The Complex Intracellular Lifecycle of Staphylococcus aureus Contributes to Reduced Antibiotic Efficacy and Persistent Bacteremia
Abstract
1. Introduction
2. The Clinical Challenges of Persistent Staphylococcus aureus Bacteremia (SAB)
3. Treatment Approaches for Persistent SAB
4. Small-Colony-Variant S. aureus Results in Reduced Antibiotic Efficacy and Infection Persistence
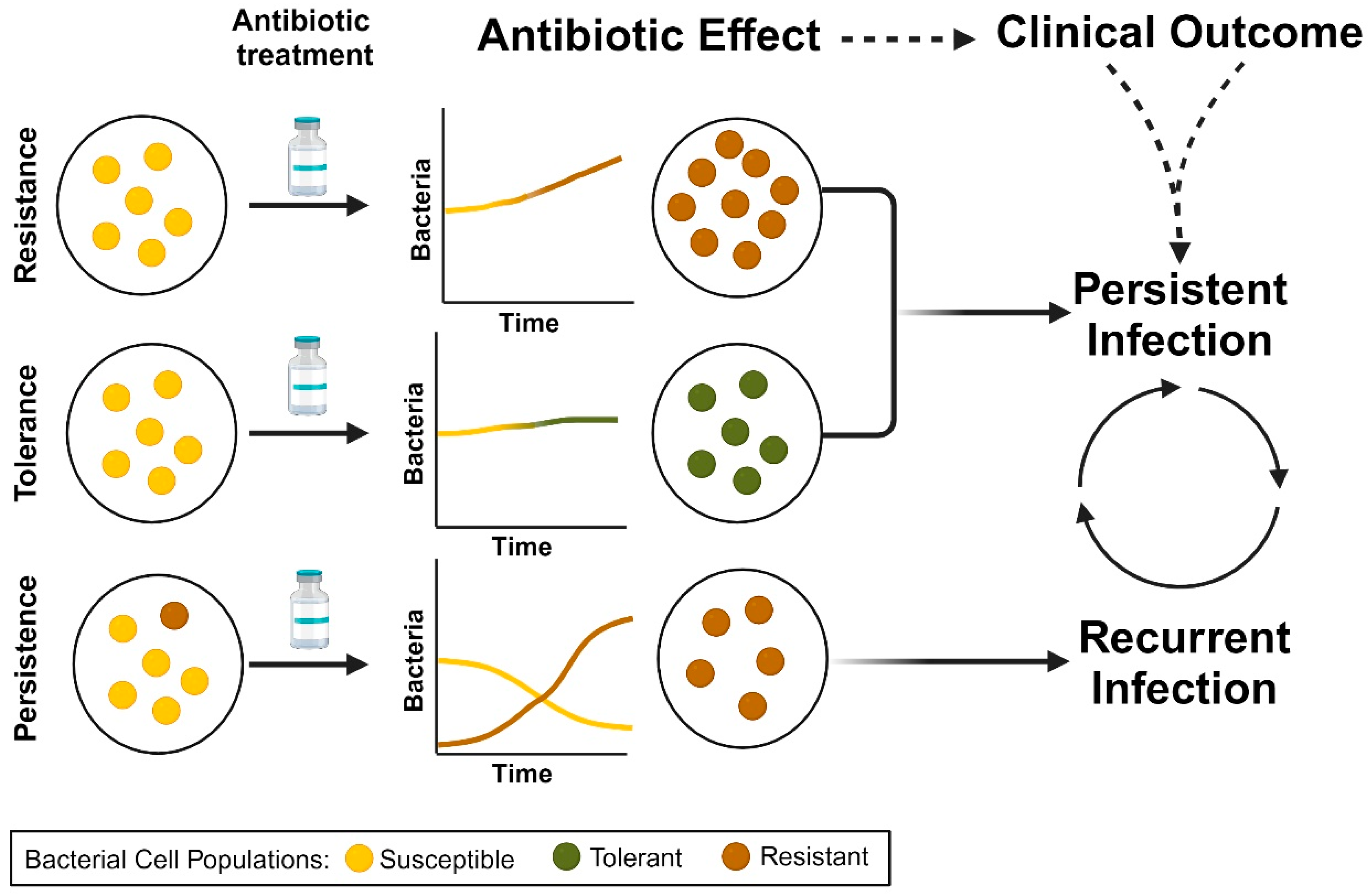
5. Antimicrobial Resistance, Tolerance, and Persistence in S. aureus Contribute to Persistent Bacteremia
6. Proclivity of Staphylococcus aureus for Intracellular Growth and Persistence
7. Intracellular S. aureus Reduces Immune Recognition and Activation
8. Anaerobic Metabolism of S. aureus Correlates to Intracellular Growth
9. Intracellular S. aureus Is More Resistant to the Effects of Antibiotics through Multifaceted Mechanisms
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodvold, K.A.; McConeghy, K.W. Methicillin-Resistant Staphylococcus aureus Therapy: Past, Present, and Future. Clin. Infect. Dis. 2014, 58, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.L.; Bayer, A.S.; Fowler, V.G. Persistent Methicilin-Resistant Staphylococcus aureus Bacteremia: Resetting the Clock for Optimal Management. Clin. Infect. Dis. 2022, 75, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Sakoulas, G.; Deresinski, S.; van Hal, S.J. When sepsis persists: A review of MRSA bacteraemia salvage therapy. J. Antimicrob. Chemother. 2015, 71, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; Perez, K.K.; Forrest, G.N.; Goff, D.A. Review of rapid diagnostic tests used by antimicrobial stewardship pro-grams. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S134–S145. [Google Scholar] [CrossRef]
- Patel, R.; Fang, F.C. Diagnostic Stewardship: Opportunity for a Laboratory–Infectious Diseases Partnership. Clin. Infect. Dis. 2018, 67, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, N.; Quinn, E.L.; Saravolatz, L.D. Trimethoprim-Sulfamethoxazole Compared with Vancomycin for the Treatment of Staphylococcus aureus Infection. Ann. Intern. Med. 1992, 117, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.P.; Fromm, B.S.; Reddy, B.R. Slow Response to Vancomycin or Vancomycin plus Rifampin in Methicillin-resistant Staphylococcus aureus Endocarditis. Ann. Intern. Med. 1991, 115, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Eickhoff, J.C.; Shukla, S.K.; Pantrangi, M.; Rooijakkers, S.; Cosgrove, S.E.; Nizet, V.; Sakoulas, G. Elevated Serum Interleukin-10 at Time of Hospital Admission Is Predictive of Mortality in Patients with Staphylococcus aureus Bacteremia. J. Infect. Dis. 2012, 206, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; McKinnell, J.A.; Sakoulas, G. Avoiding the Perfect Storm: The Biologic and Clinical Case for Reevaluating the 7-Day Expectation for Methicillin-Resistant Staphylococcus aureus Bacteremia Before Switching Therapy. Clin. Infect. Dis. 2014, 59, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Shukla, S.K.; Berti, A.D.; Hayney, M.S.; Henriquez, K.M.; Ranzoni, A.; Cooper, M.A.; Proctor, R.A.; Nizet, V.; Sakoulas, G. Increased Endovascular Staphylococcus aureus Inoculum Is the Link Between Elevated Serum Interleukin 10 Concentrations and Mortality in Patients with Bacteremia. Clin. Infect. Dis. 2017, 64, 1406–1412. [Google Scholar] [CrossRef]
- Kuehl, R.; Morata, L.; Boeing, C.; Subirana, I.; Seifert, H.; Rieg, S.; Bin Kim, H.; Kim, E.S.; Liao, C.-H.; Tilley, R.; et al. Defining persistent Staphylococcus aureus bacteraemia: Secondary analysis of a prospective cohort study. Lancet Infect. Dis. 2020, 20, 1409–1417. [Google Scholar] [CrossRef]
- Holland, T.L.; Cosgrove, S.E.; Doernberg, S.B.; Jenkins, T.C.; Turner, N.A.; Boucher, H.W.; Pavlov, O.; Titov, I.; Kosulnykov, S.; Atanasov, B.; et al. Ceftobiprole for Treatment of Complicated Staphylococcus aureus Bacteremia. N. Engl. J. Med. 2023, 389, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial Salvage Therapy for Persistent Staphylococcal Bacteremia Using Daptomycin Plus Ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
- Rose, W.E.; Schulz, L.T.; Andes, D.; Striker, R.; Berti, A.D.; Hutson, P.R.; Shukla, S.K. Addition of Ceftaroline to Daptomycin after Emergence of Daptomycin-Nonsusceptible Staphylococcus aureus during Therapy Improves Antibacterial Activity. Antimicrob. Agents Chemother. 2012, 56, 5296–5302. [Google Scholar] [CrossRef]
- Lin, J.C.; Aung, G.; Thomas, A.; Jahng, M.; Johns, S.; Fierer, J. The use of ceftaroline fosamil in methicillin-resistant Staphy-lococcus aureus endocarditis and deep-seated MRSA infections: A retrospective case series of 10 patients. J. Infect. Chemother. 2013, 19, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.; Fantl, M.; Geriak, M.; Nizet, V.; Sakoulas, G. Current Paradigms of Combination Therapy in Methicillin-Resistant Staphylococcus aureus (MRSA) Bacteremia: Does it Work, which Combination, and for which Patients? Clin. Infect. Dis. 2021, 73, 2353–2360. [Google Scholar] [CrossRef]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of Mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus Standard Therapy for Bacteremia and Endocarditis Caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Pujol, M.; Miro, J.M.; Shaw, E.; Aguado, J.M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Alvarez, R.; et al. Daptomycin Plus Fosfomycin versus Daptomycin Alone for Methicillin-resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. 2021, 72, 1517–1525. [Google Scholar] [CrossRef]
- Davis, J.S.; Sud, A.; O’Sullivan, M.V.; Robinson, J.O.; Ferguson, P.E.; Foo, H.; van Hal, S.J.; Ralph, A.P.; Howden, B.P.; Binks, P.M.; et al. Combination of Vancomycin and beta-Lactam Therapy for Methicillin-Resistant Staphylococcus aureus Bacteremia: A Pilot Multicenter Randomized Controlled Trial. Clin. Infect. Dis. 2016, 62, 173–180. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; et al. Effect of Vancomycin or Daptomycin with vs without an Antistaphylococcal β-Lactam on Mortality, Bacteremia, Relapse, or Treatment Failure in Patients with MRSA Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Staphylococcus aureus endocarditis: A consequence of medical progress. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Rose, W.; Schrodi, S.J. Complex host genetic susceptibility to Staphylococcus aureus infections. Trends Microbiol. 2015, 23, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef]
- Rooijakkers, S.H.; van Kessel, K.P.; van Strijp, J.A. Staphylococcal innate immune evasion. Trends Microbiol. 2005, 13, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Minejima, E.; Bensman, J.; She, R.C.; Mack, W.J.; Tuan Tran, M.; Ny, P.; Lou, M.; Yamaki, J.; Nieberg, P.; Ho, J.; et al. A Dysregulated Balance of Proinflammatory and Anti-Inflammatory Host Cytokine Response Early during Therapy Predicts Persistence and Mortality in Staphylococcus aureus Bacteremia. Crit. Care Med. 2016, 44, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Volk, C.F.; Burgdorf, S.; Edwardson, G.; Nizet, V.; Sakoulas, G.; Rose, W.E. Interleukin (IL)-1beta and IL-10 Host Responses in Patients with Staphylococcus aureus Bacteremia Determined by Antimicrobial Therapy. Clin. Infect. Dis. 2020, 70, 2634–2640. [Google Scholar] [CrossRef]
- Wozniak, J.M.; Mills, R.H.; Olson, J.; Caldera, J.; Sepich-Poore, G.D.; Carrillo-Terrazas, M.; Tsai, C.-M.; Vargas, F.; Knight, R.; Dorrestein, P.C.; et al. Mortality Risk Profiling of Staphylococcus aureus Bacteremia by Multiomic Serum Analysis Reveals Early Predictive and Pathogenic Signatures. Cell 2020, 182, 1311–1327. [Google Scholar] [CrossRef]
- Ferry, T.; Thomas, D.; Genestier, A.-L.; Bes, M.; Lina, G.; Vandenesch, F.; Etienne, J. Comparative Prevalence of Superantigen Genes in Staphylococcus aureus Isolates Causing Sepsis with and without Septic Shock. Clin. Infect. Dis. 2005, 41, 771–777. [Google Scholar] [CrossRef]
- Welsh, K.J.; Abbott, A.N.; Lewis, E.M.; Gardiner, J.M.; Kruzel, M.C.; Lewis, C.T.; Mohr, J.F.; Wanger, A.; Armitige, L.Y. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J. Clin. Microbiol. 2010, 48, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Gillet, Y.; Issartel, B.; Vanhems, P.; Fournet, J.C.; Lina, G.; Bes, M.; Vandenesch, F.; Piemont, Y.; Brousse, N.; Floret, D.; et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002, 359, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.P.; Ajao, A.O.; Aman, M.J.; Karauzum, H.; Sarwar, J.; Lydecker, A.D.; Johnson, J.K.; Nguyen, C.; Chen, W.H.; Roghmann, M.-C. Lower Antibody Levels to Staphylococcus aureus Exotoxins Are Associated with Sepsis in Hospitalized Adults with Invasive, S. aureus Infections. J. Infect. Dis. 2012, 206, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.P.; Kort, T.; Shulenin, S.; Kanipakala, T.; Ganjbaksh, N.; Roghmann, M.C.; Holtsberg, F.W.; Aman, M.J. Antibodies to S. aureus LukS-PV Attenuated Subunit Vaccine Neutralize a Broad Spectrum of Canonical and Non-Canonical Bi-component Leukotoxin Pairs. PLoS ONE 2015, 10, e0137874. [Google Scholar] [CrossRef]
- Fritz, S.A.; Tiemann, K.M.; Hogan, P.G.; Epplin, E.K.; Rodriguez, M.; Al-Zubeidi, D.N.; Wardenburg, J.B.; Hunstad, D.A. A Serologic Correlate of Protective Immunity Against Community-Onset Staphylococcus aureus Infection. Clin. Infect. Dis. 2013, 56, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- François, B.; Mercier, E.; Gonzalez, C.; Asehnoune, K.; Nseir, S.; Fiancette, M.; Desachy, A.; Plantefève, G.; Meziani, F.; de Lame, P.-A.; et al. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: First-in-human trial. Intensive Care Med. 2018, 44, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.J.; Dotel, R.; Braddick, M.; Britton, P.N.; Eisen, D.P.; Francis, J.R.; Lynar, S.; McMullan, B.; Meagher, N.; Nelson, J.; et al. Clindamycin adjunctive therapy for severe Staphylococcus aureus treatment evaluation (CASSETTE)-an open-labelled pilot randomized controlled trial. JAC Antimicrob. Resist. 2022, 4, dlac014. [Google Scholar] [CrossRef] [PubMed]
- Anpalagan, K.; Dotel, R.; MacFadden, D.R.; Smith, S.; Voss, L.; Petersiel, N.; Marks, M.; Marsh, J.; Mahar, R.K.; McGlothlin, A.; et al. Does adjunctive clindamycin have a role in Staphylococcus aureus bacteremia? A protocol for the adjunctive treatment domain of the S. aureus Network Adaptive Platform (SNAP) randomized controlled trial. Clin. Infect. Dis. 2024, ciae28. [Google Scholar] [CrossRef]
- Proctor, R.A.; Balwit, J.M.; Vesga, O. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 1994, 3, 302–312. [Google Scholar]
- von Eiff, C. Staphylococcus aureus small colony variants: A challenge to microbiologists and clinicians. Int. J. Antimicrob. Agents 2008, 31, 507–510. [Google Scholar] [CrossRef]
- Kahl, B.C.; Belling, G.; Becker, P.; Chatterjee, I.; Wardecki, K.; Hilgert, K.; Cheung, A.L.; Peters, G.; Herrmann, M. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect. Immun. 2005, 73, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Kolle, W.; Hetsch, H. Die Experimentelle Bakteriologie und die Infektionskrankheiten mit besonderer Berücksichtigung der Immunitätslehre; Urban und Schwarzenberg: Berlin, Germany, 1911; Volume 1. [Google Scholar]
- Bigger, J.W.; Boland, C.R.; O’meara, R.A.Q. Variant colonies of Staphylococcus aureus. J. Pathol. Bacteriol. 1927, 30, 261–269. [Google Scholar] [CrossRef]
- Youmans, G.P. Production of Small-Colony Variants of Staphylococcus aureus. Proc. Soc. Exp. Biol. Med. 1937, 36, 94–96. [Google Scholar] [CrossRef]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C.; Becker, K.; Löffler, B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef]
- Maduka-Ezeh, A.N.; Greenwood-Quaintance, K.E.; Karau, M.J.; Berbari, E.F.; Osmon, D.R.; Hanssen, A.D.; Steckelberg, J.M.; Patel, R. Antimicrobial susceptibility and biofilm formation of Staphylococcus epidermidis small colony variants associated with prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2012, 74, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; van Bambeke, F. Antibiotic activity against small-colony variants of Staphylococcus aureus: Review of in vitro, animal and clinical data. J. Antimicrob. Chemother. 2013, 68, 1455–1464. [Google Scholar] [CrossRef]
- Baltch, A.L.; Ritz, W.J.; Bopp, L.H.; Michelsen, P.; Smith, R.P. Activities of daptomycin and comparative antimicrobials, singly and in combination, against extracellular and intracellular Staphylococcus aureus and its stable small-colony variant in human monocyte-derived macrophages and in broth. Antimicrob. Agents Chemother. 2008, 52, 1829–1833. [Google Scholar] [CrossRef]
- Kriegeskorte, A.; Grubmuller, S.; Huber, C.; Kahl, B.C.; von Eiff, C.; Proctor, R.A.; Peters, G.; Eisenreich, W.; Becker, K. Staphylococcus aureus small colony variants show common metabolic features in central metabolism irrespective of the un-derlying auxotrophism. Front. Cell. Infect. Microbiol. 2014, 4, 141. [Google Scholar] [CrossRef]
- Mishra, N.N.; Bayer, A.S.; Weidenmaier, C.; Grau, T.; Wanner, S.; Stefani, S.; Cafiso, V.; Bertuccio, T.; Yeaman, M.R.; Nast, C.C.; et al. Phenotypic and Genotypic Characterization of Daptomycin-Resistant Methicillin-Resistant Staphylococcus aureus Strains: Relative Roles of mprF and dlt Operons. PLoS ONE 2014, 9, e107426. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Schweizer, F.; Karlowsky, J.A. Oritavancin: Mechanism of Action. Clin. Infect. Dis. 2012, 54, S214–S219. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Burillo, A. Oritavancin: A novel lipoglycopeptide active against Gram-positive pathogens including multiresistant strains. Int. J. Antimicrob. Agents 2010, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Boulet, M.L.; Isabelle, C.; Guay, I.; Brouillette, E.; Langlois, J.-P.; Jacques, P.; Rodrigue, S.; Brzezinski, R.; Beauregard, P.B.; Bouarab, K.; et al. Tomatidine Is a Lead Antibiotic Molecule That Targets Staphylococcus aureus ATP Synthase Subunit C. Antimicrob. Agents Chemother. 2018, 62, e02197-17. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.-P.; Millette, G.; Guay, I.; Dubé-Duquette, A.; Chamberland, S.; Jacques, P.; Rodrigue, S.; Bouarab, K.; Marsault, É.; Malouin, F. Bactericidal Activity of the Bacterial ATP Synthase Inhibitor Tomatidine and the Combination of Tomatidine and Aminoglycoside against Persistent and Virulent Forms of Staphylococcus aureus. Front. Microbiol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Monk, J.M.; Szubin, R.; Berti, A.D. Rapid resistance development to three antistaphylococcal therapies in anti-biotic-tolerant Staphylococcus aureus bacteremia. PLoS ONE 2021, 16, e0258592. [Google Scholar] [CrossRef] [PubMed]
- Berti, A.D.; Shukla, N.; Rottier, A.D.; McCrone, J.S.; Turner, H.M.; Monk, I.R.; Baines, S.L.; Howden, B.P.; Proctor, R.A.; Rose, W.E. Daptomycin selects for genetic and phenotypic adaptations leading to antibiotic tolerance in MRSA. J. Antimicrob. Chemother. 2018, 73, 2030–2033. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Berti, A.D.; Hirsch, E.B. Tolerance to antibiotics affects response. Science 2020, 367, 141–142. [Google Scholar] [CrossRef]
- Windels, E.M.; Michiels, J.E.; van den Bergh, B.; Fauvart, M.; Michiels, J. Antibiotics: Combatting Tolerance to Stop Resistance. mBio 2019, 10, e02095-19. [Google Scholar] [CrossRef] [PubMed]
- Meredith, E.M.; Harven, L.T.; Berti, A.D. Antimicrobial Efficacy against Antibiotic-Tolerant Staphylococcus aureus Depends on the Mechanism of Antibiotic Tolerance. Antibiotics 2022, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Peyrusson, F.; Varet, H.; Nguyen, T.K.; Legendre, R.; Sismeiro, O.; Coppee, J.Y.; Wolz, C.; Tenson, T.; van Bambeke, F. In-tracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat. Commun. 2020, 11, 2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bojer, M.S.; George, S.E.; Wang, Z.; Jensen, P.R.; Wolz, C.; Ingmer, H. Inactivation of TCA cycle enhances Staph-ylococcus aureus persister cell formation in stationary phase. Sci. Rep. 2018, 8, 10849. [Google Scholar] [CrossRef]
- Zalis, E.A.; Nuxoll, A.S.; Manuse, S.; Clair, G.; Radlinski, L.C.; Conlon, B.P.; Adkins, J.; Lewis, K. Stochastic Variation in Expression of the Tricarboxylic Acid Cycle Produces Persister Cells. mBio 2019, 10. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Loffler, B.; Proctor, R.A. Persistence of Staphylococcus aureus: Multiple Metabolic Pathways Impact the Ex-pression of Virulence Factors in Small-Colony Variants (SCVs). Front. Microbiol. 2020, 11, 1028. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Raad, I.; Hanna, H.; Jiang, Y.; Dvorak, T.; Reitzel, R.; Chaiban, G.; Sherertz, R.; Hachem, R. Comparative activities of dap-tomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 2007, 51, 1656–1660. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S. Effect of antibacterials on biofilms. Am. J. Infect. Control 2008, 36, S175.e9–S175.e11. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Theis, T.J.; Daubert, T.A.; Kluthe, K.E.; Brodd, K.L.; Nuxoll, A.S. Staphylococcus aureus persisters are associated with reduced clearance in a catheter-associated biofilm infection. Front. Cell. Infect. Microbiol. 2023, 13, 1178526. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Rolston, K.V. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006, 106, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, G.; Luo, J.; Cheng, J.; Wu, D.; Cheng, L.; Huang, X.; Fu, S.; Liu, J. Staphylococcus aureus induces mitophagy to promote its survival within bovine mammary epithelial cells. Vet. Microbiol. 2023, 280, 109697. [Google Scholar] [CrossRef] [PubMed]
- Rollin, G.; Tan, X.; Tros, F.; Dupuis, M.; Nassif, X.; Charbit, A.; Coureuil, M. Intracellular Survival of Staphylococcus aureus in Endothelial Cells: A Matter of Growth or Persistence. Front. Microbiol. 2017, 8, 1354. [Google Scholar] [CrossRef]
- Perez, K.; Patel, R. Survival of Staphylococcus epidermidis in Fibroblasts and Osteoblasts. Infect. Immun. 2018, 86, 10. [Google Scholar] [CrossRef]
- Abu-Humaidan, A.H.; Elvén, M.; Sonesson, A.; Garred, P.; Sørensen, O.E. Persistent Intracellular Staphylococcus aureus in Keratinocytes Lead to Activation of the Complement System with Subsequent Reduction in the Intracellular Bacterial Load. Front. Immunol. 2018, 9, 396. [Google Scholar] [CrossRef]
- Prajsnar, T.K.; Serba, J.J.; Dekker, B.M.; Gibson, J.F.; Masud, S.; Fleming, A.; Johnston, S.A.; Renshaw, S.A.; Meijer, A.H. The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils. Autophagy 2020, 17, 888–902. [Google Scholar] [CrossRef]
- Yang, D.; Wijenayaka, A.R.; Solomon, L.B.; Pederson, S.M.; Findlay, D.M.; Kidd, S.P.; Atkins, G.J. Novel Insights into Staphylococcus aureus Deep Bone Infections: The Involvement of Osteocytes. mBio 2018, 9, e00415-18. [Google Scholar] [CrossRef]
- Zelmer, A.R.; Nelson, R.; Richter, K.; Atkins, G.J. Can intracellular Staphylococcus aureus in osteomyelitis be treated using current antibiotics? A systematic review and narrative synthesis. Bone Res. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Clement, S.; Vaudaux, P.; Francois, P.; Schrenzel, J.; Huggler, E.; Kampf, S.; Chaponnier, C.; Lew, D.; Lacroix, J. Evidence of an Intracellular Reservoir in the Nasal Mucosa of Patients with Recurrent Staphylococcus aureus Rhinosinusitis. J. Infect. Dis. 2005, 192, 1023–1028. [Google Scholar] [CrossRef]
- Ou, J.; Bassiouni, A.; Drilling, A.; Psaltis, A.; Vreugde, S.; Wormald, P. The persistence of intracellular Staphylococcus aureus in the sinuses: A longitudinal study. Rhinol. J. 2017, 55, 305–311. [Google Scholar] [CrossRef]
- Zautner, A.E.; Krause, M.; Stropahl, G.; Holtfreter, S.; Frickmann, H.; Maletzki, C.; Kreikemeyer, B.; Pau, H.W.; Podbielski, A. Intracellular Persisting Staphylococcus aureus Is the Major Pathogen in Recurrent Tonsillitis. PLoS ONE 2010, 5, e9452. [Google Scholar] [CrossRef]
- Ellington, J.K.; Harris, M.; Webb, L.; Smith, B.; Smith, T.; Tan, K.; Hudson, M. Intracellular Staphylococcus aureus. A mech-anism for the indolence of osteomyelitis. J. Bone Jt. Surg. Br. Vol. 2003, 85, 918–921. [Google Scholar] [CrossRef]
- Torlakovic, E.; Hibbs, J.R.; Miller, J.S.; Litz, C.E. Intracellular Bacteria in Blood Smears in Patients with Central Venous Catheters. Arch. Intern. Med. 1995, 155, 1547–1550. [Google Scholar] [CrossRef]
- Grundmeier, M.; Tuchscherr, L.; Brück, M.; Viemann, D.; Roth, J.; Willscher, E.; Becker, K.; Peters, G.; Löffler, B. Staphylococcal Strains Vary Greatly in Their Ability to Induce an Inflammatory Response in Endothelial Cells. J. Infect. Dis. 2010, 201, 871–880. [Google Scholar] [CrossRef]
- Menzies, B.E.; Kourteva, I. Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol. Med. Microbiol. 2000, 29, 39–45. [Google Scholar] [CrossRef]
- Schnaith, A.; Kashkar, H.; Leggio, S.A.; Addicks, K.; Krönke, M.; Krut, O. Staphylococcus aureus Subvert Autophagy for Induction of Caspase-independent Host Cell Death. J. Biol. Chem. 2007, 282, 2695–2706. [Google Scholar] [CrossRef]
- Soong, G.; Chun, J.; Parker, D.; Prince, A. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J. Infect. Dis. 2012, 205, 1571–1579. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Medina, E.; Hussain, M.; Völker, W.; Heitmann, V.; Niemann, S.; Holzinger, D.; Roth, J.; Proctor, R.A.; Becker, K.; et al. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 2011, 3, 129–141. [Google Scholar] [CrossRef]
- Löffler, B.; Tuchscherr, L.; Niemann, S.; Peters, G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. 2014, 304, 170–176. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Löffler, B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr. Genet. 2015, 62, 15–17. [Google Scholar] [CrossRef]
- Haslinger-Loffler, B.; Kahl, B.C.; Grundmeier, M.; Strangfeld, K.; Wagner, B.; Fischer, U.; Cheung, A.L.; Peters, G.; Schulze-Osthoff, K.; Sinha, B. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell. Microbiol. 2005, 7, 1087–1097. [Google Scholar] [CrossRef]
- Ou, J.J.; Drilling, A.J.; Cooksley, C.; Bassiouni, A.; Kidd, S.P.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Reduced Innate Immune Response to a Staphylococcus aureus Small Colony Variant Compared to Its Wild-Type Parent Strain. Front. Cell. Infect. Microbiol. 2016, 6, 187. [Google Scholar] [CrossRef]
- Horst, S.A.; Hoerr, V.; Beineke, A.; Kreis, C.; Tuchscherr, L.; Kalinka, J.; Lehne, S.; Schleicher, I.; Kohler, G.; Fuchs, T.; et al. A novel mouse model of Staphylococcus aureus chronic osteo-myelitis that closely mimics the human infection: An integrated view of disease pathogenesis. Am. J. Pathol. 2012, 181, 1206–1214. [Google Scholar] [CrossRef]
- Seidl, K.; Solis, N.V.; Bayer, A.S.; Hady, W.A.; Ellison, S.; Klashman, M.C.; Xiong, Y.Q.; Filler, S.G. Divergent Responses of Different Endothelial Cell Types to Infection with Candida albicans and Staphylococcus aureus. PLoS ONE 2012, 7, e39633. [Google Scholar] [CrossRef]
- Jarry, T.M.; Cheung, A.L. Staphylococcus aureus Escapes More Efficiently from the Phagosome of a Cystic Fibrosis Bronchial Epithelial Cell Line than from Its Normal Counterpart. Infect. Immun. 2006, 74, 2568–2577. [Google Scholar] [CrossRef]
- Hudson, M.C.; Ramp, W.K.; Nicholson, N.C.; Williams, A.S.; Nousiainen, M.T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 1995, 19, 409–419. [Google Scholar] [CrossRef]
- Weinrick, B.; Dunman, P.M.; McAleese, F.; Murphy, E.; Projan, S.J.; Fang, Y.; Novick, R.P. Effect of mild acid on gene expression in Staphylococcus aureus. J. Bacteriol. 2004, 186, 8407–8423. [Google Scholar] [CrossRef]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A Potential New Pathway for Staphylococcus aureus Dissemination: The Silent Survival of S. aureus Phagocytosed by Human Monocyte-Derived Macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef]
- Pang, Y.Y.; Schwartz, J.; Thoendel, M.; Ackermann, L.W.; Horswill, A.R.; Nauseef, W.M. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J. Innate Immun. 2010, 2, 546–559. [Google Scholar] [CrossRef]
- Tranchemontagne, Z.R.; Camire, R.B.; O’Donnell, V.J.; Baugh, J.; Burkholder, K.M. Staphylococcus aureus Strain USA300 Perturbs Acquisition of Lysosomal Enzymes and Requires Phagosomal Acidification for Survival inside Macrophages. Infect. Immun. 2016, 84, 241–253. [Google Scholar] [CrossRef]
- Watkins, K.E.; Unnikrishnan, M. Evasion of host defenses by intracellular Staphylococcus aureus. Adv. Appl. Microbiol. 2020, 112, 105–141. [Google Scholar] [CrossRef]
- Lacoma, A.; Cano, V.; Moranta, D.; Regueiro, V.; Dominguez-Villanueva, D.; Laabei, M.; Gonzalez-Nicolau, M.; Ausina, V.; Prat, C.; Bengoechea, J.A. Investigating intracellular persistence of Staphylococcus aureus within a murine alveolar macro-phage cell line. Virulence 2017, 8, 1761–1775. [Google Scholar] [CrossRef]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Kuiack, R.C.; McGavin, M.J.; Heinrichs, D.E. Staphylococcus aureus Uses the GraXRS Regulatory System to Sense and Adapt to the Acidified Phagolysosome in Macrophages. mBio 2018, 9, e01143-18. [Google Scholar] [CrossRef]
- Karavolos, M.H.; Horsburgh, M.J.; Ingham, E.; Foster, S.J. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology 2003, 149, 2749–2758. [Google Scholar] [CrossRef]
- Cosgrove, K.; Coutts, G.; Jonsson, I.-M.; Tarkowski, A.; Kokai-Kun, J.F.; Mond, J.J.; Foster, S.J. Catalase (KatA) and Alkyl Hydroperoxide Reductase (AhpC) Have Compensatory Roles in Peroxide Stress Resistance and Are Required for Survival, Persistence, and Nasal Colonization in Staphylococcus aureus. J. Bacteriol. 2007, 189, 1025–1035. [Google Scholar] [CrossRef]
- Treffon, J.; Block, D.; Moche, M.; Reiss, S.; Fuchs, S.; Engelmann, S.; Becher, D.; Langhanki, L.; Mellmann, A.; Peters, G.; et al. Adaptation of Staphylococcus aureus to Airway Environments in Patients with Cystic Fibrosis by Upregulation of Super-oxide Dismutase M and Iron-Scavenging Proteins. J. Infect. Dis. 2018, 217, 1453–1461. [Google Scholar] [CrossRef]
- Treffon, J.; Chaves-Moreno, D.; Niemann, S.; Pieper, D.H.; Vogl, T.; Roth, J.; Kahl, B.C. Importance of superoxide dismutases A and M for protection of Staphylococcus aureus in the oxidative stressful environment of cystic fibrosis airways. Cell. Microbiol. 2020, 22, e13158. [Google Scholar] [CrossRef]
- Munzenmayer, L.; Geiger, T.; Daiber, E.; Schulte, B.; Autenrieth, S.E.; Fraunholz, M.; Wolz, C. Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell. Microbiol. 2016, 18, 1172–1183. [Google Scholar] [CrossRef]
- Jarry, T.M.; Memmi, G.; Cheung, A.L. The expression of alpha-haemolysin is required for Staphylococcus aureus phagosomal escape after internalization in CFT-1 cells. Cell. Microbiol. 2008, 10, 1801–1814. [Google Scholar] [CrossRef]
- Grosz, M.; Kolter, J.; Paprotka, K.; Winkler, A.C.; Schafer, D.; Chatterjee, S.S.; Geiger, T.; Wolz, C.; Ohlsen, K.; Otto, M.; et al. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenolsoluble modulin alpha. Cell. Microbiol. 2014, 16, 451–465. [Google Scholar] [CrossRef]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.H.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Geiger, T.; Francois, P.; Liebeke, M.; Fraunholz, M.; Goerke, C.; Krismer, B.; Schrenzel, J.; Lalk, M.; Wolz, C. The Stringent Response of Staphylococcus aureus and Its Impact on Survival after Phagocytosis through the Induction of Intracellular PSMs Expression. PLoS Pathog. 2012, 8, e1003016. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Joo, H.-S.; Duong, A.C.; Dieringer, T.D.; Tan, V.Y.; Song, Y.; Fischer, E.R.; Cheung, G.Y.C.; Li, M.; Otto, M. Essential Staphylococcus aureus toxin export system. Nat. Med. 2013, 19, 364–367. [Google Scholar] [CrossRef]
- Blattner, S.; Das, S.; Paprotka, K.; Eilers, U.; Krischke, M.; Kretschmer, D.; Remmele, C.W.; Dittrich, M.; Muller, T.; Schuelein-Voelk, C.; et al. Staphylococcus aureus Exploits a Non-ribosomal Cyclic Dipeptide to Modulate Survival within Epithelial Cells and Phagocytes. PLoS Pathog. 2016, 12, e1005857. [Google Scholar] [CrossRef]
- Giese, B.; Glowinski, F.; Paprotka, K.; Dittmann, S.; Steiner, T.; Sinha, B.; Fraunholz, M.J. Expression of delta-toxin by Staph-ylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of beta-toxin. Cell. Microbiol. 2011, 13, 316–329. [Google Scholar] [CrossRef]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; GÖtz, F. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability to Cope with Oxidative Stress. Infect. Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial Mechanisms of Macrophages and the Immune Evasion Strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef]
- Jubrail, J.; Morris, P.; Bewley, M.A.; Stoneham, S.; Johnston, S.A.; Foster, S.J.; Peden, A.A.; Read, R.C.; Marriott, H.M.; Dockrell, D.H. Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell. Microbiol. 2015, 18, 80–96. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell. Microbiol. 2016, 18, 514–535. [Google Scholar] [CrossRef]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Morisaki, J.H.; et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Siegmund, A.; Afzal, M.A.; Tetzlaff, F.; Keinhörster, D.; Gratani, F.; Paprotka, K.; Westermann, M.; Nietzsche, S.; Wolz, C.; Fraunholz, M.; et al. Intracellular persistence of Staphylococcus aureus in endothelial cells is promoted by the absence of phenol-soluble modulins. Virulence 2021, 12, 1186–1198. [Google Scholar] [CrossRef]
- Elgrail, M.M.; Chen, E.; Shaffer, M.G.; Srinivasa, V.; Griffith, M.P.; Mustapha, M.M.; Shields, R.K.; van Tyne, D.; Culyba, M.J. Convergent Evolution of Antibiotic Tolerance in Patients with Persistent Methicillin-Resistant Staphylococcus aureus Bacteremia. Infect. Immun. 2022, 90, e0000122. [Google Scholar] [CrossRef]
- Sriramulu, D.D.; Nimtz, M.; Romling, U. Proteome analysis reveals adaptation of Pseudomonas aeruginosa to the cystic fibrosis lung environment. Proteomics 2005, 5, 3712–3721. [Google Scholar] [CrossRef]
- Pagels, M.; Fuchs, S.; Pané-Farré, J.; Kohler, C.; Menschner, L.; Hecker, M.; McNamarra, P.J.; Bauer, M.C.; von Wachenfeldt, C.; Liebeke, M.; et al. Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Mol. Microbiol. 2010, 76, 1142–1161. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, E.; Barrientos, L.; Herrgott, L.; Marzi, S.; Toledo-Arana, A.; Moreau, K.; Vandenesch, F.; Romby, P.; Caldelari, I. The 3’UTR-derived sRNA RsaG coordinates redox homeostasis and metabolism adaptation in response to glucose-6-phosphate uptake in Staphylococcus aureus. Mol. Microbiol. 2022, 117, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.; Knight, D.; Burgess, S.; Franklin, P.; Horak, F.; Legg, J.; Moeller, A.; Stick, S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax 2004, 59, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Medina, L.M.P.; Becker, A.-K.; Michalik, S.; Surmann, K.; Hildebrandt, P.; Salazar, M.G.; Mekonnen, S.A.; Kaderali, L.; Völker, U.; van Dijl, J.M. Interaction of Staphylococcus aureus and Host Cells upon Infection of Bronchial Epithelium during Different Stages of Regeneration. ACS Infect. Dis. 2020, 6, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.D.; Snyder, D.J.; Putnam, N.E.; Valentino, M.D.; Hammer, N.D.; Lonergan, Z.R.; Hinger, S.A.; Aysanoa, E.E.; Blanchard, C.; Dunman, P.M.; et al. Bacterial Hypoxic Responses Revealed as Critical Determinants of the Host-Pathogen Outcome by TnSeq Analysis of Staphylococcus aureus Invasive Infection. PLoS Pathog. 2015, 11, e1005341. [Google Scholar] [CrossRef] [PubMed]
- Throup, J.P.; Zappacosta, F.; Lunsford, R.D.; Annan, R.S.; Carr, S.A.; Lonsdale, J.T.; Bryant, A.P.; McDevitt, D.; Rosenberg, M.; Burnham, M.K.R. The srhSR Gene Pair from Staphylococcus aureus: Genomic and Proteomic Approaches to the Identification and Characterization of Gene Function. Biochemistry 2001, 40, 10392–10401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Y.; Zhu, T.; Han, H.; Liu, H.; Xu, T.; Francois, P.; Fischer, A.; Bai, L.; Götz, F.; et al. Staphylococcus epidermidis SrrAB Regulates Bacterial Growth and Biofilm Formation Differently under Oxic and Microaerobic Conditions. J. Bacteriol. 2015, 197, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Huseby, D.L.; Brandis, G.; Hughes, D. Alternative Evolutionary Pathways for Drug-Resistant Small Colony Variant Mutants in Staphylococcus aureus. mBio 2017, 8, e00358-17. [Google Scholar] [CrossRef]
- Wickersham, M.; Wachtel, S.; Wong Fok Lung, T.; Soong, G.; Jacquet, R.; Richardson, A.; Parker, D.; Prince, A. Metabolic Stress Drives Keratinocyte Defenses against Staphylococcus aureus Infection. Cell Rep. 2017, 18, 2742–2751. [Google Scholar] [CrossRef]
- Lung, T.W.F.; Monk, I.R.; Acker, K.P.; Mu, A.; Wang, N.; Riquelme, S.A.; Pires, S.; Noguera, L.P.; Dach, F.; Gabryszewski, S.J.; et al. Staphylococcus aureus small colony variants impair host immunity by activating host cell glycolysis and inducing necroptosis. Nat. Microbiol. 2019, 5, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Kitur, K.; Wachtel, S.; Brown, A.; Wickersham, M.; Paulino, F.; Peñaloza, H.F.; Soong, G.; Bueno, S.; Parker, D.; Prince, A. Necroptosis Promotes Staphylococcus aureus Clearance by Inhibiting Excessive Inflammatory Signaling. Cell Rep. 2016, 16, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kanev, L.; Woods, S.D.; Brenner, M.; Smith, B. Managing hyperkalemia in high-risk patients in long-term care. Am. J. Manag. Care 2017, 23, S27–S36. [Google Scholar] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.D.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1578. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Novakovic, B.; ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.-C.; Wang, S.-Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Acker, K.P.; Lung, T.W.F.; West, E.; Craft, J.; Narechania, A.; Smith, H.; O’Brien, K.; Moustafa, A.M.; Lauren, C.; Planet, P.J.; et al. Strains of Staphylococcus aureus that Colonize and Infect Skin Harbor Mutations in Metabolic Genes. iScience 2019, 19, 281–290. [Google Scholar] [CrossRef]
- Gabryszewski, S.J.; Lung, T.W.F.; Annavajhala, M.K.; Tomlinson, K.L.; Riquelme, S.A.; Khan, I.N.; Noguera, L.P.; Wickersham, M.; Zhao, A.; Mulenos, A.M.; et al. Metabolic Adaptation in Methicillin-Resistant Staphylococcus aureus Pneumonia. Am. J. Respir. Cell Mol. Biol. 2019, 61, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, P.M. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 100–106. [Google Scholar] [CrossRef]
- Renard, C.; Vanderhaeghe, H.J.; Claes, P.J.; Zenebergh, A.; Tulkens, P.M. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob. Agents Chemother. 1987, 31, 410–416. [Google Scholar] [CrossRef]
- Auberttulkens, G.; Vanhoof, F.; Tulkens, P. Gentamicin-induced lysosomal phospholipidosis in cultured rat fibroblasts—Quantitative ultrastructural and biochemical-study. Lab. Investig. 1979, 40, 481–491. [Google Scholar] [PubMed]
- Cosgrove, S.E.; Vigliani, G.A.; Fowler, V.G., Jr.; Abrutyn, E.; Corey, G.R.; Levine, D.P.; Rupp, M.E.; Chambers, H.F.; Karchmer, A.W.; Boucher, H.W. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin. Infect. Dis. 2009, 48, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Barcia-Macay, M.; Seral, C.; Mingeot-Leclercq, M.-P.; Tulkens, P.M.; van Bambeke, F. Pharmacodynamic Evaluation of the Intracellular Activities of Antibiotics against Staphylococcus aureus in a Model of THP-1 Macrophages. Antimicrob. Agents Chemother. 2006, 50, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; Kahl, B.; von Eiff, C.; Vaudaux, P.E.; Lew, D.P.; Peters, G. Staphylococcal Small Colony Variants Have Novel Mechanisms for Antibiotic Resistance. Clin. Infect. Dis. 1998, 27, S68–S74. [Google Scholar] [CrossRef] [PubMed]
- Somerville, G.A.; Chaussee, M.S.; Morgan, C.I.; Fitzgerald, J.R.; Dorward, D.W.; Reitzer, L.J.; Musser, J.M. Staphylococcus aureus Aconitase Inactivation Unexpectedly Inhibits Post-Exponential-Phase Growth and Enhances Stationary-Phase Survival. Infect. Immun. 2002, 70, 6373–6382. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.; Kriegeskorte, A.; Fischer, A.; Deiwick, S.; Theimann, N.; Proctor, R.A.; Peters, G.; Herrmann, M.; Kahl, B.C. In Vivo Mutations of Thymidylate Synthase (Encoded by thyA) Are Responsible for Thymidine Dependency in Clinical Small-Colony Variants of Staphylococcus aureus. J. Bacteriol. 2008, 190, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F.; Miller, M.H. Emergence of Resistance to Cephalothin and Gentamicin During Combination Therapy for Methicillin-Resistant Staphylococcus aureus Endocarditis in Rabbits. J. Infect. Dis. 1987, 155, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Mitchell, G.; Gattuso, M.; Grondin, G.; Marsault, É.; Bouarab, K.; Malouin, F. Tomatidine Inhibits Replication of Staphylococcus aureus Small-Colony Variants in Cystic Fibrosis Airway Epithelial Cells. Antimicrob. Agents Chemother. 2011, 55, 1937–1945. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volk, C.F.; Proctor, R.A.; Rose, W.E. The Complex Intracellular Lifecycle of Staphylococcus aureus Contributes to Reduced Antibiotic Efficacy and Persistent Bacteremia. Int. J. Mol. Sci. 2024, 25, 6486. https://doi.org/10.3390/ijms25126486
Volk CF, Proctor RA, Rose WE. The Complex Intracellular Lifecycle of Staphylococcus aureus Contributes to Reduced Antibiotic Efficacy and Persistent Bacteremia. International Journal of Molecular Sciences. 2024; 25(12):6486. https://doi.org/10.3390/ijms25126486
Chicago/Turabian StyleVolk, Cecilia F., Richard A. Proctor, and Warren E. Rose. 2024. "The Complex Intracellular Lifecycle of Staphylococcus aureus Contributes to Reduced Antibiotic Efficacy and Persistent Bacteremia" International Journal of Molecular Sciences 25, no. 12: 6486. https://doi.org/10.3390/ijms25126486
APA StyleVolk, C. F., Proctor, R. A., & Rose, W. E. (2024). The Complex Intracellular Lifecycle of Staphylococcus aureus Contributes to Reduced Antibiotic Efficacy and Persistent Bacteremia. International Journal of Molecular Sciences, 25(12), 6486. https://doi.org/10.3390/ijms25126486





