Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment
Abstract
:1. Introduction
2. Exogenous dsRNA Application in Plant Protection
3. Risk Assessment and Regulatory Approaches
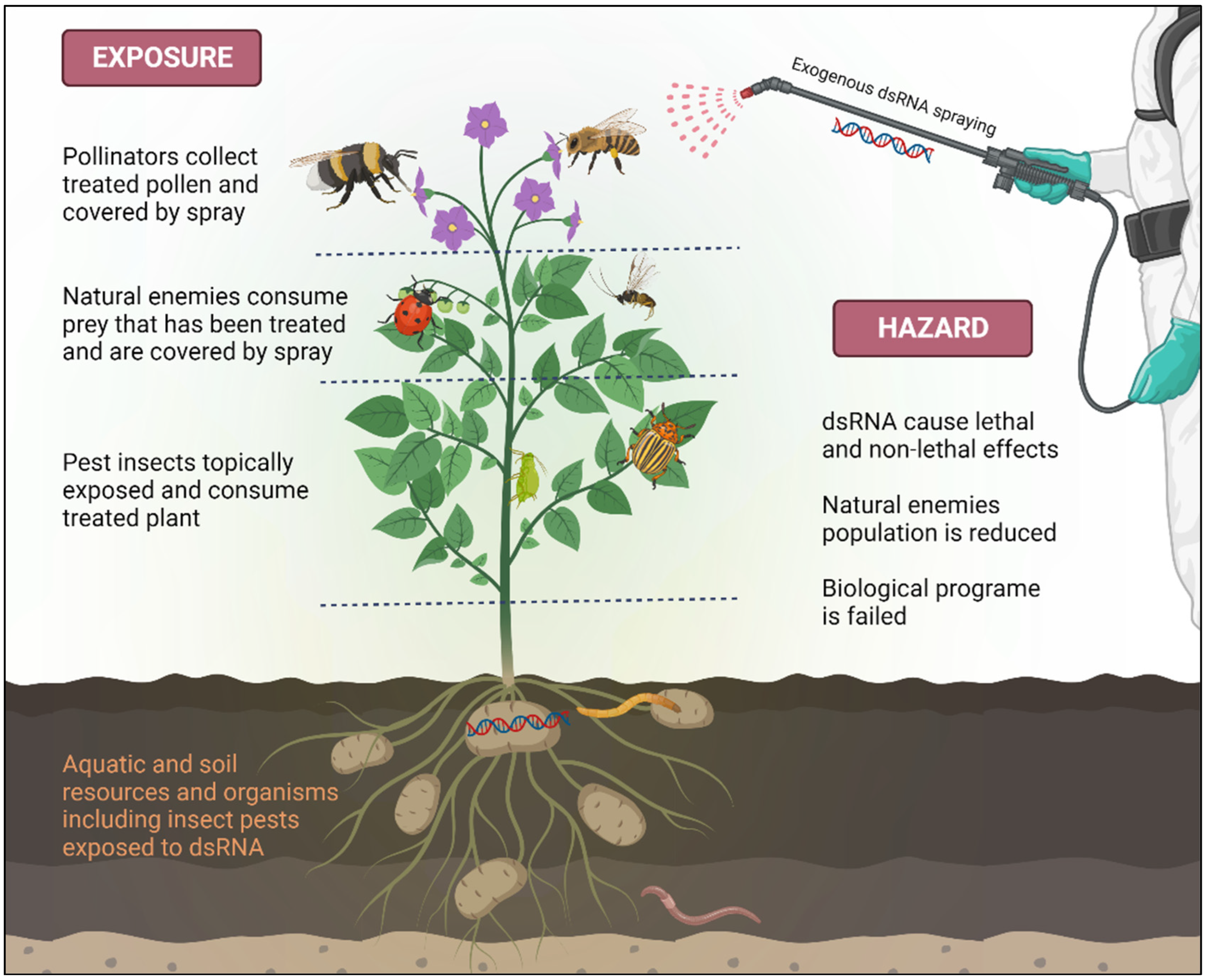
4. Safety Concerns of dsRNA Application
5. Efficiency and Perspectives of RNAi in Crop Protection
Author Contributions
Funding
Conflicts of Interest
References
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Montgomery, M.K. RNA interference: Historical overview and significance. RNA Interf. Ed. Modif. Methods Protoc. 2004, 265, 3–21. [Google Scholar]
- Christiaens, O.; Niu, J.; Nji Tizi Taning, C. RNAi in insects: A revolution in fundamental research and pest control applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Palli, S.R. RNAi turns 25: Contributions and challenges in insect science. Front. Insect Sci. 2023, 3, 1209478. [Google Scholar] [PubMed]
- Yogindran, S.; Rajam, M.V. RNAi for crop improvement. Plant Biol. Biotechnol. Vol. II Plant Genom. Biotechnol. 2015, 623–637. [Google Scholar] [CrossRef]
- Vaucheret, H.; Béclin, C.; Fagard, M. Post-transcriptional gene silencing in plants. J. Cell Sci. 2001, 114, 3083–3091. [Google Scholar] [CrossRef]
- Waterhouse, P.M.; Wang, M.-B.; Lough, T. Gene silencing as an adaptive defence against viruses. Nature 2001, 411, 834–842. [Google Scholar]
- Hernández-Soto, A.; Chacón-Cerdas, R. RNAi crop protection advances. Int. J. Mol. Sci. 2021, 22, 12148. [Google Scholar] [CrossRef]
- Leonetti, P.; Stuttmann, J.; Pantaleo, V. Regulation of plant antiviral defense genes via host RNA-silencing mechanisms. Virol. J. 2021, 18, 194. [Google Scholar]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Gordon, K.H.J.; Waterhouse, P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Rusten, T.E.; Simonsen, A. ESCRT functions in autophagy and associated disease. Cell Cycle 2008, 7, 1166–1172. [Google Scholar] [CrossRef]
- Teis, D.; Saksena, S.; Emr, S.D. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell 2008, 15, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, T.; Rusten, T.E.; Menut, L.; Nezis, I.P.; Brech, A.; Stenmark, H.; Bilder, D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 2009, 122, 2413–2423. [Google Scholar] [CrossRef]
- Lee, J.-A.; Gao, F.-B. Roles of ESCRT in autophagy-associated neurodegeneration. Autophagy 2008, 4, 230–232. [Google Scholar] [CrossRef]
- Ramaseshadri, P.; Segers, G.; Flannagan, R.; Wiggins, E.; Clinton, W.; Ilagan, O.; McNulty, B.; Clark, T.; Bolognesi, R. Physiological and Cellular Responses Caused by RNAi- Mediated Suppression of Snf7 Orthologue in Western Corn Rootworm (Diabrotica virgifera virgifera) Larvae. PLoS ONE 2013, 8, e54270. [Google Scholar] [CrossRef]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W.; et al. Characterizing the Mechanism of Action of Double-Stranded RNA Activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef]
- Romeis, J.; Widmer, F. Assessing the risks of topically applied dsRNA-based products to non-target arthropods. Front. Plant Sci. 2020, 11, 679. [Google Scholar] [PubMed]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L.-A. Prospects, challenges and current status of RNAi through insect feeding. Pest Manag. Sci. 2020, 76, 26–41. [Google Scholar] [CrossRef]
- Niu, J.; Shen, G.; Christiaens, O.; Smagghe, G.; He, L.; Wang, J. Beyond insects: Current status and achievements of RNA interference in mite pests and future perspectives. Pest Manag. Sci. 2018, 74, 2680–2687. [Google Scholar] [CrossRef]
- Li, H.; Guan, R.; Guo, H.; Miao, X. New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant. Cell Environ. 2015, 38, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest suppression. Southwest. Entomol. 2012, 37, 85–87. [Google Scholar] [CrossRef]
- Vatanparast, M.; Kim, Y. Yeast engineering to express sex pheromone gland genes of the oriental fruit moth, Grapholita molesta. J. Asia. Pac. Entomol. 2019, 22, 645–654. [Google Scholar] [CrossRef]
- Vatanparast, M.; Kim, Y. Optimization of recombinant bacteria expressing dsRNA to enhance insecticidal activity against a lepidopteran insect, Spodoptera exigua. PLoS ONE 2017, 12, e0183054. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Kazzazi, M.; Sajjadian, S.M.; Park, Y. Knockdown of Helicoverpa armigera protease genes affects its growth and mortality via RNA interference. Arch. Insect Biochem. Physiol. 2021, 108, e21840. [Google Scholar] [CrossRef]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.-W.; Broeck, J. Vanden RNA Interference in Insects: Protecting Beneficials and Controlling Pests. Front. Physiol. 2019, 9, 1912. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, K.; Scott, J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Almasi, R.; Amari, K. Harnessing environmental-RNAi to win an enduring battle: Plant versus pathogen. J. Phytopathol. 2021, 169, 649–657. [Google Scholar] [CrossRef]
- Vatanparast, M.; Kazzazi, M.; Mirzaie-asl, A.; Hosseininaveh, V. RNA interference-mediated knockdown of some genes involved in digestion and development of Helicoverpa armigera. Bull. Entomol. Res. 2017, 107, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Das, P.R.; Sherif, S.M. Application of Exogenous dsRNAs-induced RNAi in Agriculture: Challenges and Triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, J.; Huff Hartz, K.E.; Lydy, M.J.; Moon, T.S.; Sander, M.; Parker, K.M. Analysis of RNA interference (RNAi) biopesticides: Double-stranded RNA (dsRNA) extraction from agricultural soils and quantification by RT-qPCR. Environ. Sci. Technol. 2020, 54, 4893–4902. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Meghwanshi, K.K.; Shukla, N.; Shukla, J.N. Innate and adaptive resistance to RNAi: A major challenge and hurdle to the development of double stranded RNA-based pesticides. 3 Biotech 2021, 11, 498. [Google Scholar] [CrossRef]
- OECD. Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied ds-rna-Based Pesticides. Series on Pesticides, No. 104. 2020; 1–174. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2020)26&doclanguage=en (accessed on 17 March 2023).
- Andrade, E.C.; Hunter, W.B. RNAi feeding bioassay: Development of a non-transgenic approach to control Asian citrus psyllid and other hemipterans. Entomol. Exp. Appl. 2017, 162, 389–396. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in Colorado potato beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef]
- Wu, J.; Mu, L.; Kang, W.; Ze, L.; Shen, C.; Jin, L.; Anjum, A.A.; Li, G. RNA interference targeting ecdysone receptor blocks the larval–pupal transition in Henosepilachna vigintioctopunctata. Insect Sci. 2021, 28, 419–429. [Google Scholar] [CrossRef]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic spreading of exogenous applied RNA biopesticides in the crop plant Hordeum vulgare. ExRNA 2020, 2, 12. [Google Scholar]
- Kolge, H.; Kadam, K.; Galande, S.; Lanjekar, V.; Ghormade, V. New frontiers in pest control: Chitosan nanoparticles-shielded dsRNA as an effective topical RNAi spray for gram podborer biocontrol. ACS Appl. Bio Mater. 2021, 4, 5145–5157. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.; Coleman, A.D.; Maffei, M.E.; Ridout, C.J.; Hogenhout, S.A. Silencing of aphid genes by dsRNA feeding from plants. PLoS ONE 2011, 6, e25709. [Google Scholar]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.; Li, J.; Yin, M.; Ren, B.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2020, 93, 449–459. [Google Scholar]
- Huang, J.-H.; Liu, Y.; Lin, Y.-H.; Belles, X.; Lee, H.-J. Practical use of RNA interference: Oral delivery of double-stranded RNA in liposome carriers for cockroaches. J. Vis. Exp. 2018, 135, e57385. [Google Scholar]
- Christiaens, O.; Tardajos, M.G.; Martinez Reyna, Z.L.; Dash, M.; Dubruel, P.; Smagghe, G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Zhao, Y.-Q.; Zhou, Y.; Feng, H.-Y.; Gong, L.-L.; Zhang, M.-Q.; Hull, J.J.; Dewer, Y.; Roy, A.; Smagghe, G. Nanoparticle-delivered RNAi-based pesticide target screening for the rice pest white-backed planthopper and risk assessment for a natural predator. Sci. Total Environ. 2024, 926, 171286. [Google Scholar]
- Chen, X.; Li, L.; Hu, Q.; Zhang, B.; Wu, W.; Jin, F.; Jiang, J. Expression of dsRNA in recombinant Isaria fumosorosea strain targets the TLR7 gene in Bemisia tabaci. BMC Biotechnol. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Avila, L.A.; Chandrasekar, R.; Wilkinson, K.E.; Balthazor, J.; Heerman, M.; Bechard, J.; Brown, S.; Park, Y.; Dhar, S.; Reeck, G.R. Delivery of lethal dsRNAs in insect diets by branched amphiphilic peptide capsules. J. Control. Release 2018, 273, 139–146. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef] [PubMed]
- Mosa, M.A.; Youssef, K. Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar]
- Hu, D.; Chen, Z.; Zhang, C.; Ganiger, M. Reduction of Phakopsora pachyrhizi infection on soybean through host-and spray-induced gene silencing. Mol. Plant Pathol. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Islam, M.T.; Davis, Z.; Chen, L.; Englaender, J.; Zomorodi, S.; Frank, J.; Bartlett, K.; Somers, E.; Carballo, S.M.; Kester, M. Minicell-based fungal RNAi delivery for sustainable crop protection. Microb. Biotechnol. 2021, 14, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.N.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Demirer, G.S.; González-Grandío, E.; Fan, C.; Landry, M.P. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 2020, 15, 3064–3087. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019, 10, 439135. [Google Scholar] [CrossRef]
- Gan, D.; Zhang, J.; Jiang, H.; Jiang, T.; Zhu, S.; Cheng, B. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 2010, 29, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chen, Y.; Hu, Z.; Hu, M. Testing insecticidal activity of novel chemically synthesized siRNA against Plutella xylostella under laboratory and field conditions. PLoS ONE 2013, 8, e62990. [Google Scholar]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- Pallis, S.; Alyokhin, A.; Manley, B.; Rodrigues, T.; Barnes, E.; Narva, K. Effects of Low Doses of a Novel dsRNA-based Biopesticide (Calantha) on the Colorado Potato Beetle. J. Econ. Entomol. 2023, 116, 456–461. [Google Scholar] [CrossRef]
- AgroPages-US EPA Registers the World’s First Sprayable dsRNA Biopesticide-Agricultural News. Available online: https://news.agropages.com/News/NewsDetail---48705.htm (accessed on 4 January 2024).
- Yin, G.; Sun, Z.; Liu, N.; Zhang, L.; Song, Y.; Zhu, C.; Wen, F. Production of double-stranded RNA for interference with TMV infection utilizing a bacterial prokaryotic expression system. Appl. Microbiol. Biotechnol. 2009, 84, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.; de Alba, Á.-E.M.; Flores, R.; Gago, S. Double-stranded RNA interferes in a sequence-specific manner with the infection of representative members of the two viroid families. Virology 2008, 371, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Dıaz-Ruız, J.R. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef]
- Vadlamudi, T.; Patil, B.L.; Kaldis, A.; Sai Gopal, D.V.R.; Mishra, R.; Berbati, M.; Voloudakis, A. DsRNA-mediated protection against two isolates of Papaya ringspot virus through topical application of dsRNA in papaya. J. Virol. Methods 2020, 275, 113750. [Google Scholar] [CrossRef] [PubMed]
- Konakalla, N.C.; Kaldis, A.; Berbati, M.; Masarapu, H.; Voloudakis, A.E. Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 2016, 244, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic biology approach for plant protection using ds RNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef]
- Wang, M.; Dean, R.A. Movement of small RNAs in and between plants and fungi. Mol. Plant Pathol. 2020, 21, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.; Huang, H.D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Melita, O.; Kaldis, A.; Berbati, M.; Reppa, C.; Holeva, M.; Lapidot, M.; Gelbart, D.; Otten, P.; Voloudakis, A. Topical application of double-stranded RNA molecules deriving from Tomato yellow leaf curl virus reduces cognate virus infection in tomato. Biol. Plant. 2021, 65, 100–110. [Google Scholar] [CrossRef]
- Mann, C.W.G.; Sawyer, A.; Gardiner, D.M.; Mitter, N.; Carroll, B.J.; Eamens, A.L. RNA-Based Control of Fungal Pathogens in Plants. Int. J. Mol. Sci. 2023, 24, 12391. [Google Scholar] [CrossRef] [PubMed]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R. BioClayTM prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; de Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: Effectiveness of different application methods in an open-air environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gu, K.; Duan, X.; Xiao, X.; Hou, Y.; Duan, Y.; Wang, J.; Yu, N.; Zhou, M. Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mol. Plant Pathol. 2018, 19, 2543–2560. [Google Scholar] [CrossRef] [PubMed]
- Degnan, R.M.; McTaggart, A.R.; Shuey, L.S.; Pame, L.J.S.; Smith, G.R.; Gardiner, D.M.; Nock, V.; Soffe, R.; Sale, S.; Garrill, A. Exogenous double-stranded RNA inhibits the infection physiology of rust fungi to reduce symptoms in planta. Mol. Plant Pathol. 2023, 24, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.S.; Brower-Toland, B.; Jackson, A.L.; Kier, L.D. Safety assessment of food and feed from biotechnology-derived crops employing RNA-mediated gene regulation to achieve desired traits: A scientific review. Regul. Toxicol. Pharmacol. 2013, 66, 167–176. [Google Scholar] [PubMed]
- O’Neill, M.J.; Bourre, L.; Melgar, S.; O’Driscoll, C.M. Intestinal delivery of non-viral gene therapeutics: Physiological barriers and preclinical models. Drug Discov. Today 2011, 16, 203–218. [Google Scholar] [PubMed]
- Parker, K.M.; Barragán Borrero, V.; van Leeuwen, D.M.; Lever, M.A.; Mateescu, B.; Sander, M. Environmental fate of RNA interference pesticides: Adsorption and degradation of double-stranded RNA molecules in agricultural soils. Environ. Sci. Technol. 2019, 53, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental fate and dissipation of applied dsRNA in soil, aquatic systems, and plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.; Sweet, J.; Ventura, V. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Petrick, J.S. Safety considerations for humans and other vertebrates regarding agricultural uses of externally applied RNA molecules. Front. Plant Sci. 2020, 11, 407. [Google Scholar] [PubMed]
- Christiaens, O.; Sweet, J.; Dzhambazova, T.; Urru, I.; Smagghe, G.; Kostov, K.; Arpaia, S. Implementation of RNAi-based arthropod pest control: Environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 2022, 95, 1–15. [Google Scholar]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Martinez, Z.; De Schutter, K.; Van Damme, E.J.M.; Vogel, E.; Wynant, N.; Broeck, J.V.; Christiaens, O.; Smagghe, G. Accelerated delivery of dsRNA in lepidopteran midgut cells by a Galanthus nivalis lectin (GNA)-dsRNA-binding domain fusion protein. Pestic. Biochem. Physiol. 2021, 175, 104853. [Google Scholar] [CrossRef]
- Leahy, J.; Mendelsohn, M.; Kough, J.; Jones, R.; Berckes, N. Biopesticide oversight and registration at the US Environmental Protection Agency. In Biopesticides: State of the Art and Future Opportunities; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; pp. 3–18. [Google Scholar]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. EU Regulation (EC) No 1829/2003 of the European Parliament and of the council on genetically modified food and feed. Off. J. Eur. Union 2003, L 268, 1–23. Available online: http://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32003R1829 (accessed on 28 March 2024).
- EU. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009. Off. J. Eur. Union 2009, 309, 1–50. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:en:PDF (accessed on 28 March 2024).
- Schenkel, W.; Gathmann, A. Regulatory aspects of RNAi in plant production. In RNAi for Plant Improvement and Protection; CABI: Wallingford, UK, 2021; pp. 154–158. [Google Scholar]
- EU. 2013 Commission Regulation (EU) No 284/2013 of 1 March 2013 Setting Out the Data Requirements for Plant Protection Products, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protectio. Off. J. Eur. Union 2013, 93, 1–84. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32013R0283 (accessed on 6 March 2024).
- The European Commission. EC Regulation (EC) No 284/2013. Off. J. Eur. Union 2013, 93, 1–68. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:093:0085:0152:EN:PDF (accessed on 12 June 2024).
- EU. EC, 2011 Commission Regulation (EU) No 546/2011 of 10 June 2011 Implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards Uniform Principles for Evaluation and Authorisation of Plant Protection Products. Off. J. Eur. Union 2011, L155/127, 127–175. [Google Scholar]
- Bridge, A.J.; Pebernard, S.; Ducraux, A.; Nicoulaz, A.-L.; Iggo, R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 2003, 34, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Betel, D.; Miller, M.L.; Sander, C.; Leslie, C.S.; Marks, D.S. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat. Biotechnol. 2009, 27, 549–555. [Google Scholar]
- Scacheri, P.C.; Rozenblatt-Rosen, O.; Caplen, N.J.; Wolfsberg, T.G.; Umayam, L.; Lee, J.C.; Hughes, C.M.; Shanmugam, K.S.; Bhattacharjee, A.; Meyerson, M. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 1892–1897. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, A.; Anderson, E.M.; Reynolds, A.; Ilsley-Tyree, D.; Leake, D.; Fedorov, Y.; Baskerville, S.; Maksimova, E.; Robinson, K.; Karpilow, J. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods 2006, 3, 199–204. [Google Scholar] [CrossRef]
- Jackson, A.L.; Bartz, S.R.; Schelter, J.; Kobayashi, S.V.; Burchard, J.; Mao, M.; Li, B.; Cavet, G.; Linsley, P.S. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003, 21, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Dzhambazova, T.; Kostov, K.; Arpaia, S.; Joga, M.; Urru, I.; Sweet, J.; Smagghe, G. Literature review of baseline information on RNAi to support the environmental risk assessment of RNAi-based GM plants. EFSA Support. Publ. 2018, 15, 1424E. [Google Scholar] [CrossRef]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar] [CrossRef]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and innate immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef]
- Amari, K.; Niehl, A. Nucleic acid-mediated PAMP-triggered immunity in plants. Curr. Opin. Virol. 2020, 42, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Papadopoulou, K.K. Epigenetic modifications: An unexplored facet of exogenous RNA application in plants. Plants 2020, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M. Revisiting RNA-directed DNA methylation. RNA Biol. 2013, 10, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K. V Exogenous RNAs for gene regulation and plant resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Mat Jalaluddin, N.S.; Othman, R.Y.; Harikrishna, J.A. Global trends in research and commercialization of exogenous and endogenous RNAi technologies for crops. Crit. Rev. Biotechnol. 2019, 39, 67–78. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Jin, S.; Singh, N.D.; Li, L.; Zhang, X.; Daniell, H. Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa armigera larval development and pupation. Plant Biotechnol. J. 2015, 13, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.G.; Kaplanoglu, E.; Kolotilin, I.; Menassa, R.; Donly, C. RNA interference in the tobacco hornworm, Manduca sexta, using plastid-encoded long double-stranded RNA. Front. Plant Sci. 2019, 10, 442068. [Google Scholar] [CrossRef] [PubMed]
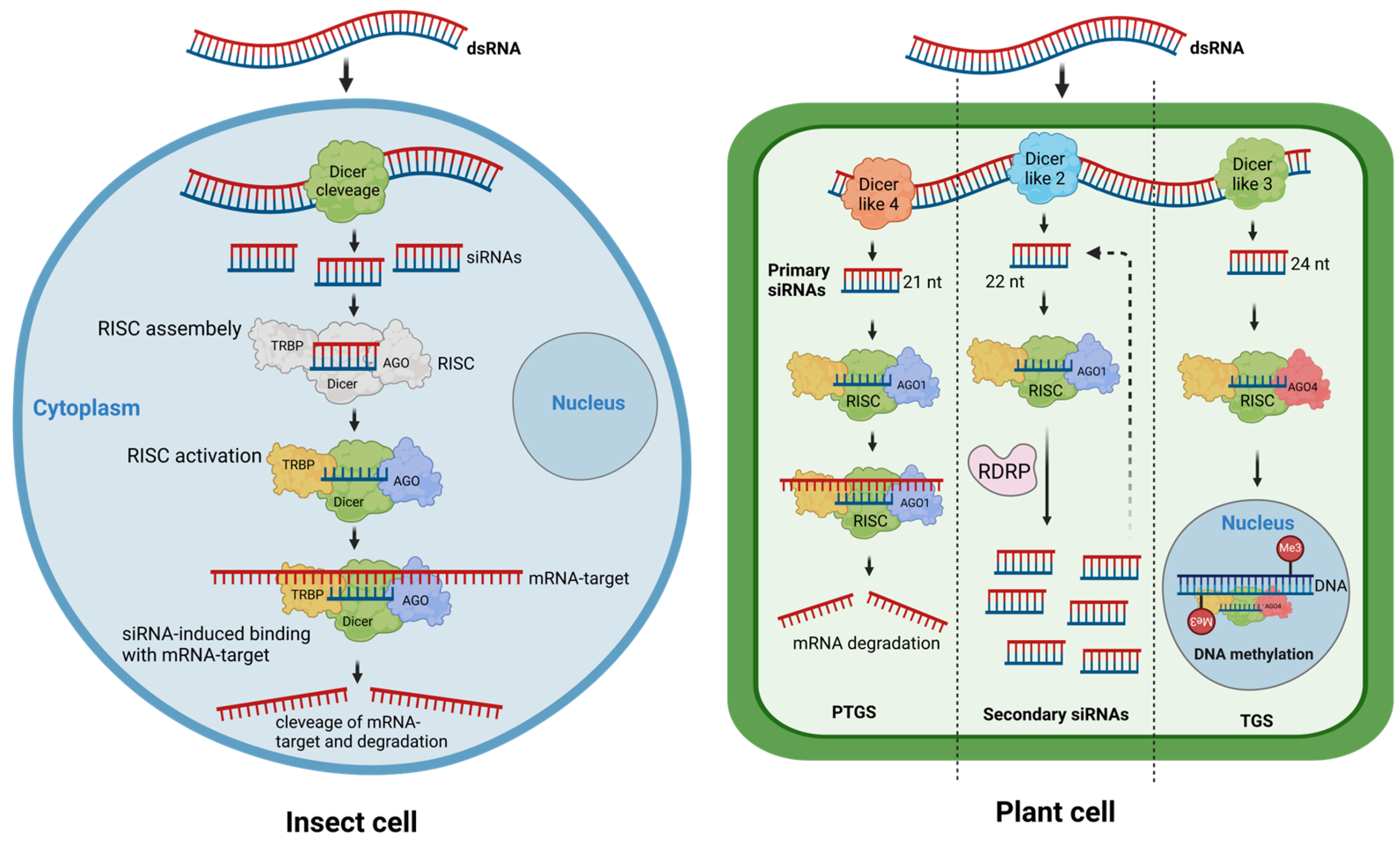
| Group | Target Organism | Gene | Plant | Application Method | Formulation | dsRNA Amount | Efficacies of Formulation | Durability | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Insects | Leptinotarsa decemlineata | Proteasome subunit beta 5 | Solanum tuberosum | Sprayable dsRNA-biopesticide | Unknown | 25 × 10−6 g/L | 90% mortality after 6 days of exposure | Increased mortality up to 9 d. after treatment | [42] |
| Acyrthosiphon pisum | vATPase | Vicia faba | Feeding dsRNA via artificial diet | Naked dsRNA | 1000 ng/μL | High levels of mortality | 6–7 days | [13] | |
| Henosepilachana vigintioctopunctata | Ecdysone receptor | Solanum tuberosum | dsEcR-E. coli directly sprayed to the foliage | Escherichia coli HT115 | 0.5 μg/mL | 40% reduced larvae pupation, 60% showed defective phenotype | Up to 10 days | [43] | |
| Sitobian avenae | Salivary sheath protein | Hordeum vulgare | Foliar spray on leaves | Naked dsRNA | 20 ng/μL | 60% reduction in transcript levels of target gene and disease resistance | Effect: 5 days; dsRNA detectable: 3–9 days | [44] | |
| Helioverpa armigera | Juvenile hormone methyltransferase, Acetylcholine esterase | Cicer arietinum | Hand-held mist sprayer | Chitosan nanoparticles | 200 μg | 100% insect mortality | Up to 5 days | [45] | |
| Myzus persicae | MpC002, Rack1 | Nicotiana benthamiana and Arabidopsis thaliana | Host induced gene silencing via transgenic plants) | Naked dsRNA produced by transgenic plants | 15–20 ng | Target gene expression knockdown by 60%, reduced aphid fecundity | 17 days after feeding | [46] | |
| Aphis glycines | TREH, ATPD, ATPE, and CHS1 | Glycine max | Transdermal dsRNA delivery system | Star polymer (SPc) nanoparticles | 500 ng/μL SPc and dsRNA | Significant gene silencing 48 h after treatment. | Significant mortality increase 4 day after treatment | [47] | |
| Blattella germanica | α-Tubulin | - | Oral delivery | Liposomes | 0.25 μg/μL of dsRNA lipoplexes | 24–60% increased mortality | Increase of mortality for 16 days | [48] | |
| Spodoptera exigua | Chitin synthase B | - | In vivo feeding bioassays | Guanidine-containing polymers | 500 ng/μL | Mortality with formulation: 53%; without formulation: 16% | Prevention of larvae pupation up to 15 days | [49] | |
| Sogatella furcifera | Vacuolar-type (H+)-ATPase | Rice | Spraying and injection | SPc | 1000 ng/μL | More than 97% | 7 days post treatment | [50] | |
| Bemisia tabaci | Toll-like receptor 7 | Hibiscus rosasinensis | Recombinant Isaria fumosorosea, leaf immersion method | Fungi conidia | 2 × 107 spore/mL | 90.33% | 8 days after treatment | [51] | |
| Tribolium castaneum | BiP, Armet | Insect rearing on wheat flour | Diet containing dsRNA-BAPCs nanoparticles | Branched amphiphilic peptide capsules (BAPCs) | 500 ng | Ingestion of dsRNA/ BAPC complexes increased mortality from 40% to 75% | Up to 40 days | [52] | |
| Fungi | Fusarium garminearum | Cytochrome P450 lanosterol C-14α-demethylases | Hordeum vulgare | Foliar spray on leaves | Naked dsRNA | 20 ng/μL | Reduced transcript levels of target gene of 75% | Effect detectable 6–8 post spraying; unprocessed ds/siRNA accumulation detectable 24 h post spraying | [53] |
| Fusarium oxysporum | CYP51, chitin synthase 1, Elongation factor 2 | Solanum lycopersicum | Topical delivery | Layered double hydroxide clay nanosheets | 100 μg/mL | Reduced fungal growth; 93% reduction in transcript abundance | Up to 8 days | [54] | |
| Phakopsora pachyrhizi | Acetyl-CoA acyltransferase 40S ribisomal protein S16, Glycine cleavage system H-protein | Glycine max | Spraying | Diethylpyrocarbonate | 20 μg | <73% reduction in fungal infection-symptoms, 75% reduction in biomass accumulation | 2 weeks | [55] | |
| Botryotiania fuckeliana | Chitin synthase class III, DCL 1, DCL2 | Fragaria ananassa | Topical spray application | Escherichia coli-derivedanucleated minicells | 125–1000 ng/mL | Knockdown of target genes, leading to significant reduction in fungal growth | Up to 12 days | [56] | |
| Plants | Nicotiana benthamiana | Green flourecent protein (GFP), 21–24 nt | Nicotiana benthamiana | High-pressure spraying method | Naked dsRNA | 10 µM | Local and systemic silencing | 20 dpa | [57] |
| Nicotiana benthamiana | GFP, siRNA loading | Nicotiana benthamiana | 1-mL needleless syringe | DNA nanostructures | 100 nM | 40–59% reduction in mRNA- and protein-level | Up to 7 days | [58] | |
| Viruses | Bean common mosaic virus | Nuclear inclusion b-protein | Nicotiana benthamiana | Manual inoculation via coborundum | Layered double hydroxide clay nanosheets | 100 μg | Reduction of infection up to 45% | Up to 10 days | [59] |
| Sugarcane Mosaic virus | Coat protein | Zea mays | Spraying bacteria-produced dsRNA | Escherichia coli HT115 | 3 μg/L | Total inhibition of virus infection | 30 dpi | [60] | |
| Pepper mild mottle virus (PMMoV) and cucumber mosaic virus (CMV) | replicase gene of PMMoV14; 2b suppressor of plant antiviral RNA silencing defense encoded by CMV25 | Arabidopsis thaliana, Vigna unguiculata, Nicotiana tabacum | Topical application as spray | Layered double hydroxide (LDH) clay nanosheets (BioClay) | 1.25 μg | spray extended virus protection from 5–7 days to <20 days | Significantly increased resistance to virus infection: up to 20 days post spraying | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatanparast, M.; Merkel, L.; Amari, K. Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. Int. J. Mol. Sci. 2024, 25, 6530. https://doi.org/10.3390/ijms25126530
Vatanparast M, Merkel L, Amari K. Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. International Journal of Molecular Sciences. 2024; 25(12):6530. https://doi.org/10.3390/ijms25126530
Chicago/Turabian StyleVatanparast, Mohammad, Lisa Merkel, and Khalid Amari. 2024. "Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment" International Journal of Molecular Sciences 25, no. 12: 6530. https://doi.org/10.3390/ijms25126530
APA StyleVatanparast, M., Merkel, L., & Amari, K. (2024). Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. International Journal of Molecular Sciences, 25(12), 6530. https://doi.org/10.3390/ijms25126530






