From Inflammation to Oncogenesis: Tracing Serum DCLK1 and miRNA Signatures in Chronic Liver Diseases
Abstract
1. Introduction
2. Results
2.1. DCLK1 Levels Are Elevated in the Blood Serum of Patients with Liver Disease
2.2. Serum from Patients with Fibrosis, Cirrhosis, or HCC Display Unique miRNAs
2.3. Differential Expression of miRNAs in Liver Disease Progression
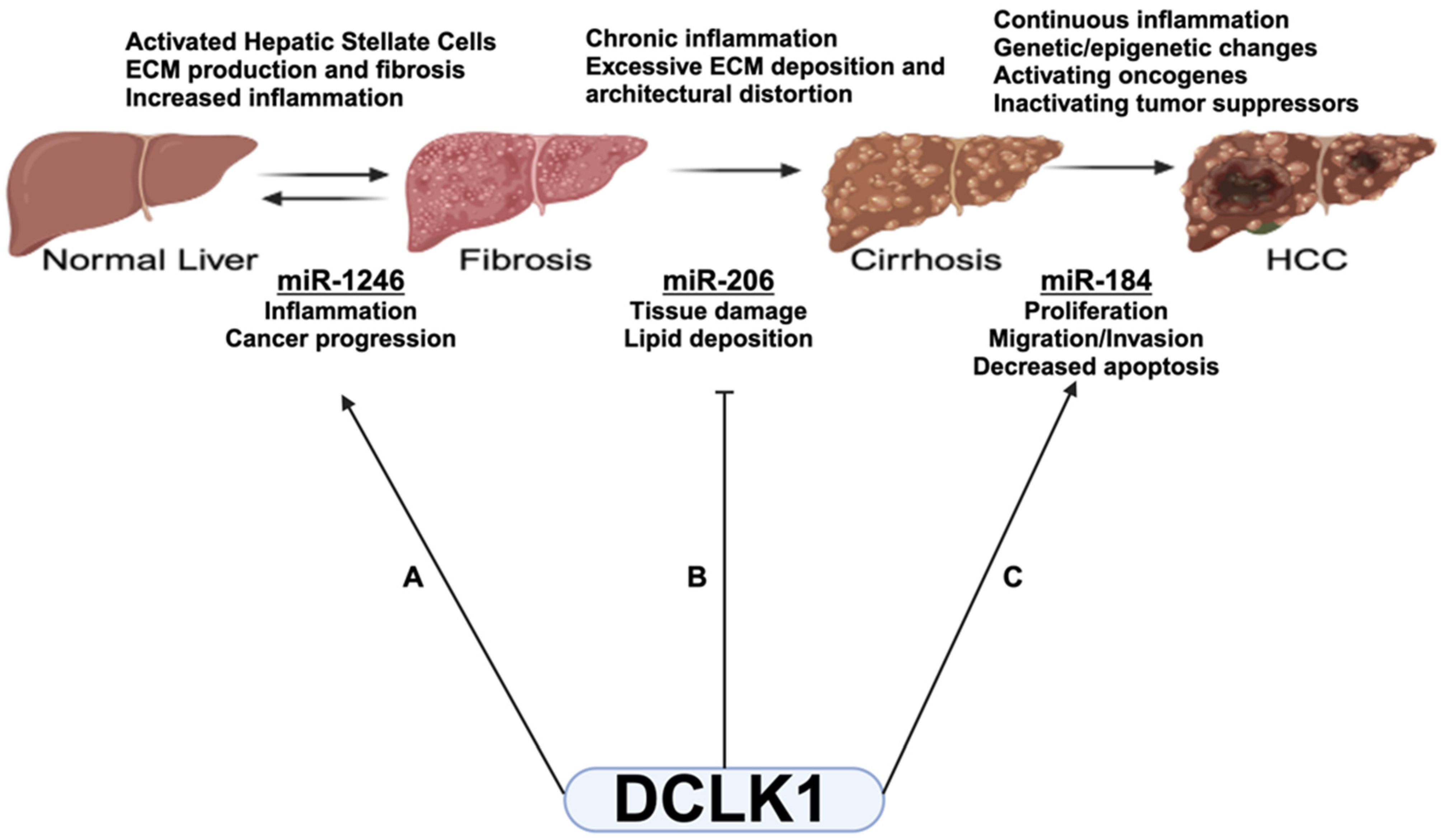
2.4. Differentially Expressed (DE) miRNAs Show DCLK1-Specific Differences in Liver Disease
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Collection and Separation
4.3. Enzyme-Linked Immunosorbent Assays (ELISA)
4.4. RNA Isolation and miRNA seqRNA
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| AFP | Alpha-fetoprotein |
| DCLK1 | Doublecortin-like kinase 1 |
| DE | Differentially expressed |
| EMT | Epithelial to mesenchymal transition |
| FDR | False Discovery Rate |
| GLM | Generalized Linear Model |
| HCC | Hepatocellular carcinoma |
| miRNA | microRNA |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| TGF | Transforming growth factor |
References
- Tacke, F. Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo. Fibrogenesis Tissue Repair 2012, 5, S27. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273.e1261. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.R.; Karalis, J.D.; Chansard, M.; Augustine, M.M.; Mortensen, E.; Wang, S.C.; Porembka, M.R.; Zeh, H.J., 3rd; Yopp, A.C.; Polanco, P.M. Variation of Hepatocellular Carcinoma Treatment Patterns and Survival Across Geographic Regions in a Veteran Population. Ann. Surg. Oncol. 2022, 29, 8413–8420. [Google Scholar] [CrossRef]
- Hanif, H.; Ali, M.J.; Susheela, A.T.; Khan, I.W.; Luna-Cuadros, M.A.; Khan, M.M.; Lau, D.T. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J. Gastroenterol. 2022, 28, 216–229. [Google Scholar] [CrossRef]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Force, M.; Park, G.; Chalikonda, D.; Roth, C.; Cohen, M.; Halegoua-DeMarzio, D.; Hann, H.W. Alpha-Fetoprotein (AFP) and AFP-L3 Is Most Useful in Detection of Recurrence of Hepatocellular Carcinoma in Patients after Tumor Ablation and with Low AFP Level. Viruses 2022, 14, 775. [Google Scholar] [CrossRef]
- Desquilles, L.; Cano, L.; Ghukasyan, G.; Mouchet, N.; Landreau, C.; Corlu, A.; Clément, B.; Turlin, B.; Désert, R.; Musso, O. Well-differentiated liver cancers reveal the potential link between ACE2 dysfunction and metabolic breakdown. Sci. Rep. 2022, 12, 1859. [Google Scholar] [CrossRef]
- Feng, H.; Wei, X.; Pang, L.; Wu, Y.; Hu, B.; Ruan, Y.; Liu, Z.; Liu, J.; Wang, T. Prognostic and Immunological Value of Angiotensin-Converting Enzyme 2 in Pan-Cancer. Front. Mol. Biosci. 2020, 7, 189. [Google Scholar] [CrossRef]
- Starzl, T.E.; Fung, J.J. Themes of liver transplantation. Hepatology 2010, 51, 1869–1884. [Google Scholar] [CrossRef]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The Role of miRNAs in the Pathophysiology of Liver Diseases and Toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Wei, R.; Ye, T.; Zhou, J.; Wen, M.; Men, R.; Aqeilan, R.I.; Peng, Y.; Yang, L. Twist1-induced miR-199a-3p promotes liver fibrosis by suppressing caveolin-2 and activating TGF-β pathway. Signal Transduct. Target. Ther. 2020, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Messner, C.J.; Schmidt, S.; Özkul, D.; Gaiser, C.; Terracciano, L.; Krähenbühl, S.; Suter-Dick, L. Identification of miR-199a-5p, miR-214-3p and miR-99b-5p as Fibrosis-Specific Extracellular Biomarkers and Promoters of HSC Activation. Int. J. Mol. Sci. 2021, 22, 9799. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Yang, Y.L.; Wang, F.S. The Role of miR-29a in the Regulation, Function, and Signaling of Liver Fibrosis. Int. J. Mol. Sci. 2018, 19, 1889. [Google Scholar] [CrossRef] [PubMed]
- Gumilas, N.S.A.; Widodo, I.; Ratnasari, N.; Heriyanto, D.S. Potential relative quantities of miR-122 and miR-150 to differentiate hepatocellular carcinoma from liver cirrhosis. Non-Coding RNA Res. 2022, 7, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kan Changez, M.I.; Mubeen, M.; Zehra, M.; Samnani, I.; Abdul Rasool, A.; Mohan, A.; Wara, U.U.; Tejwaney, U.; Kumar, V. Role of microRNA in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A comprehensive review. J. Int. Med. Res. 2023, 51, 03000605231197058. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.; Vengadassalapathy, S.; Venkadassalapathy, S.; Balachandran, V.; Umapathy, V.R.; Veeraraghavan, V.P.; Jayaraman, S.; Patil, S.; Iyaswamy, A.; Palaniyandi, K.; et al. Pleiotropic effects of DCLK1 in cancer and cancer stem cells. Front. Mol. Biosci. 2022, 9, 965730. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Mondalek, F.G.; Qu, D.; Ponnurangam, S.; Pantazis, P.; Anant, S.; Ramanujam, R.P.; Houchen, C.W. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J. Nanobiotechnol. 2011, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; May, R.; Lightfoot, S.A.; Hoskins, A.B.; Lerner, M.; Brackett, D.J.; Postier, R.G.; Ramanujam, R.; Mohammed, A.; Rao, C.V.; et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011, 71, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Chandrakesan, P.; Weygant, N.; May, R.; Qu, D.; Chinthalapally, H.R.; Sureban, S.M.; Ali, N.; Lightfoot, S.A.; Umar, S.; Houchen, C.W. DCLK1 facilitates intestinal tumor growth via enhancing pluripotency and epithelial mesenchymal transition. Oncotarget 2014, 5, 9269–9280. [Google Scholar] [CrossRef]
- Tsui, Y.M.; Chan, L.K.; Ng, I.O. Cancer stemness in hepatocellular carcinoma: Mechanisms and translational potential. Br. J. Cancer 2020, 122, 1428–1440. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Nguyen, C.B.; Chandrakesan, P.; Wolf, R.F.; Qu, D.; May, R.; Goretsky, T.; Fazili, J.; Barrett, T.A.; Li, M.; et al. Doublecortin-like kinase 1 promotes hepatocyte clonogenicity and oncogenic programming via non-canonical β-catenin-dependent mechanism. Sci. Rep. 2020, 10, 10578. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network (Lead Contact David A. Wheeler). Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e1323. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shen, S.; Han, X.; Li, W.; Luo, W.; Lin, L.; Xu, M.; Wang, Y.; Huang, W.; Wu, G.; et al. Macrophage DCLK1 promotes atherosclerosis via binding to IKKbeta and inducing inflammatory responses. EMBO Mol. Med. 2023, 15, e17198. [Google Scholar] [CrossRef] [PubMed]
- Undi, R.B.; Ahsan, N.; Larabee, J.L.; Darlene-Reuter, N.; Papin, J.; Dogra, S.; Hannafon, B.N.; Bronze, M.S.; Houchen, C.W.; Huycke, M.M.; et al. Blocking of doublecortin-like kinase 1-regulated SARS-CoV-2 replication cycle restores cell signaling network. J. Virol. 2023, 97, e0119423. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, R.; Xiao, Z.; Huang, X.; Yao, J.; Liu, J.; An, G.; Ge, Y. Targeting DCLK1 attenuates tumor stemness and evokes antitumor immunity in triple-negative breast cancer by inhibiting IL-6/STAT3 signaling. Breast Cancer Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Allam, H.; May, R.; Sureban, S.M.; Bronze, M.S.; Bader, T.; Umar, S.; Anant, S.; Houchen, C.W. Hepatitis C virus-induced cancer stem cell-like signatures in cell culture and murine tumor xenografts. J. Virol. 2011, 85, 12292–12303. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Chandrakesan, P.; Nguyen, C.B.; Husain, S.; Gillaspy, A.F.; Huycke, M.; Berry, W.L.; May, R.; Qu, D.; Weygant, N.; et al. Inflammatory and oncogenic roles of a tumor stem cell marker doublecortin-like kinase (DCLK1) in virus-induced chronic liver diseases. Oncotarget 2015, 6, 20327–20344. [Google Scholar] [CrossRef] [PubMed]
- Sureban, S.M.; Madhoun, M.F.; May, R.; Qu, D.; Ali, N.; Fazili, J.; Weygant, N.; Chandrakesan, P.; Ding, K.; Lightfoot, S.A.; et al. Plasma DCLK1 is a marker of hepatocellular carcinoma (HCC): Targeting DCLK1 prevents HCC tumor xenograft growth via a microRNA-dependent mechanism. Oncotarget 2015, 6, 37200–37215. [Google Scholar] [CrossRef]
- Karere, G.M.; Glenn, J.P.; Li, G.; Konar, A.; VandeBerg, J.L.; Cox, L.A. Potential miRNA biomarkers and therapeutic targets for early atherosclerotic lesions. Sci. Rep. 2023, 13, 3467. [Google Scholar] [CrossRef]
- Chandrakesan, P.; Panneerselvam, J.; Qu, D.; Weygant, N.; May, R.; Bronze, M.S.; Houchen, C.W. Regulatory Roles of Dclk1 in Epithelial Mesenchymal Transition and Cancer Stem Cells. J. Carcinog. Mutagen. 2016, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Modulation of TGF-beta signaling during progression of chronic liver diseases. FBL 2009, 14, 2923–2934. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Tarao, K.; Nozaki, A.; Ikeda, T.; Sato, A.; Komatsu, H.; Komatsu, T.; Taguri, M.; Tanaka, K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019, 8, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Kaiyuan, D.; Lijuan, H.; Xueyuan, S.; Yunhui, Z. The role and underlying mechanism of miR-1299 in cancer. Future Sci. OA 2021, 7, FSO693. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; He, S.; Cheng, Z.; Chen, X.; Ren, X.; Bai, Y. DNAJA1 Stabilizes EF1A1 to Promote Cell Proliferation and Metastasis of Liver Cancer Mediated by miR-205-5p. J. Oncol. 2022, 2022, 2292481. [Google Scholar] [CrossRef]
- Khalilian, S.; Hosseini Imani, S.Z.; Ghafouri-Fard, S. Emerging roles and mechanisms of miR-206 in human disorders: A comprehensive review. Cancer Cell Int. 2022, 22, 412. [Google Scholar] [CrossRef] [PubMed]
- Iacob, D.G.; Rosca, A.; Ruta, S.M. Circulating microRNAs as non-invasive biomarkers for hepatitis B virus liver fibrosis. World J. Gastroenterol. 2020, 26, 1113–1127. [Google Scholar] [CrossRef]
- Morishita, A.; Oura, K.; Tadokoro, T.; Fujita, K.; Tani, J.; Masaki, T. MicroRNAs in the Pathogenesis of Hepatocellular Carcinoma: A Review. Cancers 2021, 13, 514. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, G.; Rastogi, A.; Trehanpati, N.; Sen, B.; Khosla, R.; Sarin, S.K. From cirrhosis to hepatocellular carcinoma: New molecular insights on inflammation and cellular senescence. Liver Cancer 2013, 2, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, R.; Arena, U.; Tassi, R. Hepatocellular carcinoma: Where are we? World J. Exp. Med. 2016, 6, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Edoo, M.I.A.; Chutturghoon, V.K.; Wusu-Ansah, G.K.; Zhu, H.; Zhen, T.Y.; Xie, H.Y.; Zheng, S.S. Serum Biomarkers AFP, CEA and CA19-9 Combined Detection for Early Diagnosis of Hepatocellular Carcinoma. Iran. J. Public Health 2019, 48, 314–322. [Google Scholar] [PubMed]
- Qi, J.; Wang, J.; Katayama, H.; Sen, S.; Liu, S.M. Circulating microRNAs (cmiRNAs) as novel potential biomarkers for hepatocellular carcinoma. Neoplasma 2013, 60, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Sheth, K.A.; Adarsh, C.K. Elevated alpha fetoprotein, no hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2013, 3, 162–164. [Google Scholar] [CrossRef]
- Fares, S.; Wehrle, C.J.; Hong, H.; Sun, K.; Jiao, C.; Zhang, M.; Gross, A.; Allkushi, E.; Uysal, M.; Kamath, S.; et al. Emerging and Clinically Accepted Biomarkers for Hepatocellular Carcinoma. Cancers 2024, 16, 1453. [Google Scholar] [CrossRef]
- Qu, D.; Johnson, J.; Chandrakesan, P.; Weygant, N.; May, R.; Aiello, N.; Rhim, A.; Zhao, L.; Zheng, W.; Lightfoot, S.; et al. Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells. PLoS ONE 2015, 10, e0118933. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Weygant, N.; Yao, J.; Chandrakesan, P.; Berry, W.L.; May, R.; Pitts, K.; Husain, S.; Lightfoot, S.; Li, M.; et al. Overexpression of DCLK1-AL Increases Tumor Cell Invasion, Drug Resistance, and KRAS Activation and Can Be Targeted to Inhibit Tumorigenesis in Pancreatic Cancer. J. Oncol. 2019, 2019, 6402925. [Google Scholar] [CrossRef]
- Sureban, S.M.; Berahovich, R.; Zhou, H.; Xu, S.; Wu, L.; Ding, K.; May, R.; Qu, D.; Bannerman-Menson, E.; Golubovskaya, V.; et al. DCLK1 Monoclonal Antibody-Based CAR-T Cells as a Novel Treatment Strategy against Human Colorectal Cancers. Cancers 2019, 12, 54. [Google Scholar] [CrossRef]
- Weygant, N.; Qu, D.; May, R.; Tierney, R.M.; Berry, W.L.; Zhao, L.; Agarwal, S.; Chandrakesan, P.; Chinthalapally, H.R.; Murphy, N.T.; et al. DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget 2015, 6, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Whorton, J.; Sureban, S.M.; May, R.; Qu, D.; Lightfoot, S.A.; Madhoun, M.; Johnson, M.; Tierney, W.M.; Maple, J.T.; Vega, K.J.; et al. DCLK1 is detectable in plasma of patients with Barrett’s esophagus and esophageal adenocarcinoma. Dig. Dis. Sci. 2015, 60, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.Y.; Jeon, S.E.; Choi, J.H.; Lee, C.J.; Jang, T.Y.; Yun, H.J.; Lee, Y.; Kim, P.; Cho, S.H.; et al. DCLK1 promotes colorectal cancer stemness and aggressiveness via the XRCC5/COX2 axis. Theranostics 2022, 12, 5258–5271. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.H.; Likhter, M.; Jogunoori, W.; Belkin, M.; Ohshiro, K.; Mishra, L. TGF-β signaling in liver and gastrointestinal cancers. Cancer Lett. 2016, 379, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Damaskos, C.; Garmpis, N.; Dimitroulis, D.; Garmpi, A.; Psilopatis, I.; Sarantis, P.; Koustas, E.; Kanavidis, P.; Prevezanos, D.; Kouraklis, G.; et al. Targeted Therapies for Hepatocellular Carcinoma Treatment: A New Era Ahead—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14117. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.S.; Simões, E.S.A.C. Serum levels of angiotensin converting enzyme as a biomarker of liver fibrosis. World J. Gastroenterol. 2017, 23, 8439–8442. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Benz, F.; Luedde, T.; Roderburg, C. The role of miRNAs in the regulation of inflammatory processes during hepatofibrogenesis. Hepatobiliary Surg. Nutr. 2015, 4, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Coenen-Stass, A.M.; Betts, C.A.; Wood, M.J. Detection and quantification of extracellular microRNAs in murine biofluids. Biol. Proced. Online 2014, 16, 5. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Benz, F.; Roderburg, C.; Vargas Cardenas, D.; Vucur, M.; Gautheron, J.; Koch, A.; Zimmermann, H.; Janssen, J.; Nieuwenhuijsen, L.; Luedde, M.; et al. U6 is unsuitable for normalization of serum miRNA levels in patients with sepsis or liver fibrosis. Exp. Mol. Med. 2013, 45, e42. [Google Scholar] [CrossRef]
- Szabo, G.; Bala, S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Mollnow, T.; Bongaerts, B.; Elfimova, N.; Vargas Cardenas, D.; Berger, K.; Zimmermann, H.; Koch, A.; Vucur, M.; Luedde, M.; et al. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS ONE 2012, 7, e32999. [Google Scholar] [CrossRef] [PubMed]
- Anadol, E.; Schierwagen, R.; Elfimova, N.; Tack, K.; Schwarze-Zander, C.; Eischeid, H.; Noetel, A.; Boesecke, C.; Jansen, C.; Dold, L.; et al. Circulating microRNAs as a marker for liver injury in human immunodeficiency virus patients. Hepatology 2015, 61, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Benz, F.; Vargas Cardenas, D.; Koch, A.; Janssen, J.; Vucur, M.; Gautheron, J.; Schneider, A.T.; Koppe, C.; Kreggenwinkel, K.; et al. Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver Int. Off. J. Int. Assoc. Study Liver 2014, 35, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Guo, C.J.; Xu, Q.Y.; Wang, J.Z.; Li, H.; Fang, C.H. miR-16 integrates signal pathways in myofibroblasts: Determinant of cell fate necessary for fibrosis resolution. Cell Death Dis. 2020, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Ding, F.; Huang, C.Y.; Xiao, H.; Fei, F.Y.; Li, J. Role of miR-16-5p in the proliferation and metastasis of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xiao, C.; Lu, H.; Yu, H.; Hong, H.; Guo, C.; Wu, Z. Effect of miR-12136 on Drug Sensitivity of Drug-Resistant Cell Line Michigan Cancer Foundation-7/Doxorubicin by Regulating ATP Binding Cassette Subfamily B Member 1. J. Biomater. Tissue Eng. 2020, 10, 1431–1435. [Google Scholar]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Taheri, M.; Samadian, M. A Review on the Role of miR-1246 in the Pathoetiology of Different Cancers. Front. Mol. Biosci. 2022, 8, 771835. [Google Scholar] [CrossRef]
- Bott, A.; Erdem, N.; Lerrer, S.; Hotz-Wagenblatt, A.; Breunig, C.; Abnaof, K.; Wörner, A.; Wilhelm, H.; Münstermann, E.; Ben-Baruch, A.; et al. miRNA-1246 induces pro-inflammatory responses in mesenchymal stem/stromal cells by regulating PKA and PP2A. Oncotarget 2017, 8, 43897–43914. [Google Scholar] [CrossRef]
- Xu, Y.F.; Hannafon, B.N.; Ding, W.Q. microRNA regulation of human pancreatic cancer stem cells. Stem Cell Investig. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, H. Icariin alleviates high-fat diet-induced nonalcoholic fatty liver disease via up-regulating miR-206 to mediate NF-kappaB and MAPK pathways. J. Biochem. Mol. Toxicol. 2024, 38, e23566. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, H.Z.; Li, X.; Wu, Z.; Han, Y.; Li, Y.; Chen, G.; Xie, X.; Huang, Y.; Du, Z.; et al. MicroRNA-184 inhibits cell proliferation and invasion, and specifically targets TNFAIP2 in Glioma. J. Exp. Clin. Cancer Res. 2015, 34, 27. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Z.; Deng, X.; Lu, Y.; Huang, X.; Lin, J.; Lan, X.; Su, Q.; Wang, C. Circular RNA CircITCH (has-circ-0001141) suppresses hepatocellular carcinoma (HCC) progression by sponging miR-184. Cell Cycle 2022, 21, 1557–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Deng, Y.; Liang, J.; Hu, Z.; Li, X.; Liu, H.; Wang, G.; Fu, B.; Zhang, T.; Zhang, Q.; et al. Circular RNA circ-102,166 acts as a sponge of miR-182 and miR-184 to suppress hepatocellular carcinoma proliferation and invasion. Cell. Oncol. 2021, 44, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Gao, K.; Li, L.; Huang, Z.; Lin, L. miR-184 functions as an oncogenic regulator in hepatocellular carcinoma (HCC). Biomed. Pharmacother. 2014, 68, 143–148. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.F.; Carithers, R.L., Jr. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005, 41, 1407–1432. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
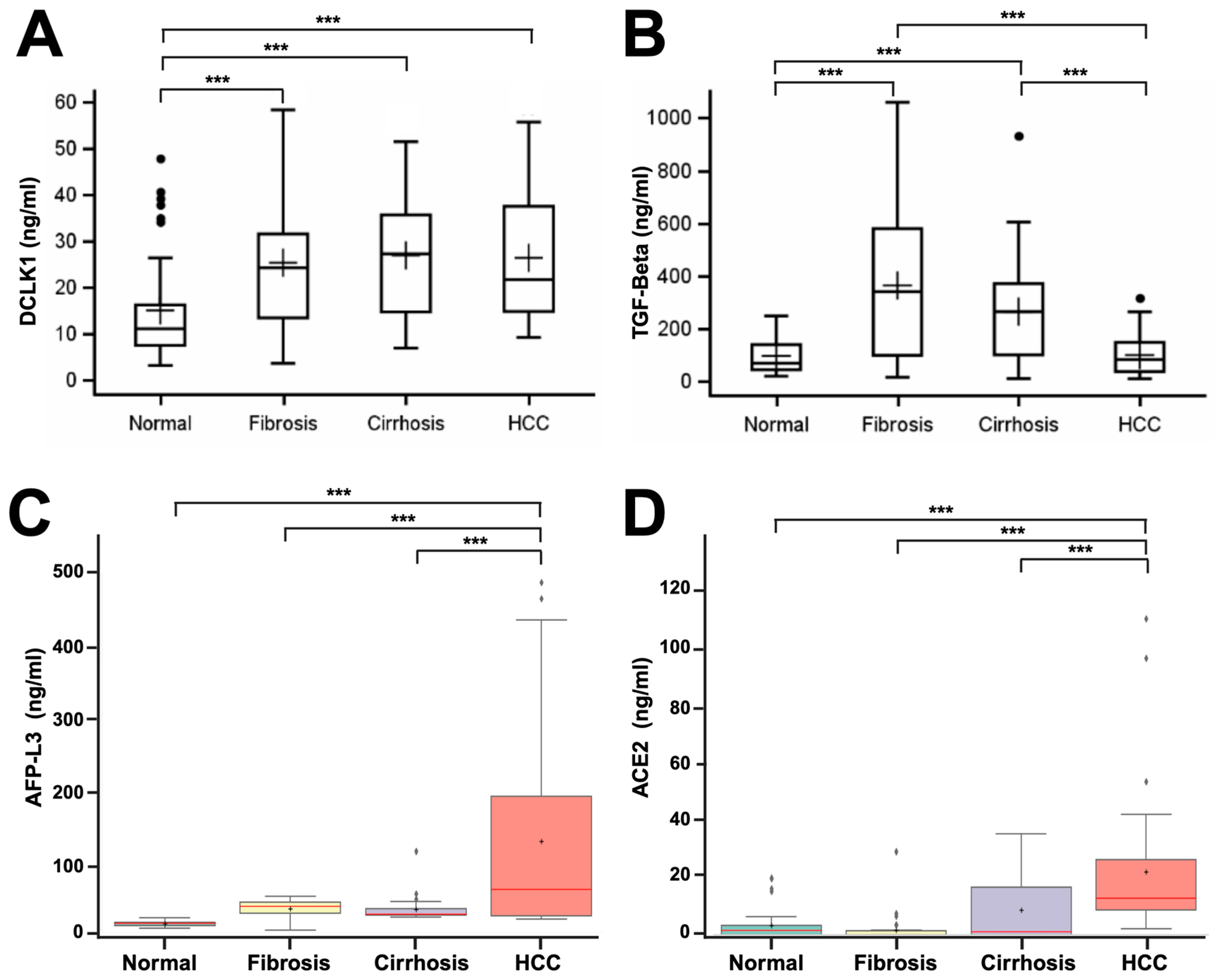
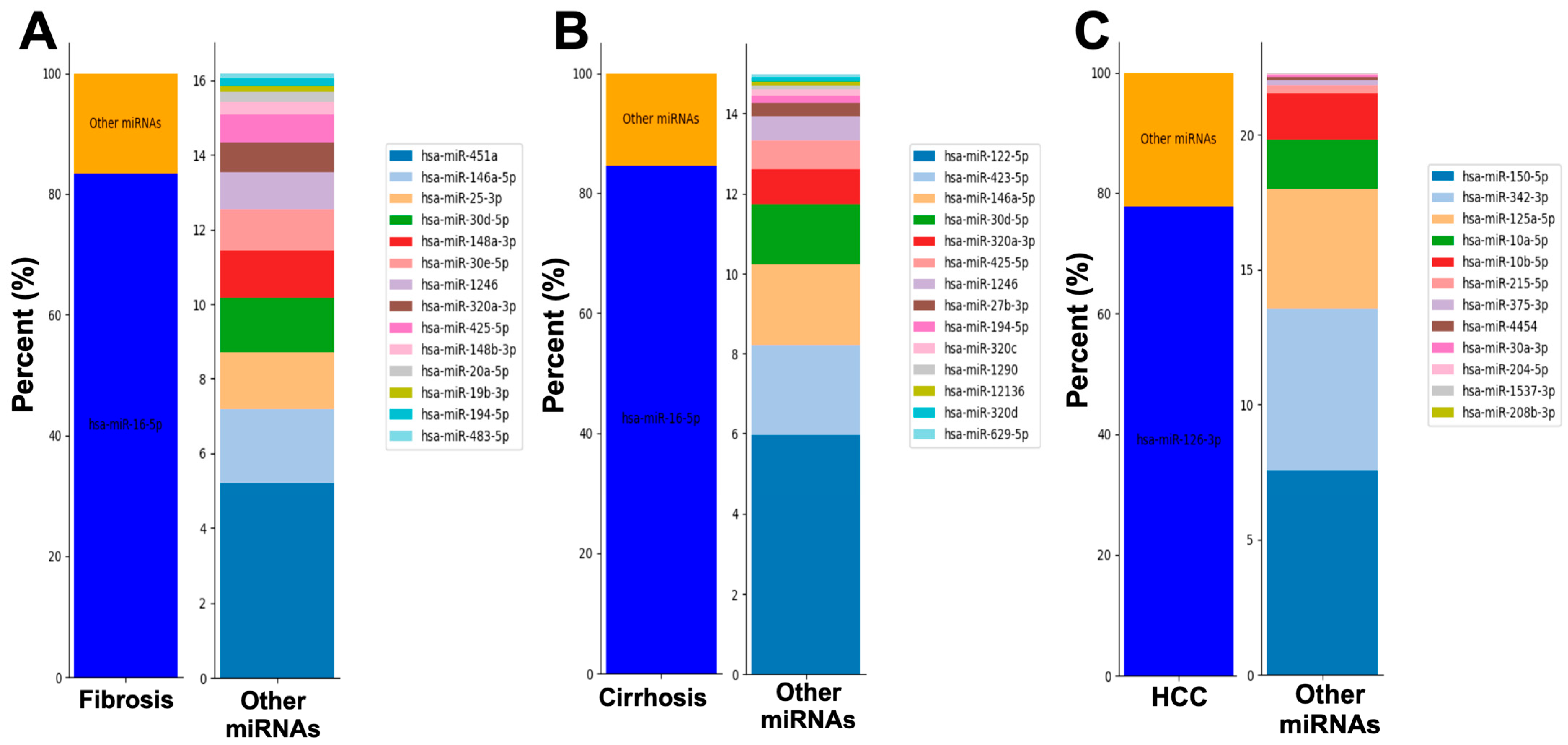
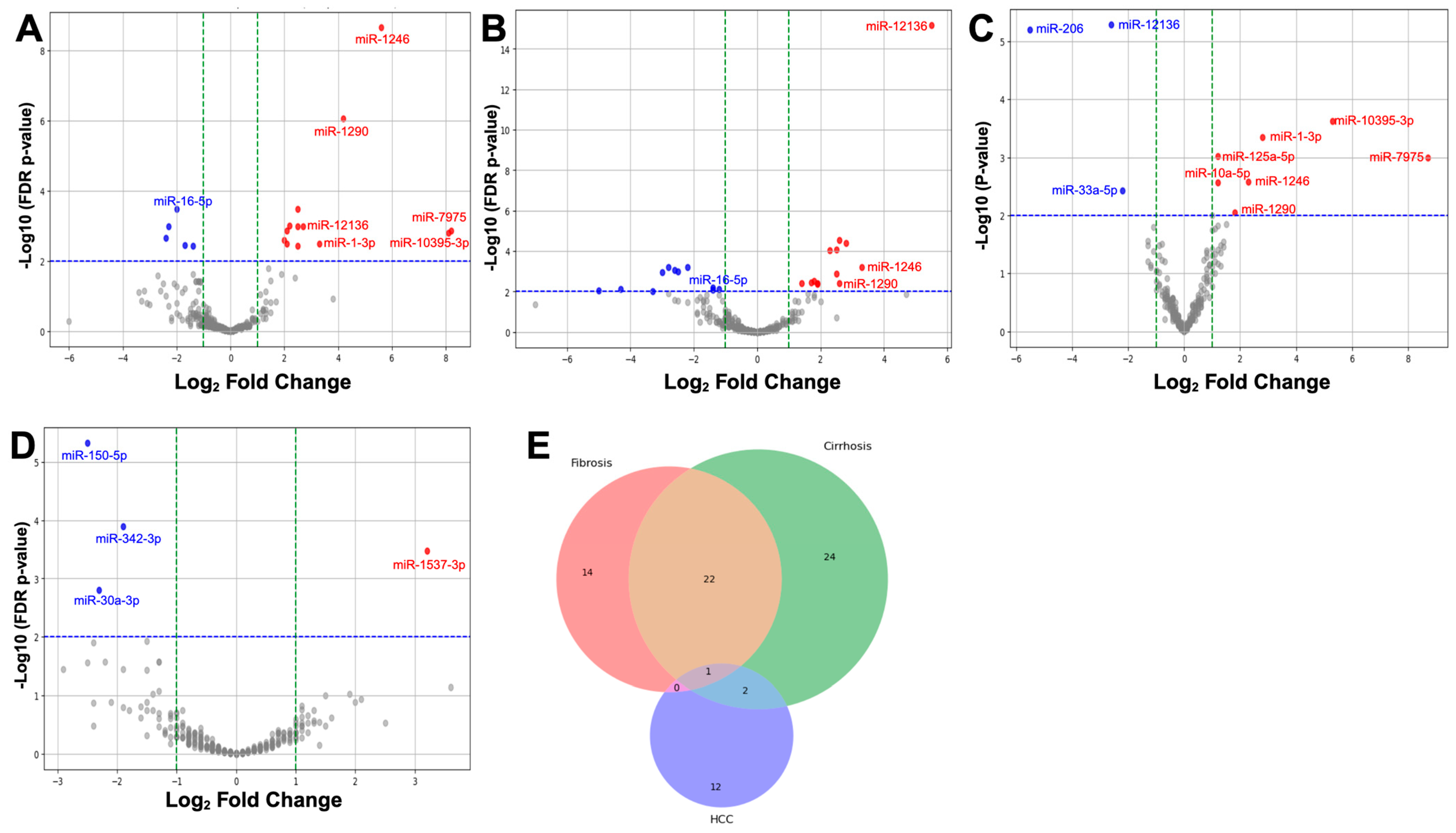

| miRNA | Expression (Fold Change (FC)) vs. Control | Fibrosis vs. Cirrhosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Fibrosis | Cirrhosis | HCC | ||||||
| log2 FC | FDR p Value | log2 FC | FDR p Value | log2 FC | FDR p Value | log2 FC | FDR p Value | |
| hsa-miR-7975 | 8.2 | 0.0014 | −0.4 | 0.9741 | 2.3 | 0.6744 | 8.7 | 0.0971 |
| hsa-miR-10395-3p | 8.1 | 0.0016 | 2.8 | 0.5049 | −0.5 | 0.9483 | 5.3 | 0.0454 |
| hsa-miR-3667-5p | 5.6 | 3.85 × 10−5 | 4.4 | 0.0010 | 3.1 | 0.0998 | 1.3 | 0.6963 |
| hsa-miR-1246 | 5.6 | 2.12 × 10−9 | 3.3 | 0.0006 | 0.0 | 0.9892 | 2.3 | 0.1708 |
| hsa-miR-1290 | 4.2 | 8.54 × 10−7 | 2.6 | 0.0040 | 0.5 | 0.8371 | 1.8 | 0.3955 |
| hsa-miR-1-3p | 3.3 | 0.0032 | 0.6 | 0.7926 | 1.0 | 0.5772 | 2.8 | 0.0654 |
| hsa-miR-12136 | 2.7 | 0.0010 | 5.5 | 6.56 × 10−19 | 0.4 | 0.8482 | −2.6 | 0.0018 |
| hsa-miR-122-3p | 2.7 | 0.0448 | 1.1 | 0.5664 | 1.4 | 0.4709 | 1.8 | 0.5825 |
| hsa-miR-320d | 2.5 | 0.0003 | 2.5 | 8.09 × 10−5 | 0.6 | 0.6744 | 0.1 | 0.9729 |
| hsa-miR-27a-5p | 2.5 | 0.0448 | 2.2 | 0.0293 | 2.0 | 0.1304 | 0.4 | 0.9447 |
| hsa-miR-3679-5p | 2.5 | 0.0010 | 1.8 | 0.0147 | 0.8 | 0.5216 | 0.8 | 0.7621 |
| hsa-miR-642a-3p | 2.5 | 0.0036 | 1.1 | 0.2885 | −0.8 | 0.6105 | 1.5 | 0.5027 |
| hsa-miR-483-5p | 2.4 | 0.0306 | 1.3 | 0.2812 | −0.8 | 0.6684 | 1.3 | 0.6207 |
| hsa-miR-125a-3p | 2.3 | 0.0343 | 1.0 | 0.4715 | 0.9 | 0.5302 | 1.4 | 0.5825 |
| hsa-miR-3960 | 2.2 | 0.0010 | 2.3 | 9.47 × 10−5 | −0.3 | 0.8637 | 0.0 | 0.9803 |
| hsa-miR-627-5p | 2.1 | 0.0032 | 2.6 | 2.78 × 10−5 | 0.1 | 0.9462 | −0.4 | 0.8832 |
| hsa-miR-320c | 2.1 | 0.0014 | 1.8 | 0.0029 | 0.1 | 0.9554 | 0.4 | 0.8832 |
| hsa-miR-320b | 2.0 | 0.0025 | 1.6 | 0.0125 | 0.0 | 0.9938 | 0.5 | 0.8255 |
| hsa-miR-4429 | 1.9 | 0.0238 | 1.9 | 0.0043 | 0.1 | 0.9676 | 0.1 | 0.9729 |
| hsa-miR-629-5p | 1.5 | 0.0355 | 1.7 | 0.0037 | 0.7 | 0.4360 | −0.1 | 0.9729 |
| hsa-miR-320a-3p | 1.4 | 0.0162 | 1.4 | 0.0040 | −0.4 | 0.6744 | 0.1 | 0.9729 |
| hsa-miR-30e-5p | −1.1 | 0.0379 | −0.6 | 0.2654 | 0.0 | 0.9880 | −0.3 | 0.8255 |
| hsa-miR-148b-3p | −1.1 | 0.0405 | −0.9 | 0.0991 | 0.0 | 0.9720 | −0.1 | 0.9571 |
| hsa-miR-20a-5p | −1.2 | 0.0379 | −0.4 | 0.6584 | −0.1 | 0.9148 | −0.7 | 0.6176 |
| hsa-miR-425-5p | −1.2 | 0.0377 | −1.1 | 0.0283 | −0.3 | 0.7935 | 0.0 | 0.9818 |
| hsa-miR-146a-5p | −1.2 | 0.0379 | −1.4 | 0.0063 | −0.3 | 0.7528 | 0.2 | 0.9447 |
| hsa-miR-19b-3p | −1.3 | 0.0386 | −0.3 | 0.7926 | 0.4 | 0.6760 | −0.8 | 0.5825 |
| hsa-miR-148a-3p | −1.3 | 0.0383 | −0.9 | 0.1440 | 0.2 | 0.8482 | −0.2 | 0.9447 |
| hsa-miR-25-3p | −1.4 | 0.0238 | −0.8 | 0.2350 | −0.6 | 0.5162 | −0.4 | 0.8255 |
| hsa-miR-30d-5p | −1.4 | 0.0037 | −1.2 | 0.0075 | −0.4 | 0.5640 | 0.0 | 0.9803 |
| hsa-miR-451a | −1.7 | 0.0036 | −0.8 | 0.2812 | −0.8 | 0.3722 | −0.8 | 0.6176 |
| hsa-miR-16-5p | −2.0 | 0.0003 | −1.4 | 0.0079 | −1.0 | 0.1979 | −0.4 | 0.8255 |
| hsa-miR-499a-5p | −2.5 | 0.0434 | −3.0 | 0.0011 | −1.5 | 0.1811 | 0.7 | 0.8889 |
| hsa-miR-16-5p | −2.0 | 0.0003 | −1.4 | 0.0079 | −1.0 | 0.1979 | −0.4 | 0.8255 |
| hsa-miR-200a-3p | −2.1 | 0.0448 | −2.6 | 0.0009 | −1.4 | 0.1811 | 0.7 | 0.8255 |
| hsa-miR-194-5p | −2.3 | 0.0010 | −2.2 | 0.0006 | −1.0 | 0.3269 | 0.1 | 0.9803 |
| miRNA | Expression (Fold Change (FC)) vs. Control | Cirrhosis vs. HCC Fibrosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Fibrosis | Cirrhosis | HCC | ||||||
| log2 FC | FDR p Value | log2 FC | FDR p Value | log2 FC | FDR p Value | log2 FC | FDR p Value | |
| hsa-miR-184 | 3.8 | 0.1178 | 4.7 | 0.0140 | 1.4 | 0.7145 | 3.2 | 0.0896 |
| hsa-miR-1297 | 1.4 | 0.3037 | 2.8 | 4.01 × 10−5 | 0.2 | 0.9127 | 2.6 | 7.20 × 10−5 |
| hsa-miR-4488 | 1.7 | 0.1471 | 2.5 | 0.0013 | −0.4 | 0.8482 | 2.8 | 8.46 × 10−5 |
| hsa-miR-206 | −3.1 | 0.1620 | 2.5 | 0.1879 | −2.4 | 0.3269 | 4.8 | 0.0007 |
| hsa-miR-4508 | 1.2 | 0.4321 | 2.0 | 0.0319 | −1.0 | 0.5143 | 2.9 | 0.0002 |
| hsa-miR-576-3p | 1.2 | 0.2533 | 1.9 | 0.0040 | 0.9 | 0.3926 | 0.9 | 0.1556 |
| hsa-miR-3605-5p | 1.3 | 0.3328 | 1.8 | 0.0195 | −0.5 | 0.8062 | 2.3 | 0.0026 |
| hsa-miR-4516 | 1.2 | 0.2508 | 1.6 | 0.0261 | −0.3 | 0.8421 | 1.9 | 0.0027 |
| hsa-miR-651-5p | 1.3 | 0.0742 | 1.3 | 0.0318 | 0.6 | 0.6105 | 0.8 | 0.2316 |
| hsa-miR-423-5p | 1.1 | 0.0874 | 1.2 | 0.0293 | 0.1 | 0.9608 | 1.1 | 0.0236 |
| hsa-miR-27b-3p | −0.6 | 0.1568 | −1.2 | 0.0147 | −0.6 | 0.0978 | −0.6 | 0.0852 |
| hsa-miR-598-3p | −0.9 | 0.5319 | −1.6 | 0.0286 | −0.6 | 0.5640 | −1.0 | 0.2140 |
| hsa-miR-132-3p | −0.6 | 0.7290 | −1.7 | 0.0261 | −0.8 | 0.4654 | −0.9 | 0.2907 |
| hsa-miR-141-3p | −1.3 | 0.3132 | −1.7 | 0.0319 | −1.5 | 0.1314 | −0.2 | 0.8700 |
| hsa-miR-34a-5p | −1.2 | 0.3394 | −1.8 | 0.0195 | −0.3 | 0.8868 | −1.6 | 0.0403 |
| hsa-miR-483-3p | −0.6 | 0.7307 | −1.9 | 0.0273 | −1.6 | 0.0273 | −0.3 | 0.2842 |
| hsa-miR-205-5p | −1.3 | 0.3095 | −2.5 | 0.0010 | −1.3 | 0.2030 | −1.2 | 0.1555 |
| hsa-miR-429 | −0.7 | 0.7321 | −2.5 | 0.0293 | −0.5 | 0.8208 | −2.1 | 0.0751 |
| hsa-miR-885-5p | −1.1 | 0.6561 | −2.6 | 0.0261 | −0.4 | 0.8963 | −2.3 | 0.0457 |
| hsa-miR-122-5p | −1.8 | 0.0773 | −2.8 | 0.0006 | −0.9 | 0.5216 | −1.9 | 0.0251 |
| hsa-miR-200b-3p | −0.9 | 0.6838 | −2.8 | 0.0122 | −0.6 | 0.7145 | −2.3 | 0.0494 |
| hsa-miR-296-5p | −0.5 | 0.7767 | −3.3 | 0.0097 | −0.6 | 0.6882 | −2.7 | 0.0403 |
| hsa-miR-208b-3p | −3.3 | 0.1386 | −4.3 | 0.0076 | −2.9 | 0.0361 | −1.5 | 0.4826 |
| hsa-miR-1299 | −3.0 | 0.1775 | −5.0 | 0.0093 | −2.4 | 0.1320 | −2.6 | 0.2324 |
| hsa-miR-885-3p | −3.4 | 0.0773 | −7.0 | 0.0421 | −0.3 | 0.8679 | −6.6 | 0.0443 |
| miRNA | Expression (Fold Change (FC)) vs. Control | Fibrosis vs. HCC | Cirrhosis vs. HCC Fibrosis | |||
|---|---|---|---|---|---|---|
| HCC | ||||||
| log2 FC | FDR p Value | log2 FC | FDR p Value | log2 FC | FDR p Value | |
| hsa-miR-132-5p | 3.6 | 0.0280 | -- | -- | −2.9 | 0.0618 |
| hsa-miR-1537-3p | 3.2 | 0.0003 | −3.4 | 0.0262 | −2.9 | 0.0025 |
| hsa-miR-126-3p | −1.3 | 0.0267 | 1.9 | 1.20 × 10−5 | 1.2 | 0.0157 |
| hsa-miR-125a-5p | −1.3 | 0.0267 | 2.2 | 1.69 × 10−7 | 1.0 | 0.0333 |
| hsa-miR-10b-5p | −1.5 | 0.0372 | 2.0 | 0.0003 | 0.9 | 0.1599 |
| hsa-miR-10a-5p | −1.5 | 0.0120 | 1.8 | 0.0002 | 0.6 | 0.2907 |
| hsa-miR-342-3p | −1.9 | 0.0001 | 2.2 | 4.38 × 10−7 | 1.3 | 0.0093 |
| hsa-miR-4454 | −1.9 | 0.0361 | 0.0 | 0.9991 | 1.3 | 0.1332 |
| hsa-miR-204-5p | −2.2 | 0.0267 | 2.8 | 0.0008 | 2.0 | 0.0242 |
| hsa-miR-30a-3p | −2.3 | 0.0016 | 3.3 | 1.77 × 10−7 | 2.3 | 0.0005 |
| hsa-miR-215-5p | −2.4 | 0.0125 | 2.6 | 0.0014 | 1.3 | 0.1700 |
| hsa-miR-150-5p | −2.5 | 4.68 × 10−6 | 3.4 | 9.96 × 10−12 | 2.3 | 3.88 × 10−6 |
| hsa-miR-375-3p | −2.5 | 0.0273 | 0.0 | 0.9868 | 0.3 | 0.8306 |
| miRNA | Fibrosis | Cirrhosis | HCC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Log2 Fold Change | Fold Change | FDR p Value | Log2 Fold Change | Fold Change | FDR p Value | Log2 Fold Change | Fold Change | FDR p Value | |
| hsa-miR-184 | −4.6 | −25 | N.D. | 4.2 | 18.4 | 0.3693 | −6.9 | −121.7 | 0.0305 |
| hsa-miR-1246 | −3.8 | −13.6 | 0.0151 | −1.3 | −2.4 | 0.9102 | −0.9 | −1.8 | 0.9983 |
| hsa-miR-206 | 0.1 | 1.1 | 0.9431 | 5.7 | 50.7 | 0.028 | −1.8 | −3.4 | 0.9983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, L.L.; Qu, D.; Sureban, S.; Mitchell, S.; Pitts, K.; Cooper, N.; Fazili, J.; Harty, R.; Oseini, A.; Ding, K.; et al. From Inflammation to Oncogenesis: Tracing Serum DCLK1 and miRNA Signatures in Chronic Liver Diseases. Int. J. Mol. Sci. 2024, 25, 6481. https://doi.org/10.3390/ijms25126481
Moore LL, Qu D, Sureban S, Mitchell S, Pitts K, Cooper N, Fazili J, Harty R, Oseini A, Ding K, et al. From Inflammation to Oncogenesis: Tracing Serum DCLK1 and miRNA Signatures in Chronic Liver Diseases. International Journal of Molecular Sciences. 2024; 25(12):6481. https://doi.org/10.3390/ijms25126481
Chicago/Turabian StyleMoore, Landon L., Dongfeng Qu, Sripathi Sureban, Stephanie Mitchell, Kamille Pitts, Nasya Cooper, Javid Fazili, Richard Harty, Abdul Oseini, Kai Ding, and et al. 2024. "From Inflammation to Oncogenesis: Tracing Serum DCLK1 and miRNA Signatures in Chronic Liver Diseases" International Journal of Molecular Sciences 25, no. 12: 6481. https://doi.org/10.3390/ijms25126481
APA StyleMoore, L. L., Qu, D., Sureban, S., Mitchell, S., Pitts, K., Cooper, N., Fazili, J., Harty, R., Oseini, A., Ding, K., Bronze, M., & Houchen, C. W. (2024). From Inflammation to Oncogenesis: Tracing Serum DCLK1 and miRNA Signatures in Chronic Liver Diseases. International Journal of Molecular Sciences, 25(12), 6481. https://doi.org/10.3390/ijms25126481







