A Novel 10-Base Pair Deletion in the First Exon of GmHY2a Promotes Hypocotyl Elongation, Induces Early Maturation, and Impairs Photosynthetic Performance in Soybean
Abstract
:1. Introduction
2. Results
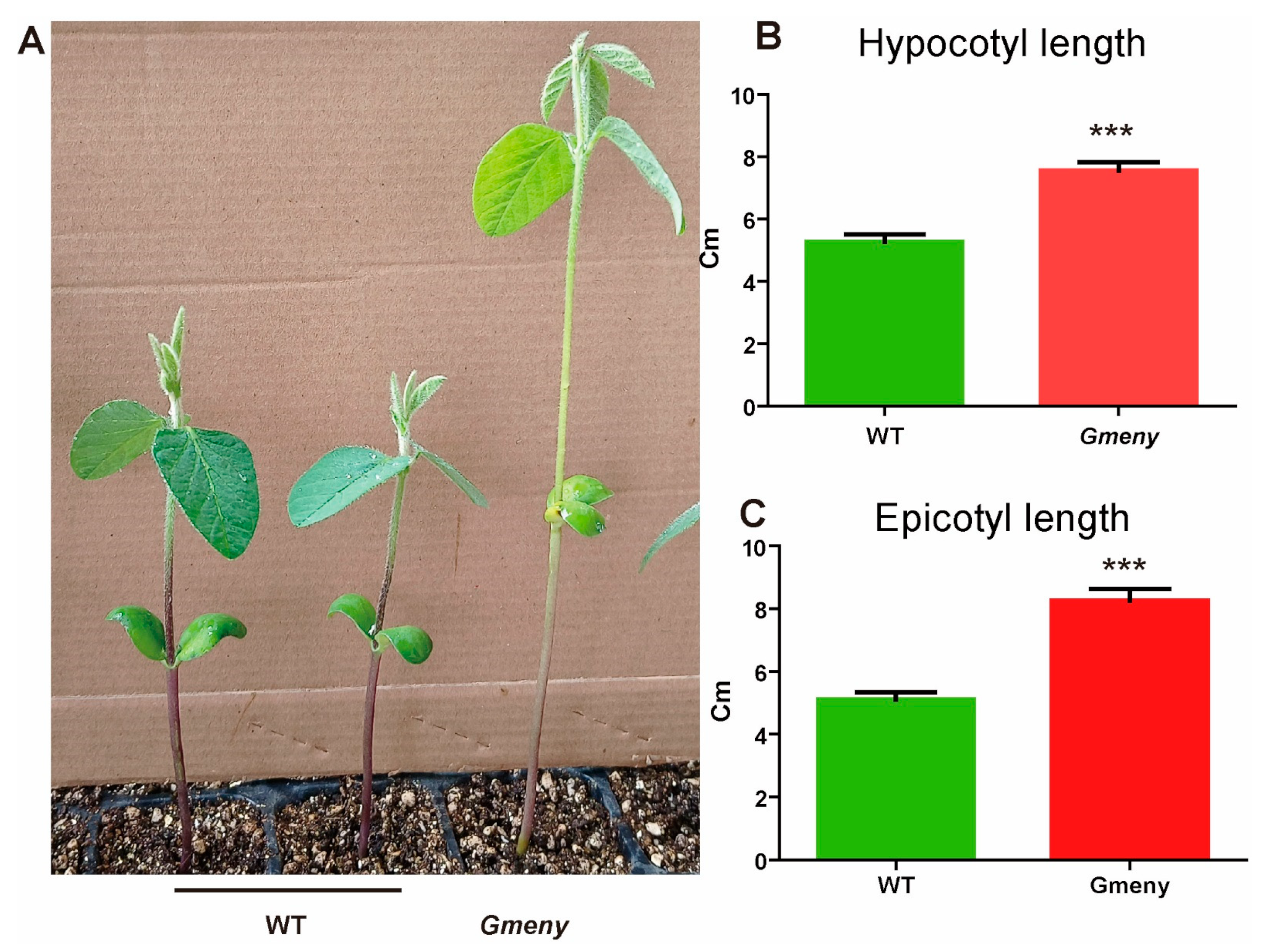
2.1. The Gmeny Mutant Displays Yellowing Leaves and Elongated Nodes
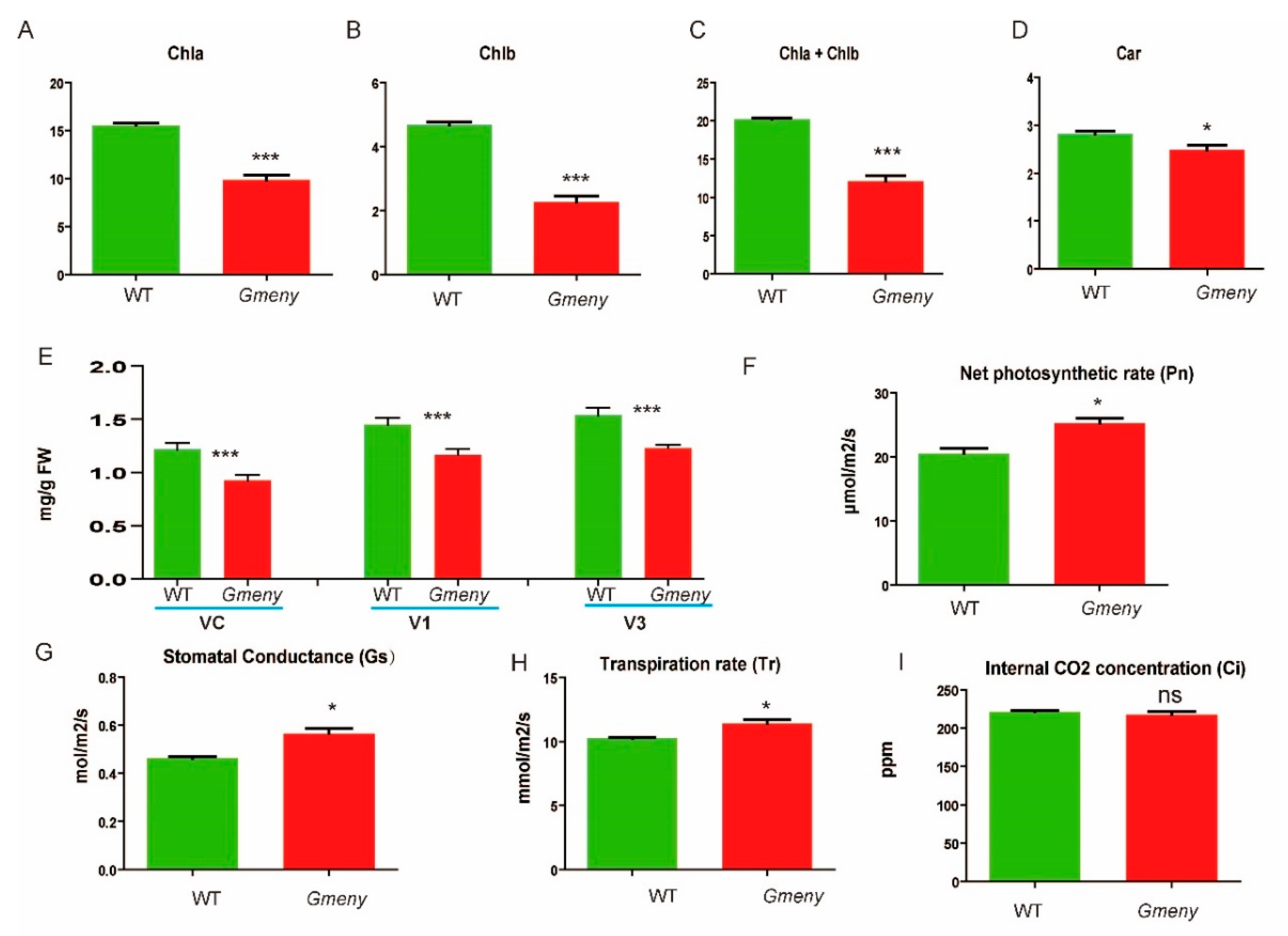
2.2. Yellowing Leaves of Gmeny Have Decreased Chlorophyll Contents and Changes in Photosynthetic Capacity
2.3. The BVF-IGV Pipeline Identified Glyma.02G304700 as the Candidate Gene for the Gmeny Phenotype
2.4. Functional Prediction and Evolutionary Analysis of Glyma.02G204700 in Soybean
2.5. Haplotypes of Glyma.02G204700
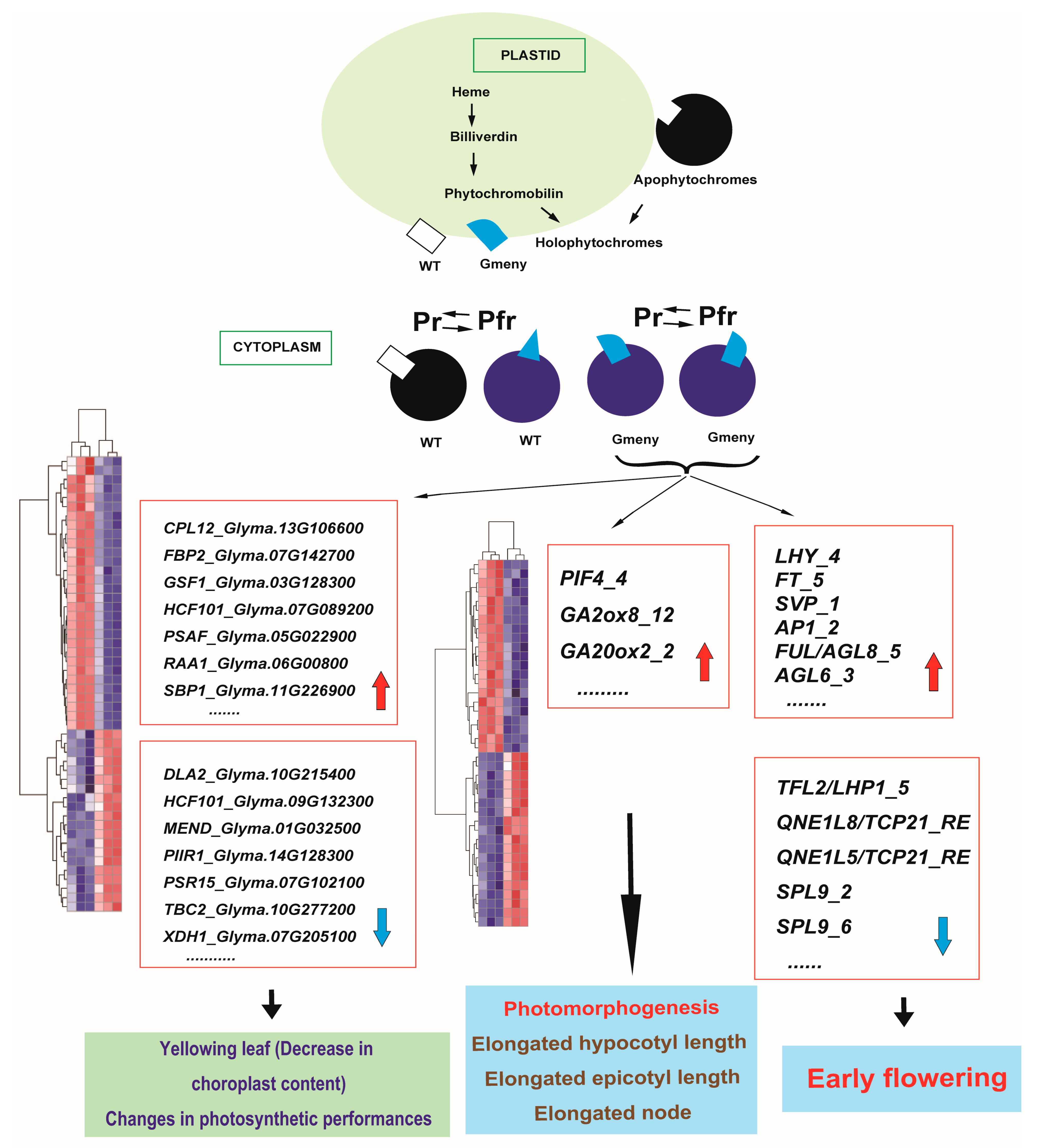
2.6. Transcriptome Analysis
3. Discussion
3.1. Mutation of a GmHY2a Gene Affects PΦB Biosynthesis, Inactivating the Photopigment System and Causing Diverse Changes in Photosynthesis, Photomorphogenesis, and Flowering Time
3.2. The BVF-IGV Pipeline Is Capable of Identifying the Causal Mutation for the Gmeny Mutant in One Step
4. Materials and Methods
4.1. Mutant Library Generation and Phenotyping
4.2. Measurement of Photosynthetic Pigments and Parameters
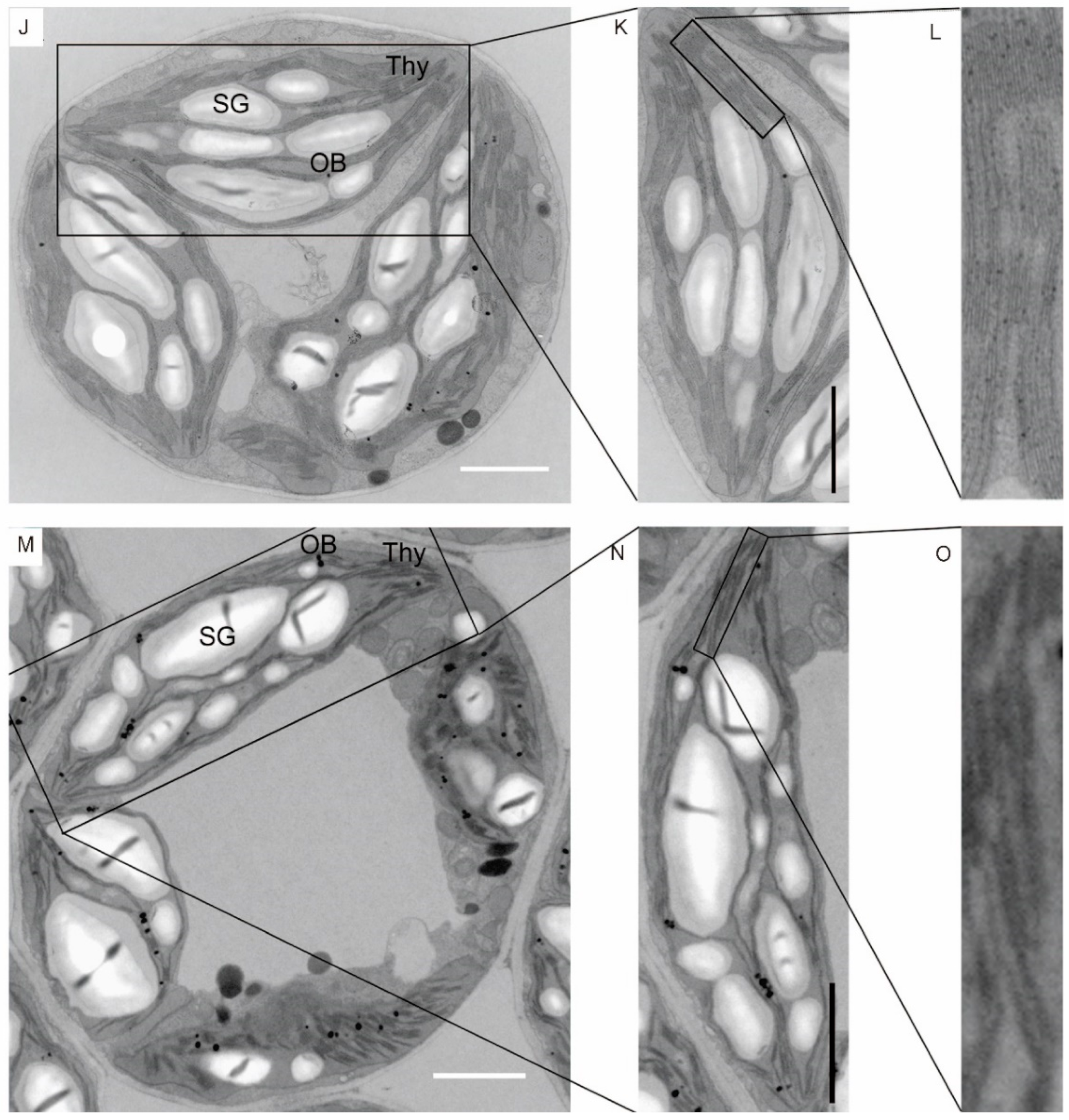
4.3. Transmission Electron Microscopy
4.4. Identification of the Causal Gene Using MutMap and BVF-IGV
4.5. Generation of the F2 Population and Association Analysis
4.6. Multiple-Sequence Alignment and Phylogenetic Analysis
4.7. Haplotype Analysis
4.8. RNA Sequencing and Transcriptome Analysis
4.9. Quantitative RT-PCR
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lashkari, M.; Madani, H.; Ardakani, M.R.; Golzardi, F.; Zargari, K. Effect of plant density on yield and yield components of different corn (Zea mays L.) hybrids. Educ. Manag. Adm. Leadersh. 2011, 10, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J. Photoreceptor Signaling Networks in Plant Responses to Shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y. Signaling Mechanisms of Higher Plant Photoreceptors: A Structure-Function Perspective. Curr. Top. Dev. Biol. 2005, 68, 227–261. [Google Scholar] [PubMed]
- Jing, Y.J.; Lin, R.C. Transcriptional Regulatory Network of the Light Signaling Pathways. New Phytol. 2020, 227, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Whitelam, G.C.; Johnson, E.; Peng, J.R.; Carol, P.; Anderson, M.L.; Cowl, J.S.; Harberd, N.P. Phytochrome a Null Mutants of Arabidopsis Display a Wild-Type Phenotype in White Light. Plant Cell 1993, 5, 757–768. [Google Scholar] [PubMed]
- Reed, J.W.; Nagpal, P.; Poole, D.S.; Furuya, M.; Chory, J. Mutations in the Gene for the Red Far-Red Light Receptor Phytochrome B Alter Cell Elongation and Physiological Responses throughout Arabidopsis Development. Plant Cell 1993, 5, 147–157. [Google Scholar] [PubMed]
- Burgie, E.S.; Vierstra, R.D. Phytochromes: An Atomic Perspective on Photoactivation and Signaling. Plant Cell 2014, 26, 4568–4583. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Ince, Y.Ç.; Fankhauser, C. Molecular Mechanisms Underlying Phytochrome-Controlled Morphogenesis in Plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.J.; Wahleithner, J.A.; Lagarias, J.C. Biosynthesis of the Plant Photoreceptor Phytochrome. Arch. Biochem. Biophys. 1993, 306, 1–15. [Google Scholar] [CrossRef]
- Kohchi, T.; Mukougawa, K.; Frankenberg, N.; Masuda, M.; Yokota, A.; Lagarias, J.C. The Arabidopsis HY2 Gene Encodes Phytochromobilin Synthase, a Ferredoxin-Dependent Biliverdin Reductase. Plant Cell 2001, 13, 425–436. [Google Scholar] [CrossRef]
- Weller, J.L.; Murfet, I.C.; Reid, J.B. Pea Mutants with Reduced Sensitivity to Far-Red Light Define an Important Role for Phytochrome a in Day-Length Detection. Plant Physiol. 1997, 114, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, Y.; Yokoo, T.; Saito, H.; Tsukiyama, T.; Quan, X.; Zikihara, K.; Katsura, H.; Tokutomi, S.; Aboshi, T.; Mori, N.; et al. The Effects of Phyto-chrome-Mediated Light Signals on the Developmental Acquisition of Photoperiod Sensitivity in Rice. Sci. Rep. 2015, 5, 7709. [Google Scholar] [CrossRef]
- Hu, L.L.; Liu, P.; Jin, Z.S.; Sun, J.; Weng, Y.Q.; Chen, P.; Du, S.L.; Wei, A.M.; Li, Y.H. A mutation in CsHY2 encoding a phytochromobilin (PΦB) synthase leads to an elongated hypocotyl 1 (elh1) phenotype in Cucumber (Cucumis sativus L). Theor. Appl. Genet. 2021, 134, 2639–2652. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Yang, S.X.; Wang, Q.S.; Yu, H.; Zhao, B.F.; Wu, T.; Tang, K.Q.; Ma, J.J.; Yang, X.J.; Feng, X.Z. Soybean Gmhy2a Encodes a Phytochromobilin Synthase That Regulates Internode Length and Flowering Time. J. Exp. Bot. 2022, 73, 6646–6662. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santangelo, S.; Napier, N.; Robson, F.; Weller, J.L.; Bond, D.M.; Macknight, R.C. A Point Mutation in Phytochromobilin synthase Alters the Circadian Clock and Photoperiodic Flowering of Medicago truncatula. Plants 2022, 11, 239. [Google Scholar] [CrossRef]
- Terry, M.J.; Kendrick, R.E. Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol. 1999, 119, 143–152. [Google Scholar] [CrossRef]
- Xiong, S.S.; Guo, D.D.; Wan, Z.; Quan, L.; Lu, W.T.; Xue, Y.G.; Liu, B.H.; Zhai, H. Regulation of Soybean Stem Growth Habit: A Ten-Year Progress Report. Crop J. 2023, 11, 1642–1648. [Google Scholar] [CrossRef]
- De Lucas, M.; Davière, J.M.; Rodríguez-Falcón, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blázquez, M.A.; Titarenko, E.; Prat, S. A Molecular Framework for Light and Gibberellin Control of Cell Elongation. Nature 2008, 451, 480–484. [Google Scholar] [CrossRef]
- Feng, S.H.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.L.; Wang, F.; Chen, L.Y.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated Regulation of Development by Light and Gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef]
- Achard, P.; Liao, L.L.; Jiang, C.F.; Desnos, T.; Bartlett, J.; Fu, X.D.; Harberd, N.P. Dellas Contribute to Plant Photomorphogenesis. Plant Physiol. 2007, 143, 1163–1172. [Google Scholar] [CrossRef]
- Li, K.L.; Yu, R.B.; Fan, L.M.; Wei, N.; Chen, H.D.; Deng, X.W. Della-Mediated Pif Degradation Contributes to Coordination of Light and Gibberellin Signalling In Arabidopsis. Nat. Commun. 2016, 7, 11868. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops That Feed the World 2. Soybean—Worldwide Production, Use, and Constraints Caused by Pathogens and Pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Abe, A.; Kosugi, S.; Yoshida, K.; Natsume, S.; Takagi, H.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Mitsuoka, C.; Tamiru, M.; et al. Genome Sequencing Reveals Agronomically Important Loci in Rice Using Mutmap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, J.L.; Zhai, H.; Xu, K.; Zhu, X.B.; Wu, H.Y.; Zhang, W.J.; Wu, S.H.; Chen, X.; Xia, Z.J. Dysfunction of an Anaphase-Promoting Complex Subunit 8 Homolog Leads to Super-Short Petioles and Enlarged Petiole Angles in Soybean. Int. J. Mol. Sci. 2023, 24, 11024. [Google Scholar] [CrossRef] [PubMed]
- Monte, E.; Tepperman, J.M.; Al-Sady, B.; Kaczorowski, K.A.; Alonso, J.M.; Ecker, J.R.; Li, X.; Zhang, Y.L.; Quail, P.H. The Phytochrome-Interacting Transcription Factor, Pif3, Acts Early, Selectively, and Positively in Light-Induced Chloroplast Development. Proc. Natl. Acad. Sci. USA 2004, 101, 16091–16098. [Google Scholar] [CrossRef]
- Bayer, P.E.; Valliyodan, B.; Hu, H.F.; Marsh, J.I.; Yuan, Y.X.; Vuong, T.D.; Patil, G.; Song, Q.J.; Batley, J.; Varshney, R.K.; et al. Sequencing the Usda Core Soybean Collection Reveals Gene Loss During Domestication and Breeding. Plant Genome 2022, 15, e20109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Jiang, H.; Hu, Z.B.; Song, Q.J.; An, Y.Q.C. Development of a Versatile Resource for Post-Genomic Research through Consolidating and Characterizing 1500 Diverse Wild and Cultivated Soybean Genomes. BMC Genom. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Kafri, M.; Patena, W.; Martin, L.; Wang, L.Y.; Gomer, G.; Ergun, S.L.; Sirkejyan, A.K.; Goh, A.; Wilson, A.T.; Gavrilenko, S.E.; et al. Systematic Identification and Characterization of Genes in the Regulation and Biogenesis of Photosynthetic Machinery. Cell 2023, 186, 5638–5655.e25. [Google Scholar] [CrossRef]
- Xia, Z.J.; Zhai, H.; Zhang, Y.F.; Wang, Y.Y.; Wang, L.; Xu, K.; Wu, H.Y.; Zhu, J.L.; Jiao, S.; Wan, Z.; et al. QNE1 Is a Key Flowering Regulator Determining the Length of the Vegetative Period in Soybean Cultivars. Sci. China Life Sci. 2022, 65, 2472–2490. [Google Scholar] [CrossRef]
- Zhang, S.R.; Wang, H.; Wang, Z.Y.; Ren, Y.; Niu, L.F.; Liu, J.; Liu, B. Photoperiodism Dynamics during the Domestication and Improvement of Soybean. Sci. China Life Sci. 2017, 60, 1416–1427. [Google Scholar] [CrossRef]
- Moon, J.; Zhu, L.; Shen, H.; Huq, E. Pif1 Directly and Indirectly Regulates Chlorophyll Biosynthesis to Optimize the Greening Process In Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 9433–9438. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, P.G.; Fankhauser, C.; Terry, M.J. Pif3 Is a Repressor of Chloroplast Development. Proc. Natl. Acad. Sci. USA 2009, 106, 7654–7659. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, P.G.; Terry, M.J. Light Signalling Pathways Regulating the Mg-Chelatase Branchpoint of Chlorophyll Synthesis During De-Etiolation In Arabidopsis thaliana. Photochem. Photobiol. Sci. 2008, 7, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jafari, F.; Wang, H. Integration of light and hormone signaling pathways in the regulation of plant shade avoidance syndrome. aBioTech 2021, 2, 131–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galstyan, A.; Cifuentes-Esquivel, N.; Bou-Torrent, J.; Martinez-Garcia, J.F. The Shade Avoidance Syndrome in Arabidopsis: A Fundamental Role for Atypical Basic Helix-Loop-Helix Proteins as Transcriptional Cofactors. Plant J. 2011, 66, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Hornitschek, P.; Lorrain, S.; Zoete, V.; Michielin, O.; Fankhauser, C. Inhibition of the Shade Avoidance Response by Formation of Non-DNA Binding Bhlh Heterodimers. Embo J. 2009, 28, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, H.B.; Ma, M.D.; Li, Q.Q.; Kong, D.X.; Sun, J.; Ma, X.J.; Wang, B.B.; Chen, C.X.; Xie, Y.R.; et al. Arabidopsis Fhy3 and Far1 Regulate the Balance between Growth and Defense Responses under Shade Conditions. Plant Cell 2019, 31, 2089–2106. [Google Scholar] [CrossRef]
- Crocco, C.D.; Locascio, A.; Escudero, C.M.; Alabadi, D.; Blázquez, M.A.; Botto, J.F. The Transcriptional Regulator Bbx24 Impairs Della Activity to Promote Shade Avoidance In Arabidopsis thaliana. Nat. Commun. 2015, 6, 6202. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.J.; Li, J.; Zhu, D.M.; Deng, X.W. Noncanonical Role of Cop1/Spa Complex in Repressing Bin2-Mediated Pif3 Phosphorylation and Degradation in Darkness. Proc. Natl. Acad. Sci. USA 2017, 114, 3539–3544. [Google Scholar] [CrossRef]
- Blanco-Touriñán, N.; Legris, M.; Minguet, E.G.; Costigliolo-Rojas, C.; Nohales, M.A.; Iniesto, E.; García-León, M.; Pacin, M.; Heucken, N.; Bloemeir, T.; et al. Cop1 Destabilizes Della Proteins In Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 13792–13799. [Google Scholar] [CrossRef]
- Xia, Z.J.; Watanabe, S.; Yamada, T.; Tsubokura, Y.; Nakashima, H.; Zhai, H.; Anai, T.; Sato, S.; Yamazaki, T.; Lü, S.X.; et al. Positional Cloning and Characterization Reveal the Molecular Basis for Soybean Maturity Locus E1 That Regulates Photoperiodic Flowering. Proc. Natl. Acad. Sci. USA 2012, 109, E2155–E2164. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Wan, Z.; Jiao, S.; Zhou, J.W.; Xu, K.; Nan, H.Y.; Liu, Y.X.; Xiong, S.S.; Fan, R.; Zhu, J.L.; et al. GmMDE Genes Bridge the Maturity Gene E1 and Florigens in Photoperiodic Regulation of Flowering in Soy-bean. Plant Physiol. 2022, 189, 1021–1036. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Wigge, P.A. Ft Protein Acts as a Long-Range Signal In Arabidopsis. Curr. Biol. 2007, 17, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts—Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Feng, X.X.; Yang, S.X.; Tang, K.Q.; Zhang, Y.H.; Leng, J.T.; Ma, J.J.; Wang, Q.; Feng, X.Z. GmPGL1, a Thiamine Thiazole Synthase, Is Required for the Biosynthesis of Thiamine in Soybean. Front. Plant Sci. 2019, 10, 1546. [Google Scholar] [CrossRef]
- Tomsone, L.; Kruma, Z. Spectrophotometric Analysis of Pigments in Horseradish by Using Various Extraction Solvents. In Proceedings of the Foodbalt 2019: 13th Baltic Conference on Food Science and Technology, Jelgava, Latvia, 2–3 May 2019; pp. 210–215. [Google Scholar]
- Zhang, C.Y.; Li, Y.S.; Yu, Z.H.; Wang, G.H.; Liu, X.B.; Liu, J.J.; Liu, J.D.; Zhang, X.M.; Yin, K.D.; Jin, J. Co-Elevation of Atmospheric [CO2] and Temperature Alters Photosynthetic Capacity and Instantaneous Water Use Efficiency in Rice Cultivars in a Cold-Temperate Region. Front. Plant Sci. 2022, 13, 1037720. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Q.S.; Zhang, Z.R.; Wu, T.; Yang, X.J.; Zhu, X.B.; Ye, Y.H.; Leng, J.T.; Yang, S.X.; Feng, X.Z. Genetic Mapping of the Gmpgl3 Mutant Reveals the Function of Gmtic110a in Soybean Chloroplast Development. Front. Plant Sci. 2022, 13, 892077. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.B.; Zheng, K.J.; Lu, L.; Yu, H.; Wang, F.W.; Yang, X.J.; Bhat, J.A.; Zhao, B.F.; Wang, Y.; Li, H.Y.; et al. Disruption of Chorismate Synthase1 Leads to Yellow-Green Variegation in Soybean Leaves. J. Exp. Bot. 2023, 74, 4014–4030. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. Ngs Qc Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Chiang, C.; Layer, R.M.; Faust, G.G.; Lindberg, M.R.; Rose, D.B.; Garrison, E.P.; Marth, G.; Quinlan, A.; Hall, I.M. SpeedSeq: Ultra-fast personal genome analysis and interpretation. Nat. Methods 2015, 12, 966–968. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.Y.; Ruden, D.M. A program for annotatingand predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The Clustal_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- French, N.; Yu, S.; Biggs, P.; Holland, B.; Fearnhead, P.; Binney, B.; Fox, A.; Grove-White, D.; Leigh, J.W.; Miller, W.; et al. Evolution of Campylobacter species in New Zealand. In Campylobacter Ecology and Evolution; Sheppard, S.K., Méric, G., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 221–240. [Google Scholar]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. Cluego: A Cytoscape Plug-in to Decipher Functionally Grouped Gene Ontology and Pathway Annotation Networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time Pcr Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wang, H.; Li, Y.; Rao, D.; Wang, F.; Gao, Y.; Zhong, W.; Zhao, Y.; Wu, S.; Chen, X.; et al. A Novel 10-Base Pair Deletion in the First Exon of GmHY2a Promotes Hypocotyl Elongation, Induces Early Maturation, and Impairs Photosynthetic Performance in Soybean. Int. J. Mol. Sci. 2024, 25, 6483. https://doi.org/10.3390/ijms25126483
Zhu X, Wang H, Li Y, Rao D, Wang F, Gao Y, Zhong W, Zhao Y, Wu S, Chen X, et al. A Novel 10-Base Pair Deletion in the First Exon of GmHY2a Promotes Hypocotyl Elongation, Induces Early Maturation, and Impairs Photosynthetic Performance in Soybean. International Journal of Molecular Sciences. 2024; 25(12):6483. https://doi.org/10.3390/ijms25126483
Chicago/Turabian StyleZhu, Xiaobin, Haiyan Wang, Yuzhuo Li, Demin Rao, Feifei Wang, Yi Gao, Weiyu Zhong, Yujing Zhao, Shihao Wu, Xin Chen, and et al. 2024. "A Novel 10-Base Pair Deletion in the First Exon of GmHY2a Promotes Hypocotyl Elongation, Induces Early Maturation, and Impairs Photosynthetic Performance in Soybean" International Journal of Molecular Sciences 25, no. 12: 6483. https://doi.org/10.3390/ijms25126483




