Evaluating Single-Nucleotide Polymorphisms in Inflammasome Proteins and Serum Levels of IL-18 and IL-1β in Kidney Interstitial Damage in Anti-Neutrophilic Cytoplasmic Antibody-Associated Vasculitis
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Serum and Urinary IL-18 and IL-1β Levels
2.3. Allele and Genotype Distribution
2.4. IL-18 SNPs, sIL-18 Levels, and Lesions in the Kidney Biopsy
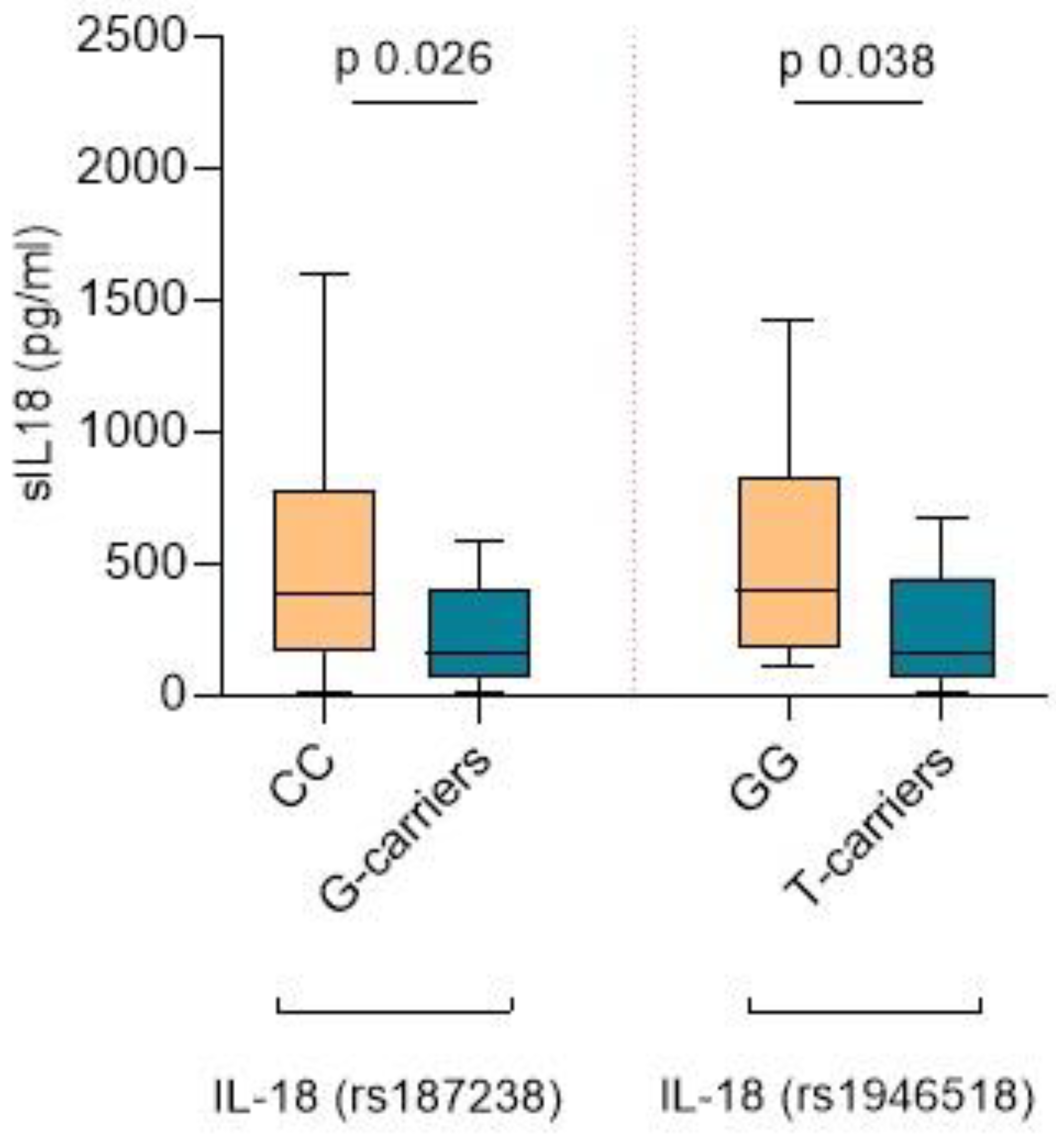
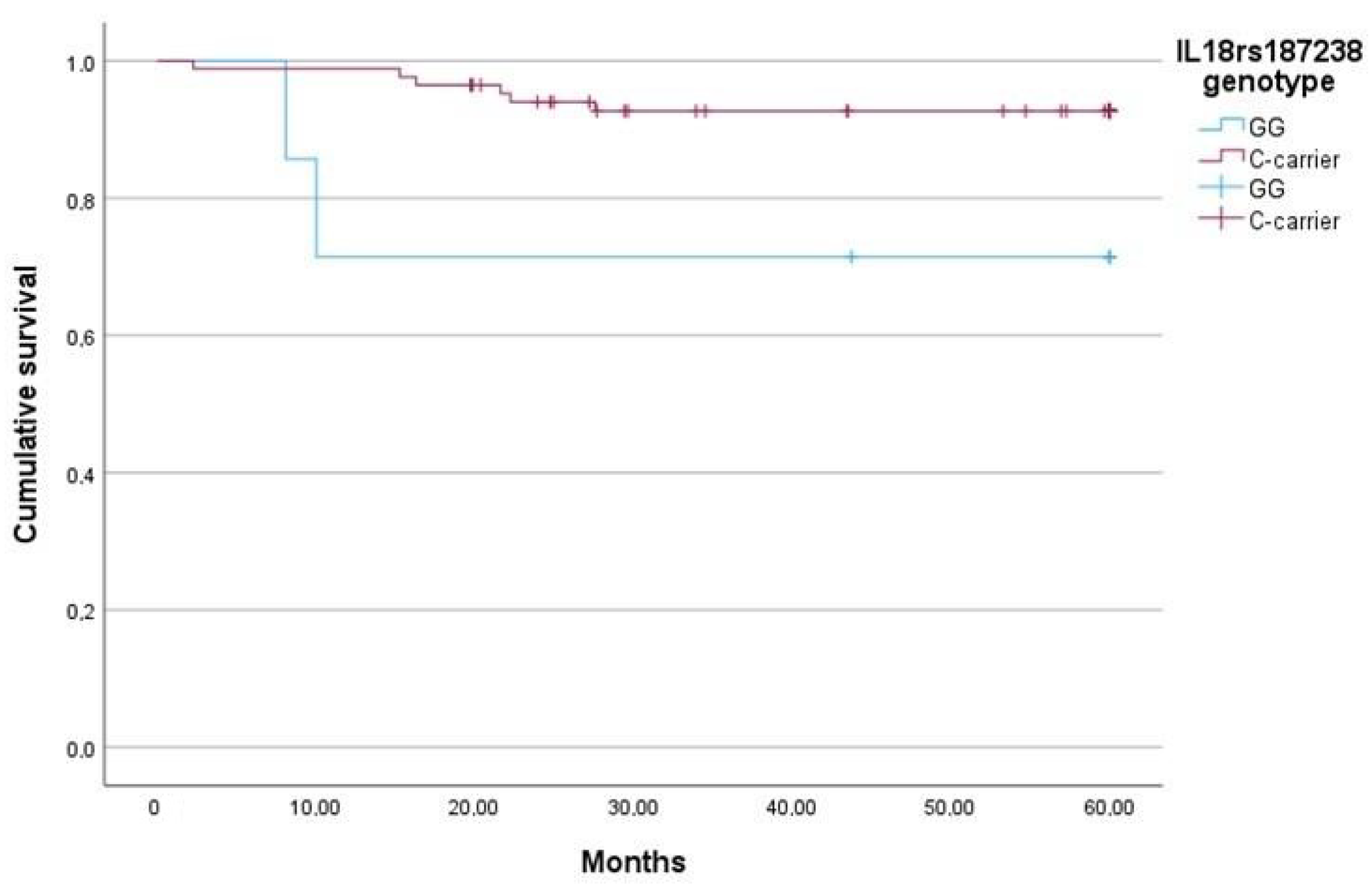
2.5. The IL-18 rs187238 G Allele Is Associated with Lower sIL-18, More Severe Fibrosis, and Higher Mortality
2.6. The IL-18 rs1946518 T Allele Is Associated with Lower sIL-18, Lower eGFR Levels during Follow-Up, and a Higher Risk of Relapse within the First Year after Diagnosis
2.7. Association of SNPs and Organ Involvement in AAV
3. Discussion
4. Methods
4.1. Study Population
4.2. Clinical and Laboratory Data
4.3. Sampling, Genotyping Assays, and Cytokine Assessment
4.4. Selection of SNPs
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Anton-Pampols, P.; Diaz-Requena, C.; Martinez-Valenzuela, L.; Gomez-Preciado, F.; Fulladosa, X.; Vidal-Alabro, A.; Torras, J.; Lloberas, N.; Draibe, J. The Role of Inflammasomes in Glomerulonephritis. Int. J. Mol. Sci. 2022, 23, 4208. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.K.; Tang, K.T.; Chen, D.Y. The NLRP3 Inflammasome as a Pathogenic Player Showing Therapeutic Potential in Rheumatoid Arthritis and Its Comorbidities: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 626. [Google Scholar] [CrossRef]
- Li, R.N.; Ou, T.T.; Lin, C.H.; Lin, Y.Z.; Fang, T.J.; Chen, Y.J.; Tseng, C.-C.; Sung, W.-Y.; Wu, C.-C.; Yen, J.-H. NLRP3 Gene Polymorphisms in Rheumatoid Arthritis and Primary Sjogren’s Syndrome Patients. Diagnostics 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alamino, R.; Cuchacovich, R.; Espinoza, L.R.; Porretta, C.P.; Zea, A.H. Role of Inflammasome Activation in Systemic Lupus Erythematosus: Are Innate Immune Cells Activated? Reumatol. Clin. 2021, 17, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, Z.; Cao, L.; Zou, H.; Zhu, X. Role of NLRP3 inflammasome in systemic sclerosis. Arthritis Res. Ther. 2022, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.; Watts, R. ANCA-associated vasculitis. Clin. Med. 2017, 17, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Xiao, Z.; Lin, W.; Zhang, Y.; Meng, T.; Ning, J.; Xu, H.; Tang, R.; Xiao, X. Acute interstitial nephritis caused by ANCA-associated vasculitis: A case based review. Clin. Rheumatol. 2024, 43, 1227–1244. [Google Scholar] [CrossRef] [PubMed]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.-H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic Classification of ANCA-Associated Glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef]
- Brix, S.R.; Noriega, M.; Tennstedt, P.; Vettorazzi, E.; Busch, M.; Nitschke, M.; Jabs, W.J.; Özcan, F.; Wendt, R.; Hausberg, M.; et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018, 94, 1177–1188. [Google Scholar] [CrossRef]
- Hewins, P.; Morgan, M.D.; Holden, N.; Neil, D.; Williams, J.M.; Savage, C.O.S.; Harper, L. IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int. 2006, 69, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Sasatomi, Y.; Watanabe, R.; Watanabe, M.; Miyake, K.; Abe, Y.; Yasuno, T.; Ito, K.; Ueki, N.; Hamauchi, A.; et al. IL-1β promotes tubulointerstitial injury in MPO-ANCA-associated glomerulonephritis. Clin. Nephrol. 2016, 86, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Sun, X.J.; Chen, M.; Zhao, M.H. The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis. J. Transl. Med. 2019, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Lundtoft, C.; Knight, A.; Meadows, J.R.S.; Karlsson, Å.; Rantapää-Dahlqvist, S.; Berglin, E.; Palm, Ø.; Haukeland, H.; Gunnarsson, I.; Bruchfeld, A.; et al. The HLA region in ANCA-associated vasculitis: Characterisation of genetic associations in a Scandinavian patient population. RMD Open 2024, 10, e004039. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, A.; Rani, L.; Kaur, N.; Anand, S.; Saikia, B.; Jha, S.; Nada, R.; Minz, R.W. Distinct HLA and non-HLA associations in different subtypes of ANCA-associated vasculitides in North India. Int. J. Rheum. Dis. 2020, 23, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Casal Moura, M.; Deng, Z.; Brooks, S.R.; Tew, W.; Fervenza, F.C.; Kallenberg, C.G.M.; Langford, C.A.; Merkel, P.A.; Monach, P.A.; Seo, P.; et al. Risk of relapse of ANCA-associated vasculitis among patients homozygous for the proteinase 3 gene Val119Ile polymorphism. RMD Open 2023, 9, e002935. [Google Scholar] [CrossRef] [PubMed]
- Husmann, C.A.; Holle, J.U.; Moosig, F.; Mueller, S.; Wilde, B.; Tervaert, J.W.C.; Harper, L.; Assmann, G.; Gross, W.L.; Epplen, J.T.; et al. Genetics of toll like receptor 9 in ANCA associated vasculitides. Ann. Rheum. Dis. 2014, 73, 890–896. [Google Scholar] [CrossRef]
- Martorana, D.; Maritati, F.; Malerba, G.; Bonatti, F.; Alberici, F.; Oliva, E.; Sebastio, P.; Manenti, L.; Brugnano, R.; Catanoso, M.G.; et al. PTPN22 R620W polymorphism in the ANCA-associated vasculitides. Rheumatology 2012, 51, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Niederer, H.A.; Williams, J.; Harper, L.; Watts, R.A.; Lyons, P.A.; Smith, K.G. Confirmation of the genetic association of CTLA4 and PTPN22 with ANCA-associated vasculitis. BMC Med. Genet. 2009, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Ceballos-Olvera, I.; del Barrio, L.; Re, F. Role of the Inflammasome, IL-1 β, and IL-18 in Bacterial Infections. Sci. World J. 2011, 11, 2037–2050. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.I.; Lee, K.H.; Joo, Y.H.; Lee, J.M.; Jeon, J.; Jung, H.J.; Shin, M.; Cho, S.; Kim, T.H.; Park, S.; et al. Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review. J. Autoimmun. 2019, 103, 102299. [Google Scholar] [CrossRef]
- Masutani, K.; Tokumoto, M.; Nakashima, H.; Tsuruya, K.; Kashiwagi, M.; Kudoh, Y.; Fukuda, K.; Kanai, H.; Akahoshi, M.; Otsuka, T.; et al. Strong polarization toward Th1 immune response in ANCA-associated glomerulonephritis. Clin. Nephrol. 2003, 59, 395–405. [Google Scholar] [CrossRef]
- Anders, H.J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Dong, F.; Guo, L.; Hou, Y.; Yi, F.; Liu, J.; Xu, D. Interleukin-1β increases permeability and upregulates the expression of vascular endothelial-cadherin in human renal glomerular endothelial cells. Mol. Med. Rep. 2015, 11, 3708–3714. [Google Scholar] [CrossRef] [PubMed]
- Timoshanko, J.R.; Kitching, A.R.; Iwakura, Y.; Holdsworth, S.R.; Tipping, P.G. Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor I to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am. J. Pathol. 2004, 164, 1967–1977. [Google Scholar] [CrossRef]
- Pawluczyk, I.Z.A.; Harris, K.P.G. Cytokine interactions promote synergistic fibronectin accumulation by mesangial cells. Kidney Int. 1998, 54, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik-Kułak, P.; Michalska-Jakubus, M.; Kowal, M.; Krasowska, D. Serum Levels of Selected IL-1 Family Cytokines in Patients with Morphea. J. Clin. Med. 2022, 11, 6375. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Manfrere, K.C.G.; Torrealba, M.P.; Ferreira, F.M.; de Sousa, E.S.A.; Miyashiro, D.; Teixeira, F.M.E.; Custódio, R.W.A.; Nakaya, H.I.; Ramos, Y.A.L.; Sotto, M.N.; et al. Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome. Int. J. Mol. Sci. 2023, 24, 4674. [Google Scholar] [CrossRef] [PubMed]
- Mende, R.; Vincent, F.B.; Kandane-Rathnayake, R.; Koelmeyer, R.; Lin, E.; Chang, J.; Hoi, A.Y.; Morand, E.F.; Harris, J.; Lang, T. Analysis of Serum Interleukin (IL)-1β and IL-18 in Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, O.; Andersson, B.; Hahn-Zoric, M.; Almroth, G. Serum concentration of interleukin-18 is up-regulated in patients with ANCA-associated vasculitis. Autoimmunity 2007, 40, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance. Open Life Sci. 2024, 19, 20220823. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Ni, Z.; Cao, L.; Zhou, M.; Mou, S.; Wang, Q.; Zhang, M.; Fang, W.; Yan, Y.; Qian, J. Serum IL-18 is closely associated with renal tubulointerstitial injury and predicts renal prognosis in IgA nephropathy. Mediat. Inflamm. 2012, 2012, 728417. [Google Scholar] [CrossRef] [PubMed]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Khripko, O.P.; Sennikova, N.S.; Lopatnikova, J.A.; Khripko, J.I.; Filipenko, M.L.; Khrapov, E.A.; Gelfgat, E.L.V.; Yakushenko, E.V.; Kozlov, V.A.; Sennikov, S.V. Association of single nucleotide polymorphisms in the IL-18 gene with production of IL-18 protein by mononuclear cells from healthy donors. Mediat. Inflamm. 2008, 2008, 309721. [Google Scholar]
- Kim, C.D.; Ryu, H.M.; Choi, J.Y.; Choi, H.J.; Choi, H.J.; Cho, J.H.; Park, S.H.; Won, D.I.; Kim, Y.L. Association of G-137C IL-18 promoter polymorphism with acute allograft rejection in renal transplant recipients. Transplantation 2008, 86, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Cho, J.H.; Lim, J.H.; Yu, C.H.; Choi, J.Y.; Yoon, S.H.; Park, S.H.; Kim, Y.L.; Kim, C.D. Impact of gene polymorphisms of interleukin-18, transforming growth factor-β, and vascular endothelial growth factor on development of IgA nephropathy and thin glomerular basement membrane disease. Kidney Res. Clin. Pract. 2012, 31, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Giedraitis, V.; He, B.; Huang, W.X.; Hillert, J. Cloning and mutation analysis of the human IL-18 promoter: A possible role of polymorphisms in expression regulation. J. Neuroimmunol. 2001, 112, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Tan, Y.L.; Chen, D.C.; Tan, S.P.; Malouta, M.Z.; Bernard, J.D.; Combs, J.L.; Bhatti, S.; Davis, M.C.; Kosten, T.R.; et al. Serum IL-18 level, clinical symptoms and IL-18-607A/C polymorphism among chronic patients with schizophrenia in a Chinese Han population. Psychoneuroendocrinology 2016, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Feng, W.; Li, Y.; Shi, Y.; Cai, B.; Liao, Y.; Zhang, J.; Huang, Z.; Wang, L. Interleukin 18 -607 A/C Gene Polymorphism is Associated with Susceptibility to IgA Nephropathy in a Chinese Han Population. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, J.; Sierocka, A.; Tejchman, K.; Ziȩtek, Z.; Romanowski, M.; Pawlik, A.; Sieńko, J.; Żukowski, M.; Ciechanowski, K.; Ostrowski, M.; et al. The impact of interleukin 12B (1188A>C), interleukin 16 (-295T>C), and interleukin 18 (607C>A, 137G>C) gene polymorphisms on long-term renal transplant function and recipient outcomes. Transplant. Proc. 2014, 46, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Jabir, N.R.; Zughaibi, T.A.; Suhail, M. Association of interleukin-18 promoter polymorphism with comorbid conditions of cardiovascular disease. J. King Saud. Univ. Sci. 2023, 35, 102440. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, X.; Huang, E.; Wang, Q.; Wen, C.; Yang, G.; Lu, L.; Cui, D. Inflammasome and Its Therapeutic Targeting in Rheumatoid Arthritis. Front. Immunol. 2022, 12, 816839. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Yang, Y.X.; Meng, X.; Luo, X.Y.; Li, X.M.; Shuai, Z.W.; Ye, D.-Q.; Pan, H.-F. NLRP3: A promising therapeutic target for autoimmune diseases. Autoimmun. Rev. 2018, 17, 694–702. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Mukhtyar, C.; Flossmann, O.; Alberici, F.; Baslund, B.; Batra, R.; Brown, D.; Holle, J.; Hruskova, Z.; Jayne, D.R.W.; et al. A cross-sectional study of the Birmingham vasculitis activity score version 3 in systemic vasculitis. Rheumatology 2011, 50, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Sadr, M.; Rezaei, A.; Shahkarami, S.; Daryani, N.E.; Bidoki, A.; Rezaei, N. Association of NLRP3 single nucleotide polymorphisms with ulcerative colitis: A case-control study. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.; Brandao, L.; Guimaraes, R.; Segat, L.; Araujo, J.; Crovella, S. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity 2010, 43, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.; Vendramin, A.; Catamo, E.; Fabris, A.; Crovella, S. The Missense Variation Q705K in CIAS1 / NALP3 / NLRP3 Gene and an NLRP1 Haplotype Are Associated with Celiac Disease. Am. J. Gastroenterol. 2011, 106, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.-C. Association between functional NLRP3 polymorphisms and susceptibility to autoimmune and inflammatory diseases: A meta-analysis. Lupus 2016, 25, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Addobbati, C.; da Cruz, H.L.A.; Adelino, J.E.; Ramos, A.L.M.T.; Fragoso, T.S.; Domingues, A.; Duarte, L.B.P.; Oliveira, R.D.R.; Louzada-Júnior, P.; Donadi, E.A.; et al. Polymorphisms and expression of inflammasome genes are associated with the development and severity of rheumatoid arthritis in Brazilian patients. Inflamm. Res. 2017, 67, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Saito, W.; Kitaichi, N.; Miura, T.; Ishida, S.; Ohno, S. Evaluation of NLRP1 gene polymorphisms in Vogt-Koyanagi-Harada disease. Jpn. J. Ophthalmol. 2011, 55, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Li, H.; Fang, Y.; Liu, Y.; Li, M.; Zhong, B.; Yang, F.; Zou, Q.; Wu, Y. NLRP1 gene polymorphism influences gene transcription and is a risk factor for rheumatoid arthritis in Han Chinese. Arthritis Rheum. 2011, 64, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Huang, Y.; Zhang, H.; Liu, R. MMP-2, TNF-α and NLRP1 polymorphisms in Chinese patients with ankylosing spondylitis and rheumatoid arthritis. Mol. Biol. Rep. 2013, 40, 6303–6308. [Google Scholar] [CrossRef] [PubMed]
- Dieudé, P.; Guedj, M.; Wipff, J.; Ruiz, B.; Riemekasten, G.; Airo, P.; Melchers, I.; Hachulla, E.; Cerinic, M.M.; Diot, E.; et al. NLRP1 influences the systemic sclerosis phenotype: A new clue for the contribution of innate immunity in systemic sclerosis-related fibrosing alveolitis pathogenesis. Ann. Rheum. Dis. 2010, 70, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, A.; Girardelli, M.; Kamada, A.J.; Pancotto, J.A.; Donadi, E.A.; Crovella, S.; Sandrin-Garcia, P. Polimorphisms in Inflammasome Genes Are Involved in the Predisposition to Systemic Lupus Erythematosus. Autoimmunity 2012, 45, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, M.; Laddha, N.; Mansuri, M.; Marfatia, Y.; Begum, R. Association of NLRP1 genetic variants and mRNA overexpression with generalized vitiligo and disease activity in a Gujarat population. Br. J. Dermatol. 2013, 169, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, K.S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Association of Nod-like receptor protein-1 (rs2670660) and Toll-like receptor-4 (rs4986790) with non-segmental vitiligo: A case–control study in South Indian population. Int. J. Immunogenet. 2019, 46, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sharab, L.Y.; Morford, L.A.; Dempsey, J.; Falcão-Alencar, G.; Mason, A.; Jacobson, E.; Kluemper, G.T.; Macri, J.V.; Hartsfield, J.K. Genetic and treatment-related risk factors associated with external apical root resorption (EARR) concurrent with orthodontia. Orthod. Craniofacial Res. 2015, 18, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamad, M.; Cornelis, F.; Marzouk, S.; Chabchoub, G.; Bahloul, Z.; Rebai, A.; Fakhfakh, F.; Ayadi, H.; Petit-Teixeira, E.; Maalej, A. Association study of CARD8 (p.C10X) and NLRP3 (p.Q705K) variants with rheumatoid arthritis in French and Tunisian populations. Int. J. Immunogenet. 2011, 39, 131–136. [Google Scholar] [CrossRef]
- Yi, M.; Shao, X.; Ma, J.; Tian, B.; Zhang, Y.; Liu, S. rs2043211 polymorphism in CARD8 is not associated with Tourette syndrome in a family-based association study in the Chinese Han population. Int. J. Psychiatry Med. 2015, 49, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-T.; Ma, X.-J.; Zong, Y.; Du, X.-M.; Hu, J.-H.; Lu, G.-C. Is the CARD8 rs2043211 polymorphism associated with susceptibility to Crohn’s disease? A meta-analysis. Autoimmunity 2015, 48, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, S.-S.; Oh, D.H.; Park, D.-J.; Kim, H.-S.; Choi, J.R.; Chae, S.-C.; Yun, K.J.; Chung, W.T.; Choe, J.-Y.; et al. Genetic Association for P2X7R rs3751142 and CARD8 rs2043211 Polymorphisms for Susceptibility of Gout in Korean Men: Multi-Center Study. J. Korean Med. Sci. 2016, 31, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, D.S.; Goricar, K.; Hovnik, T.; Mendez, A.; Bratina, N.; Brecelj, J.; Vidan-Jeras, B.; Battelino, T.; Dolzan, V. Dual Role of PTPN22 but Not NLRP3 Inflammasome Polymorphisms in Type 1 Diabetes and Celiac Disease in Children. Front. Pediatr. 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, Z.; Akbarian, M.; Mirkazemi, S.; Shahlaee, A.; Alizadeh, Z.; Amirzargar, A.A.; Jamshidi, A.R.; Ghoroghi, S.; Poursani, S.; Nourijelyani, K.; et al. Interleukin-1 gene cluster and IL-1 receptor polymorphisms in Iranian patients with systemic lupus erythematosus. Rheumatol. Int. 2013, 33, 2591–2596. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Farazmand, A.; Mahmoudi, M.; Ashraf-Ganjouei, A.; Javinani, A.; Nazari, B.; Kavosi, H.; Amirzargar, A.A.; Jamshidi, A.R.; Gharibdoost, F. IL-1A rs1800587, IL-1B rs1143634 and IL-1R1 rs2234650 polymorphisms in Iranian patients with systemic sclerosis. Int. J. Immunogenet. 2015, 42, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kapelski, P.; Skibinska, M.; Maciukiewicz, M.; Wilkosc, M.; Frydecka, D.; Groszewska, A.; Narozna, B.; Dmitrzak-Weglarz, M.; Czerski, P.; Pawlak, J.; et al. Association study of functional polymorphisms in interleukins and interleukin receptors genes: IL1A, IL1B, IL1RN, IL6, IL6R, IL10, IL10RA and TGFB1 in schizophrenia in Polish population. Schizophr. Res. 2015, 169, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Assari, R.; Aghighi, Y.; Ziaee, V.; Sadr, M.; Rahmani, F.; Rezaei, A.; Sadr, Z.; Moradinejad, M.H.; Raeeskarami, S.R.; Rezaei, N. Pro-inflammatory cytokine single nucleotide polymorphisms in Kawasaki disease. Int. J. Rheum. Dis. 2016, 21, 1120–1126. [Google Scholar] [CrossRef]
- Shehjar, F.; Afroze, D.; Misgar, R.A.; Malik, S.A.; Laway, B.A. Association of polymorphic variants of IL-1β and IL-1RN genes in the development of Graves’ disease in Kashmiri population (North India). Hum. Immunol. 2018, 79, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Panah, P.-S.; Moravvej, H.; Sadaf, Z.; Babaei, H.; Geranmayeh, M.; Hajmanouchehri, S.; Karimi, A.; Sajjadi, F.; Arghand, F.; Ludwig, R.J.; et al. Proinflammatory Cytokine Gene Polymorphisms in Bullous Pemphigoid. Front. Immunol. 2019, 10, 636. [Google Scholar] [CrossRef]
- Pawlik, A.; Kurzawski, M.; Drozdzik, M.; Dziedziejko, V.; Safranow, K.; Herczynska, M. Interleukin-18 gene (IL18) promoter polymorphisms in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2009, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Tavares, N.A.; Santos, M.M.; Moura, R.; Araújo, J.; Guimarães, R.; Crovella, S.; Brandão, L. Interleukin 18 (IL18) gene promoter polymorphisms are associated with type 1 diabetes mellitus in Brazilian patients. Cytokine 2013, 62, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, W.G.A.D.; Cilião, D.A.; Genre, J.; Gondim, D.D.; Alves, R.G.; Hassan, N.D.; Lima, F.P.; Pereira, M.G.; Donadi, E.A.; Crispim, J.C.d.O. Genetic polymorphisms of Interleukin-18 are not associated with allograft function in kidney transplant recipients. Genet. Mol. Biol. 2014, 37, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.D.; Ates, O. Genetic analysis of interleukin 18 gene polymorphisms in alopecia areata. J. Clin. Lab. Anal. 2018, 32, e22386. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; Dandana, M.; Magdoud, K.; Meddeb, S.; Ben Slama, N.; Hizem, S.; Mahjoub, T. Interleukin-18 promoter polymorphisms and risk of idiopathic recurrent pregnancy loss in a Tunisian population. J. Reprod. Immunol. 2012, 93, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, Y.; Mendes, A.K.B.; Souza, E.M.; Alberton, D.; Rego, F.G.M.; Valdameri, G.; Picheth, G. Research Article Interleukin-18 (rs187238) and glucose transporter 4 (rs5435) polymorphisms in Euro-Brazilians with type 1 diabetes. Evolution 2017, 16. [Google Scholar] [CrossRef] [PubMed]
| Gender (% male) | 44.56 |
| Median age (IQR) (years) | 68 (60–76.75) |
| Organ involvement (%) | 65.51 |
| ANCA type (%MPO) | 74.70 |
| Median ANCA titer (IQR) (karbU/L) | 13 (1.70–54) |
| Median serum creatinine IQR (µmol/L) | 145 (104.51–180) |
| Mean eGFR ± SD (mL/min) | 38.5 ± 18.63 |
| Mean proteinuria ± SD (g/mol) | 0.53 ± 0.60 |
| Median serum CRP (IQR) mg/L | 4 (1.40–11.92) |
| Berden Histopathologic Classification (% patients) | |
| Focal | 18.47 |
| Crescentic | 26.08 |
| Mixed | 31.52 |
| Sclerotic | 11.95 |
| N/A | 10.86 |
| Induction treatment (% patients) | |
| Methylprednisolone pulses | 40.21 |
| Plasma exchange | 34.78 |
| Cyclophosphamide | 42.39 |
| Rituximab | 28.26 |
| sIL-18 (pg/mL) | sIL-1β (pg/mL) | |||||
|---|---|---|---|---|---|---|
| Absent–Mild (Grades 0–1) | Moderate–Severe (Grades 2–3) | p-Value | Absent–Mild (Grades 0–1) | Moderate–Severe (Grades 2–3) | p-Value | |
| Fibrosis | 567.93 ± 599.11 | 191.34 ± 250.34 | 0.013 | 1.36 ± 1.34 | 0.42 ± 0.59 | 0.002 |
| Atrophy | 474.78 ± 523.43 | 166.25 ± 268.31 | 0.062 | 1.38 ± 1.49 | 0.2 ± 0.25 | 0.004 |
| Inflammatory Infiltrate | 507.80 ± 513.59 | 370.27 ± 487.39 | 0.20 | 3.27 ± 8.64 | 0.95 ± 1.29 | 0.18 |
| Gene | SNP | Hardy–Weinberg Equilibrium | Minor Allele in the Study Population | MAF in the Study Population | MAF (European Ancestry) | |
|---|---|---|---|---|---|---|
| Chi-Square | p-Value | |||||
| IL-1β | rs1143634 | 0.237 | 0.88 | A | 0.19 | A allele, 0.24 |
| NLRP3 | rs4612666 | 0.096 | 0.853 | T | 0.27 | T allele, 0.24 |
| IL-18 | rs1946518 | 2.51 | 0.285 | G | 0.48 | T allele, 0.42 |
| IL-18 | rs187238 | 1.152 | 0.562 | G | 0.32 | G allele, 0.28 |
| NLRP1 | rs878329 | 0.509 | 0.775 | C | 0.44 | C allele, 0.46 |
| CARD8 | rs2043211 | 0.182 | 0.913 | T | 0.33 | T allele, 0.33 |
| NLRP3 | rs10754558 | 1.872 | 0.392 | G | 0.36 | G allele, 0.46 |
| CASP1 | rs530537 | 0.163 | 0.922 | C | 0.48 | C allele, 0.44 |
| IL-18 rs187238 | IL-18 rs1946518 | |||||
|---|---|---|---|---|---|---|
| GG n = 7 (7.6%) | C-Carriers n = 85 (92.4%) | p-Value | T-Carriers n = 67 (72.8%) | GG n = 25 (27.2%) | p-Value | |
| Mean sCreatinine ± SD (µmol/L) | 363.68 ± 288.19 | 206.71 ± 110.35 | 0.16 | 360.04 ± 238.12 | 347.82 ± 296.61 | 0.90 |
| Mean eGFR ± SD (mL/min) | 23.37 ± 18.99 | 33 ± 21.15 | 0.20 | 20.93 ± 18.70 | 25.17 ± 19.44 | 0.38 |
| Mean proteinuria ± SD (g/day) | 0.78 ± 1.15 | 0.53 ± 0.78 | 0.64 | 0.69 ± 0.58 | 0.79 ± 1.28 | 0.75 |
| Mean sCRP ± SD (mg/mL) | 68.3 ± 77.98 | 61.17 ± 73.90 | 0.83 | 67.37 ± 66.37 | 67.80 ± 81.35 | 0.98 |
| Mean ANCA titer (KarbU/L) | 494.58 ± 882.16 | 469.58 ± 611.73 | 0.47 | 809.88 ± 1197.76 | 371.74 ± 668.78 | 0.12 |
| Gene | SNP | Chromosome Location | Functional Consequence | Alleles | MAF (European Ancestry) |
|---|---|---|---|---|---|
| IL-1β | rs1143634 | chr2:112832813 | Coding sequence variant, synonymous variant | G/A, transition substitution | A allele, 0.24 |
| NLRP3 | rs4612666 | chr1:247435768 | Intron variant | C/T, transition substitution | T allele, 0.24 |
| IL-18 | rs1946518 | chr11:112164735 | Upstream transcript variant | T/G, transversion substitution | T allele, 0.42 |
| IL-18 | rs187238 | chr11:112164265 | Upstream transcript variant | C/G, transversion substitution | G allele, 0.28 |
| NLRP1 | rs878329 | chr17:5649930 | None | G/C, transversion substitution | C allele, 0.46 |
| CARD8 | rs2043211 | chr19:48234449 | Stop gained variant | A/T, transversion substitution | T allele, 0.33 |
| NLRP3 | rs10754558 | chr1:247448734 | 3′UTR variant | C/G, transversion substitution | G allele, 0.46 |
| CASP1 | rs530537 | chr11:105027786 | Intron variant | C/T, transition substitution | C allele, 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Valenzuela, L.; Vidal-Alabró, A.; Rubio, B.; Antón-Pàmpols, P.; Gómez-Preciado, F.; Fulladosa, X.; Cruzado, J.M.; Torras, J.; Lloberas, N.; Draibe, J. Evaluating Single-Nucleotide Polymorphisms in Inflammasome Proteins and Serum Levels of IL-18 and IL-1β in Kidney Interstitial Damage in Anti-Neutrophilic Cytoplasmic Antibody-Associated Vasculitis. Int. J. Mol. Sci. 2024, 25, 6479. https://doi.org/10.3390/ijms25126479
Martinez Valenzuela L, Vidal-Alabró A, Rubio B, Antón-Pàmpols P, Gómez-Preciado F, Fulladosa X, Cruzado JM, Torras J, Lloberas N, Draibe J. Evaluating Single-Nucleotide Polymorphisms in Inflammasome Proteins and Serum Levels of IL-18 and IL-1β in Kidney Interstitial Damage in Anti-Neutrophilic Cytoplasmic Antibody-Associated Vasculitis. International Journal of Molecular Sciences. 2024; 25(12):6479. https://doi.org/10.3390/ijms25126479
Chicago/Turabian StyleMartinez Valenzuela, Laura, Anna Vidal-Alabró, Belén Rubio, Paula Antón-Pàmpols, Francisco Gómez-Preciado, Xavier Fulladosa, Josep Maria Cruzado, Juan Torras, Nuria Lloberas, and Juliana Draibe. 2024. "Evaluating Single-Nucleotide Polymorphisms in Inflammasome Proteins and Serum Levels of IL-18 and IL-1β in Kidney Interstitial Damage in Anti-Neutrophilic Cytoplasmic Antibody-Associated Vasculitis" International Journal of Molecular Sciences 25, no. 12: 6479. https://doi.org/10.3390/ijms25126479
APA StyleMartinez Valenzuela, L., Vidal-Alabró, A., Rubio, B., Antón-Pàmpols, P., Gómez-Preciado, F., Fulladosa, X., Cruzado, J. M., Torras, J., Lloberas, N., & Draibe, J. (2024). Evaluating Single-Nucleotide Polymorphisms in Inflammasome Proteins and Serum Levels of IL-18 and IL-1β in Kidney Interstitial Damage in Anti-Neutrophilic Cytoplasmic Antibody-Associated Vasculitis. International Journal of Molecular Sciences, 25(12), 6479. https://doi.org/10.3390/ijms25126479







