Integrative Analysis of Metabolome and Transcriptome of Carotenoid Biosynthesis Reveals the Mechanism of Fruit Color Change in Tomato (Solanum lycopersicum)
Abstract
1. Introduction
2. Results
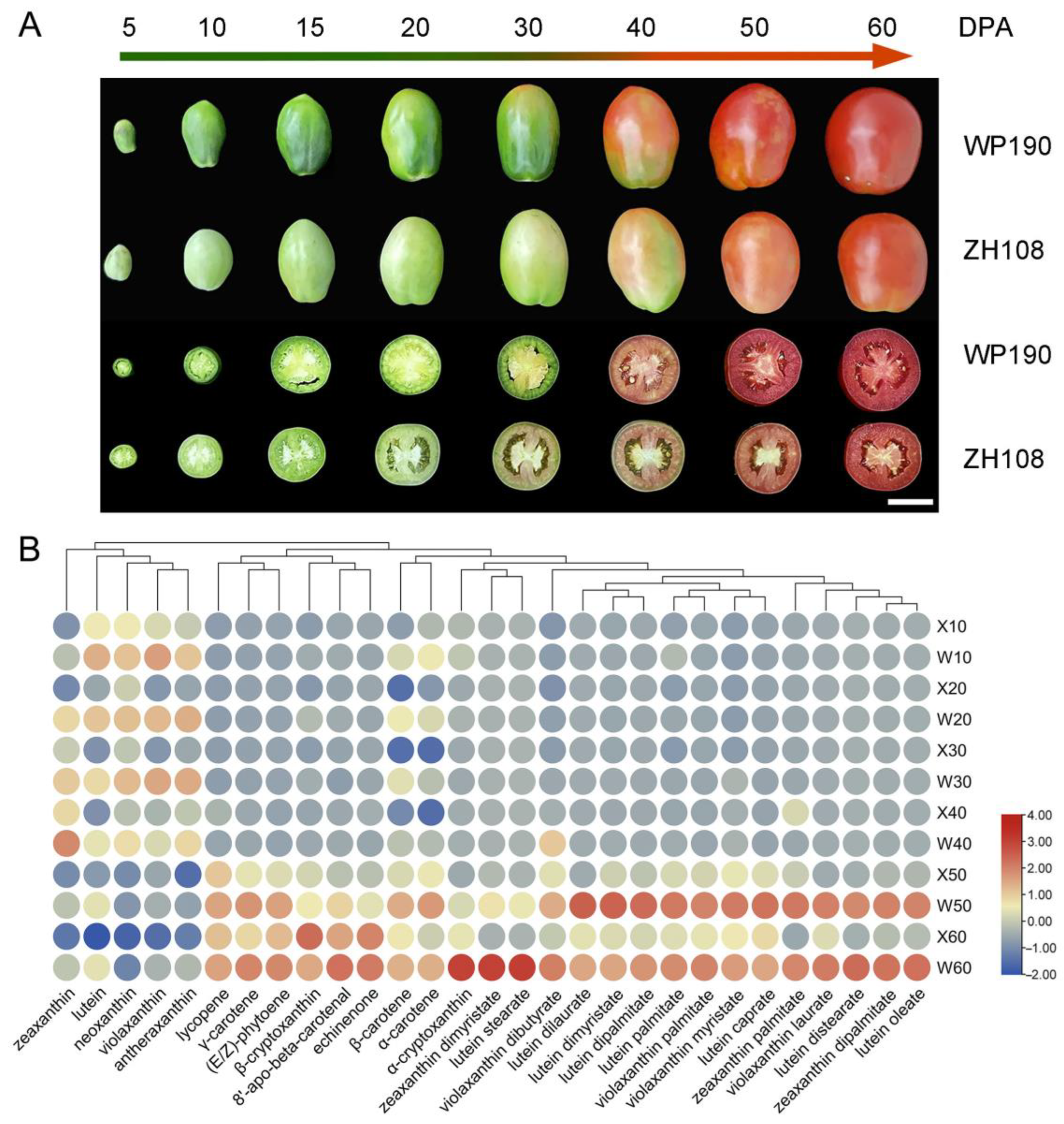
2.1. Metabolic Analysis of Carotenoid Synthesis in Tomato Fruits
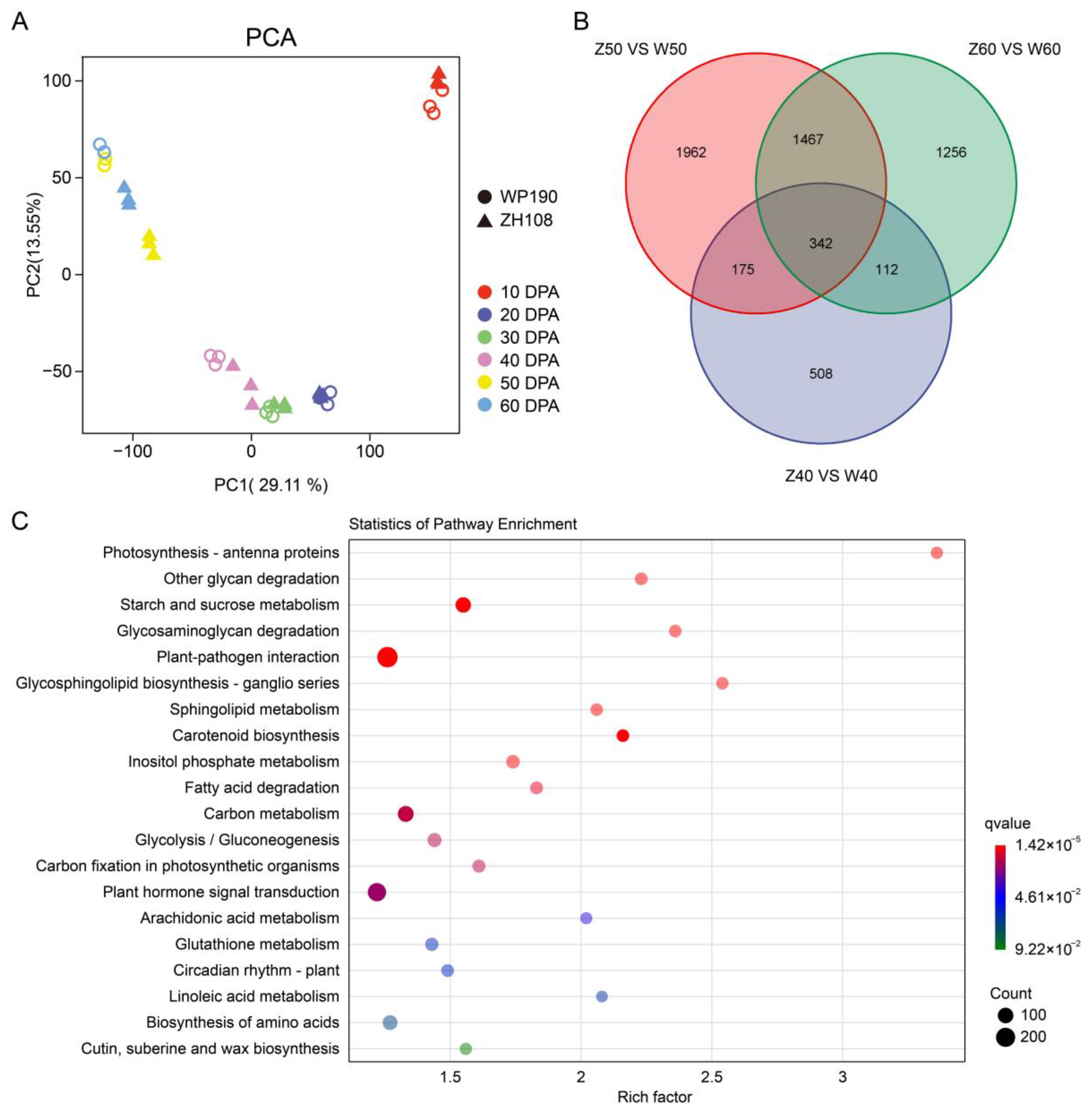
2.2. An Overview of the Transcriptomic Data in Tomato Fruit
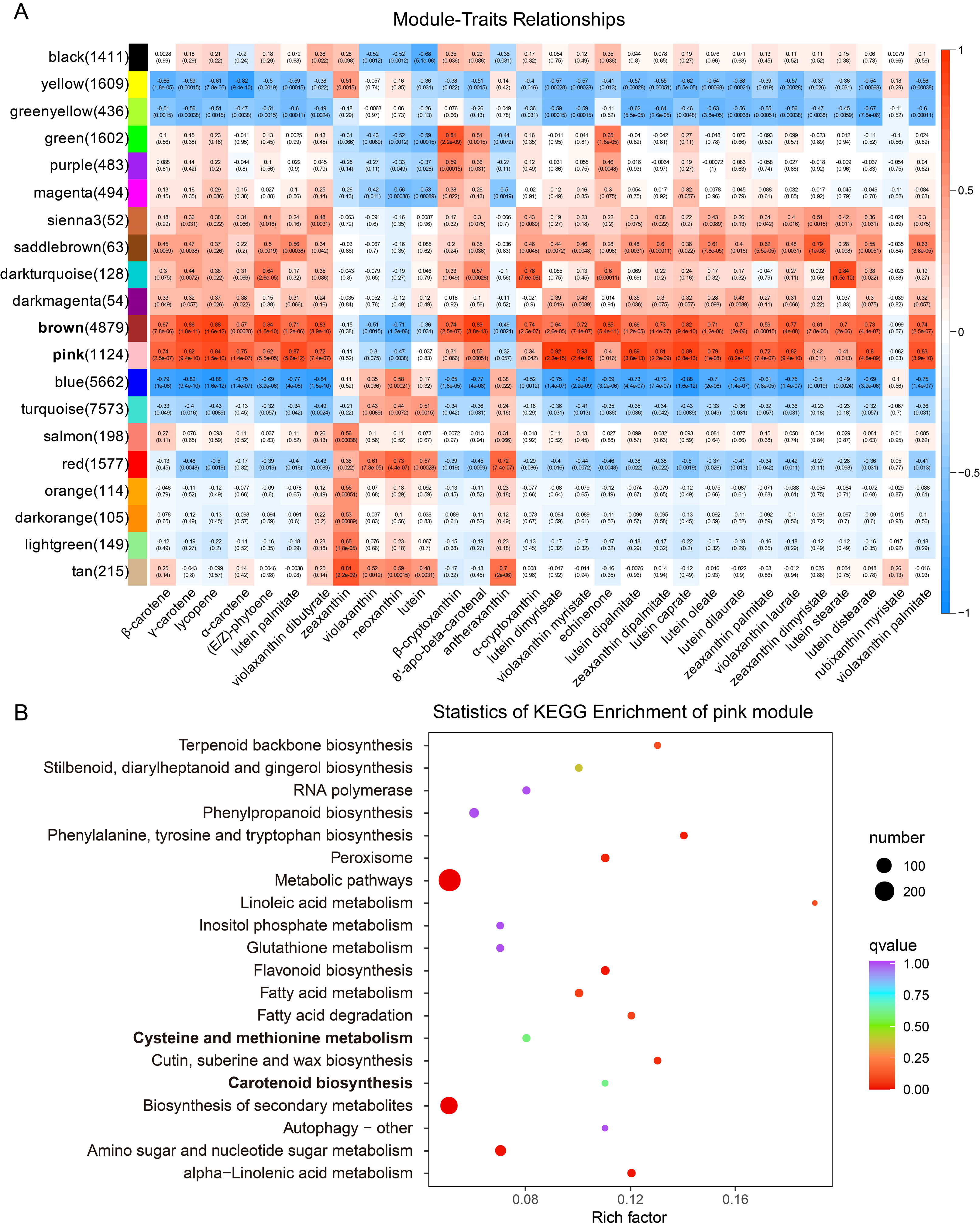
2.3. Gene Screening Using WGCNA Analysis
2.4. Expression Patterns and Validation of Genes in the Carotenoid Biosynthesis and Signal Transduction Pathway
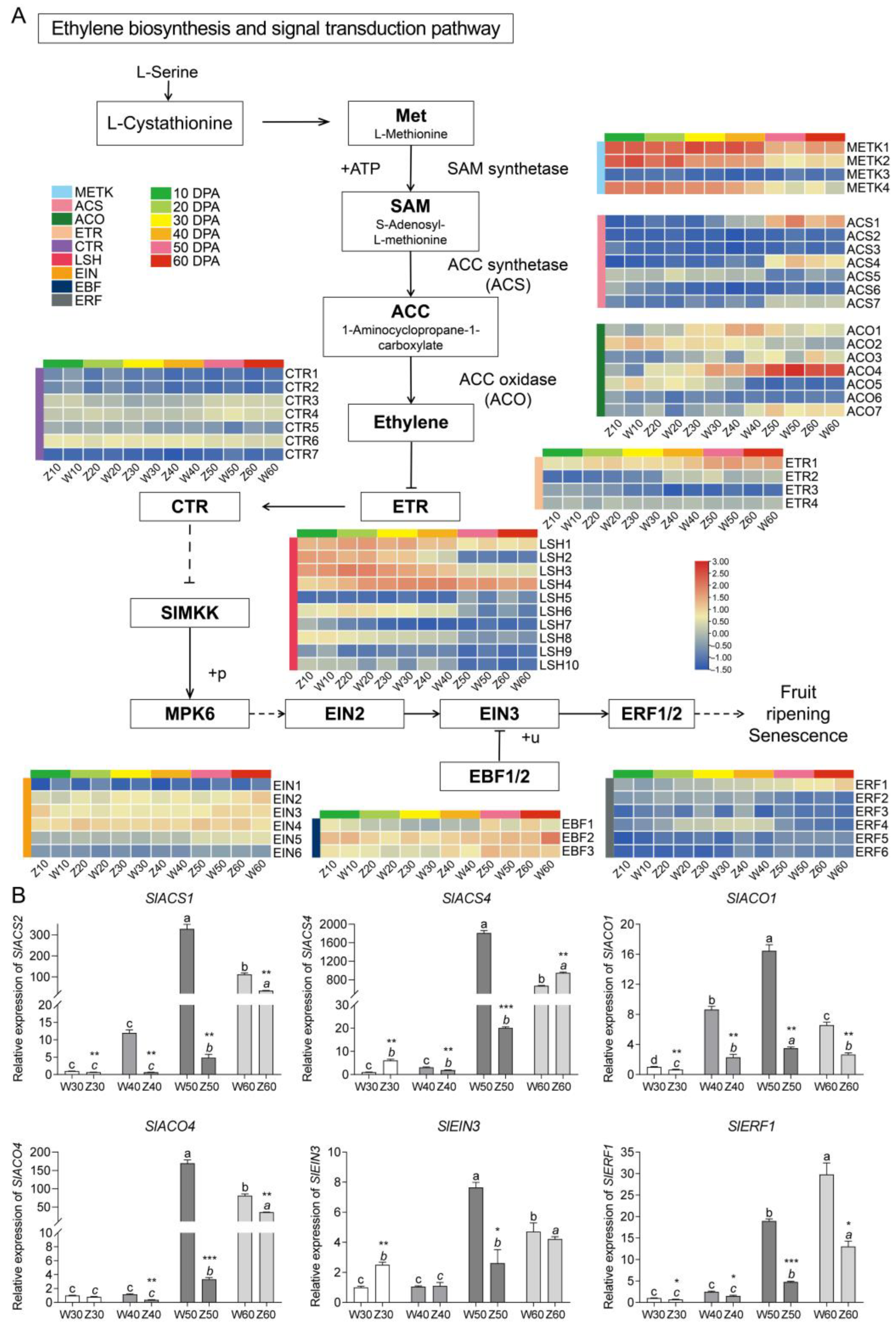
2.5. Expression Patterns and Validation of Genes in the Ethylene Biosynthesis and Signal Transduction Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Preparation
4.2. Carotenoid Identification and Quantification
4.3. RNA-Seq Analysis
4.4. Co-Expression Network Analysis for Construction of Modules
4.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Andres, A.I.; Petron, M.J.; Delgado-Adamez, J.; Lopez, M.; Timon, M. Effect of Tomato Pomace Extracts on the Shelf-Life of Modified Atmosphere-Packaged Lamb Meat. J. Food Process. Preserv. 2017, 41, e13018. [Google Scholar] [CrossRef]
- Wang, R.; Angenent, G.C.; Seymour, G.; de Maagd, R.A. Revisiting the Role of Master Regulators in Tomato Ripening. Trends Plant Sci. 2020, 25, 291–301. [Google Scholar] [CrossRef]
- Ecarnot, M.; Baogonekczyk, P.; Tessarotto, L.; Chervin, C. Rapid Phenotyping of the Tomato Fruit Model, Micro-Tom, Withaportable VIS-NIR Spectrometer. Plant Physiol. Biochem. 2013, 70, 159–163. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Barja, M.V.; Ezquerro, M.; Beretta, S.; Diretto, G.; Florez-Sarasa, I.; Feixes, E.; Fiore, A.; Karlova, R.; Fernie, A.R.; Beekwilder, J.; et al. Several Geranylgeranyl Diphosphate Synthase Isoforms Supply Metabolic Substrates for Carotenoid Biosynthesis in Tomato. New Phytol. 2021, 231, 255–272. [Google Scholar] [CrossRef]
- Gady, A.L.F.; Vriezen, W.H.; Van de Wal, M.H.B.J.; Huang, P.; Bovy, A.G.; Visser, R.G.F.; Bachem, C.W.B. Induced Point Mutations in the Phytoene Synthase 1 Gene Cause Differences in Carotenoid Content during Tomato Fruit Ripening. Mol. Breed. 2012, 29, 801–812. [Google Scholar] [CrossRef]
- Beltrán, J.; Kloss, B.; Hosler, J.P.; Geng, J.; Liu, A.; Modi, A.; Dawson, J.H.; Sono, M.; Shumskaya, M.; Ampomah-Dwamena, C.; et al. Control of Carotenoid Biosynthesis through a Heme-Based Cis-Trans Isomerase. Nat. Chem. Biol. 2015, 11, 598–605. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, F.; Xun, S.; Chang, J.; Zheng, A.; Jiang, M.; Miao, H.; Wang, Q.; Tang, H. Molecular Cloning and Expression Analysis of the ζ-Carotene Desaturase Gene in Chinese Kale (Brassica oleracea Var. Alboglabra bailey). Hortic. Plant J. 2018, 4, 94–102. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Giorio, G.; Marino, I.; Merendino, A.; Petrozza, A.; Salfi, L.; Stigliani, A.L.; Cellini, F. Virtually Complete Conversion of Lycopene into β-Carotene in Fruits of Tomato Plants Transformed with the Tomato Lycopene β-Cyclase (Tlcy-b) CDNA. Plant Sci. 2004, 166, 207–214. [Google Scholar] [CrossRef]
- Zhu, C.; Peng, C.; Qiu, D.; Zeng, J. Metabolic Profiling and Transcriptional Analysis of Carotenoid Accumulation in a Red-Fleshed Mutant of Pummelo (Citrus grandis). Molecules 2022, 27, 4595. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, K.; Tanaka, M.; Kurata, R.; Kai, Y. Comparative Transcriptome Analysis Implied a ZEP Paralog Was a Key Gene Involved in Carotenoid Accumulation in Yellow-Fleshed Sweetpotato. Sci. Rep. 2020, 10, 20607. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Fu, Y.; Zhou, Y.; Cheng, M.; Deng, M.; Li, M.; Zhu, T.; Yang, J.; Guo, X.; Gui, L.; et al. Myb10-D Confers PHS-3D Resistance to Pre-Harvest Sprouting by Regulating NCED in ABA Biosynthesis Pathway of Wheat. New Phytol. 2021, 230, 1940–1952. [Google Scholar] [CrossRef]
- Huang, W.; Hu, N.; Xiao, Z.; Qiu, Y.; Yang, Y.; Yang, J.; Mao, X.; Wang, Y.; Li, Z.; Guo, H. A Molecular Framework of Ethylene-Mediated Fruit Growth and Ripening Processes in Tomato. Plant Cell 2022, 34, 3280–3300. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid Accumulation during Tomato Fruit Ripening Is Modulated by the Auxin-Ethylene Balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Bai, S.; Kusano, M.; Ezura, H.; Wang, N. Increased ACS Enzyme Dosage Causes Initiation of Climacteric Ethylene Production in Tomato. Int. J. Mol. Sci. 2022, 23, 10788. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.J.; Pang, S.Q.; Zheng, Q.; Quan, S.W.; Liu, Y.F.; Xu, T.; Liu, Y.D.; Qi, M.F. Function Analysis of the ERF and DREB Subfamilies in Tomato Fruit Development and Ripening. Front. Plant Sci. 2022, 13, 849048. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, Y.; Liu, Z.; Liu, Z.; Shu, P.; Wang, R.; Hao, Y.; Su, D.; Pirrello, J.; Liu, Y.; et al. SlERF.F12 Modulates the Transition to Ripening in Tomato Fruit by Recruiting the Co-Repressor TOPLESS and Histone Deacetylases to Repress Key Ripening Genes. Plant Cell 2022, 34, 1250–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, M.; Zhang, M.; Yang, M.; Dai, S.; Meng, Q.; Lv, W.; Zhuang, K. ETHYLENE-INSENSITIVE 3-LIKE 2 Regulates β-Carotene and Ascorbic Acid Accumulation in Tomatoes during Ripening. Plant Physiol. 2023, 192, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Tu, Y.; Wang, Y.; Cheng, W.; Yang, Y. Overexpression of an EIN3-Binding F-Box Protein2-like Gene Caused Elongated Fruit Shape and Delayed Fruit Development and Ripening in Tomato. Plant Sci. 2018, 272, 131–141. [Google Scholar] [CrossRef]
- Wang, L.C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant Cell 2002, 14, 51–131. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, P.; Singh, V.; Parida, A.; Raghuvanshi, U.; Kumar, R.; Sharma, A.K. Ethylene Response Factor ERF.D7 Activates Auxin Response Factor 2 Paralogs to Regulate Tomato Fruit Ripening. Plant Physiol. 2022, 190, 2775–2796. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Li, Z.; Liu, M. Role of Ethylene Response Factors (ERFs) in Fruit Ripening. Food Qual. Saf. 2020, 4, 15–20. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Keith Harris, G. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Frusciante, S.; Fabbri, C.; Schauer, N.; Busta, L.; Wang, Z.; Matas, A.J.; Fiore, A.; Rose, J.K.C.; Fernie, A.R.; et al. Manipulation of β-Carotene Levels in Tomato Fruits Results in Increased ABA Content and Extended Shelf Life. Plant Biotechnol. J. 2020, 18, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, X.; Tang, Z.; Zhang, X.; Lei, F.; Hou, L.; Li, M. Combined Analysis of Carotenoid Metabolites and the Transcriptome to Reveal the Molecular Mechanism Underlying Fruit Colouration in Zucchini (Cucurbita pepo L.). Food Chem. Mol. Sci. 2021, 2, 100021. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, L. Toward the ‘Golden’ Era: The Status in Uncovering the Regulatory Control of Carotenoid Accumulation in Plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of Carotenoid Metabolism in Tomato. Mol. Plant 2014, 8, 28–39. [Google Scholar] [CrossRef]
- Cruz, A.B.; Bianchetti, R.E.; Alves, F.R.R.; Purgatto, E.; Peres, L.E.P.; Rossi, M.; Freschi, L. Light, Ethylene and Auxin Signaling Interaction Regulates Carotenoid Biosynthesis during Tomato Fruit Ripening. Front. Plant Sci. 2018, 9, 1370. [Google Scholar] [CrossRef]
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating Colors: Regulation of Carotenoid Biosynthesis and Accumulation by Light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of Transgenic Tomato Plants Expressing an Additional Phytoene Synthase in a Fruit-Specific Manner. Natl. Acad. Sci. 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Efremov, G.I.; Slugina, M.A.; Shchennikova, A.V.; Kochieva, E.Z. Differential Regulation of Phytoene Synthase Psy1 during Fruit Carotenogenesis in Cultivated and Wild Tomato Species (Solanum Section Lycopersicon). Plants 2020, 9, 1169. [Google Scholar] [CrossRef] [PubMed]
- Giorio, G.; Stigliani, A.L.; D’Ambrosio, C. Phytoene Synthase Genes in Tomato (Solanum lycopersicum L.)—New Data on the Structures, the Deduced Amino Acid Sequences and the Expression Patterns. FEBS J. 2008, 275, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Hwang, I.; Goswami, G.; Jung, H.J.; Nath, U.K.; Yoo, H.J.; Lee, J.M.; Nou, I.S. Molecular Insights Reveal Psy1, SGR, and SlMYB12 Genes Are Associated with Diverse Fruit Color Pigments in Tomato (Solanum lycopersicum L.). Molecules 2017, 22, 2180. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Welsch, R.; Camagna, M.; Kohlen, W.; Balcke, G.U.; Tissier, A.; Walter, M.H. Strigolactone Levels in Dicot Roots Are Determined by an Ancestral Symbiosis-Regulated Clade of the PHYTOENE SYNTHASE Gene Family. Front. Plant Sci. 2018, 9, 334441. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Stauder, R.; Tissier, A. Evolution of Root-Specific Carotenoid Precursor Pathways for Apocarotenoid Signal Biogenesis. Plant Sci. 2015, 233, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Namitha, K.K.; Archana, S.N.; Negi, P.S. Expression of Carotenoid Biosynthetic Pathway Genes and Changes in Carotenoids during Ripening in Tomato (Lycopersicon esculentum). Food Funct. 2011, 2, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of Tomato Lycopene Biosynthesis through Virus-Induced Gene Silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of Tangerine from Tomato Reveals a Carotenoid Isomerase Essential for the Production of β-Carotene and Xanthophylls in Plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid Metabolism and Regulation in Horticultural Crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Pandurangaiah, S.; Ravishankar, K.V.; Shivashankar, K.S.; Sadashiva, A.T.; Pillakenchappa, K.; Narayanan, S.K. Differential Expression of Carotenoid Biosynthetic Pathway Genes in Two Contrasting Tomato Genotypes for Lycopene Content. J. Biosci. 2016, 41, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Gupta, S.; Sarma, S.; Rai, M.; Sreelakshmi, Y.; Sharma, R. Mutations in Tomato 1-Aminocyclopropane Carboxylic Acid Synthase 2 Uncover Its Role in Development beside Fruit Ripening. Plant J. 2021, 106, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Gapper, N.E.; McQuinn, R.P.; Giovannoni, J.J. Molecular and Genetic Regulation of Fruit Ripening. Plant Mol. Biol. 2013, 82, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Van de Poel, B.; Bulens, I.; Markoula, A.; Hertog, M.L.A.T.M.; Dreesen, R.; Wirtz, M.; Vandoninck, S.; Oppermann, Y.; Keulemans, J.; Hell, R.; et al. Targeted Systems Biology Profiling of Tomato Fruit Reveals Coordination of the Yang Cycle and a Distinct Regulation of Ethylene Biosynthesis during Postclimacteric Ripening. Plant Physiol. 2012, 160, 1498–1514. [Google Scholar] [CrossRef] [PubMed]
- Oeller, P.W.; Min-Wong, L.; Taylor, L.P.; Pike, D.A.; Theologis, A.A. Reversible Inhibition of Tomato Fruit Senescence by Antisense RNA. Science 1991, 254, 437–439. [Google Scholar] [CrossRef]
- Ito, Y.; Kitagawa, M.; Ihashi, N.; Yabe, K.; Kimbara, J.; Yasuda, J.; Ito, H.; Inakuma, T.; Hiroi, S.; Kasumi, T. DNA-Binding Specificity, Transcriptional Activation Potential, and the Rin Mutation Effect for the Tomato Fruit-Ripening Regulator RIN. Plant J. 2008, 55, 212–223. [Google Scholar] [CrossRef]
- Itkin, M.; Seybold, H.; Breitel, D.; Rogachev, I.; Meir, S.; Aharoni, A. TOMATO AGAMOUS-LIKE 1 is a Component of the Fruit Ripening Regulatory Network. Plant J. 2009, 60, 1081–1095. [Google Scholar] [CrossRef]
- Li, S.; Zhu, B.; Pirrello, J.; Xu, C.; Zhang, B.; Bouzayen, M.; Chen, K.; Grierson, D. Roles of RIN and Ethylene in Tomato Fruit Ripening and Ripening-Associated Traits. New Phytol. 2020, 226, 460–475. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, Q.; Qin, L.; Pan, C.; Lamin-Samu, A.T.; Lu, G. Tomato AUXIN RESPONSE FACTOR 5 Regulates Fruit Set and Development via the Mediation of Auxin and Gibberellin Signaling. Sci. Rep. 2018, 8, 2971. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene Signaling in Plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Tieman, D.M.; Ciardi, J.A.; Taylor, M.G.; Klee, H.J. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J. 2001, 26, 47–58. [Google Scholar] [CrossRef]
- Lee, J.M.; Joung, J.G.; McQuinn, R.; Chung, M.Y.; Fei, Z.; Tieman, D.; Klee, H.; Giovannoni, J. Combined Transcriptome, Genetic Diversity and Metabolite Profiling in Tomato Fruit Reveals That the Ethylene Response Factor SlERF6 Plays an Important Role in Ripening and Carotenoid Accumulation. Plant J. 2012, 70, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Diretto, G.; Pirrello, J.; Roustan, J.P.; Li, Z.; Giuliano, G.; Regad, F.; Bouzayen, M. The Chimeric Repressor Version of an Ethylene Response Factor (ERF) Family Member, SlERF.B3, Shows Contrasting Effects on Tomato Fruit Ripening. New Phytol. 2014, 203, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gomes, B.L.; Mila, I.; Purgatto, E.; Peres, L.E.P.; Frasse, P.; Maza, E.; Zouine, M.; Roustan, J.P.; Bouzayen, M.; et al. Comprehensive Profiling of Ethylene Response Factor Expression Identifies Ripening-Associated ERF Genes and Their Link to Key Regulators of Fruit Ripening in Tomato. Plant Physiol. 2016, 170, 1732–1744. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, J.; Muhammad, T.; Yang, T.; Li, N.; Yang, H.; Yu, Q.; Wang, B. Integrative Analysis of Metabolome and Transcriptome of Carotenoid Biosynthesis Reveals the Mechanism of Fruit Color Change in Tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2024, 25, 6493. https://doi.org/10.3390/ijms25126493
Hu J, Wang J, Muhammad T, Yang T, Li N, Yang H, Yu Q, Wang B. Integrative Analysis of Metabolome and Transcriptome of Carotenoid Biosynthesis Reveals the Mechanism of Fruit Color Change in Tomato (Solanum lycopersicum). International Journal of Molecular Sciences. 2024; 25(12):6493. https://doi.org/10.3390/ijms25126493
Chicago/Turabian StyleHu, Jiahui, Juan Wang, Tayeb Muhammad, Tao Yang, Ning Li, Haitao Yang, Qinghui Yu, and Baike Wang. 2024. "Integrative Analysis of Metabolome and Transcriptome of Carotenoid Biosynthesis Reveals the Mechanism of Fruit Color Change in Tomato (Solanum lycopersicum)" International Journal of Molecular Sciences 25, no. 12: 6493. https://doi.org/10.3390/ijms25126493
APA StyleHu, J., Wang, J., Muhammad, T., Yang, T., Li, N., Yang, H., Yu, Q., & Wang, B. (2024). Integrative Analysis of Metabolome and Transcriptome of Carotenoid Biosynthesis Reveals the Mechanism of Fruit Color Change in Tomato (Solanum lycopersicum). International Journal of Molecular Sciences, 25(12), 6493. https://doi.org/10.3390/ijms25126493






