Therapeutic Potential and Challenges of Mesenchymal Stem Cell-Derived Exosomes for Peripheral Nerve Regeneration: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Systematic Review
3.2. Liu et al. [60]: UMSC-Derived Exosomes
3.3. Yang et al. [59] and Chen et al. [58]: ASC-Derived Exosomes
3.4. Ikumi et al. [64]: Purified Exosome Product (PEP)
3.5. Li et al. [61]: BMSC-Derived Exosomes
3.6. Rao et al. [62]: GMSC-Derived Exosomes
3.7. Pan et al. [63]: Induced Human Pluripotent Stem Cell (iPCS)-Derived Exosomes
4. Discussion
5. Challenges in the Field and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, L.; Mee, T.; Zhou, X.; Jia, X. Augmenting Peripheral Nerve Regeneration with Adipose-Derived Stem Cells. Stem Cell Rev. Rep. 2021, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef] [PubMed]
- Madison, R.D.; Robinson, G.A.; Chadaram, S.R. The specificity of motor neurone regeneration (preferential reinnervation). Acta Physiol. 2007, 189, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Rodríguez, F.J.; Guzmán, M.S.; Jaramillo, J.; Navarro, X. Effects of motor and sensory nerve transplants on amount and specificity of sciatic nerve regeneration. J. Neurosci. Res. 2007, 85, 2800–2812. [Google Scholar] [CrossRef] [PubMed]
- Lubińska, L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977, 130, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Rotshenker, S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflammation 2011, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.-L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Scheib, J.; Höke, A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration. Front. Cell Neurosci. 2021, 15, 820216. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, R.; Xu, Y.; Ma, X.; Zhou, B. The Mechanisms of Peripheral Nerve Preconditioning Injury on Promoting Axonal Regeneration. Neural Plast. 2021, 2021, 6648004. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.R.; Gordon, T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery 2009, 65, A105–A114. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef] [PubMed]
- Tse, R.; Ko, J.H. Nerve Glue for Upper Extremity Reconstruction. Hand Clin. 2012, 28, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Lien, B.V.; Brown, N.J.; Ransom, S.C.; Lehrich, B.M.; Shahrestani, S.; Tafreshi, A.R.; Ransom, R.C.; Sahyouni, R. Enhancing peripheral nerve regeneration with neurotrophic factors and bioengineered scaffolds: A basic science and clinical perspective. J. Peripher. Nerv. Syst. 2020, 25, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, H.; Qing, L.; Yang, X.; Jia, X. Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomater. Sci. 2018, 6, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Belanger, K.; Dinis, T.M.; Taourirt, S.; Vidal, G.; Kaplan, D.L.; Egles, C. Recent Strategies in Tissue Engineering for Guided Peripheral Nerve Regeneration. Macromol. Biosci. 2016, 16, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, H. A Comprehensive Review of Concentrated Growth Factors and Their Novel Applications in Facial Reconstructive and Regenerative Medicine. Aesthetic Plast. Surg. 2020, 44, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, L.; Sun, Y.; Sun, X.; Wen, C.; Shahmoradi, M.; Zhou, Y. Concentrated growth factor increases Schwann cell proliferation and neurotrophic factor secretion and promotes functional nerve recovery in vivo. Int. J. Mol. Med. 2016, 37, 493–500. [Google Scholar] [CrossRef]
- Madduri, S.; di Summa, P.; Papaloïzos, M.; Kalbermatten, D.; Gander, B. Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials 2010, 31, 8402–8409. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, M.; Wu, Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef] [PubMed]
- Strauch, B.; Rodriguez, D.M.; Diaz, J.; Yu, H.-L.; Kaplan, G.; Weinstein, D.E. Autologous Schwann Cells Drive Regeneration through a 6-cm Autogenous Venous Nerve Conduit. J. Reconstr. Microsurg. 2001, 17, 589–598. [Google Scholar] [CrossRef]
- Levi, A.D.; Burks, S.S.; Anderson, K.D.; Dididze, M.; Khan, A.; Dietrich, W.D. The Use of Autologous Schwann Cells to Supplement Sciatic Nerve Repair with a Large Gap: First in Human Experience. Cell Transplant. 2016, 25, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.L.; Kirk, C.J.; Lerner, M.A.; Tennekoon, G.I. Purification and expansion of human Schwann cells in vitro. Nat. Med. 1995, 1, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, J.; Lee, D.Y.; Kim, Y.-D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Rep. 2017, 8, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Guilak, F. Differentiation Potential of Adipose Derived Adult Stem (ADAS) Cells. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2003; pp. 137–160. Available online: https://www.sciencedirect.com/science/article/pii/S007021530358005X (accessed on 22 June 2022).
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Hayashi, T.; Kitada, M.; Kohama, M.; Matsue, D.; Teramoto, N.; Ose, T.; Itokazu, Y.; Koshino, K.; Watabe, H.; et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp. Neurol. 2010, 223, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Taghi, G.M.; Ghasem Kashani Maryam, H.; Taghi, L.; Leili, H.; Leyla, M. Characterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biol. Int. 2012, 36, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Salamon, A.; Herzmann, N.; Adam, S.; Kleine, H.-D.; Matthiesen, I.; Ueberreiter, K.; Peters, K. Isolation and Differentiation Potential of Human Mesenchymal Stem Cells From Adipose Tissue Harvested by Water Jet-Assisted Liposuction. Aesthet. Surg. J. 2015, 35, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Xu, Y.; Liu, X.; Xu, S.; Zhang, Y.; Zhu, Z.; He, B. Effect of Induction Time on the Proliferation and Differentiation of Induced Schwann-Like Cells from Adipose-Derived Stem Cells. Cell Mol. Neurobiol. 2020, 40, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Faroni, A.; Smith, R.J.P.; Lu, L.; Reid, A.J. Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. Eur. J. Neurosci. 2016, 43, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Faroni, A.; Terenghi, G.; Reid, A.J. Adipose-derived stem cells and nerve regeneration: Promises and pitfalls. Int. Rev. Neurobiol. 2013, 108, 121–136. [Google Scholar] [PubMed]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. [Google Scholar] [CrossRef] [PubMed]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration-In vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.Y.; Clavijo-Alvarez, J.; Brayfield, C.; Rubin, J.P.; Marra, K.G. Delivery of Adipose-Derived Precursor Cells for Peripheral Nerve Repair. Cell Transplant. 2009, 18, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sowa, Y.; Kishida, T.; Imura, T.; Numajiri, T.; Nishino, K.; Tabata, Y.; Mazda, O. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast. Reconstr. Surg. 2016, 137, 318e–330e. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Castiglione, G.; Turano, E.; Bissolotti, G.; Angiari, S.; Farinazzo, A.; Constantin, G.; Bedogni, G.; Bedogni, A.; Bonetti, B. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng. Part A 2012, 18, 1264–1272. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Ma, T.; Li, Y. Preconditioning stem cells for in vivo delivery. Biores Open Access 2014, 3, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.; Mantovani, C.; Kalbermatten, D.F.; Pierer, G.; Terenghi, G.; Kingham, P.J. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, e811–e817. [Google Scholar] [CrossRef] [PubMed]
- Thirabanjasak, D.; Tantiwongse, K.; Thorner, P.S. Angiomyeloproliferative lesions following autologous stem cell therapy. J. Am. Soc. Nephrol. JASN 2010, 21, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Saffari, S.; Saffari, T.M.; Ulrich, D.J.O.; Hovius, S.E.R.; Shin, A.Y. The interaction of stem cells and vascularity in peripheral nerve regeneration. Neural Regen. Res. 2021, 16, 1510–1517. [Google Scholar] [PubMed]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. Biomed. Res. Int. 2019, 2019, 6458237. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, 1802896. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xu, Y.; Ahmad, M.A.; Javed, R.; Hagiwara, H.; Tian, X. Exosomes as a Promising Therapeutic Strategy for Peripheral Nerve Injury. Curr. Neuropharmacol. 2021, 19, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Namini, M.S.; Daneshimehr, F.; Beheshtizadeh, N.; Mansouri, V.; Ai, J.; Jahromi, H.K.; Ebrahimi-Barough, S. Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem Cell Res Ther. 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Bucan, V.; Vaslaitis, D.; Peck, C.-T.; Strauß, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Yin, G.; Sun, Y.-D.; Lin, Y.-F.; Xie, Z.; English, A.W.; Li, Q.-F.; Lin, H.-D. Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neurosci. Ther. 2020, 26, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.-S.; Kuo, P.-J.; Wu, S.-C.; Huang, L.-H.; Lu, T.-H.; Wu, Y.-C.; Wu, C.-J.; Lin, C.-W.; Tsai, C.-W.; Hsieh, C.-H. Enhanced Nerve Regeneration by Exosomes Secreted by Adipose-Derived Stem Cells with or without FK506 Stimulation. Int. J. Mol. Sci. 2021, 22, 8545. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: The role of secretomes. Exp Neurol. 2022, 354, 114069. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, S.; Duscher, D.; Kang, Y.; Liu, Y.; Wang, C.; Yuan, M.; Guo, G.; Xiong, H.; Zhan, P.; et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell Physiol. 2019, 234, 23097–23110. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Xu, Y.; Jiang, W.; Shao, Y.; Xing, J.; Chen, Y.; Han, Y. Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res. Ther. 2021, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tong, H.; Li, J.; Wang, L.; Fan, X.; Song, H.; Yang, M.; Wang, H.; Jiang, X.; Zhou, X.; et al. Low-Stiffness Hydrogels Promote Peripheral Nerve Regeneration Through the Rapid Release of Exosomes. Front. Bioeng. Biotechnol. 2022, 10, 922570. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, S.-Y.; Zhang, M.; Pi, W.; Wang, B.; Li, Q.-C.; Lu, C.-F.; Zhang, P.-X. Sustained release of exosomes loaded into polydopamine-modified chitin conduits promotes peripheral nerve regeneration in rats. Neural Regen. Res. 2022, 17, 2050–2057. [Google Scholar] [PubMed]
- Rao, F.; Zhang, D.; Fang, T.; Lu, C.; Wang, B.; Ding, X.; Wei, S.; Zhang, Y.; Pi, W.; Xu, H.; et al. Exosomes from Human Gingiva-Derived Mesenchymal Stem Cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019, 2019, 2546367. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhao, M.; Yi, X.; Tao, J.; Li, S.; Jiang, Z.; Cheng, B.; Yuan, H.; Zhang, F. Acellular nerve grafts supplemented with induced pluripotent stem cell-derived exosomes promote peripheral nerve reconstruction and motor function recovery. Bioact. Mater. 2022, 15, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Ikumi, A.; Gingery, A.; Toyoshima, Y.; Zhao, C.; Moran, S.L.; Livia, C.; Rolland, T.; Peterson, T.; Sabbah, M.S.; Boroumand, S.; et al. Administration of Purified Exosome Product in a Rat Sciatic Serve Reverse Autograft Model. Plast. Reconstr. Surg. 2021, 148, 200e–211e. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Chen, H.; Tang, J.; Jia, X. Exosomes and Their MicroRNA Cargo: New Players in Peripheral Nerve Regeneration. Neurorehabil Neural Repair. 2018, 32, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Tan, X.-H.; Zhao, J.-H.; Zhang, Q.-P.; Zhang, X.-F.; Ma, Z.-J.; Peng, Y.-N.; Liu, Q.-B.; Zhang, H.-Y.; Li, Y.-Q.; et al. Bone marrow mesenchymal stem cell-derived exosome uptake and retrograde transport can occur at peripheral nerve endings. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ding, Y.; He, R.; Huang, K.; Liu, L.; Jiang, C.; Liu, Z.; Wang, Y.; Yan, X.; Cao, F.; et al. Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury. Stem Cell Res. Ther. 2020, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Goo, J.; Lee, Y.; Lee, J.; Kim, I.-S.; Jeong, C. Extracellular Vesicles in Therapeutics: A Comprehensive Review on Applications, Challenges, and Clinical Progress. Pharmaceutics 2024, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Dhar, R.; Devi, A. Stem Cell-Derived Exosomes: An Advanced Horizon to Cancer Regenerative Medicine. ACS Appl. Bio Mater. 2024, 7, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, M.; Takahashi, K. Present and future challenges of induced pluripotent stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140367. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Liu, Q.; Reisdorf, R.L.; Boroumand, S.; Behfar, A.; Moran, S.L.; Amadio, P.C.; Gingery, A.; Zhao, C. Characterization of a purified exosome product and its effects on canine flexor tenocyte biology. J. Orthop. Res. 2020, 38, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, S.; Wang, Y.; Jacobson, D.S.; Reisdorf, R.L.; Kuroiwa, T.; Behfar, A.; Moran, S.L.; Steinmann, S.P.; Zhao, C. Effects of purified exosome product on rotator cuff tendon-bone healing in vitro and in vivo. Biomaterials 2021, 276, 121019. [Google Scholar] [CrossRef] [PubMed]
- Monte-Raso, V.V.; Barbieri, C.H.; Mazzer, N.; Yamasita, A.C.; Barbieri, G. Is the Sciatic Functional Index always reliable and reproducible? J. Neurosci. Methods 2008, 170, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H.; Mao, H.-Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraiah, S.; Kumbar, S.G. Review: Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2019, 4, 107–113. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Sanchez Rezza, A.; Kulahci, Y.; Gorantla, V.S.; Zor, F.; Drzeniek, N.M. Implantable Biomaterials for Peripheral Nerve Regeneration-Technology Trends and Translational Tribulations. Front. Bioeng. Biotechnol. 2022, 10, 863969. [Google Scholar] [CrossRef]
- Koopman, J.E.; Duraku, L.S.; de Jong, T.; de Vries, R.B.M.; Michiel Zuidam, J.; Hundepool, C.A. A systematic review and meta-analysis on the use of fibrin glue in peripheral nerve repair: Can we just glue it? J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1018–1033. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, B. A new fibrin seal: Functional evaluation of sensory regeneration following primary repair of peripheral nerves. J. Hand Surg. 1994, 19, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.; Miears, H.; Cox, C.; MacKay, B. Fibrin Glue and Its Alternatives in Peripheral Nerve Repair. Ann. Plast. Surg. 2021, 86, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xu, B.; Jin, X.; Chen, F.; Liu, S.; Liu, S.; Wang, S.; Zhang, F.; Song, K.; Wang, J.; et al. Nerve Guide Conduits Integrated with Fisetin-Loaded Chitosan Hydrogels for Reducing Oxidative Stress, Inflammation, and Nerve Regeneration. Macromol. Biosci. 2024, 24, e2300476. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Kao, H.-K.; Wun, J.-R.; Chou, P.-Y.; Chen, Z.-Y.; Chen, S.-H.; Hsieh, S.-T.; Fang, H.-W.; Lin, F.-H. Thermosensitive hydrogel carrying extracellular vesicles from adipose-derived stem cells promotes peripheral nerve regeneration after microsurgical repair. APL Bioeng. 2022, 6, 046103. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Nydick, J.A.; Means, K.R.; Merrell, G.A.; Ilyas, A.; Levin, L.S.; RECON Study Group. A Multicenter Prospective Randomized Comparison of Conduits Versus Decellularized Nerve Allograft for Digital Nerve Repairs. J. Hand Surg. 2023, 48, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Gao, X.; Qian, J.; Li, S.; Yu, B.; Hao, Y.; Wei, B.; Ma, T.; Wu, H.; Yang, S.; et al. A Novel Superparamagnetic Multifunctional Nerve Scaffold: A Remote Actuation Strategy to Boost In Situ Extracellular Vesicles Production for Enhanced Peripheral Nerve Repair. Adv. Mater. 2024, 36, 2305374. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, X.; Kou, Y.; Han, N. Engineering strategies and optimized delivery of exosomes for theranostic application in nerve tissue. Theranostics 2023, 13, 4266–4286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, Q.; Jiang, Y.; Tang, H.; Zhang, L.; Li, D.; Xu, Y. Emerging technologies for engineering of extracellular vesicles. Front. Bioeng. Biotechnol. 2023, 11, 1298746. [Google Scholar] [CrossRef] [PubMed]
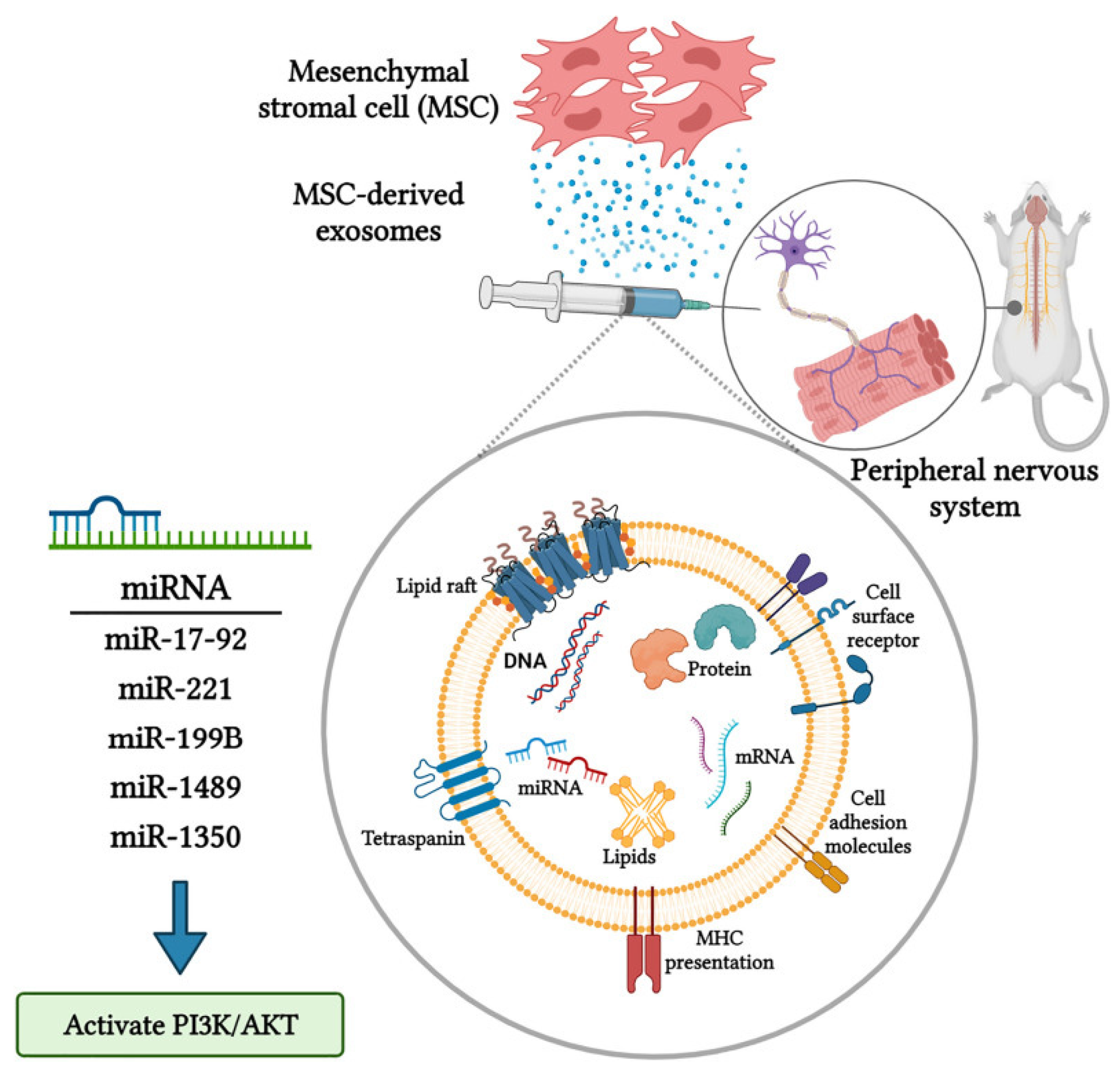
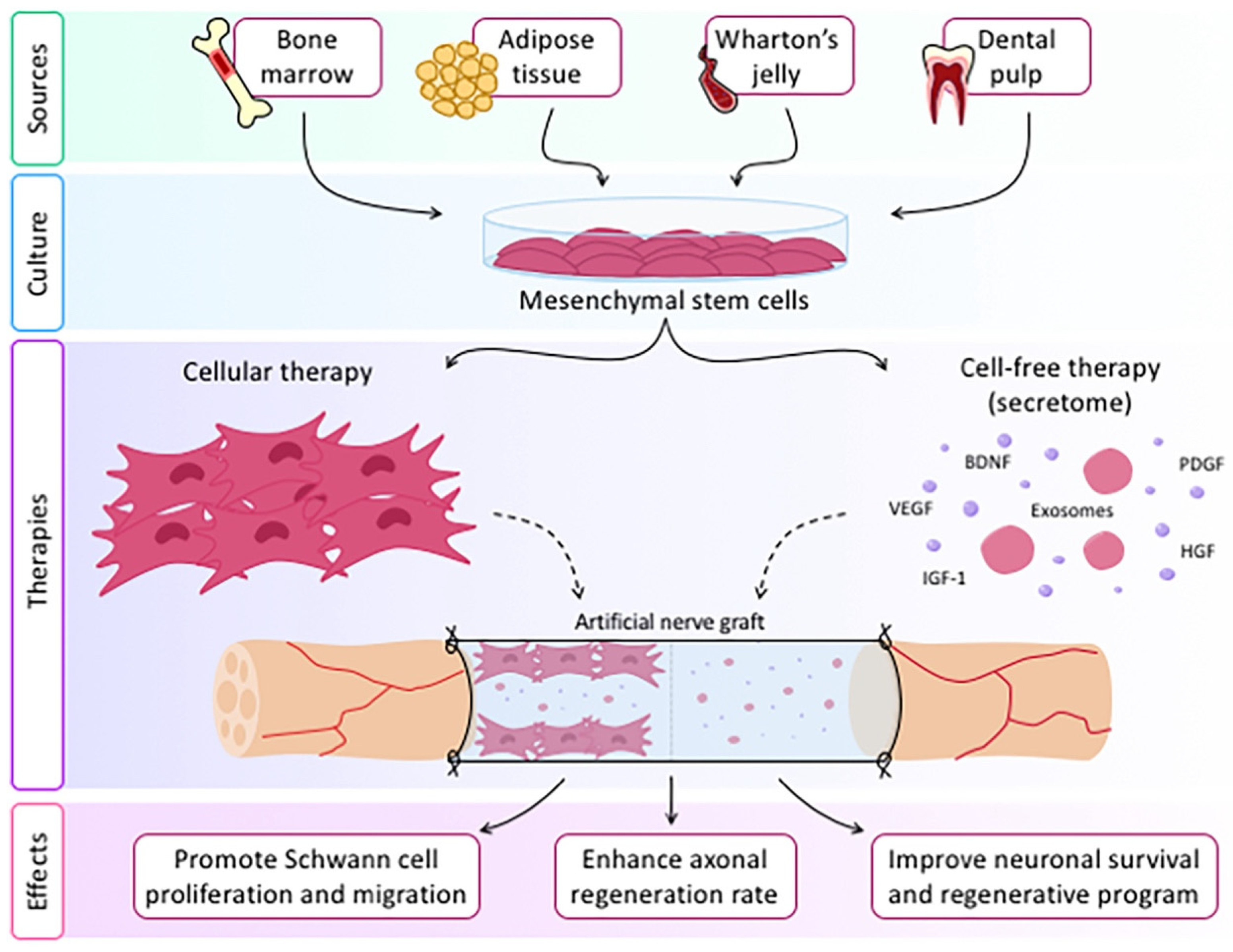

| Exosomes | Scaffold | Study Model | Defect | Outcome | Author | Year | Reference |
|---|---|---|---|---|---|---|---|
| UMSC [0.5 μg/μL] | Hydrogel (HAMA) (stiff: 100 μg/μL; soft: 40 μg/μL) | in vivo and in vitro rat sciatic nerve | Crush injury | The stiffness of the hydrogel changed the dynamic of exosome release. Soft hydrogel showed better PNR and functional recovery. | Liu et al. | 2022 | [60] |
| hASC [0.5 μg/μL] | Matrigel in a silicone scaffold | in vivo and in vitro rat sciatic nerve | 7 mm | ASC Exo promoted PNR via SC upregulation, with better myelinization and axonal regeneration. | Chen et al. | 2019 | [58] |
| ASC (+NT3) [0.5 μg/μL] | Alginate hydrogel in a silicone scaffold | in vivo and in vitro rat sciatic nerve | 10 mm | Exo NT3 improved the PNR, with no difference between the Exo and non-Exo groups. | Yang et al. | 2021 | [59] |
| PEP (5%) | Reverse autograft with fibrin glue | in vivo and in vitro rat sciatic nerve | 10 mm | PEP improved motor outcome and nerve regeneration and changed the gene profile. | Ikumi et al. | 2021 | [64] |
| BMSC Chitosan 364 μg | Chitosan (+/− PDA modified) | in vivo and in vitro rat sciatic nerve | 2 mm | The chitosan PDA Exo group was more efficient than its chitosan counterpart on PNR; chistosan PDA Exo released Exo in a slower pattern. | Li et al. | 2022 | [61] |
| GMSC [1 μg/μL] | Chitin | in vivo and in vitro rat sciatic nerve | 10 mm | Exosomes provided better motor function, axon regeneration, myelinization and nerve conduction as well as higher diameter. Autografts provided better results. | Rao et al. | 2019 | [62] |
| iPSC | Acellular nerve grafts (ANGs) | in vivo rat sciatic nerve | 15 mm | Motor function, axon diameter and muscle reinnervation were comparable to autografts. | Pan et al. | 2022 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogny, C.; André-Lévigne, D.; Kalbermatten, D.F.; Madduri, S. Therapeutic Potential and Challenges of Mesenchymal Stem Cell-Derived Exosomes for Peripheral Nerve Regeneration: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6489. https://doi.org/10.3390/ijms25126489
Dogny C, André-Lévigne D, Kalbermatten DF, Madduri S. Therapeutic Potential and Challenges of Mesenchymal Stem Cell-Derived Exosomes for Peripheral Nerve Regeneration: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(12):6489. https://doi.org/10.3390/ijms25126489
Chicago/Turabian StyleDogny, Clelia, Dominik André-Lévigne, Daniel F. Kalbermatten, and Srinivas Madduri. 2024. "Therapeutic Potential and Challenges of Mesenchymal Stem Cell-Derived Exosomes for Peripheral Nerve Regeneration: A Systematic Review" International Journal of Molecular Sciences 25, no. 12: 6489. https://doi.org/10.3390/ijms25126489





