Optimization of Liquid Crystalline Mixtures Enantioseparation on Polysaccharide-Based Chiral Stationary Phases by Reversed-Phase Chiral Liquid Chromatography
Abstract
1. Introduction
- -
- polysaccharides,
- -
- Pirkle-type phases,
- -
- cyclodextrins,
- -
- crown ethers,
- -
- ligand exchange phases,
- -
- ion-exchange phases,
- -
- protein phases,
- -
- macrocyclic antibiotics.
- -
- volume ratio of solvents used,
- -
- chemical structure of the studied racemic mixtures,
- -
- the type of elution,
- -
- the type of chiral selector.
2. Results and Discussion
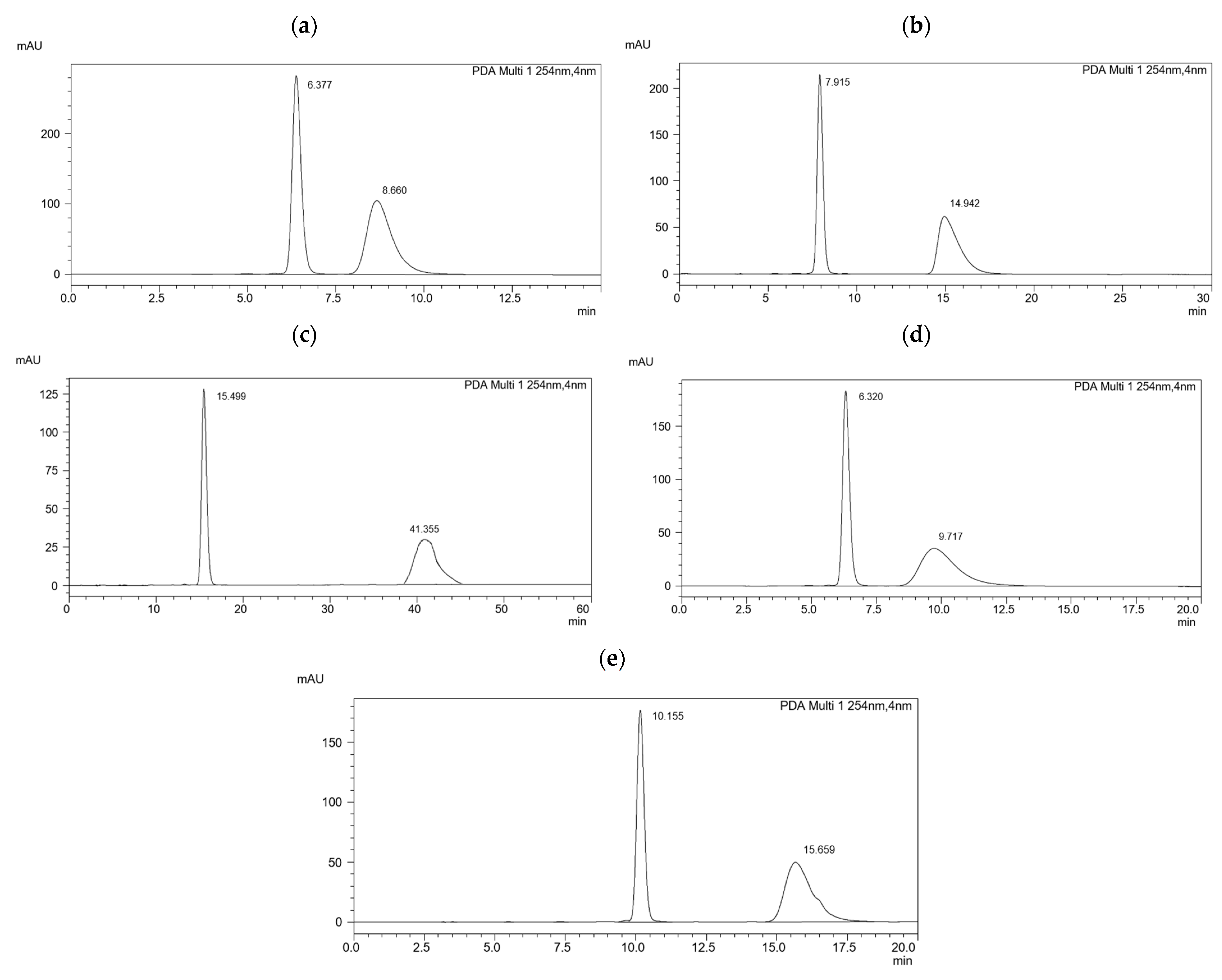
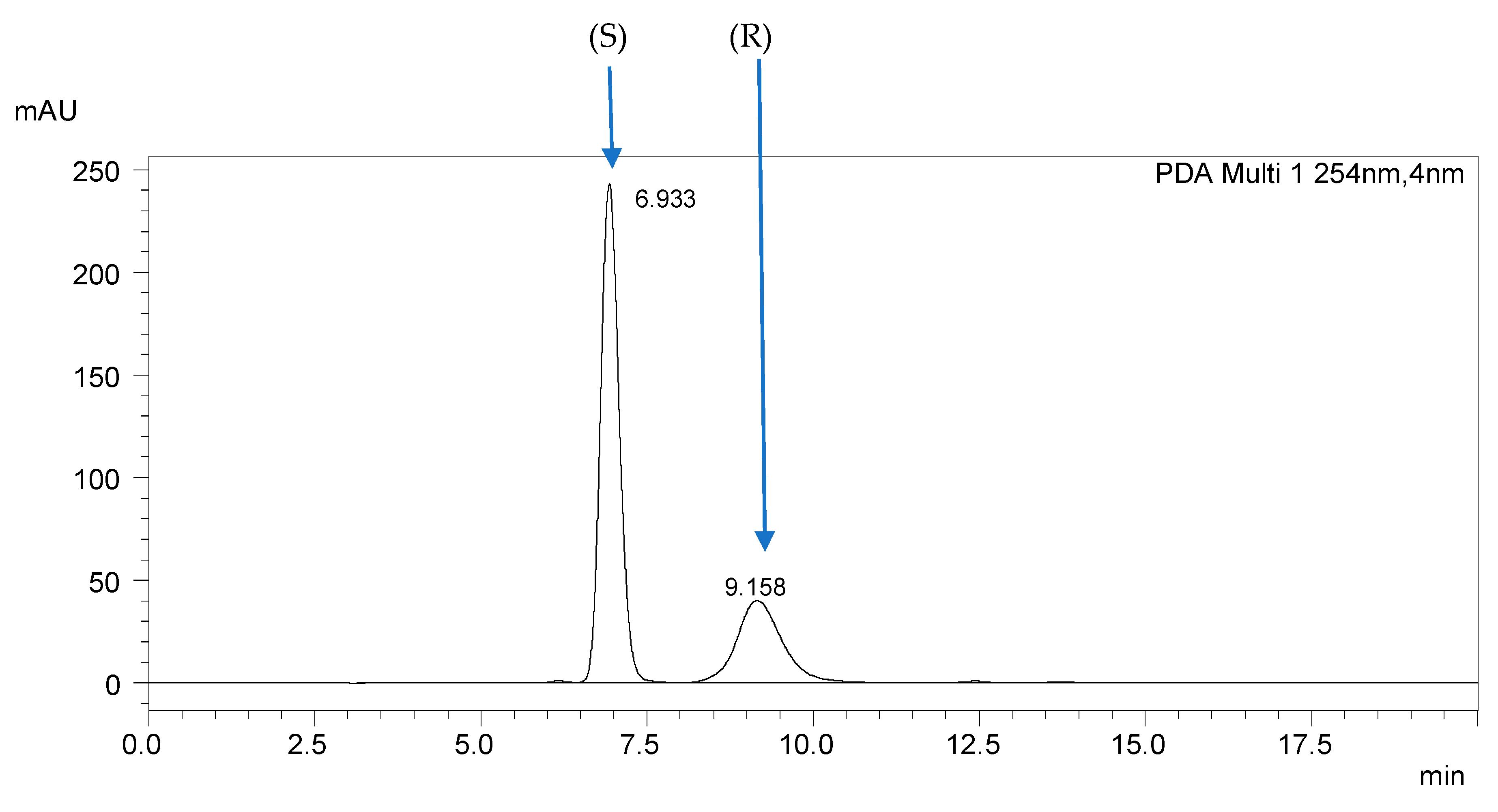
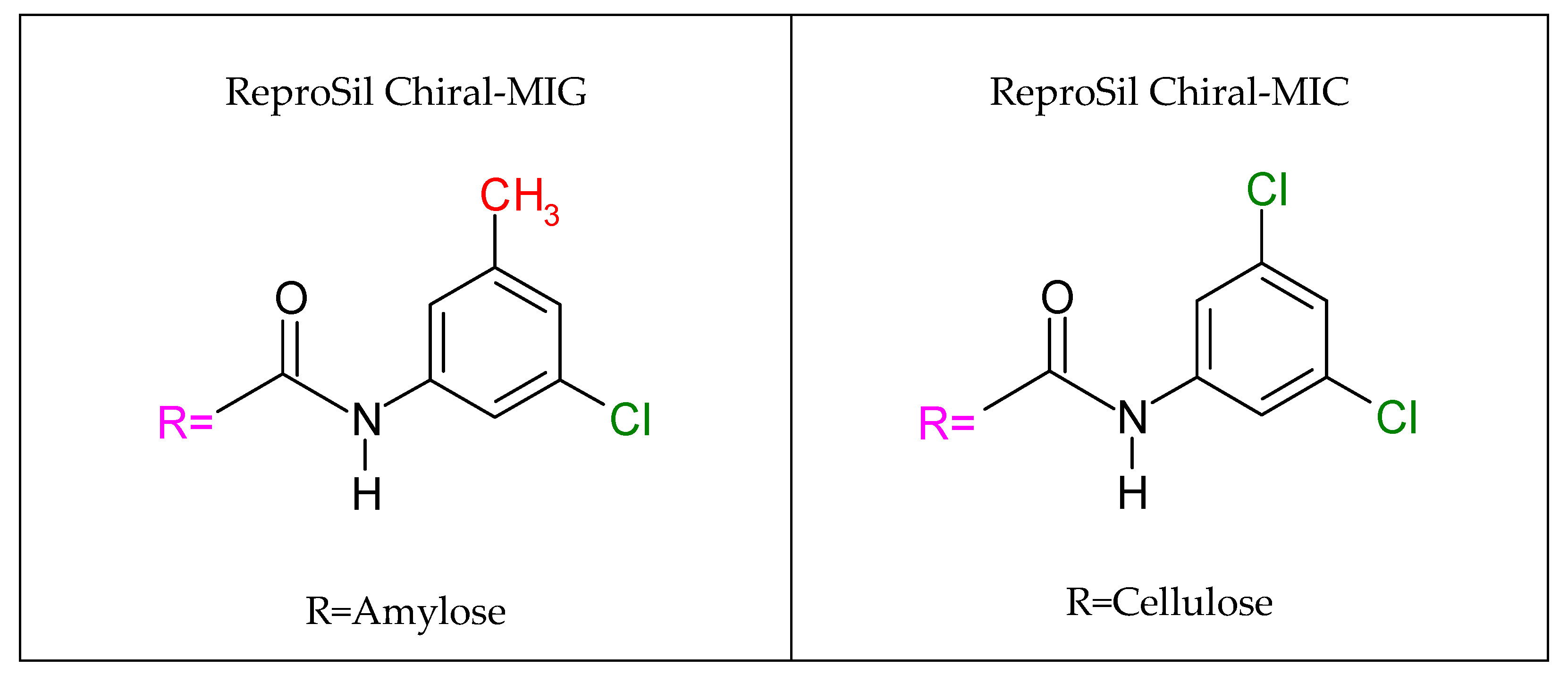
2.1. Optimization on the ReproSil Chiral-MIG Column
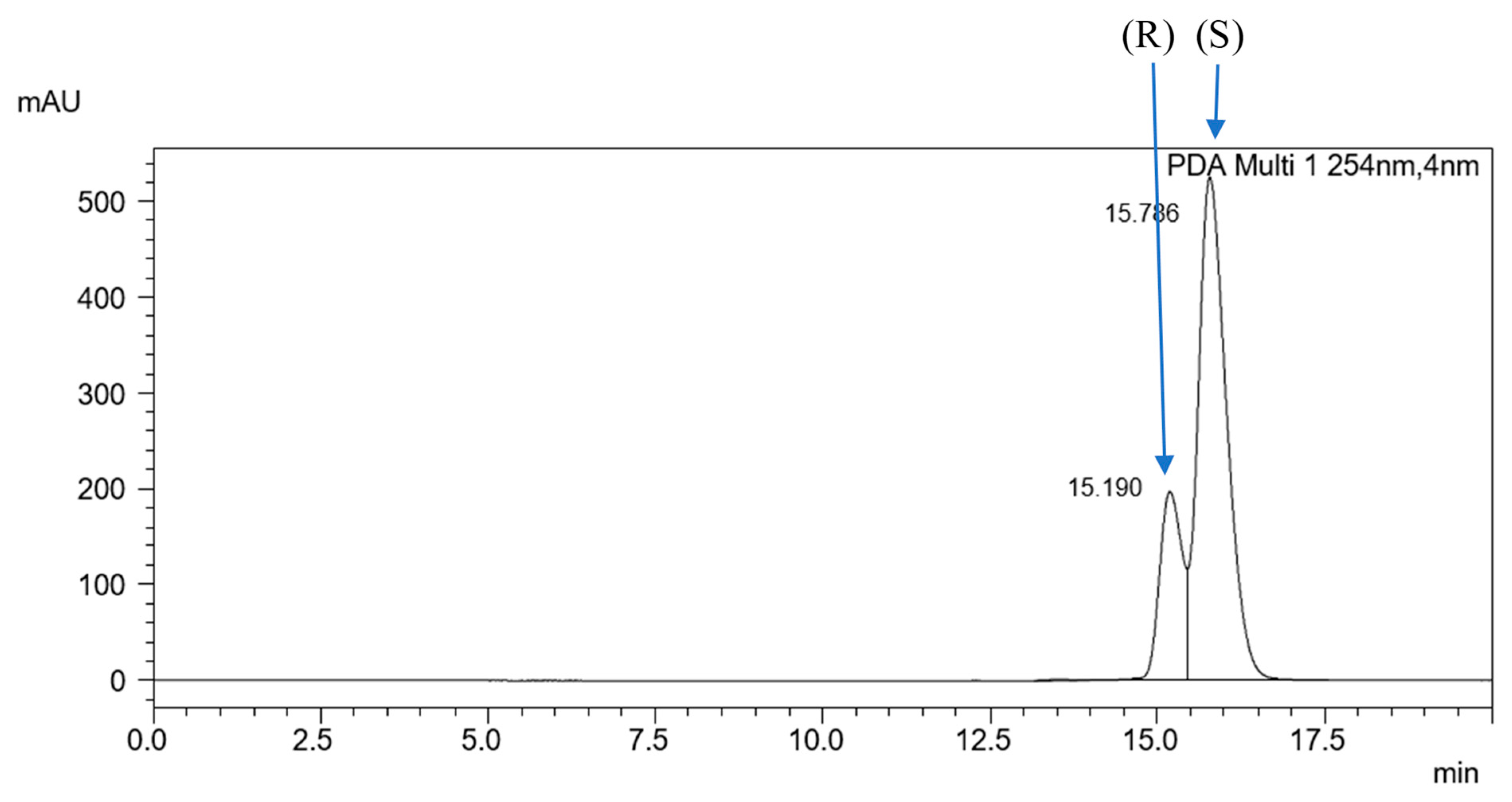
2.2. Optimization on the ReproSil Chiral-MIC Column
3. Materials and Methods
3.1. Racemic Mixtures
3.2. Equipment
3.3. Chromatographic Conditions and Calculations
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clayden, J.; Greeves, N.; Warren, S.G. Organic Chemistry; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Clark, A.; Kitson, R.R.A.; Mistry, N.; Taylor, P.; Taylor, M.; Lloyd, M.; Akamune, C. Introduction to Stereochemistry; Royal Society of Chemistry: Cambridge, UK, 2021. [Google Scholar]
- Mislow, K.; Siegel, J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 1984, 106, 3319–3328. [Google Scholar] [CrossRef]
- van Brakel, J. Substances: The Ontology of Chemistry. Phil. Chem. 2012, 6, 191–229. [Google Scholar]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing…Only Different. Toxic. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Knoche, B.; Blaschke, G. Investigations on the in vitro racemization of thalidomide by high-performance liquid chromatography. J. Chrom. A 1994, 666, 235–240. [Google Scholar] [CrossRef]
- Drayer, D.E. Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview. Clin. Pharm. Ther. 1986, 40, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Fabro, S.; Smith, R.L.; Williams, R.T. Toxicity and Teratogenicity of Optical Isomers of Thalidomide. Nature 1967, 215, 296. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A. An Extraordinary State of Matter. Liquid Crystals; Polish Publishing House: Warsaw, Poland, 1979. [Google Scholar]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics; Taylor & Francis: London, UK, 1997. [Google Scholar]
- Żmija, J.; Zieliński, J.; Parka, J.; Nowinowski-Kruszelnicki, E. Liquid crystal displays. Physics, Technology and Applications; Polish Scientific Publishing House: Warsaw, Poland, 1993. [Google Scholar]
- Vojtylová, T.; Niezgoda, I.; Galewski, Z.; Hamplová, V.; Sykora, D. A new approach to the chiral separation of novel diazenes. J. Sep. Sci. 2015, 38, 4211–4215. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.A.; Mangelings, D.; Heyden, Y.V. Chiral separations in reversed-phase liquid chromatography: Evaluation of several polysaccharide-based chiral stationary phases for a separation strategy update. J. Chrom. A 2012, 1269, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Vojtylová, T.; Kašpar, M.; Hamplová, V.; Novotná, V.; Sýkora, D. Chiral HPLC for a study of the optical purity of new liquid crystalline materials derived from lactic acid. Phase Trans. 2014, 87, 758–769. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, G.; Han, Q.; Hu, X.; Zhang, Q.; He, Z. Methylamidation and normal phase chromatography with silica-hydride-based stationary phase for N-glycosylation characterization of monoclonal antibody drugs. Chin. J. Chrom. 2018, 36, 615–620. [Google Scholar] [CrossRef]
- Vojtylová-Jurkovičová, T.; Vaňkátová, P.; Urbańska, M.; Hamplová, V.; Sýkora, D.; Bubnov, A. Effective control of optical purity by chiral HPLC separation for ester-based liquid crystalline materials forming anticlinic smectic phases. Liq. Cryst. 2021, 48, 43–53. [Google Scholar] [CrossRef]
- Vojtylová, T.; Hamplová, V.; Galewski, Z.; Korbecka, I.; Sýkora, D. Chiral separation of novel diazenes on a polysaccharide-based stationary phase in the reversed-phase mode. J. Sep. Sci. 2017, 40, 1465–1469. [Google Scholar] [CrossRef]
- Poryvai, A.; Vojtylová-Jurkovičová, T.; Šmahel, M.; Kolderová, N.; Tomášková, P.; Sýkora, D.; Kohout, M. Determination of optical purity of lactic acid-based chiral liquid crystals and corresponding building blocks by chiral high-performance liquid chromatography and supercritical fluid chromatography. Molecules 2019, 24, 1099. [Google Scholar] [CrossRef] [PubMed]
- Vojtylová, T.; Żurowska, M.; Milewska, K.; Hamplová, V.; Sýkora, D. Chiral HPLC and physical characterisation of orthoconic antiferroelectric liquid crystals. Liq. Cryst. 2016, 43, 1244–1250. [Google Scholar] [CrossRef]
- Brown, C.L.; Dornan, L.M.; Muldoon, M.J.; Hembre, R.T.; Stevenson, P.J.; Manesiotis, P. Comparison of three stationary phases in the separation of polyphenyls by liquid chromatography. J. Chrom. A 2022, 1671, 462992. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M. Separation of liquid crystalline racemic mixtures obtained on the basis of (R,S)-2-hexanol on amylose tris(3-chloro-5-methylphenylcarbamate) covalently immobilised on silica in high-performance liquid chromatography. Liq. Cryst. 2023, 50, 1893–1901. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Momotko, M.; Szarmańska, J.; Boczkaj, G.; Kamiński, M. Types of chiral stationary phases and its applications to liquid chromatography (LC)—A mini review. Cam. Sep. 2015, 7, 1–30. [Google Scholar]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Pol. Env. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chrom. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Xiaoming, C.; Yamamoto, C.; Okamoto, Y. Polysaccharide derivatives as useful chiral stationary phases in high-performance liquid chromatography. Pure Appl. Chem. 2007, 79, 1561–1573. [Google Scholar] [CrossRef]
- Ikai, T.; Yamamoto, C.; Kamigaito, M.; Okamoto, Y. Immobilized Polysaccharide-Based Chiral Stationary Phases for HPLC. Polym. J. 2006, 38, 91–108. [Google Scholar] [CrossRef]
- Yashima, E. Polysaccharide-based chiral stationary phases for high-performance liquid chromatographic enantioseparation. J. Chrom. A 2001, 906, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Yashima, E. Polysaccharide Derivatives for Chromatographic Separation of Enantiomers. Angew. Chem. Int. Ed. 1998, 37, 1020–1043. [Google Scholar] [CrossRef]
- Tachibana, K.; Ohnishi, A. Reversed-phase liquid chromatographic separation of enantiomers on polysaccharide type chiral stationary phases. J. Chrom. A 2001, 906, 127–154. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y. Chiral Polymers for Resolution of Enantiomers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1731–1970. [Google Scholar] [CrossRef]
- Kazusaki, M.; Kawabata, H.; Matsukura, H. Comparative study of amylose and cellulose derivatized chiral stationary phases in the reversed-phase mode. J. Liq. Chrom. Rel. Technol. 2000, 23, 2819–2828. [Google Scholar] [CrossRef]
- Fan, X.; Cao, L.; Geng, L.; Ma, L.; Wei, Y.; Wang, Y. Polysaccharides as separation media for the separation of proteins, peptides and stereoisomers of amino acids. Inter. J. Biol. Macromol. 2021, 186, 616–638. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Ali, I. Optimization strategies for HPLC enantioseparation of racemic drugs using polysaccharides and macrocyclic glycopeptide antibiotic chiral stationary phases. Il Farm. 2002, 57, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Ellington, J.J.; Evans, J.J.; Prickett, K.B.; Champion, W.L., Jr. High-performance liquid chromatographic separation of the enantiomers of organophosphorus pesticides on polysaccharide chiral stationary phases. J. Chrom. A 2001, 928, 145–154. [Google Scholar] [CrossRef]
- Kasat, R.B.; Wang, N.H.L.; Franses, E.I. Effects of backbone and side chain on the molecular environments of chiral cavities in polysaccharide-based biopolymers. Biomacromolecules 2007, 8, 1676–1685. [Google Scholar] [CrossRef]
- Chiyo, Y.; Yoshio, O. Optically active polymers for chiral separation. Bull. Chem. Soc. Jpn. 2004, 77, 227–257. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kaida, Y. Resolution by high-performance liquid chromatography using polysaccharide carbamates and benzoates as chiral stationary phases. J. Chrom. A 1994, 666, 403–419. [Google Scholar] [CrossRef]
- Dierking, I. Chiral Liquid Crystals: Structures, Phases, Effects. Symmetry 2014, 6, 444–472. [Google Scholar] [CrossRef]
- Ando, J.K.; Collings, P.J. A chiral–racemic lyotropic chromonic liquid crystal system. Soft Matt. 2021, 17, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://dr-maisch.com/dr-maisch-phases/reprosil-chiral (accessed on 1 March 2024).
- Vaňkátová, P.; Šrolerová, T.; Kubíčková, A.; Kalíková, K. Fast UHPLC enantioseparation of liquid crystalline materials with chiral center based on octanol in reversed-phase and polar organic mode. Mon. Chemie–Chem. Month. 2020, 151, 1235–1240. [Google Scholar] [CrossRef]
- Urbańska, M.; Dziaduszek, J.; Strzeżysz, O.; Szala, M. Synclinic and anticlinic properties of (R,S) 4′-(1-methylheptyloxycarbonyl)biphenyl-4-yl 4-[7-(2,2,3,3,4,4,4-heptafluorobutoxy)heptyl-1-oxy]benzoates. Phase Trans. 2019, 92, 657–666. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, J.; Rejmer, W.; Czupryński, K.; Raszewski, Z.; Piecek, W. Comparison of racemic and enantiomeric 4′-(1-Methylheptyloxycarbonyl)Biphenyl-4-yl 4-[3-(2,2,3,3,4,4,4-Heptafluorobutoxy)Prop-1-Oxy]benzoates. Mol. Cryst. Liq. Cryst. 2010, 525, 219–225. [Google Scholar] [CrossRef]
- Drzewiński, W.; Dąbrowski, R.; Czupryński, K. Orthoconic antiferroelectrics. Synthesis and mesomorphic properties of optically active (S)-(+)-4-(1-methylheptyloxycarbonyl)phenyl 4′-(fluoroalkanoyloxyalkoxy)biphenyl-4-carboxylates and 4′-(alkanoyloxyalkoxy)biphenyl-4-carboxylates. Pol. J. Chem. 2002, 76, 273–284. [Google Scholar] [CrossRef]
- Dallocchio, R.; Dessì, A.; Sechi, B.; Chankvetadze, B.; Cossu, S.; Mamane, V.; Aubert, E.; Rozzo, C.; Palmieri, G.; Spissu, Y.; et al. Exploring interaction modes between polysaccharide-based selectors and biologically active 4,4′-bipyridines by experimental and computational analysis. J. Chrom. Open 2022, 2, 100030. [Google Scholar] [CrossRef]
- Ravisankar, P.; Anusha, S.; Supriya, K.; Kumar, U.A. Fundamental Chromatographic Parameters. Inter. J. Pharm. Sci. Rev. Res. 2019, 55, 46–50. [Google Scholar]
- Tsui, H.-W.; Kuo, C.-H.; Huang, Y.-C. Elucidation of retention behaviors in reversed-phase liquid chromatography as a function of mobile phase composition. J. Chrom. A 2019, 1595, 127–135. [Google Scholar] [CrossRef] [PubMed]

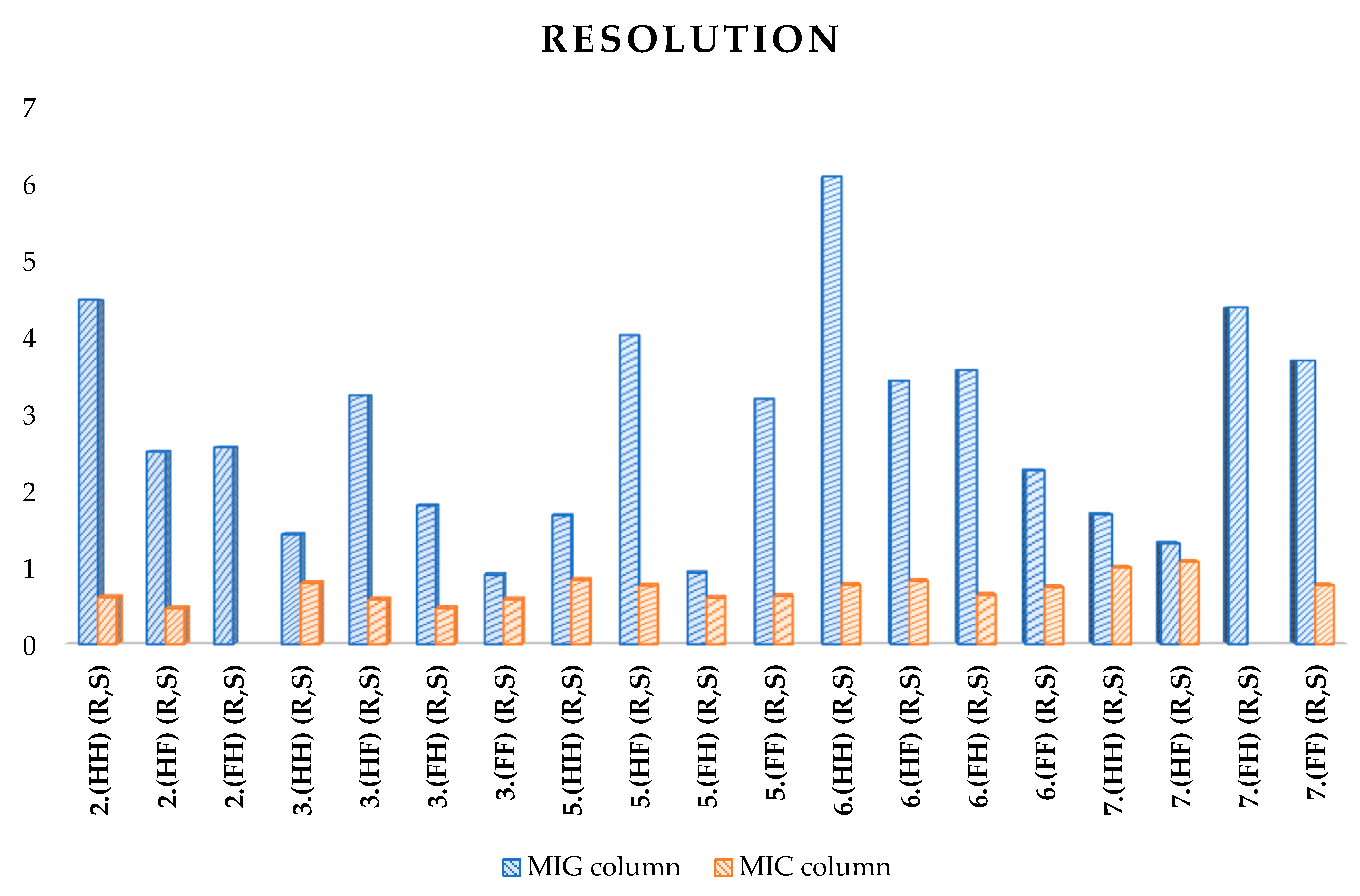


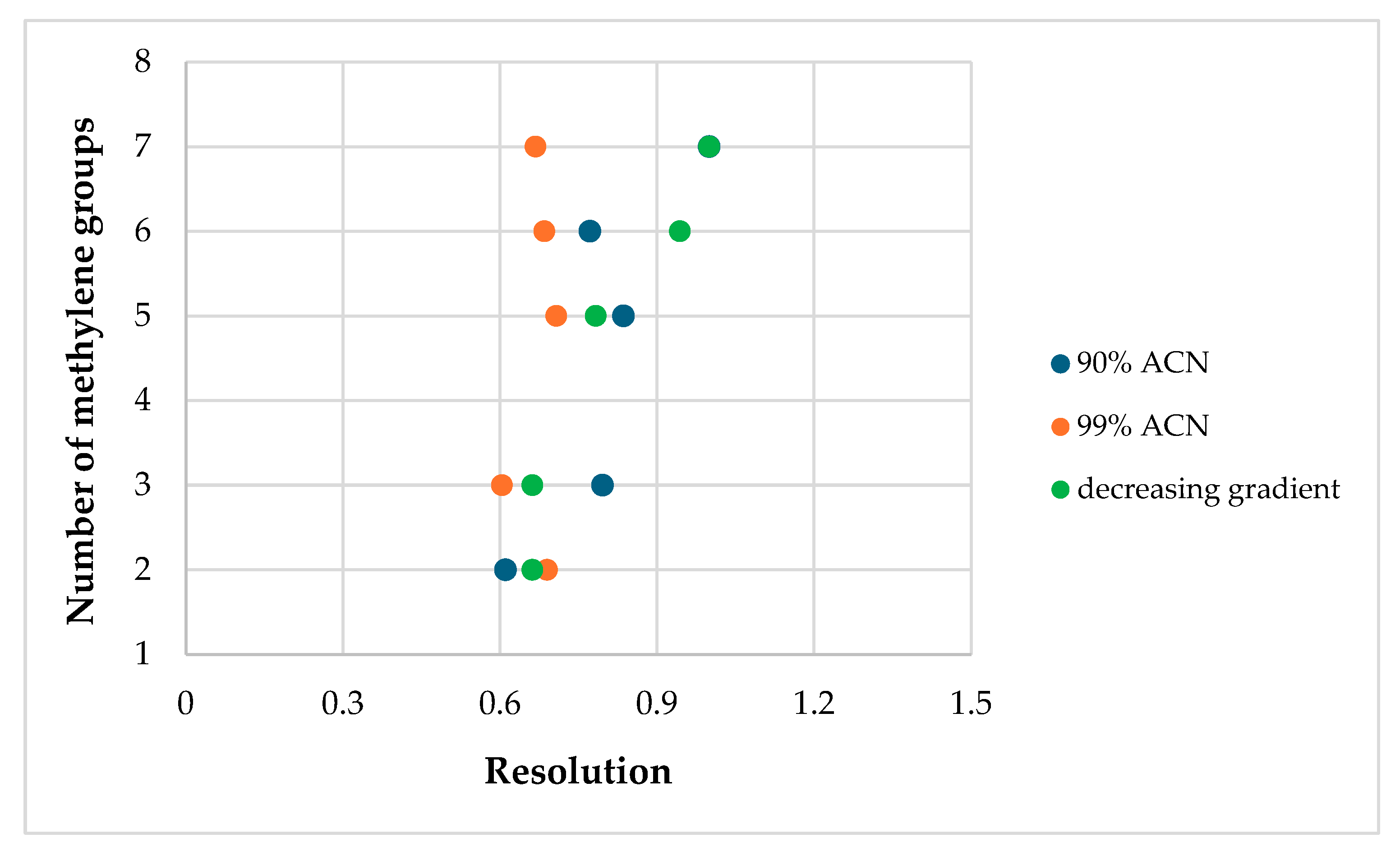
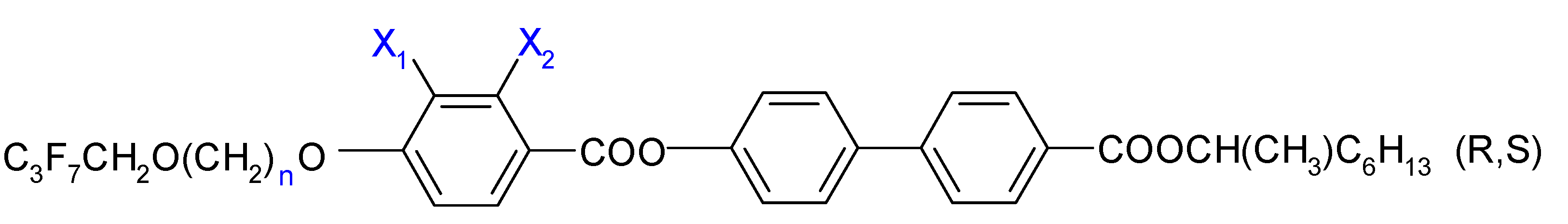
| Resolution | |||||
|---|---|---|---|---|---|
| Acronym of the Racemic Mixtures | ACN/H2O 99/1 (ν/ν) | ACN/H2O 95/5 (ν/ν) | ACN/H2O 90/10 (ν/ν) | Decreasing Gradient | Increasing–Decreasing Gradient |
| 2.(HH) (R,S) | 1.301 | 2.773 | 4.482 | 1.131 | 2.905 |
| 2.(HF) (R,S) | - | 1.301 | 2.503 | - | 1.809 |
| 2.(FH) (R,S) | 0.696 | 1.471 | 2.561 | 0.933 | 1.794 |
| 3.(HH) (R,S) | 1.262 | 2.632 | 1.428 | 1.149 | 3.021 |
| 3.(HF) (R,S) | 0.767 | 1.653 | 3.237 | 0.822 | 2.330 |
| 3.(FH) (R,S) | 0.509 | 1.080 | 1.802 | 0.554 | 1.526 |
| 3.(FF) (R,S) | - | - | 0.900 | - | 0.630 |
| 5.(HH) (R,S) | 2.498 | 4.847 | 1.675 | 2.120 | 4.391 |
| 5.(HF) (R,S) | 1.878 | 3.757 | 4.021 | 1.590 | 4.744 |
| 5.(FH) (R,S) | 1.056 | 1.672 | 0.928 | 0.880 | 3.098 |
| 5.(FF) (R,S) | 0.736 | 1.632 | 3.188 | 0.606 | 2.665 |
| 6.(HH) (R,S) | 1.942 | 4.133 | 6.083 | 2.123 | 3.795 |
| 6.(HF) (R,S) | 1.773 | 3.116 | 3.425 | 1.574 | 4.989 |
| 6.(FH) (R,S) | 1.008 | 1.637 | 3.565 | 0.953 | 2.983 |
| 6.(FF) (R,S) | 0.440 | 1.027 | 2.260 | 0.368 | 1.845 |
| 7.(HH) (R,S) | 2.845 | 5.454 | 1.689 | 2.135 | 1.536 |
| 7.(HF) (R,S) | 2.650 | 5.196 | 1.310 | 1.627 | 0.969 |
| 7.(FH) (R,S) | 1.436 | 2.353 | 4.382 | 1.348 | 3.195 |
| 7.(FF) (R,S) | 0.921 | 2.116 | 3.690 | 0.910 | 2.879 |
| Resolution, MIG Column | ||
|---|---|---|
| Acronym of the Racemic Mixtures | ACN/H2O 90/10 (ν/ν), 1 mL·min−1 | ACN/H2O 90/10 (ν/ν), 0.3 mL·min−1 |
| 3.(FF) (R,S) based on (R,S)-2-octanol | 0.900 | - |
| 3.(FF) (R,S) based on (R,S)-2-hexanol | - | 0.890 |
| 5.(HH) (R,S) based on (R,S)-2-octanol | 1.675 | - |
| 5.(HH) (R,S) based on (R,S)-2-hexanol | - | 2.254 |
| 7.(HH) (R,S) based on (R,S)-2-octanol | 1.689 | - |
| 7.(HH) (R,S) based on (R,S)-2-hexanol | - | 2.640 |
| Acronym of the Racemic Mixtures | tR [min] | α | NS | |
|---|---|---|---|---|
| Enantiomer 1 | Enantiomer 2 | |||
| 2.(HH) (R,S) | 9.371 | 17.328 | 1.849 | 2200 |
| 2.(HF) (R,S) | 7.627 | 11.007 | 1.443 | 1900 |
| 2.(FH) (R,S) | 8.165 | 11.431 | 1.400 | 1650 |
| 3.(HH) (R,S) | 6.180 | 7.037 | 1.138 | - |
| 3.(HF) (R,S) | 7.932 | 11.665 | 1.469 | 2050 |
| 3.(FH) (R,S) | 8.112 | 9.824 | 1.211 | 2150 |
| 3.(FF) (R,S) | 7.204 | 7.699 | 1.068 | - |
| 5.(HH) (R,S) | 14.933 | 17.278 | 1.157 | 5600 |
| 5.(HF) (R,S) | 7.000 | 11.223 | 1.603 | 3100 |
| 5.(FH) (R,S) | 5.918 | 6.754 | 1.141 | - |
| 5.(FF) (R,S) | 8.548 | 13.331 | 1.559 | 1850 |
| 6.(HH) (R,S) | 15.499 | 41.355 | 2.668 | 950 |
| 6.(HF) (R,S) | 6.159 | 10.098 | 1.639 | 950 |
| 6.(FH) (R,S) | 12.306 | 18.903 | 1.536 | 1700 |
| 6.(FF) (R,S) | 9.583 | 13.539 | 1.412 | 1500 |
| 7.(HH) (R,S) | 7.543 | 10.288 | 1.369 | 600 |
| 7.(HF) (R,S) | 5.970 | 7.280 | 1.219 | - |
| 7.(FH) (R,S) | 14.211 | 27.357 | 1.925 | 800 |
| 7.(FF) (R,S) | 11.297 | 20.524 | 1.816 | 2050 |
| Resolution | |||||
|---|---|---|---|---|---|
| Acronym of the Racemic Mixtures | ACN/H2O 99/1 (ν/ν) | ACN/H2O 95/5 (ν/ν) | ACN/H2O 90/10 (ν/ν) | Decreasing Gradient | Increasing–Decreasing Gradient |
| 2.(HH) (R,S) | 0.690 | - | 0.611 | 0.662 | 0.612 |
| 2.(HF) (R,S) | 0.471 | - | 0.466 | 0.434 | - |
| 2.(FH) (R,S) | - | - | - | - | - |
| 3.(HH) (R,S) | 0.604 | 0.702 | 0.796 | 0.662 | 0.502 |
| 3.(HF) (R,S) | 0.577 | - | 0.582 | - | - |
| 3.(FH) (R,S) | - | - | 0.469 | 0.425 | - |
| 3.(FF) (R,S) | - | - | 0.583 | - | - |
| 5.(HH) (R,S) | 0.708 | 0.965 | 0.836 | 0.783 | 0.736 |
| 5.(HF) (R,S) | 0.602 | 0.674 | 0.761 | 0.517 | - |
| 5.(FH) (R,S) | 0.465 | - | 0.604 | - | - |
| 5.(FF) (R,S) | 0.390 | - | 0.628 | - | - |
| 6.(HH) (R,S) | 0.685 | 1.112 | 0.772 | 0.944 | 0.597 |
| 6.(HF) (R,S) | 0.635 | - | 0.824 | 0.513 | 0.474 |
| 6.(FH) (R,S) | 0.628 | - | 0.640 | 0.454 | - |
| 6.(FF) (R,S) | 0.531 | 0.500 | 0.743 | 0.592 | 0.510 |
| 7.(HH) (R,S) | 0.668 | 0.661 | 1.000 | 1.000 | 0.549 |
| 7.(HF) (R,S) | 0.725 | 0.588 | 1.073 | 0.734 | 0.740 |
| 7.(FH) (R,S) | 0.595 | 0.433 | - | 0.672 | 0.487 |
| 7.(FF) (R,S) | 0.482 | 0.852 | 0.768 | 0.696 | - |
| 2.(HH) (R,S) | 3.(HH) (R,S) | 5.(HH) (R,S) | 6.(HH) (R,S) | 7.(HH) (R,S) |
| 2.(HF) (R,S) | 3.(HF) (R,S) | 5.(HF) (R,S) | 6.(HF) (R,S) | 7.(HF) (R,S) |
| 2.(FH) (R,S) | 3.(FH) (R,S) | 5.(FH) (R,S) | 6.(FH) (R,S) | 7.(FH) (R,S) |
| - | 3.(FF) (R,S) | 5.(FF) (R,S) | 6.(FF) (R,S) | 7.(FF) (R,S) |
| No. | v/v | Flow Rate [mL·min−1] | Injection Volume [µL] | |
|---|---|---|---|---|
| ACN | H2O | |||
| 1. | 99 | 1 | 1 | 15 |
| 2. | 95 | 5 | 1 | 15 |
| 3. | 90 | 10 | 1 | 15 |
| Decreasing Gradient | Increasing–Decreasing Gradient | ||||
|---|---|---|---|---|---|
| Time [min] | ACN/H2O (v/v) | Injection Volume [µL] | Time [min] | ACN/H2O (v/v) | Injection Volume [µL] |
| 0.01 | 99/1 | 10 | 0.01 | 92/8 | 10 |
| 5 | 96/4 | 3 | 94/6 | ||
| 10 | 93/7 | 6 | 96/4 | ||
| 15 | 90/10 | 9 | 98/2 | ||
| 20 | STOP | 12 | 95/5 | ||
| - | - | 15 | 92/8 | ||
| - | - | 20 | STOP | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbańska, M. Optimization of Liquid Crystalline Mixtures Enantioseparation on Polysaccharide-Based Chiral Stationary Phases by Reversed-Phase Chiral Liquid Chromatography. Int. J. Mol. Sci. 2024, 25, 6477. https://doi.org/10.3390/ijms25126477
Urbańska M. Optimization of Liquid Crystalline Mixtures Enantioseparation on Polysaccharide-Based Chiral Stationary Phases by Reversed-Phase Chiral Liquid Chromatography. International Journal of Molecular Sciences. 2024; 25(12):6477. https://doi.org/10.3390/ijms25126477
Chicago/Turabian StyleUrbańska, Magdalena. 2024. "Optimization of Liquid Crystalline Mixtures Enantioseparation on Polysaccharide-Based Chiral Stationary Phases by Reversed-Phase Chiral Liquid Chromatography" International Journal of Molecular Sciences 25, no. 12: 6477. https://doi.org/10.3390/ijms25126477
APA StyleUrbańska, M. (2024). Optimization of Liquid Crystalline Mixtures Enantioseparation on Polysaccharide-Based Chiral Stationary Phases by Reversed-Phase Chiral Liquid Chromatography. International Journal of Molecular Sciences, 25(12), 6477. https://doi.org/10.3390/ijms25126477







