The Long-Distance Transport of Jasmonates in Salt-Treated Pea Plants and Involvement of Lipid Transfer Proteins in the Process
Abstract
1. Introduction
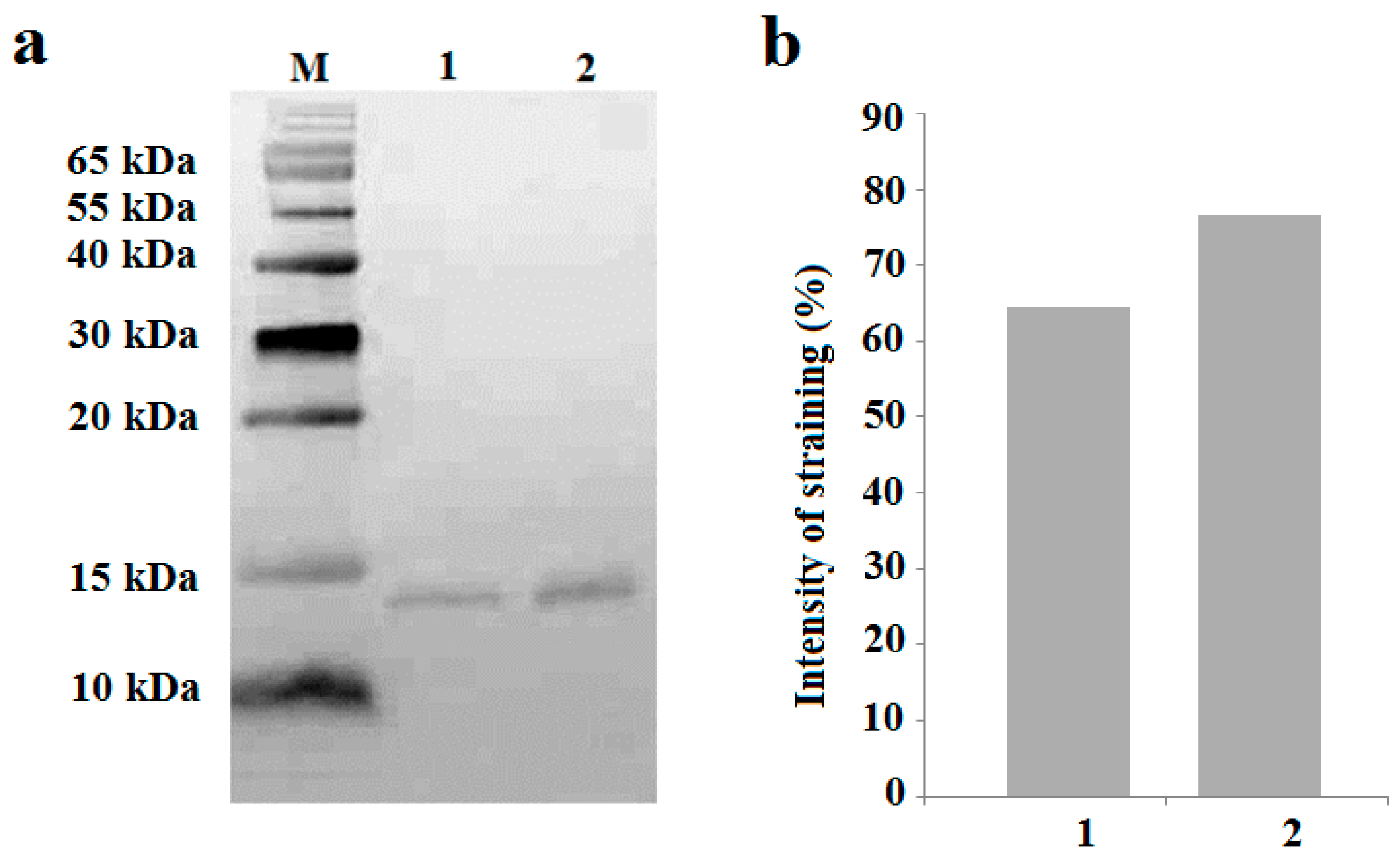
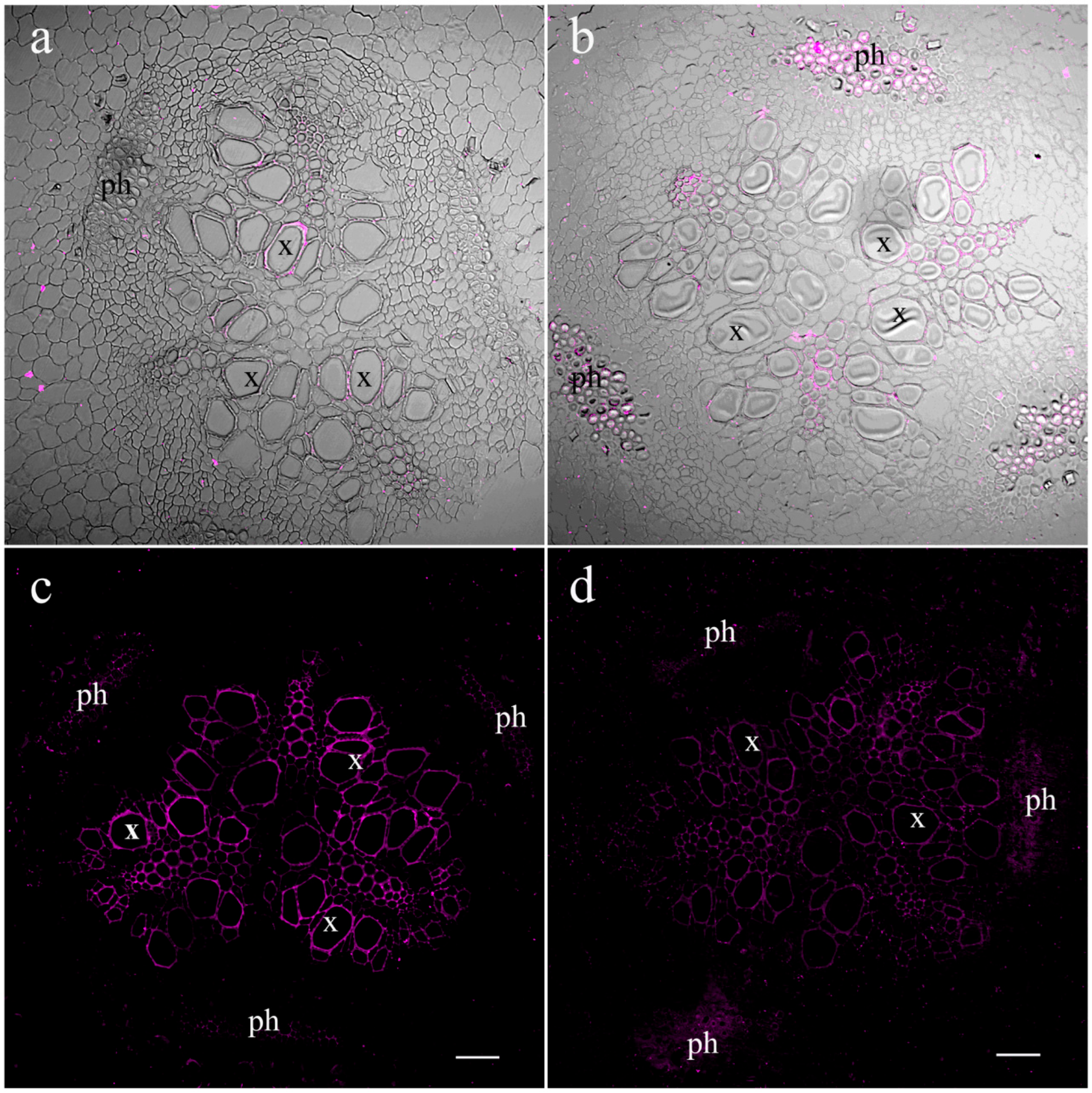
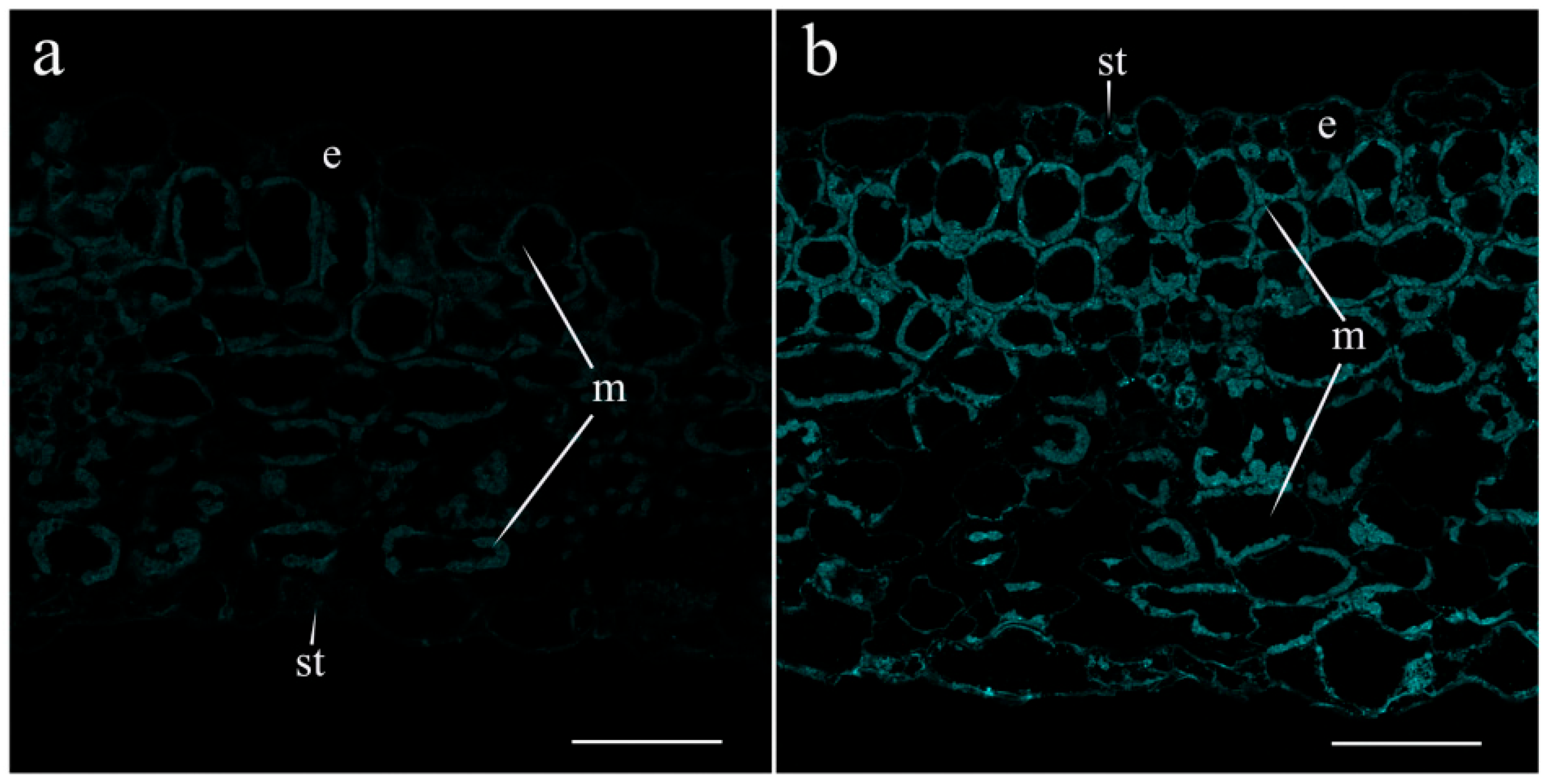
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Growth and the Collection of Plant Material
4.2. SDS Electrophoresis
4.3. Western Blotting
4.4. Methyl Jasmonate Treatment
4.5. Immunoassay of Jasmonates
4.6. Immunohistochemistry
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasternack, C.; Strnad, M. Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M. Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci. 1997, 2, 22–28. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Lacombe, B.; Achard, P. Long-distance transport of phytohormones through the plant vascular system. Curr. Opin. Plant. Biol. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef]
- Ladeynova, M.; Kuznetsova, D.; Mudrilov, M.; Vodeneev, V. Integration of electrical signals and phytohormones in the control of systemic response. Int. J. Mol. Sci. 2023, 24, 847. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F.; Li, S.; Yu, G.; Wang, L.; Li, Q.; Zhu, X.; Li, Z.; Yuan, L.; Liu, P. Importers drive leaf-to-leaf jasmonic acid transmission in wound-induced systemic immunity. Mol. Plant. 2020, 13, 1485–1498. [Google Scholar] [CrossRef]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef]
- Ahmad, B.; Raina, A.; Naikoo, M.I.; Khan, S. Role of methyl jasmonates in salt stress tolerance in crop plants. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–384. [Google Scholar]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.-T.; Figueroa, C.R. Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.R.; Sun, G.W.; Dong, L.J.; Yang, L.Q.; Yu, S.N.; Zhang, S.L.; Liu, J.F. Physiological and molecular responses to drought and salinity in soybean. Biol. Plant. 2017, 61, 557–564. [Google Scholar] [CrossRef]
- Akhiyarova, G.; Vafina, G.; Veselov, D.; Kudoyarova, G. Immunolocalization of jasmonates and auxins in pea roots in connection with inhibition of root growth under salinity conditions. Int. J. Mol. Sci. 2023, 24, 15148. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.; Shin, D.; Park, S.; Jang, S.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.-K.; Lee, I.-J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Modarres Sanavy, S.A.M.; Allahdadi, I. The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci. 2011, 5, 726–734. [Google Scholar]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J. Exp. Bot. 2004, 55, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Förster, S.; Schmidt, L.K.; Kopic, E.; Anschütz, U.; Huang, S.; Schlücking, K.; Köster, P.; Waadt, R.; Larrieu, A.; Batistič, O.; et al. Wounding-induced stomatal closure requires jasmonate-mediated activation of gork K+ channels by a Ca2+sensor-kinase CBL1-CIPK5 complex. Dev. Cell. 2019, 48, 87–99.e6. [Google Scholar] [CrossRef]
- De Ollas, C.; Arbona, V.; Gómez-Cadenas, A.; Dodd, I.C. Attenuated accumulation of jasmonates modifies stomatal responses to water deficit. J. Exp. Bot. 2018, 69, 2103–2116. [Google Scholar] [CrossRef]
- Xin, P.; Guo, Q.; Li, B.; Cheng, S.; Yan, J.; Chu, J. A tailored high-efficiency sample pretreatment method for simultaneous quantification of 10 classes of known endogenous phytohormones. Plant Comm. 2020, 1, 100047. [Google Scholar] [CrossRef]
- Da Silva, P.; Landon, C.; Industri, B.; Marais, A.; Marion, D.; Ponchet, M.; Vovelle, F. Solution structure of a tobacco lipid transfer protein exhibiting new biophysical and biological features. Proteins 2005, 59, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Lipid transfer proteins as components of the plant innate immune system: Structure, functions, and applications. Acta Naturae 2016, 8, 47–61. [Google Scholar] [CrossRef]
- Gao, H.; Ma, K.; Ji, G.; Pan, L.; Zhou, Q. Lipid transfer proteins involdaved in plant–pathogen interactions and their molecular mechanisms. Mol. Plant Pathol. 2022, 23, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.N.; Finkina, E.I.; Bogdanov, I.V.; Tagaev, A.A.; Ovchinnikova, T.V. Features and possible applications of plant lipid-binding and transfer proteins. Membranes 2023, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Akhiyarova, G.R.; Ivanov, R.S.; Ivanov, I.I.; Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Nuzhnaya, T.; Ovchinnikova, T.V.; Veselov, D.S.; Kudoyarova, G.R. Effects of salinity and abscisic acid on lipid transfer protein accumulation, suberin deposition and hydraulic conductance in pea roots. Membranes 2021, 11, 762. [Google Scholar] [CrossRef]
- Djordjevic, M.A.; Oakes, M.; Li, D.X.; Hwang, C.H.; Hocart, C.H.; Gresshoff, P.M. The glycinemax xylem sap and apoplast proteome. J. Proteome Res. 2007, 6, 3771–3779. [Google Scholar] [CrossRef]
- Popova, A.V.; Borisova, P.; Vasilev, D. Response of pea plants (Pisum sativum cv. Ran 1) to NaCl treatment in regard to membrane stability and photosynthetic activity. Plants 2023, 12, 324. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fragoso, V.; Rothe, E.; Baldwin, I.T.; Kim, S.G. Root jasmonic acid synthesis and perception regulate folivore-induced shoot metabolites and increase Nicotiana attenuata resistance. New Phytol. 2014, 202, 1335–1345. [Google Scholar] [CrossRef]
- Vellosillo, T.; Martínez, M.; López, M.A.; Vicente, J.; Cascón, T.; Dolan, L.; Hamberg, M.; Castresana, G. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Grebner, W.; Stingl, N.E.; Oenel, A.; Mueller, M.J.; Berger, S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol. 2013, 161, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lai, J.; Wu, Q.; Zhang, S.; Chen, L.; Dai, Y.-S.; Wang, C.; Du, J.; Xiao, S.; Yang, C. Jasmonate complements the function of Arabidopsis lipoxygenase3 in salinity stress response. Plant Sci. 2016, 244, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, J.; Feron, R.; Huisman, B.A.H.; Fasolino, A.; Hilbers, C.W.; Derksen, J.; Mariani, C. Lipid transfer proteins enhance cell wall extension in tobacco. Plant Cell 2005, 17, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Zamora, O.; Schulze, S.; Azoulay-Shemer, T.; Parik, H.; Unt, J.; Brosché, M.; Schroeder, J.I.; Yarmolinsky, D.; Kollist, H. Jasmonic acid and salicylic acid play minor roles in stomatal regulation by CO2, abscisic acid, darkness, vapor pressure deficit and ozone. Plant J. 2021, 108, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Rakwal, R.; Agrawa, G.K. Interplant communication: Airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem. Biophys. Res. Commun. 2008, 376, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U. Most commonly used discontinuous buffer system for SDS electrophoresis. Nature 1970, 227, 680–686. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, I.V.; Shenkarev, Z.O.; Finkina, E.I.; Melnikova, D.N.; Rumynskiy, E.I.; Arseniev, A.S.; Ovchinnikova, T.V. A novel lipid transfer protein from the pea Pisum sativum: Isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic properties. BMC Plant Biol. 2016, 16, 107. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Trekozova, A.W.; Kudoyarova, G.R. Effect of phosphorus starvation on hormone content and growth of barley plants. Acta Physiol. Plant. 2016, 38, 108. [Google Scholar] [CrossRef]
- Veselov, S.U.; Kudoyarova, G.R.; Egutkin, N.L.; Gyuli-Zade, V.G.; Mustafina, A.R.; Kof, E.K. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole 3-acetic acid. Physiol. Plant. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Korobova, A.; Ivanov, R.; Timergalina, L.; Vysotskaya, L.; Nuzhnaya, T.; Akhiyarova, G.; Kusnetsov, V.; Veselov, D.; Kudoyarova, G. Effect of low light stress on distribution of auxin (indole-3-acetic acid) between shoot and roots and development of lateral roots in barley plants. Biology 2023, 12, 787. [Google Scholar] [CrossRef]



| Treatments | Time after the Start of Treatment, h | |||
|---|---|---|---|---|
| 1.5 | 4.5 | |||
| Concentration | Flow Rate | Concentration | Flow Rate | |
| Control | 12 ± 4 | 0.97 ± 0.1 | 36 ± 5 | 0.41 ± 0.1 |
| NaCl treatment | 206 ± 19 * | 6.9 ± 0.7 * | 224 ± 30 * | 0.62 ± 0.1 * |
| Treatments | Time after the Start of Treatment, h | |
|---|---|---|
| 1.5 | 4.5 | |
| Control | 119 ± 3 c | 118 ± 3 c |
| NaCl treatment | 99 ± 1 b | 90 ± 1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafina, G.; Akhiyarova, G.; Korobova, A.; Finkina, E.I.; Veselov, D.; Ovchinnikova, T.V.; Kudoyarova, G. The Long-Distance Transport of Jasmonates in Salt-Treated Pea Plants and Involvement of Lipid Transfer Proteins in the Process. Int. J. Mol. Sci. 2024, 25, 7486. https://doi.org/10.3390/ijms25137486
Vafina G, Akhiyarova G, Korobova A, Finkina EI, Veselov D, Ovchinnikova TV, Kudoyarova G. The Long-Distance Transport of Jasmonates in Salt-Treated Pea Plants and Involvement of Lipid Transfer Proteins in the Process. International Journal of Molecular Sciences. 2024; 25(13):7486. https://doi.org/10.3390/ijms25137486
Chicago/Turabian StyleVafina, Gulnara, Guzel Akhiyarova, Alla Korobova, Ekaterina I. Finkina, Dmitry Veselov, Tatiana V. Ovchinnikova, and Guzel Kudoyarova. 2024. "The Long-Distance Transport of Jasmonates in Salt-Treated Pea Plants and Involvement of Lipid Transfer Proteins in the Process" International Journal of Molecular Sciences 25, no. 13: 7486. https://doi.org/10.3390/ijms25137486
APA StyleVafina, G., Akhiyarova, G., Korobova, A., Finkina, E. I., Veselov, D., Ovchinnikova, T. V., & Kudoyarova, G. (2024). The Long-Distance Transport of Jasmonates in Salt-Treated Pea Plants and Involvement of Lipid Transfer Proteins in the Process. International Journal of Molecular Sciences, 25(13), 7486. https://doi.org/10.3390/ijms25137486








