction between SPARC and GRP78 Interferes with ER Stress Signaling and Potentiates Apoptosis via PERK/EIF2α and IRE1α/XBP-1 in Colorectal Cancer. Cell Death Dis. 2019, 10, 504. [Google Scholar] [CrossRef]
- Chetty, C.; Dontula, R.; Ganji, P.N.; Gujrati, M.; Lakka, S.S. SPARC Expression Induces Cell Cycle Arrest via STAT3 Signaling Pathway in Medulloblastoma Cells. Biochem. Biophys. Res. Commun. 2012, 417, 874–879. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cros, J.; Faivre, S.; Hammel, P.; Raymond, E. Stromal Expression of SPARC in Pancreatic Adenocarcinoma. Cancer Metast. Rev. 2013, 32, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Pohl, N.M.; Qian, Z.; Yang, G.R.; Gou, Y.; Guzman, G.; Kajdacsy-Balla, A.; Iozzo, R.V.; Yang, W. Decorin-Mediated Inhibition of Colorectal Cancer Growth and Migration Is Associated with E-Cadherin in Vitro and in Mice. Carcinogenesis 2012, 33, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, E.; Levkau, B.; Schaefer, L.; Kresse, H.; Walsh, K. Decorin-Mediated Signal Transduction in Endothelial Cells. J. Biol. Chem. 2001, 276, 40687–40692. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.G.; Bhattacharyya, I.; Lydiatt, W.M.; Vishwanatha, J.K. Aberrant Expression and Localization of Decorin in Human Oral Dysplasia and Squamous Cell Carcinoma. Cancer Res. 2003, 63, 7769–7776. [Google Scholar] [PubMed]
- Reed, C.C.; Gauldie, J.; Iozzo, R.V. Suppression of Tumorigenicity by Adenovirus-Mediated Gene Transfer of Decorin. Oncogene 2002, 21, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.S.; Yenisey, C.; Rose, R.W.; Tootell, M.; Santra, M.; Iozzo, R.V. Decorin Suppresses Tumor Cell-Mediated Angiogenesis. Oncogene 2002, 21, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Tong, C.; Dockendorff, A.; Bancroft, L.; Gallagher, L.; Guzman, G.; Iozzo, R.V.; Augenlicht, L.H.; Yang, W. Genetic Deficiency of Decorin Causes Intestinal Tumor Formation through Disruption of Intestinal Cell Maturation. Carcinogenesis 2008, 29, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, H.; Owens, R.T.; Wu, J.; Chen, Y.Q.; Berquin, I.M.; Perry, D.; O’Flaherty, J.T.; Edwards, I.J. Decorin Suppresses Prostate Tumor Growth through Inhibition of Epidermal Growth Factor and Androgen Receptor Pathways. Neoplasia 2009, 11, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Z.-X.; Wang, R. MicroRNA-21: A Novel Therapeutic Target in Human Cancer. Cancer Biol. Ther. 2010, 10, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Soria-Valles, C.; Gutiérrez-Fernández, A.; Guiu, M.; Mari, B.; Fueyo, A.; Gomis, R.R.; López-Otín, C. The Anti-Metastatic Activity of Collagenase-2 in Breast Cancer Cells Is Mediated by a Signaling Pathway Involving Decorin and MiR-21. Oncogene 2014, 33, 3054–3063. [Google Scholar] [CrossRef]
- Liang, S.; Xu, J.-F.; Cao, W.-J.; Li, H.-P.; Hu, C.-P. Human Decorin Regulates Proliferation and Migration of Human Lung Cancer A549 Cells. Chin. Med. J. 2013, 126, 4736–4741. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z.; Reszegi, A.; Szilák, L.; Dankó, T.; Kovalszky, I.; Baghy, K. Tumor-Specific Inhibitory Action of Decorin on Different Hepatoma Cell Lines. Cell Signal. 2019, 62, 109354. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, S.; D’Alessandro, G.; Chece, G.; Brau, F.; Maggi, L.; Rosa, A.; Porzia, A.; Mainiero, F.; Esposito, V.; Lauro, C.; et al. Enriched Environment Reduces Glioma Growth through Immune and Non-Immune Mechanisms in Mice. Nat. Commun. 2015, 6, 6623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Bergin, S.M.; Huang, W.; Slater, A.M.; Liu, X.; Judd, R.T.; Lin, E.-J.D.; Widstrom, K.J.; Scoville, S.D.; Yu, J.; et al. Environmental and Genetic Activation of Hypothalamic BDNF Modulates T-Cell Immunity to Exert an Anticancer Phenotype. Cancer Immunol. Res. 2016, 4, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, X.; Lin, E.-J.D.; Wang, C.; Choi, E.Y.; Riban, V.; Lin, B.; During, M.J. Environmental and Genetic Activation of a Brain-Adipocyte BDNF/Leptin Axis Causes Cancer Remission and Inhibition. Cell 2010, 142, 52–64. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent Insights into Targeting the IL-6 Cytokine Family in Inflammatory Diseases and Cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Orange, S.T.; Leslie, J.; Ross, M.; Mann, D.A.; Wackerhage, H. The Exercise IL-6 Enigma in Cancer. Trends Endocrinol. Metab. 2023, 34, 749–763. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an Energy Allocator in Muscle Tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.D.; Yu, J.J.; Spiotto, M.T.; Bartkowski, M.; Simons, J.W. Characterization of the Role of IL-6 in the Progression of Prostate Cancer. Prostate 1999, 38, 199–207. [Google Scholar] [CrossRef]
- Orange, S.T.; Jordan, A.R.; Odell, A.; Kavanagh, O.; Hicks, K.M.; Eaglen, T.; Todryk, S.; Saxton, J.M. Acute Aerobic Exercise-conditioned Serum Reduces Colon Cancer Cell Proliferation in Vitro through Interleukin-6-induced Regulation of DNA Damage. Int. J. Cancer 2022, 151, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Bakar-Ates, F. The Confounding Effect of Interleukin-6 on Apoptosis of MCF-7 Cells through Downregulation of MMP-2/-9 MRNA Expression. Turk. J. Biochem. 2021, 46, 549–555. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 Is Expressed and Secreted by Human Skeletal Muscle Cells. Am. J. Physiol. Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Bøhn, S.K.; Raastad, T.; Paulsen, G.; Blomhoff, R.; Kadi, F. Differences in the Inflammatory Plasma Cytokine Response Following Two Elite Female Soccer Games Separated by a 72-h Recovery. Scand. J. Med. Sci. Sports 2010, 20, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhu, Z.; Xiao, H.; Wakefield, M.R.; Ding, V.A.; Bai, Q.; Fang, Y. The Role of IL-7 in Immunity and Cancer. Anticancer Res. 2017, 37, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, L.; Kim, S.; Lee, S.; Oh, S.; Jo, S.; Jang, I.; Kim, T.-D. The Role of IL-7 and IL-7R in Cancer Pathophysiology and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 10412. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Wheatley-Guy, C.M.; Rosenthal, A.C.; Gastineau, D.A.; Katsanis, E.; Johnson, B.D.; Simpson, R.J. Exercise and the Immune System: Taking Steps to Improve Responses to Cancer Immunotherapy. J. Immunother. Cancer 2021, 9, e001872. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Shimizu, T.; Lotze, M.T. Interleukin-10 Promotes the Maintenance of Antitumor CD8+ T-Cell Effector Function in Situ. Blood 2001, 98, 2143–2151. [Google Scholar] [CrossRef]
- Huang, S.; Ullrich, S.E.; Bar-Eli, M. Regulation of Tumor Growth and Metastasis by Interleukin-10: The Melanoma Experience. J. Interferon Cytokine Res. 1999, 19, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Calarota, S.A.; Vidali, F.; MacDonald, T.T.; Corazza, G.R. Role of IL-15 in Immune-Mediated and Infectious Diseases. Cytokine Growth Factor Rev. 2011, 22, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.E.; Giri, J.G.; Lindemann, M.J.; Linett, M.L.; Ahdieh, M.; Paxton, R.; Anderson, D.; Eisenmann, J.; Grabstein, K.; Caligiuri, M.A. Interleukin (IL) 15 Is a Novel Cytokine That Activates Human Natural Killer Cells via Components of the IL-2 Receptor. J. Exp. Med. 1994, 180, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, C.A.; Finkelstein, S.E.; Surman, D.R.; Lichtman, M.K.; Gattinoni, L.; Theoret, M.R.; Grewal, N.; Spiess, P.J.; Antony, P.A.; Palmer, D.C.; et al. IL-15 Enhances the in Vivo Antitumor Activity of Tumor-Reactive CD8+ T Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, S.; Hwang, I.; Tough, D.F.; Sprent, J. Potent and Selective Stimulation of Memory-Phenotype CD8+ T Cells In Vivo by IL-15. Immunity 1998, 8, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.F.; Goldusky, J.; Bhimalli, P.; Marzo, A.L.; Schneider, J.R. Tracking Fluorescently Labeled IL-15 and Anti-PD-1 in the Tumor Microenvironment and Draining Lymph Nodes. J. Immunol. Methods 2022, 505, 113253. [Google Scholar] [CrossRef]
- Roy, M.; Fowler, A.M.; Ulaner, G.A.; Mahajan, A. Molecular Classification of Breast Cancer. PET Clin. 2023, 18, 441–458. [Google Scholar] [CrossRef]
- Stravokefalou, V.; Stellas, D.; Karaliota, S.; Nagy, B.A.; Valentin, A.; Bergamaschi, C.; Dimas, K.; Pavlakis, G.N. Heterodimeric IL-15 (HetIL-15) Reduces Circulating Tumor Cells and Metastasis Formation Improving Chemotherapy and Surgery in 4T1 Mouse Model of TNBC. Front. Immunol. 2023, 13, 1014802. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, M.D.; Dubois, S.P.; Müller, J.R.; Bryant, B.; Ma, E.; Conlon, K.C.; Waldmann, T.A. Interleukin-15 Augments NK Cell–Mediated ADCC of Alemtuzumab in Patients with CD52+ T-Cell Malignancies. Blood Adv. 2023, 7, 384–394. [Google Scholar] [CrossRef]
- Luo, Z.; He, Z.; Qin, H.; Chen, Y.; Qi, B.; Lin, J.; Sun, Y.; Sun, J.; Su, X.; Long, Z.; et al. Exercise-Induced IL-15 Acted as a Positive Prognostic Implication and Tumor-Suppressed Role in Pan-Cancer. Front Pharmacol. 2022, 13, 1053137. [Google Scholar] [CrossRef]
- Laron, Z. The Somatostatin-GHRH-GH-IGF-1 Axis. In Growth Hormone, IGF_1 and Growth: New Views and Old Concepts. Modern Endocrinology and Diabetes; Merimee, T., Laron, Z., Eds.; Freund Publishing House Ltd.: London, UK; Tel Aviv, Israel, 1996; pp. 5–10. [Google Scholar]
- Kineman, R.D.; del Rio-Moreno, M.; Sarmento-Cabral, A. 40 YEARS of IGF1: Understanding the Tissue-Specific Roles of IGF1/IGF1R in Regulating Metabolism Using the Cre/LoxP System. J. Mol. Endocrinol. 2018, 61, T187–T198. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Baylink, D. IGF-Binding Proteins Are Multifunctional and Act via IGF-Dependent and -Independent Mechanisms. J. Endocrinol. 2002, 175, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Weng, X.; Huang, X.; Peng, Y.; Guo, H.; Xu, Y. IGFBP2 in Cancer: Pathological Role and Clinical Significance (Review). Oncol. Rep 2020, 45, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Li, M.; Zhang, S.; Lu, Y.; Liang, Y.; Zhao, K.; Li, Y. Insulin-like Growth Factor 1/Insulin-like Growth Factor 1 Receptor Signaling Protects against Cell Apoptosis through the PI3K/AKT Pathway in Glioblastoma Cells. Exp. Ther. Med. 2018, 16, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. Role of the Insulin-Like Growth Factor Family in Cancer Development and Progression. J. Natl. Cancer Inst. 2000, 92, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Rajah, R.; Valentinis, B.; Cohen, P. Insulin-like Growth Factor (IGF)-Binding Protein-3 Induces Apoptosis and Mediates the Effects of Transforming Growth Factor-Β1 on Programmed Cell Death through a P53- and IGF-Independent Mechanism. J. Biol. Chem. 1997, 272, 12181–12188. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Peehl, D.M.; Lamson, G.; Rosenfeld, R.G. Insulin-Like Growth Factors (IGFs), IGF Receptors, and IGF-Binding Proteins in Primary Cultures of Prostate Epithelial Cells. J. Clin. Endocrinol. Metab. 1991, 73, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Weng, X.-F.; Huang, B.-L.; Guo, H.-P.; Xu, Y.-W.; Peng, Y.-H. IGFBP-1 in Cancer: Expression, Molecular Mechanisms, and Potential Clinical Implications. Am. J. Transl. Res. 2021, 13, 813–832. [Google Scholar]
- Kuhn, H.; Frille, A.; Petersen, M.A.; Oberhuber-Kurth, J.; Hofmann, L.; Gläser, A.; Taubenheim, S.; Klagges, S.; Kraemer, S.; Broschewitz, J.; et al. IGFBP3 Inhibits Tumor Growth and Invasion of Lung Cancer Cells and Is Associated with Improved Survival in Lung Cancer Patients. Transl. Oncol. 2023, 27, 101566. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like Growth Factors and Neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Le, H.T.; Lee, H.J.; Cho, J.; Min, H.-Y.; Lee, J.-S.; Lee, S.-J.; Lee, H.-Y. Insulin-Like Growth Factor Binding Protein-3 Exerts Its Anti-Metastatic Effect in Aerodigestive Tract Cancers by Disrupting the Protein Stability of Vimentin. Cancers 2021, 13, 1041. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Ruan, B.; Wu, J.; Wang, J.; Shang, R.; Sun, W.; Li, X.; Dou, K.; Wang, D.; Li, Y. Insulin-like Growth Factor Binding Protein-1 Inhibits Cancer Cell Invasion and Is Associated with Poor Prognosis in Hepatocellular Carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 5645–5654. [Google Scholar]
- Figueroa, J.A.; Sharma, J.; Jackson, J.G.; McDermott, M.J.; Hilsenbeck, S.G.; Yee, D. Recombinant Insulin-like Growth Factor Binding Protein-1 Inhibits IGF-I, Serum, and Estrogen-dependent Growth of MCF-7 Human Breast Cancer Cells. J. Cell Physiol. 1993, 157, 229–236. [Google Scholar] [CrossRef] [PubMed]
- McGuire, W.L.; Jackson, J.G.; Figueroa, J.A.; Shimasaki, S.; Powell, D.R.; Yee, D. Regulation of Insulin-Like Growth Factor-Binding Protein (IGFBP) Expression by Breast Cancer Cells: Use of IGFBP-1 as as Inhibitor of Insulin-like Growth Factor Action. J. Natl. Cancer Inst. 1992, 84, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.; Jackson, J.G.; Kozelsky, T.W.; Figueroa, J.A. Insulin-like Growth Factor Binding Protein 1 Expression Inhibits Insulin-like Growth Factor I Action in MCF-7 Breast Cancer Cells. Cell Growth Differ. 1994, 5, 73–77. [Google Scholar] [PubMed]
- Ngo, T.H.; Barnard, R.J.; Leung, P.-S.; Cohen, P.; Aronson, W.J. Insulin-like Growth Factor I (IGF-I) and IGF Binding Protein-1 Modulate Prostate Cancer Cell Growth and Apoptosis: Possible Mediators for the Effects of Diet and Exercise on Cancer Cell Survival. Endocrinology 2003, 144, 2319–2324. [Google Scholar] [CrossRef]
- Major, J.M.; Laughlin, G.A.; Kritz-Silverstein, D.; Wingard, D.L.; Barrett-Connor, E. Insulin-Like Growth Factor-I and Cancer Mortality in Older Men. J. Clin. Endocrinol. Metab. 2010, 95, 1054–1059. [Google Scholar] [CrossRef]
- Rowlands, M.; Gunnell, D.; Harris, R.; Vatten, L.J.; Holly, J.M.P.; Martin, R.M. Circulating Insulin-like Growth Factor Peptides and Prostate Cancer Risk: A Systematic Review and Meta-analysis. Int. J. Cancer 2009, 124, 2416–2429. [Google Scholar] [CrossRef]
- Endogenous Hormones and Breast Cancer Collobaorative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like Growth Factor 1 (IGF1), IGF Binding Protein 3 (IGFBP3), and Breast Cancer Risk: Pooled Individual Data Analysis of 17 Prospective Studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Xu, W.; Wang, X.; Zhao, W.; Wu, J. IGF-I and IGFBP-3 and the Risk of Lung Cancer: A Meta-Analysis Based on Nested Case-Control Studies. J. Exp. Clin. Cancer Res. 2009, 28, 89. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, Z.W.; Firpo, M.A.; Repko, R.C.; Scaife, C.L.; Adler, D.G.; Boucher, K.M.; Mulvihill, S.J. Serum IGFBP2 and MSLN as Diagnostic and Prognostic Biomarkers for Pancreatic Cancer. HPB 2014, 16, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Lu, H.; Gao, W.; Wang, L.; Lu, K.; Wu, S.; Pataer, A.; Huang, M.; El-Zein, R.; Lin, T.; et al. Insulin-Like Growth Factor Binding Protein-2 Level Is Increased in Blood of Lung Cancer Patients and Associated with Poor Survival. PLoS ONE 2013, 8, e74973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.M.Y.; Wellberg, E.A.; Kopp, J.L.; Johnson, J.D. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab. J. 2021, 45, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between Hyperinsulinemia and Increased Risk of Cancer Death in Nonobese and Obese People: A Population-based Observational Study. Int. J. Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Hyperinsulinaemia in Cancer. Nat. Rev. Cancer 2020, 20, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fan, R.; Hao, Z.; Li, J.; Yang, X.; Zhang, Y.; Xia, Y. The Association between Physical Activity and Insulin Level Under Different Levels of Lipid Indices and Serum Uric Acid. Front. Physiol. 2022, 13, 809669. [Google Scholar] [CrossRef] [PubMed]
- Berg, U.; Bang, P. Exercise and Circulating Insulin-Like Growth Factor I. Horm. Res. Paediatr. 2004, 62, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Matsubara, T.; Tobina, T.; Shindo, M.; Tokuyama, K.; Tanaka, K.; Tanaka, H. Effect of Low-Intensity Aerobic Exercise on Insulin-Like Growth Factor-I and Insulin-Like Growth Factor-Binding Proteins in Healthy Men. Int. J. Endocrinol. 2010, 2010, 452820. [Google Scholar] [CrossRef]
- Kim, T.; Chang, J.S.; Kim, H.; Lee, K.H.; Kong, I.D. Intense Walking Exercise Affects Serum IGF-1 and IGFBP3. J. Lifestyle Med. 2015, 5, 21–25. [Google Scholar] [CrossRef]
- Irwin, M.L.; Varma, K.; Alvarez-Reeves, M.; Cadmus, L.; Wiley, A.; Chung, G.G.; DiPietro, L.; Mayne, S.T.; Yu, H. Randomized Controlled Trial of Aerobic Exercise on Insulin and Insulin-like Growth Factors in Breast Cancer Survivors: The Yale Exercise and Survivorship Study. Cancer Epidemiol. Biomark. Prevent. 2009, 18, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Arnarson, A.; Geirsdottir, O.G.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Insulin-like Growth Factor-1 and Resistance Exercise in Community Dwelling Old Adults. J. Nutr. Health Aging 2015, 19, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Ngo, T.H.; Leung, P.; Aronson, W.J.; Golding, L.A. A Low-fat Diet and/or Strenuous Exercise Alters the IGF Axis in Vivo and Reduces Prostate Tumor Cell Growth in Vitro. Prostate 2003, 56, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Brasel, J.A.; Mohan, S.; Wong, W.L.T.; Cooper, D.M. Increased Physical Activity and the Growth Hormone-IGF-I Axis in Adolescent Males. Am. J. Physiol. Regulat. Integrat. Compar. Physiol. 1998, 275, R308–R314. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.T.; Clemmons, D.R.; Underwood, L.E.; Ben-Ezra, V.; McMurray, R. The Effect of Exercise on Plasma Somatomedin-C/Insulinlike Growth Factor I Concentrations. Metabolism 1987, 36, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J. Growth Hormone and Exercise. Clin. Endocrinol. 1999, 50, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.R.; Durand, R.J.; Acevedo, E.O.; Johnson, L.G.; Kraemer, G.R.; Hebert, E.P.; Castracane, V.D. Rigorous Running Increases Growth Hormone and Insulin-Like Growth Factor-I Without Altering Ghrelin. Exp. Biol. Med. 2004, 229, 240–246. [Google Scholar] [CrossRef] [PubMed]
- de Souza Vale, R.G.; de Oliveira, R.D.; Pernambuco, C.S.; de Meneses, Y.P.d.S.F.; Novaes, J.d.S.; de Andrade, A.d.F.D. Effects of Muscle Strength and Aerobic Training on Basal Serum Levels of IGF-1 and Cortisol in Elderly Women. Arch. Gerontol. Geriatr. 2009, 49, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Borst, S.E.; De Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of Resistance Training on Insulin-like Growth Factor-I and IGF Binding Proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef]
- Ballard, T.L.; Clapper, J.A.; Specker, B.L.; Binkley, T.L.; Vukovich, M.D. Effect of Protein Supplementation during a 6-Mo Strength and Conditioning Program on Insulin-like Growth Factor I and Markers of Bone Turnover in Young Adults1–3. Am. J. Clin. Nutr. 2005, 81, 1442–1448. [Google Scholar] [CrossRef]
- Devin, J.L.; Hill, M.M.; Mourtzakis, M.; Quadrilatero, J.; Jenkins, D.G.; Skinner, T.L. Acute High Intensity Interval Exercise Reduces Colon Cancer Cell Growth. J. Physiol. 2019, 597, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Augsten, M.; Strömberg, A.; Rullman, E.; Mijwel, S.; Kharaziha, P.; Panaretakis, T.; Gustafsson, T.; Östman, A. Effect of Acute Exercise on Prostate Cancer Cell Growth. PLoS ONE 2013, 8, e67579. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Lillelund, C.; Midtgaard, J.; Andersen, C.; Pedersen, B.K.; Christensen, J.F.; Hojman, P. Exercise Regulates Breast Cancer Cell Viability: Systemic Training Adaptations versus Acute Exercise Responses. Breast Cancer Res. Treat. 2016, 159, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Maruszewska, A.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. The Potential Importance of CXCL1 in the Physiological State and in Noncancer Diseases of the Cardiovascular System, Respiratory System and Skin. Int. J. Mol. Sci. 2022, 24, 205. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and Osteoprotegerin Are Type II Muscle Specific Myokines Protecting Pancreatic Beta-Cells against Proinflammatory Cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.P.; Drazick, A.T.; Gao, H.; Li, Y. Skeletal Muscle Secretion of IL-6 Is Muscle Type Specific: Ex Vivo Evidence. Biochem. Biophys. Res. Commun. 2018, 505, 146–150. [Google Scholar] [CrossRef]
- Mita, Y.; Zhu, H.; Furuichi, Y.; Hamaguchi, H.; Manabe, Y.; Fujii, N.L. R-Spondin3 Is a Myokine That Differentiates Myoblasts to Type I Fibres. Sci. Rep. 2022, 12, 13020. [Google Scholar] [CrossRef]
- Kuhnen, G.; Guedes Russomanno, T.; Murgia, M.; Pillon, N.J.; Schönfelder, M.; Wackerhage, H. Genes Whose Gain or Loss of Function Changes Type 1, 2A, 2X, or 2B Muscle Fibre Proportions in Mice—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 12933. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional Co-Activator PGC-1α Drives the Formation of Slow-Twitch Muscle Fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Kang, C. Role of PGC-1α in sarcopenia: Etiology and potential intervention—A mini-review. Gerontology 2015, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Dunlap, K.R.; Rosa-Caldwell, M.E.; Haynie, W.S.; Jansen, L.T.; Washington, T.A.; Greene, N.P. Comparative Plasma Proteomics in Muscle Atrophy during Cancer-cachexia and Disuse: The Search for Atrokines. Physiol. Rep. 2020, 8, e14608. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal Muscle FOXO1 (FKHR) Transgenic Mice Have Less Skeletal Muscle Mass, Down-Regulated Type I (Slow Twitch/Red Muscle) Fiber Genes, and Impaired Glycemic Control. J. Biolog. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [PubMed]
- Walters, Z.S.; Villarejo-Balcells, B.; Olmos, D.; Buist, T.W.S.; Missiaglia, E.; Allen, R.; Al-Lazikani, B.; Garrett, M.D.; Blagg, J.; Shipley, J. JARID2 Is a Direct Target of the PAX3-FOXO1 Fusion Protein and Inhibits Myogenic Differentiation of Rhabdomyosarcoma Cells. Oncogene 2014, 33, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Kitamura, Y.I.; Funahashi, Y.; Shawber, C.J.; Castrillon, D.H.; Kollipara, R.; DePinho, R.A.; Kitajewski, J.; Accili, D. A Foxo/Notch Pathway Controls Myogenic Differentiation and Fiber Type Specification. J. Clin. Investig. 2007, 117, 2477–2485. [Google Scholar] [CrossRef]
- Sasako, T.; Umehara, T.; Soeda, K.; Kaneko, K.; Suzuki, M.; Kobayashi, N.; Okazaki, Y.; Tamura-Nakano, M.; Chiba, T.; Accili, D.; et al. Deletion of Skeletal Muscle Akt1/2 Causes Osteosarcopenia and Reduces Lifespan in Mice. Nat. Commun. 2022, 13, 5655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kump, D.S. Mechanisms Underlying the Rarity of Skeletal Muscle Cancers. Int. J. Mol. Sci. 2024, 25, 6480. https://doi.org/10.3390/ijms25126480
Kump DS. Mechanisms Underlying the Rarity of Skeletal Muscle Cancers. International Journal of Molecular Sciences. 2024; 25(12):6480. https://doi.org/10.3390/ijms25126480
Chicago/Turabian StyleKump, David S. 2024. "Mechanisms Underlying the Rarity of Skeletal Muscle Cancers" International Journal of Molecular Sciences 25, no. 12: 6480. https://doi.org/10.3390/ijms25126480
APA StyleKump, D. S. (2024). Mechanisms Underlying the Rarity of Skeletal Muscle Cancers. International Journal of Molecular Sciences, 25(12), 6480. https://doi.org/10.3390/ijms25126480





