History of the Development of Knowledge about the Neuroendocrine Control of Ovulation—Recent Knowledge on the Molecular Background
Abstract
1. Introduction
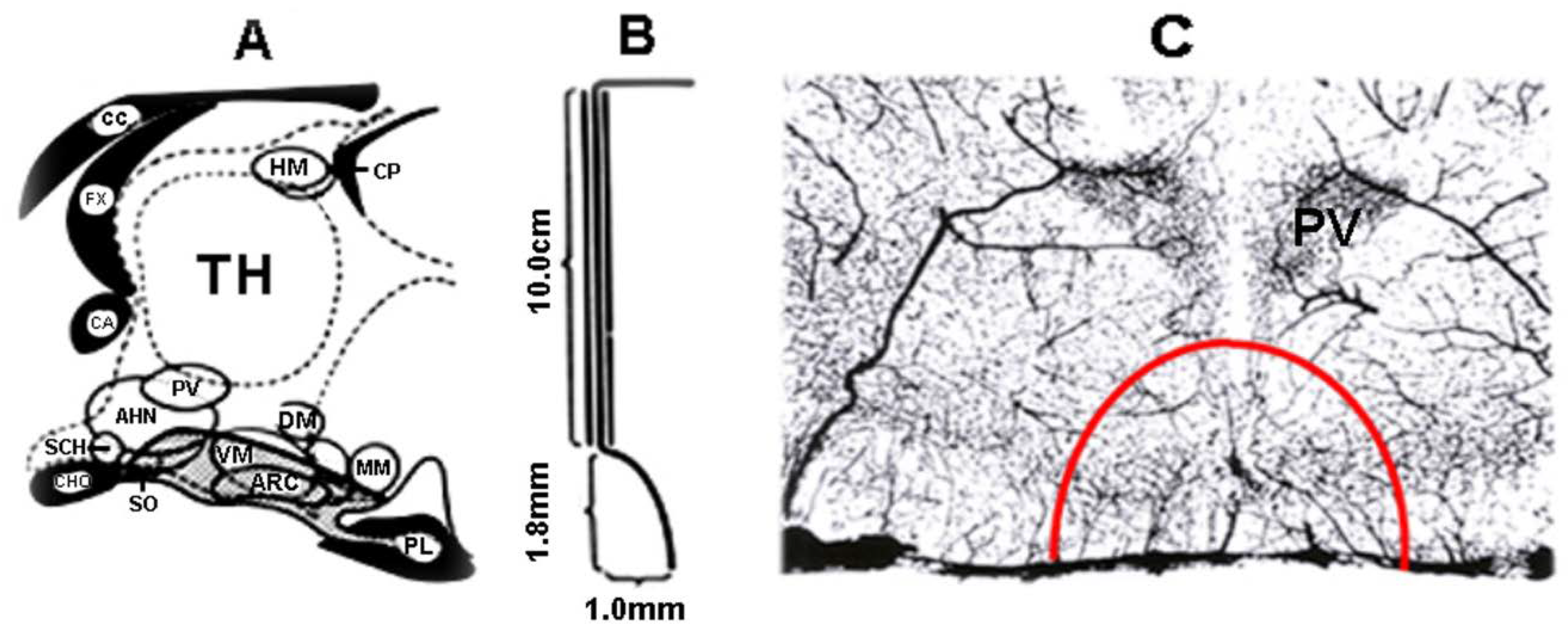
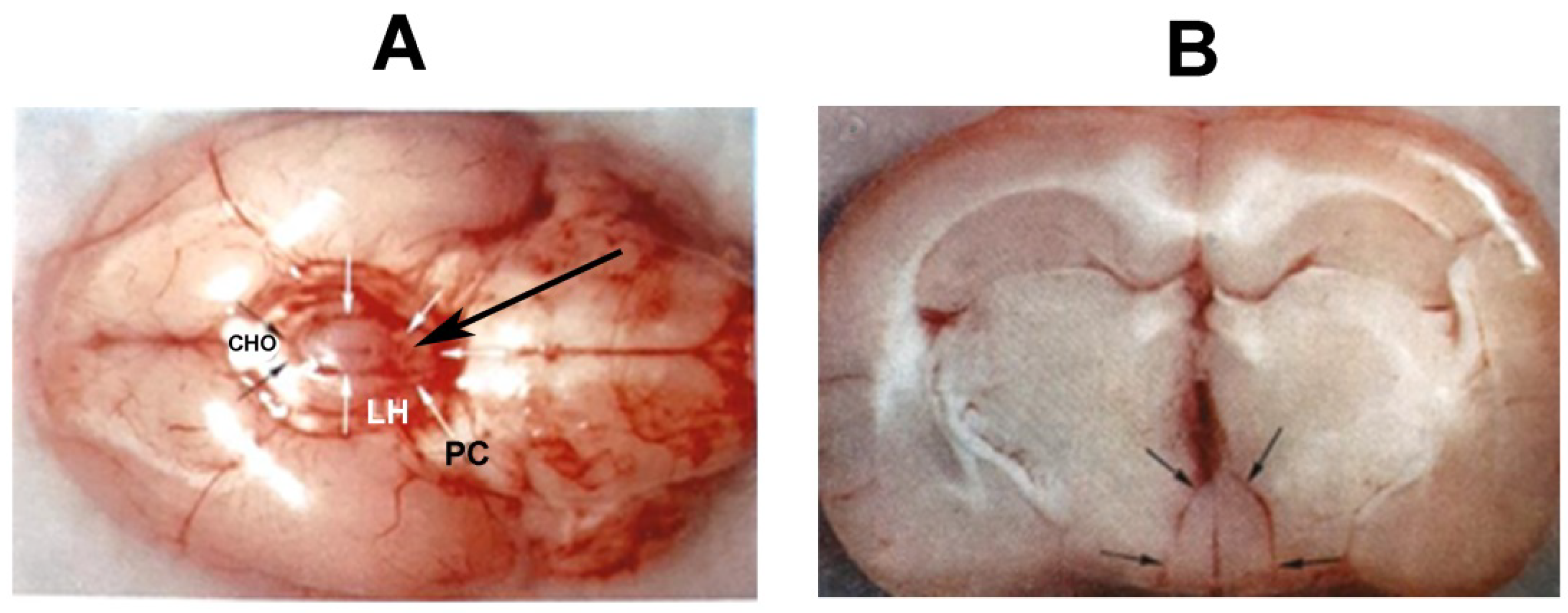
2. Blood Supply of the Pituitary Gland
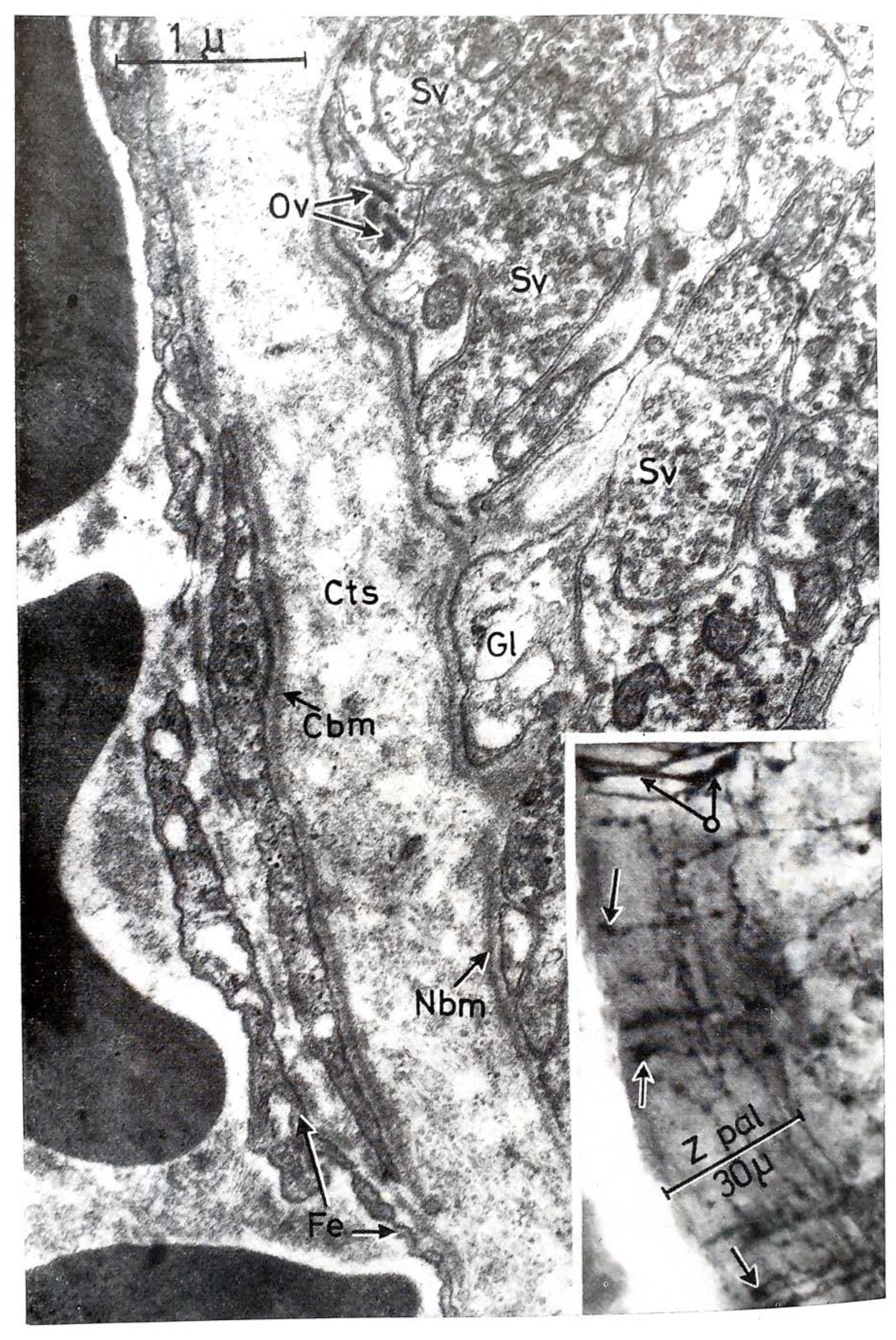
3. Structure of the ME
4. Ovarian Hormones Involved in Ovulation: Estrogen (E) and Progesterone (P)
5. Anterior Pituitary Cell Composition, Pituitary Hormones Involved in Ovulation: LH, FSH, and PRL
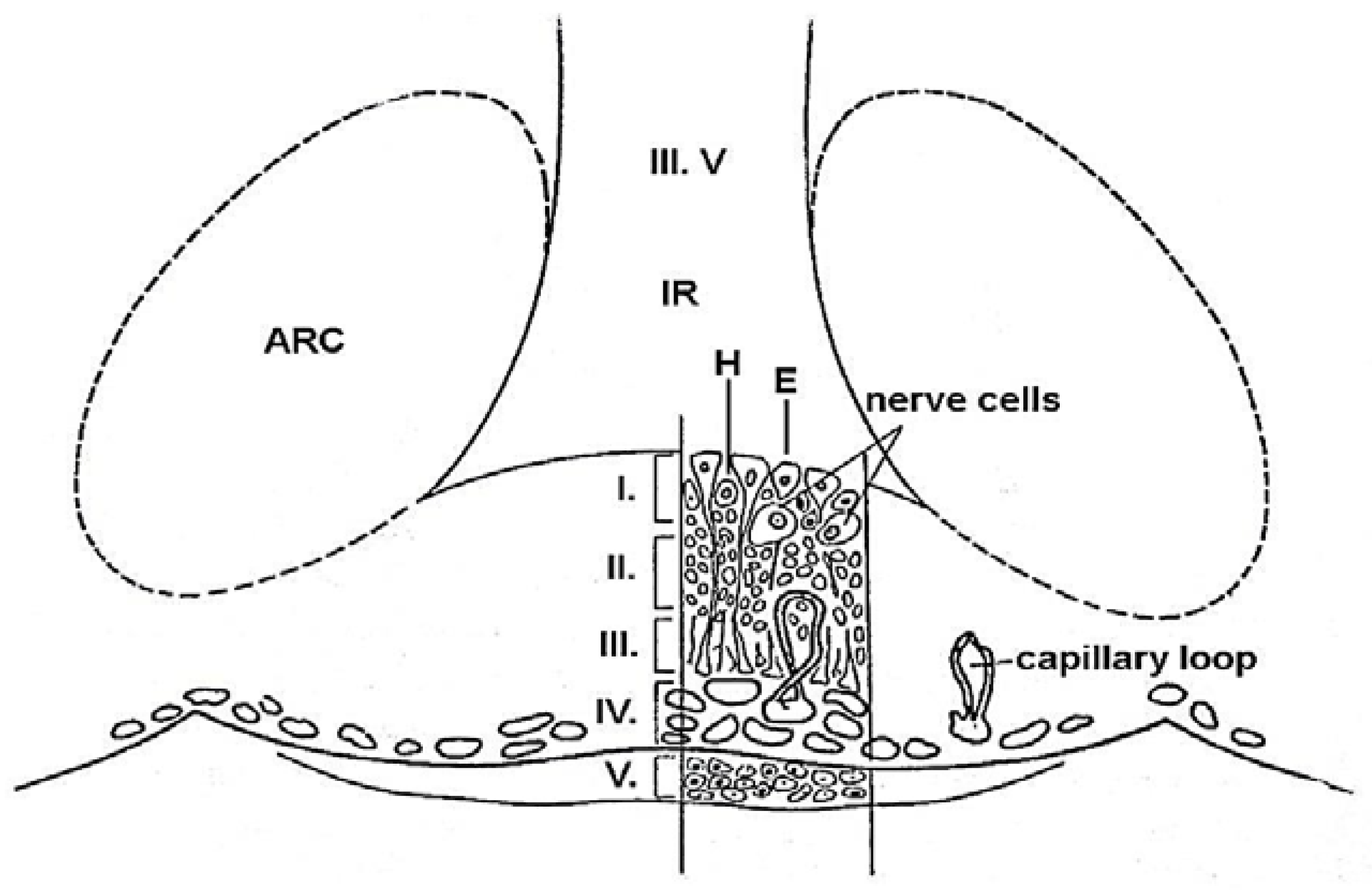
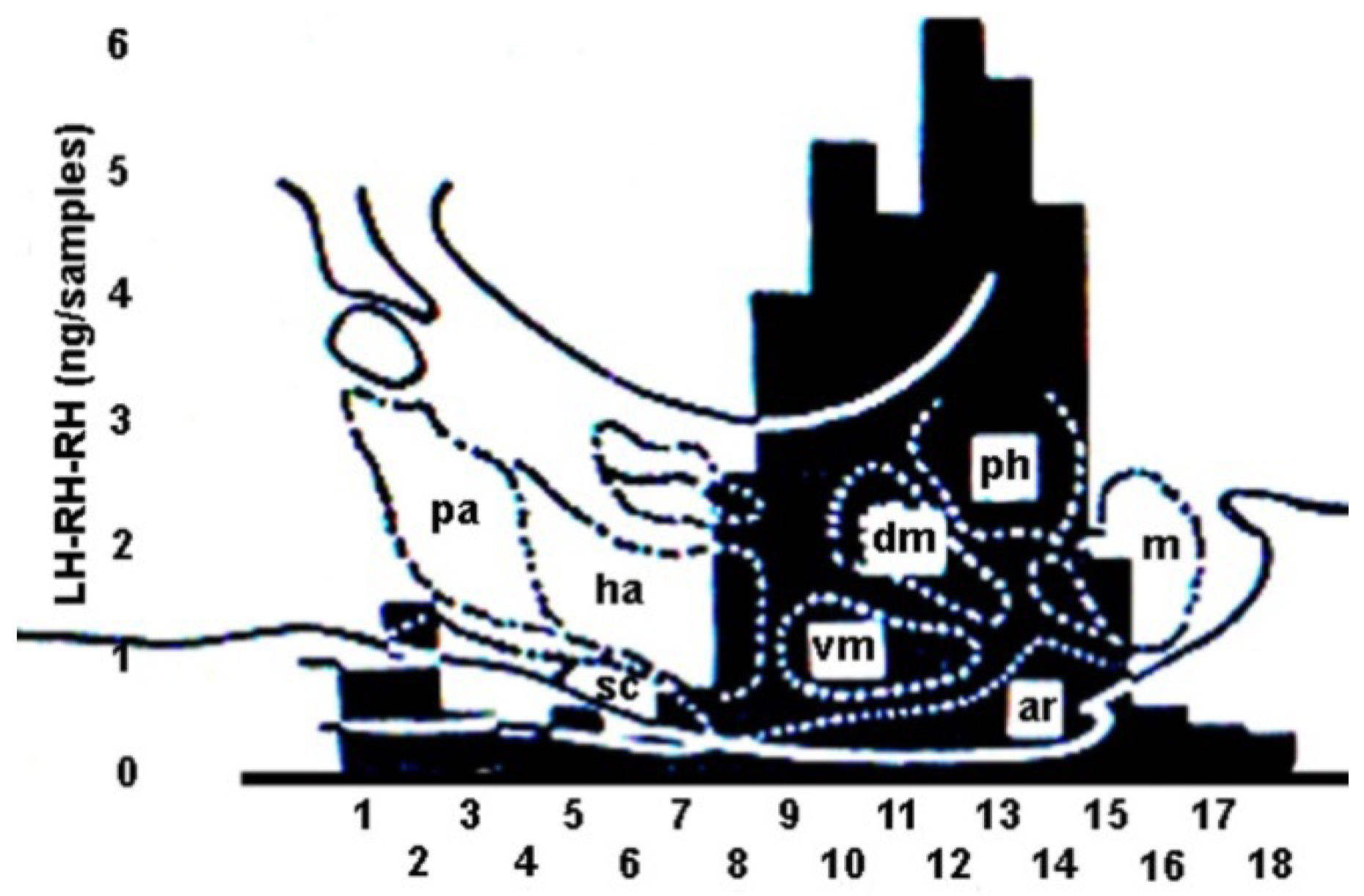
6. Hypophysiotropic Area
7. Discovery of Releasing and Inhibiting Hormones
8. Origin and Development of GnRH Neurons
9. Pulsatility of GnRH
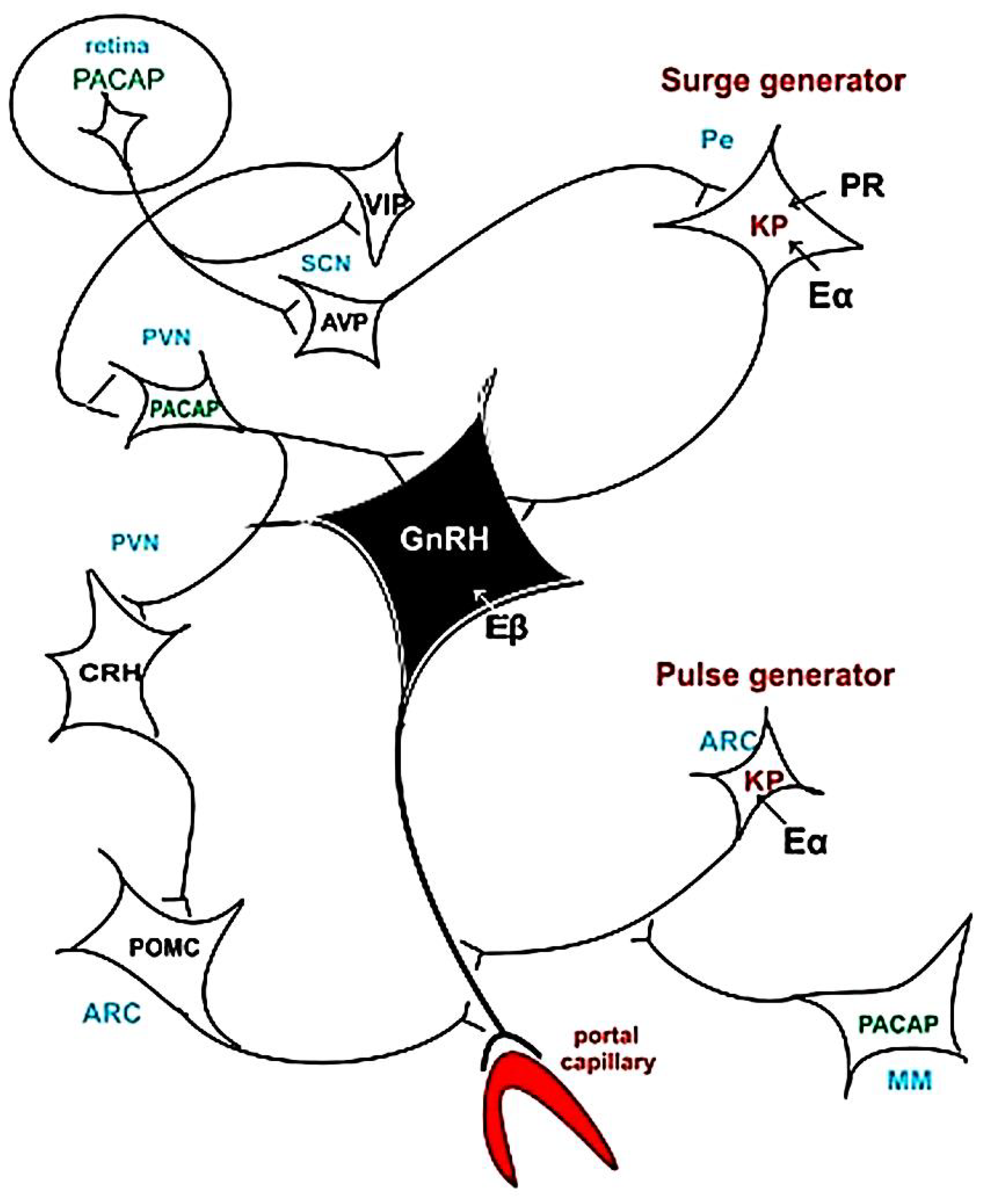
10. Feedback Mechanism and Pulse and Surge Generators
11. Discovery of the Gonadotropin-Inhibiting Hormone
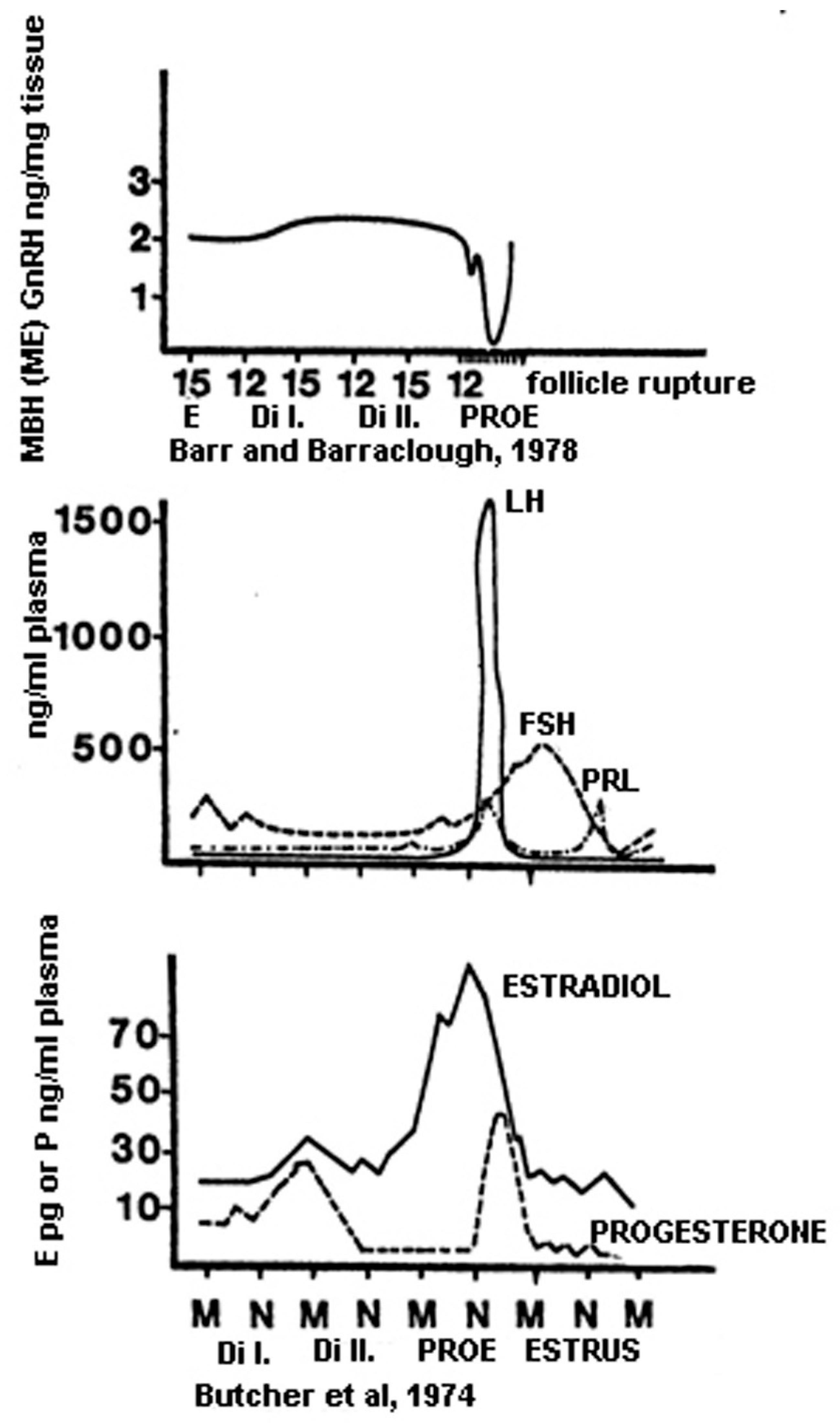
12. The Sequence of Hormonal Events during Ovulation
13. Infertility and Assisted Reproduction
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| AMH | anti-Müllerian hormone |
| AVP | arginine vasopressin |
| BBB | blood–brain barrier |
| BMP4 | bone morphogenetic protein |
| C | corticotropes |
| CNS | central nervous system |
| CRH | corticotropic-hormone-releasing hormone |
| Dy | dynorphin |
| ELISA | enzyme-linked immunosorbent assay |
| E | estrogen |
| E2 | 17-β estradiol |
| Erα | estradiol receptor α |
| Erβ | estradiol receptor β |
| FGF | fibroblast growth factor protein |
| FSH G | follicle-stimulating hormone gonadotropes |
| GH | growth hormone |
| GnIH | gonadotropin-release-inhibiting hormone |
| GnRH | gonadotropin-releasing hormone |
| GnRHR | GnRH receptor |
| GPR KP | G-protein-coupled receptor kisspeptin |
| KNDY | neurons expressing all three peptides: KP, NKB and Dy |
| KO | knockout |
| ERKO | E2 receptor knockout |
| LH | luteinizing hormone |
| LHRH | luteinizing hormone-releasing hormone |
| LIM | homeobox subfamily |
| M | melanocyte |
| MBH | medial basal hypothalamus |
| ME | median eminence |
| MSH | melanocyte-stimulating hormone |
| NKB | neurokinin B |
| NPY | neuropeptide-Y |
| P | progesterone |
| PACAP | pituitary-adenylate-cyclase-activating polypeptide |
| Pit1 | pituitary-specific transcription factor |
| PR | progesterone receptor |
| PRL | prolactin |
| PROP1 | gene encoding Pit1 |
| RIA | radioimmunoassay |
| SCN | suprachiasmatic nucleus |
| SF-1 | steroidogenic factor-1 |
| Shh | sonic hedgehog protein |
| TRH | thyroid-stimulating hormone-releasing hormone |
| TSH | thyroid-stimulating hormone |
| VEGFA | vascular endothelial growth factor A |
| VIP | vasoactive intestinal polypeptide |
| VNO | vomeronasal organ |
| Wnt5 | wingless protein |
References
- Rowan, W. On photoperiodism, reproductive periodicity, and the annual migrations of birds and certain fishes. Proc. Boston Soc. Nat. Hist. 1926, 38, 147–189. [Google Scholar]
- Rowan, W. Reproductive rhythm in birds. Nature 1928, 122, 11–12. [Google Scholar] [CrossRef]
- Rowan, W. Experiments in bird migration. I. Manipulation of the reproductive cycle: Seasonal histological changes in the gonads. Proc. Boston Soc. Nat. Hist. 1929, 39, 151–208. [Google Scholar]
- Rowan, W. Experiments in bird migration. II. Reversed migration. Proc. Nat. Acad. Sci. USA 1930, 16, 520–525. [Google Scholar] [CrossRef]
- Bissonnette, T.H. Studies on the sexual cycle in birds. IV. Experimental modification of the sexual cycle in males of the European starling (Sturnus vulgaris) by changes in the daily period of illumination and of muscular work. J. Exp. Zool. 1931, 58, 281–313. [Google Scholar] [CrossRef]
- Bissonnette, T.H. Modification of mammalian sexual cycles. III. Reversal of the cycle in male ferrets (Putorius vulgaris) by increasing periods of exposure to light between October second and March thirtieth. J. Exp. Zool. 1935, 71, 341–367. [Google Scholar] [CrossRef]
- Bissonnette, T.H. Modification of mammalian sexual cycles. IV. Delay of oestrus and induction of anoestrus in female ferrets by reduction of intensity and duration of daily light periods in the normal oestrous season. J. Exp. Biol. 1935, 12, 315–320. [Google Scholar] [CrossRef]
- Bissonnette, T.H. Modification of mammalian sexual cycles. V. The avenue of reception of sexually stimulating light. J. Comp. Psychol. 1936, 22, 93–103. [Google Scholar] [CrossRef]
- Bissonnette, T.H. Sexual photoperiodicity. Quart. Rev. Biol. 1936, 11, 371–386. [Google Scholar] [CrossRef]
- Bissonnette, T.H.; Chapnick, M.H. Studies on the sexual cycle in birds. II. The normal progressive changes in the testis from November to May in the European starling (Sturnus vulgaris), an introduced, non-migratory bird. Am. J. Anat. 1930, 45, 307–343. [Google Scholar] [CrossRef]
- Bissonnette, T.H.; Wadlund, A.P. Spermatogenesis in Sturnus vulgaris: Refractory period and acceleration in relation to wave-length and rate of increase of light ration. J. Morph. 1931, 52, 403–420. [Google Scholar] [CrossRef]
- Baker, J.R.; Ranson, R.M. Factors affecting the breeding of the field-mouse (Microtus agrestis). II. Temperature and food. Proc. R. Soc. B 1932, 112, 39–45. [Google Scholar]
- Hill, R.T.; Parkes, A.S.; White, W.E. The assay of the ovulation-producing substance. J. Physiol. 1934, 81, 335–360. [Google Scholar] [CrossRef]
- Long, J.A.; Evans, H.M. The Long and Evans Monograph (1922) on the Estrous Cycle in the Rat. Endocrinology 1991, 129, 2812–2814. [Google Scholar]
- Huston, T.M.; Nalbandov, A.V. Neurohumoral control of the pituitary in the fowl. Endocrinology 1953, 52, 149–156. [Google Scholar] [CrossRef]
- Van Tienhoven, A. Further study on the neurogenic blockage of LH release in the hen. Anat. Rec. 1953, 115, 374. [Google Scholar]
- Moore, W.W.; Nalbandov, A.V. Neurogenic effects of uterine distention on the estrous cycle of the ewe. Endocrinology 1953, 53, 1–11. [Google Scholar] [CrossRef]
- Marshall, F.; Bowden, F.P. The Effect of Irradiation with Different Wave-lengths on the Oestrous Cycle of the Ferret, with Remarks on the Factors Controlling Sexual Periodicity. J. Exp. Biol. 1934, 11, 409–422. [Google Scholar] [CrossRef]
- Popa, G.T.; Fielding, U. The vascular link between pituitary and hypothalamus. Lancet 1930, 2, 238–240. [Google Scholar] [CrossRef]
- Green, J.D.; Harris, G.W. The neurovascular link between the neural lobe and adenohypophysis. J. Endocrinol. 1947, 5, 136–146. [Google Scholar] [CrossRef]
- Harris, G.W. Neural Control of the Pituitary Gland; Edward Arnold: London, UK, 1955. [Google Scholar]
- Szentágothai, J.; Flerkó, B.; Mess, B.; Halász, B. Hypothalamic Control of the Anterior Pituitary. An Experimental-Morphological Study, 3rd ed.; Akadémiai Kiadó: Budapest, Hungary, 1968. [Google Scholar]
- Greep, R.O.; Astwood, E.B. The Pituitary Gland and Its Neuroendocrine Control/Part 2. In Handbook of Physiology, 12th ed.; Section 7 Endocrinology; Baker, M.W., Harris, V.D., Eds.; Kessinger Publishing: Whitefish, MT, USA, 2007. [Google Scholar]
- Ganten, D.; Pfaff, D. Current Topics in Neuroendocrinolgy; Springer: Berlin/Heidelberg, Germany, 1985; Volume 7. [Google Scholar]
- Freeman, M.E. The neuroendocrine control of the ovarian cycle of the rat. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven: New York, NY, USA, 1994; pp. 613–658. [Google Scholar]
- Schwartz, N.B. Neuroendocrine Regulation of Reproductive Cyclicity Chapter 8. In The Physiology of Reproduction, 2nd ed.; Raven: New York, NY, USA, 1994. [Google Scholar]
- Halász, B. Anatomy of hypothalamus. In Encyclopedia of Endocrine Diseases, 2nd ed.; Martini, L., Ed.; Elsevier: New York, NY, USA, 2004; Volume 2, pp. 81–89. [Google Scholar]
- Schwartz, N.B. Reproduction and Fertility, Chapter 24. In Endocrinology: Basic and Clinical Principles, 2nd ed.; Melmed, S., Michel Conn, P., Eds.; Humana Peress: Totowa, NJ, USA, 2005. [Google Scholar]
- Knobil, E.; Neill, J.D. (Eds.) Physiology of Reproduction, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Clark, I.J.; Campbell, R.; Smith, J.T.; Wray, S. Neuroendocrine control of reproduction. Chapter 9. In Handbook of Neuroendocrinolgy; Fink, G., Pfaff, D.W., Levine, J.E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 197–235. [Google Scholar]
- Fink, G. Neural control of the anterior lobe of the pituitary gland (pars distalis). Chapter 5. In Handbook of Neuroendocrinolgy; Fink, G., Pfaff, D.W., Levine, J.E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 97–137. [Google Scholar]
- Watts, A.G. 60 years of Neuroendocrinology: The structure of the neuroendocrine hypothalamus: The neuroanatomical legacy of Geoffrey Harris. J. Endocrinol. 2015, 226, T25–T39. [Google Scholar] [CrossRef]
- Fink, G. 60 years of neuroendocrinology. Memoir: Harris’ neuroendocrine revolution: Of portal vessels and self-priming. J. Endocrinol. 2015, 226, T13–T24. [Google Scholar] [CrossRef]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation; StatPearls Inc.: Boston, MA, USA, 2023. [Google Scholar]
- Popa, G.T.; Fielding, U. A portal circulation from the pituitary to the hypothalamic region. J. Anat. 1930, 65, 88–91. [Google Scholar]
- Pietsch, K. Aufbau und Entwicklung der Pars tuberalis des menschlichen Hirnanhangs in ihren Beziehungen zu den übrigen Hypophysenteilen. Ztschr. F. Mikr.-Anat. Forsch. 1930, 22, 227–258. [Google Scholar]
- Basir, M.A. The vascular supply of the pituitary body in the dog. J. Anat. 1932, 66, 387–398. [Google Scholar]
- Morato, M.J.X. The blood supply of the hypophysis. Anat. Rec. 1939, 74, 297–320. [Google Scholar] [CrossRef]
- Green, J.D.; Harris, G.W. Observation of the hypophyseal-portal vessels of the living rat. J. Physiol. 1949, 108, 359–361. [Google Scholar] [CrossRef]
- Xuereb, G.P.; Prichard, M.L.; Daniel, P.M. The hypophyseal portal system of vessels in man. Q. J. Exp. Physiol. Cogn. Med. Sci. 1954, 39, 219–230. [Google Scholar]
- Landsmeer, J.M.F. Vessels of the rat’s hypophysis. Acta Anat. 1957, 12, 82–109. [Google Scholar] [CrossRef]
- Török, B. Neue Angaben zum Blutkreislauf der Hypophyse, Verhandlungen des 1; Europaischen Anatomen-Kongresses: Strasbourg, France, 1962; pp. 622–629. [Google Scholar]
- Török, B. Structure of the vascular connections of the hypothalamo-hypophyseal region. Acta Anat. 1964, 59, 84–99. [Google Scholar] [CrossRef]
- Adams, J.H.; Daniel, P.M.; Prichard, M.M.L. Distribution of hypophysial portal blood in the anterior lobe of the pituitary gland. Endocrinology 1964, 75, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.M. The anatomy of the hypothalamus and pituitary gland. In Neuroendocrinology; Martini, L., Ganong, W.F., Eds.; Academic Press: New York, NY, USA; London, UK, 1966; Volume 1, pp. 15–80. [Google Scholar]
- Duvernoy, H. The vascular architecture of the median eminence. In Brain-Endocrine Interaction. Median Eminence. Structure and Function; Knigge, K.M., Scott, D.E., Weindl, A., Eds.; Int. Symp. Munichi 1971; Karger: Basel, Switzerland, 1972; pp. 79–108. [Google Scholar]
- Akmayev, I.G. Morphological aspects of the hypothalamic-hypophyseal system. III. Vascularity of the hypothalamus, with special reference to its quantitative aspects. Z. Zellforsch. 1971, 116, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ambach, G.; Palkovits, M.; Szentágothai, J. Blood supply of the rat hypothalamus. IV. Retrochiasmatic area, median eminence, arcuate nucleus. Acta Morphol. Acad. Sci. Hung. 1976, 24, 93–119. [Google Scholar]
- Mezey, E.; Palkovits, M.; de Kloet, E.R.; Verhoef, J.; de Wied, D. Evidence for pituitary-brain transport of a behaviorally potent ACTH analog. Life Sci. 1978, 22, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Mezey, E.; Kivovics, P.; Palkovits, M. Pituitary-brain retrograde transport. Trend Neurosci. 1979, 2, 57–60. [Google Scholar] [CrossRef]
- Bergland, R.M.; Page, R.B. Pituitary-brain vascular relations: A new paradigm. Science 1979, 204, 18–24. [Google Scholar] [CrossRef]
- Page, R.B. Pituitary blood flow. Am. J. Physiol. 1982, 243, E427–E442. [Google Scholar] [CrossRef]
- Monnet, F.; Elias, K.A.; Fagin, K.; Neill, A.; Goldsmith, P.; Weiner, R.I. Formation of a direct arterial blood supply to the anterior pituitary gland following complete or partial interruption of the hypophyseal portal vessels. Neuroendocrinology 1984, 39, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.A.; Weiner, R.I. Direct arterial vascularization of estrogen-induced prolactin-secreting anterior pituitary tumors. Proc. Natl. Acad. Sci. USA 1984, 81, 4549–4553. [Google Scholar] [CrossRef] [PubMed]
- Glydon, R.S. The development of the blood supply of the pituitary in the albino rat, with special reference to the portal vessels. J. Anat. 1957, 91, 237–244. [Google Scholar]
- Negm, I.M. The vascular blood supply of the pituitary and its development. Acta Anat. 1971, 80, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Thliveris, J.A.; Currie, R.W. Observations on the hypothalamo-hypophyseal portal vasculature in the developing human fetus. Am. J. Anat. 1980, 157, 441–444. [Google Scholar] [CrossRef]
- Trandafir, T.; Dionisie, C.; Repciuc, E. The development of the hypothalamo-hypophyseal portal system in human fetus. Endocrinologie 1982, 20, 127–134. [Google Scholar] [PubMed]
- Szabó, K.; Csányi, K. The vascular architecture of the developing pituitary-median eminence complex in the rat. Cell Tissue Res. 1982, 224, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K. Origin of the adenohypophyseal vessels in the rat. J. Anat. 1987, 154, 229–235. [Google Scholar] [PubMed]
- Bargmann, W.; Scharrer, E. The site of origin of the hormones of the posterior pituitary. Am. Sci. 1951, 39, 255–259. [Google Scholar] [PubMed]
- Szentágotai, J.; Flerkó, B.; Mess, B.; Halász, B. Hypothalamic Control of the Anterior Pituitary. An Experimental-Morphological Study, 1st ed.; Akadémiai Kiadó: Budapest, Hungary, 1962. [Google Scholar]
- Szentágothai, J.; Halász, B. Regulation des Endokrinen Systems über Hypothalamus, in: Die Nervenphysiologie in egenwartiger Sicht. Bericht über die Jahresversammlung der Deutschen Akademie der Naturforscher Leopldina, Halle/Saale (ed. R. Zaunick). Nova Acta Leopoldina N F 1963, 28, 227–248. [Google Scholar]
- Röhlich, P.; Vigh, B.; Teichmann, I.; Aros, B. Electron microscopy of the median eminence of the rat. Acta Biol. Acad. Sci. Hung. 1965, 15, 431–457. [Google Scholar]
- Rinne, U.K. Ultrastructure of the median eminence of the rat. Z. Zellforsch. Mikrosk. Anat. 1966, 74, 98–122. [Google Scholar] [CrossRef]
- Knigge, K.M.; Scott, D.E. Structure and function of the median eminence. Am. J. Anat. 1970, 129, 223–243. [Google Scholar] [CrossRef]
- Clattenburg, R.E. Ultrastructure of hypothalamic neurons and of the median eminence. Can. J. Neurol. Sci. 1974, 1, 40–58. [Google Scholar] [CrossRef]
- Yin, W.; Gore, A.C. The hypothalamic median eminence and its role in reproductive aging. Ann. N. Y. Acad. Sci. 2010, 1204, 113–122. [Google Scholar] [CrossRef]
- Réthelyi, M. Neurons in the subependymal layer of the rat median eminence. Neuroendocrinology 1975, 17, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Ciofi, P.; Garret, M.; Lapirot, O.; Lafon, P.; Loyens, A.; Prévot, V.; Levine, J.E. Brain-endocrine interactions: A microvascular route in the mediobasal hypothalamus. Endocrinology 2009, 150, 5509–5519. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; González, C.B.; Delannoy, L. Cellular organization of the lateral and postinfundibular regions of the median eminence in the rat. Cell Tissue Res. 1979, 201, 377–408. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, A. Blood-brain barrier overview: Structural and functional correlation. Hindawi Neural Plast. 2021, 6564585. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Doisy, E.A. An ovarian hormone: Preliminary report on its localization, extraction and partial purification, and action in test animals. JAMA 1923, 81, 819–821. [Google Scholar] [CrossRef]
- Corner, G.W.; Allen, W.M. Physiology of the corpus luteum, II: Production of a special uterine reaction (progestational proliferation) by extracts of the corpus luteum. Am. J. Physiol.-Leg. Content 1929, 88, 326–339. [Google Scholar] [CrossRef]
- Drummond, A.E. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 2006, 4, 16. [Google Scholar] [CrossRef]
- Jensen, E.V.; Desombre, E.R.; Kawashima, T.; Suzuki, T.; Kyser, K.; Jungblut, P.W. Estrogen-binding substances of target tissues. Science 1967, 158, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.V.; Suzuki, T.; Kawashima, T.; Stumpf, W.E.; Jungblut, P.W.; DeSombre, E.R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc. Natl. Acad. Sci. USA 1968, 59, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Walter, P.; Kumar, V.; Krust, A.; Bornert, J.M.; Argos, P.; Chambon, P. Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature 1986, 320, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Thomas, P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 2012, 153, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Madeo, A.; Maggiolini, M. Nuclear alternate estrogen receptor GPR30 mediates 17β-estradiol-induced gene expression and migration in breast cancer-associated fibroblasts. Cancer Res. 2010, 70, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Krust, A.; Gansmuller, A.; Dierich, A.; Chambon, P.; Mark, M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 2000, 127, 4277–4291. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Levin, E.R. Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 2006, 20, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Williams, M.J.; Kew, K.A.; Converse, A.; Thomas, P.; Zhu, Y. Reduced Vitellogenesis and Female Fertility in Gper Knockout Zebrafish. Front. Endocrinol. 2021, 12, 637691. [Google Scholar] [CrossRef]
- Heldering, N.; Pike, A.; Andrsson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- O’Malley, B.W.; Sherman, M.R.; Toft, D.O. Progesterone “receptors” in the cytoplasm and nucleus of chick oviduct target tissue. Proc. Natl. Acad. Sci. USA 1970, 67, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.C.; Hardy, D.B.; Kovaric, K.; Mendelson, C.R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 2006, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Conneely, O.M.; Mulac-Jericevic, B.; DeMayo, F.; Lydon, J.P.; O’Malley, B.W. Reproductive functions of progesterone receptors. Recent Prog. Horm. Res. 2002, 57, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.K.; Grider, M.H. Physiology, Progesteron; StatPearls: Boston, MA, USA, 2023. [Google Scholar]
- Baulieu, E.E.; Robel, P. Neurosteroids: A new brain function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 2022, 132, 793–817. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, G.N.; Traut, H.F. The diagnostic value of vaginal smears in carcinoma of the uterus. Am. J. Obstet. Gynecol. 1941, 42, 193. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Assay of plasma insulin in human subjects by immunological methods. Nature 1959, 184, 1648–1649. [Google Scholar] [CrossRef]
- Abraham, G.E. Solid-phase radioimmunoassay of estradiol-17 beta. J. Clin. Endocrinol. Metab. 1969, 29, 866–870. [Google Scholar] [CrossRef]
- Rosner, W.; Hankinson, S.E.; Sluss, P.M.; Vesper, H.W.; Wierman, M.E. Challenges to the measurement of estradiol: An endocrine society position statement. J. Clin. Endocrinol. Metab. 2013, 98, 1376–1387. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Zhen, H.; Chen, X.; Sheng, M.; Li, K.; Xue, W.; Zhao, H.; Meng, S.; Cao, G. Determination of estrogens and estrogen mimics by solid-phase extraction with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2021, 1168, 122559. [Google Scholar] [CrossRef]
- Casals, G.; Costa, R.F.; Rull, E.U.; Escobar-Morreale, H.F.; Argente, J.; Sesmilo, G.; Biagetti, B. Recommendations for the measurement of sexual steroids in clinical practice. A position statement of SEQCML/SEEN/SEEP. Adv. Lab. Med. 2023, 4, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Csenki-Bakos, K. Development of a Biomarker Showing Estrogen Effect. Ph.D. Thesis, Szent István University, Gödöllő, Hungary, 2014. [Google Scholar]
- Midgley, A.R., Jr.; Niswender, G.D. Radioimmunoassay of steroids. Acta Endocrinol. 1970, 147, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dray, F.; Andrieu, J.M.; Renaud, F. Enzyme immunoassay of progesterone at the picogram level using beta-galactosidase as label. Biochim. Biophys. Acta 1975, 403, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, C.J.; Lee, G. Measurement of progesterone in human serum by isotope dilution liquid chromatography-tandem mass spectrometry and comparison with the commercial chemiluminescence immunoassay. Anal. Bioanal. Chem. 2010, 396, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Lieutaud, J. Essais Anatomiques; Pierre-Michel Huart: Paris, France, 1742. [Google Scholar]
- Treier, M.; Gleiberman, A.S.; O’Connell, S.M.; Szeto, D.P.; McMahon, J.A.; McMahon, A.P.; Rosenfeld, M.G. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998, 12, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Ezzat, S. Molecular basis of pituitary development and cytogenesis. Front. Horm. Res. 2004, 32, 1–19. [Google Scholar] [PubMed]
- Dasen, J.S.; Martinez Barbera, J.P.; Herman, T.S.; Connell, S.O.; Olson, L.; Ju, B.; Tollkuhn, J.; Baek, S.H.; Rose, D.W.; Rosenfeld, M.G. Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev. 2001, 15, 3193–3207. [Google Scholar] [CrossRef] [PubMed]
- Breier, G.; Albrecht, U.; Sterrer, S.; Risau, W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bakke, M.; Krimkevich, Y.; Cushman, L.J.; Parlow, A.F.; Sally, A.; Camper, S.A.; Parker, K.L. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 2001, 128, 147–154. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Ju, B.G.; Rosenfeld, M.G. Signaling and epigenetic regulation of pituitary development. Curr. Opin. Cell Biol. 2007, 19, 605–611. [Google Scholar] [CrossRef]
- Santiago-Andres, Y.; Golan, M.; Fiordelisio, T. Functional pituitary networks in vertebrates. Front. Endocrinol. 2021, 11, 619352. [Google Scholar] [CrossRef]
- Himes, A.D.; Raetzman, L.T. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev. Biol. 2009, 325, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, T.; Yoshida, M.; Dohra, H.; Suzuki, M.; Tanaka, S. Gene expression of vascular endothelial growth factor-A in the pituitary during formation of the vascular system in the hypothalamic-pituitary axis of the rat. Cell Tissue Res. 2006, 324, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Ellsworth, B.S.; Peréz Millan, M.I.; Gergics, P.; Schade, V.; Foyouzi, N.; Brinkmeier, M.L.; Mortensen, A.H.; Camper, S.A. Pituitary gland development and disease: From stem cell to hormone production. Curr. Top. Dev. Biol. 2013, 106, 1–47. [Google Scholar]
- Korsisaari, N.; Ross, J.; Wu, X.; Kowanetz, M.; Pal, N.; Hall, L.; Eastham-Anderson, J.; Forrest, W.F.; Van Bruggen, N.; Peale, F.V.; et al. Blocking vascular endothelial growth factor-A inhibits the growth of pituitary adenomas and lowers serum prolactin level in a mouse model of multiple endocrine neoplasia type 1. Clin. Cancer Res. 2008, 14, 249–258. [Google Scholar] [CrossRef]
- Japón, M.A.; Rubinstein, M.; Low, M.J. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J. Histochem. Cytochem. 1994, 42, 1117–1125. [Google Scholar] [CrossRef]
- Li, C.H.; Geschwind, I.I.; Levy, A.; Harris, J.I.; Dixon, J.S.; Pon, N.G.; Porath, J.O. Isolation and properties of alpha-corticotrophin from sheep pituitary glands. Nature 1954, 173, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Starman, B. Human Pituitary Growth Hormone. IX. Molecular weight of the monomer. Biochim. Biophys. Acta 1964, 86, 175–176. [Google Scholar] [CrossRef]
- Li, C.H.; Dixon, J.S.; Lo, T.B.; Pankov, Y.A.; Schmidt, K.D. Amino-acid sequence of ovine lactogenic hormone. Nature 1969, 224, 695–696. [Google Scholar] [CrossRef]
- Friesen, H.; Guyda, H.; Hardy, J. The biosynthesis of human growth hormone and prolactin. J. Clin. Endocrinol. Metabol. 1970, 31, 611–624. [Google Scholar] [CrossRef]
- Pierce, J.G. Eli Lilly lecture. The subunits of pituitary thyrotropin--their relationship to other glycoprotein hormones. Endocrinology 1971, 89, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.K.; Ward, D.N. The purification and chemistry of pituitary glycoprotein hormones. Pharmacol. Ther. 1975, 1, 545–570. [Google Scholar] [CrossRef] [PubMed]
- Halász, B.; Pupp, L.; Uhlarik, S. Hypophysiotrophic area in the hypothalamus. J. Endocrinol. 1962, 25, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Halász, B.; Pupp, L.; Uhlarik, S.; Tima, L. Further studies on the hormone secretion of the anterior pituitary transplanted into the hypophyseotrophic area of the rat hypothalamus. Endocrinology 1965, 77, 343–355. [Google Scholar] [CrossRef]
- Halász, B.; Pupp, L. Hormone secretion of the anterior pituitary gland after physical interruption of all nervous pathways to the hypophysiotropic area. Endocrinology 1965, 77, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Nikitovitch-Winer, M.; Everett, J.W. Functional restitution of pituitary grafts re-transplanted from kidney to median eminence. Endocrinology 1958, 63, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Nikitovitch-Winer, M.; Everett, J.W. Histologic changes in grafts of rat pituitary on the kidney and upon retransplantation under the diencephalon. Endocrinology 1959, 65, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Nikitovitch-Winer, M.; Everett, J.W. Comparative study of luteotropin secretion by hypophysial autotransplants in the rat; effects of site and stages of the estrus cycle. Endocrinology 1958, 62, 522–532. [Google Scholar] [CrossRef]
- Shiino, M.; Ishikawa, H.; Rennels, E.G. In vitro and in vivo studies on cytodifferentiation of pituitary clonal cells derived from the epithelium of Rathke’s pouch. Cell Tissue Res. 1977, 181, 473–485. [Google Scholar] [CrossRef]
- Marshall, F.H.; Verney, E.B. The occurrence of ovulation and pseudo-pregnancy in the rabbit as a result of central nervous stimulation. J. Physiol. 1936, 86, 327–336. [Google Scholar] [CrossRef]
- Harris, G.W. The Induction of ovulation in the rabbit, by electrical stimulation of the hypothalamo-hypophysial mechanism. Proc. Roy. Soc. B 1937, 122, 374–394. [Google Scholar]
- Marshall, F.H.A.; Bowden, F.P. Further Effects of Irradiation on the Oestrous Cycle of the Ferret. J. Exp. Biol. 1936, 13, 383–386. [Google Scholar] [CrossRef]
- Marshall, F.H.; Verney, E.B.; Vogt, M. The occurrence of ovulation in the rabbit as a result of stimulation of the central nervous system by drugs. J. Physiol. 1939, 97, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.W. Electrical stimulation of the hypothalamus and the mechanism of neural control of the adenohypophysis. J. Physiol. 1948, 107, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Evans, H.M. The Estrous Cycle in the Rat and Its Associated Phenomena; University of California Press: Berkeley, CA, USA, 1922; Volume 6, pp. 1–148. [Google Scholar]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Terasawa, E.; Wiegand, S.J. Effects of hypothalamic deafferentation on ovulation and estrous cyclicity in the female guinea pig. Neuroendocrinology 1978, 26, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Köves, K.; Halász, B. Location of neural structures triggering ovulation in the rat. Neuroendocrinology 1970, 6, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Halász, B.; Gorski, R.A. Gonadotrophic hormone secretion in female rats after partial or total interruption of neural afferents to the medial basal hypothalamus. Endocrinology 1967, 80, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.P.; Velasco, M.E.; Sawyer, C.H. Influences of hypothalamic deafferentation on pituitary FSH release and estrogen feedback in immature female parabiotic rats. Neuroendocrinology 1970, 6, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.P.; Saporta, S. The release of pituitary LH and sprouting of LH releasing hormone (LHRH)-containing neurons after anterior hypothalamic deafferentation. Brain Res. 1988, 454, 188–204. [Google Scholar] [CrossRef]
- Guillemin, R. The adenohypophysis and its hypothalamic control. Annu. Rev. Physiol. 1967, 29, 313–348. [Google Scholar] [CrossRef] [PubMed]
- Burgus, R.; Dunn, T.F.; Desiderio, D.; Guillemin, R. Molecular structure of the hypothalamic hyophysiotropic TRH factor of ovine origin: Mass spectrometry demonstration of the PCA-His-Pro-NH2 sequence. Comptes Rendus Hebd. Seances L‘academie Sci. Ser. D Sci. Nat. 1969, 269, 1870–1873. [Google Scholar]
- Schally, A.V.; Redding, T.V.; Bowers, C.J.; Barett, J.F. Isolation and properties of porcine thyrotropin-releasing hormone. J. Biol. Chem. 1969, 244, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Boler, J.; Enzmann, F.; Folkers, K.; Bowers, C.Y.; Schally, A.V. The identity of chemical and hormonal properties of thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem. Biophys. Res. Commun. 1969, 37, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Arimura, A.; Baba, Y.; Nair, R.M.G.; Matsuo, H.; Redding, T.W.; Debeljuk, L.; White, W.F. Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 1971, 43, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Baba, Y.; Nair, R.M.G.; Arimura, A.; Schally, A.V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem. Biophys. Res. Commun. 1971, 43, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Amoss, M.; Burgus, R.; Blackwell, R.; Vale, W.; Fellows, R.; Guillemin, R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem. Biophys. Res. Commun. 1971, 44, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Hasegawa, Y.; Minegishi, T.; Nomura, M.; Takahashi, Y.; Igarashi, M.; Kangawa, K.; Matsuo, H. Isolation and characterization of luteinizing hormone-releasing hormone. Biochem. Biophys. Res. Commun. 1982, 107, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Schally, A.V.; Gual, C.; Arimura, A. Release of LH and FSH after administration of synthetic LH-releasing hormone. J. Clin. Endocrinol. Metab. 1972, 34, 753–756. [Google Scholar] [CrossRef]
- Berson, S.A.; Yalow, R.S. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J. Clin. Investig. 1959, 38, 1996–2016. [Google Scholar] [CrossRef]
- Arimura, A.; Sato, H.; Kumasaka, T.; Fun Worobec, R.B.; Debeljuk, L.; Dunn, J.; Schally, A.V. Production of antiserum to LH-releasing hormone (LH-RH) associated with gonadal atrophy in rabbits: Development of radioimmunoassays for LH-RH. Endocrinology 1973, 93, 1092–1103. [Google Scholar] [CrossRef]
- King, J.C.; Williams, T.H.; Arimura, A. Localization of luteinizing hormone-releasing hormone in rat hypothalamus using radioimmunoassay. J. Anat. 1975, 120, 275–288. [Google Scholar] [PubMed]
- Scharrer, E. The final common path in neuroendocrine integration. Arch. D’anatomie Microsc. Morphol. Exp. 1965, 54, 359–370. [Google Scholar]
- Critchlow, V. Ovulation induced by hypothalamic stimulation in the anesthetized rat. Am. J. Physiol. 1958, 195, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, M.J.; Arimura, A.; Schally, A.V.; Palkovits, M.; Kizer, J.S. The effect of surgical isolation of the hypothalamus on its luteinizing hormone-releasing hormone content. Endocrinology 1976, 98, 662–665. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Parsons, J.A.; Erlandsen, S.L.; Arimura, A. Luteinizing hormone-releasing hormone (LH-RH) pathway of the rat hypothalamus revealed by the unlabeled antibody peroxidase-antiperoxidase method. Cell Tissue Res. 1974, 153, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.; Dubois, M.P.; Poulain, P. LRF-producing cells of the mammalian hypothalamus: A fluorescent antibody study. Z. Zellforsch. Mikrosc. Anat. 1973, 146, 351. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.L.; Dermody, W.C.; Reel, J.R. Localization of luteinizing hormone-releasing hormone in the mammalian hypothalamus. Supported in part by NIH grant HD 03159-06. Am. J. Anat. 1974, 139, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Sétáló, G.; Vigh, A.V.; Schally, A.; Arimura, B.; Flerkó. LH-RH-containing neural elements in the rat hypothalamus. Endocrinology 1975, 96, 135–142. [Google Scholar] [CrossRef]
- Merchenthaler, I.G.; Kovács, G.; Lovász, G.; Sétáló, G. The preoptico-infundibular LH-RH tract of the rat. Brain Res. 1980, 198, 63–74. [Google Scholar] [CrossRef]
- King, J.C.; Tobet, S.A.; Snavely, F.L.; Arimura, A. The LHRH system in normal and neonatally androgenized female rats. Peptides 1980, 1 (Suppl. S1), 85–100. [Google Scholar] [CrossRef]
- King, J.C.; Rubin, B.S. Dynamic alterations in luteinizing hormone-releasing hormone (LHRH) neuronal cell bodies and terminals of adult rats. Cell Mol. Neurobiol. 1995, 15, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.K.; Chiappa, S.A.; Fink, G.; Sherwood, N.M. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 1976, 264, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Tsou, R.C.; Dailey, R.A.; McLanahan, C.S.; Parent, A.D.; Tindall, G.T.; Neill, J.D. Luteinizing hormone releasing hormone (LHRH) levels in pituitary stalk plasma during the preovulatory gonadotropin surge of rabbits. Endocrinology 1977, 101, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Carmel, P.W.; Araki, S.; Ferin, M. Pituitary stalk portal blood collection in rhesus monkeys: Evidence for pulsatile release of gonadotropin-releasing hormone (GnRH). Endocrinology 1976, 99, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Moenter, S.M.; Evans, N.P. Gonadotropin-releasing hormone (GnRH) measurements in pituitary portal blood: A history. J. Neuroendocrinol. 2022, 34, e13065. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.O.; Hansson, H.A.; Sjöstrand, J. Effect of colchicine on axonal transport and morphology of retinal ganglion cells. Z. Zellforsch. 1971, 115, 265–283. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Berghorn, K.A. Gonadotropin-releasing hormone neurons: Their structure and function. Semin. Reprod Endocrinol. 1997, 15, 5–17. [Google Scholar] [CrossRef]
- Campbell, R.E.; Coolen, L.M.; Hoffman, G.E.; Hrabovszky, E. Highlights of neuroanatomical discoveries of the mammalian gonadotropin-releasing hormone system. J. Neuroendocrinol. 2022, 34, e13115. [Google Scholar] [CrossRef]
- Merchenthaler, I.; Sétaló, G.; Csontos, C.; Petrusz, P.; Flerkó, B.; Negro-Vilar, A. Combined retrograde tracing and immunocytochemical identification of luteinizing hormone-releasing hormone- and somatostatin-containing neurons projecting to the median eminence of the rat. Endocrinology 1989, 125, 2812–2821. [Google Scholar] [CrossRef]
- Anthony, E.L.; King, J.C.; Stopa, E.G. Immunocytochemical localization of LHRH in the median eminence, infundibular stalk, and neurohypophysis. Evidence for multiple sites of releasing hormone secretion in humans and other mammals. Cell Tissue Res. 1984, 236, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Szabó, F.; Horváth, J.; Heinzlmann, A.; Arimura, A.; Köves, K. Neonatal PACAP administration in rats delays puberty through the influence of the LHRH neuronal system. Regul. Pep. 2002, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, M. Neuropeptides in the hypothalamo-hypophyseal system: Lateral retrochiasmatic area as a common gate for neuronal fibers towards the median eminence. Peptides 1984, 5 (Suppl. S1), 35–39. [Google Scholar] [CrossRef]
- Wray, S.; Hoffman, G. A developmental study of the quantitative distribution of LHRH neurons within the central nervous system of postnatal male and female rats. J. Comp. Neurol. 1986, 252, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Merchenthaler, I.; Görcs, T.; Sétáló, G.; Petrusz, P.; Flerkó, B. Gonadotropin-releasing hormone (GnRH) neurons and pathways in the rat brain. Cell Tissue Res. 1984, 237, 15–29. [Google Scholar] [CrossRef]
- Clayton, R.N.; Catt, K.J. Gonadotropin-releasing hormone receptors: Characterization, physiological regulation, and relationship to reproductive function. Endocr. Rev. 1981, 2, 186–209. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.N.; Catt, K.J. Receptor-binding affinity of gonadotropin-releasing hormone analogs: Analysis by radioligand-receptor assay. Endocrinology 1980, 106, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Zilberstein, M.; Zakut, H.; Naor, Z. Coincidence of down-regulation and desensitization in pituitary gonadotrophs stimulated by gonadotropin releasing hormone. Life Sci. 1983, 32, 663–669. [Google Scholar] [CrossRef]
- Marian, J.; Cooper, R.L.; Conn, P.M. Regulation of the rat pituitary gonadotropin-releasing hormone receptor. Mol. Pharmacol. 1981, 19, 399–405. [Google Scholar]
- Popkin, R.; Fraser, H.M. The effects of immunoneutralization of LHRH or LH on pituitary LHRH receptors during the rat estrous cycle. Mol. Cell. Endocrinol. 1983, 33, 305–312. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Pfaff, D.W. Origin of luteinizing hormone-releasing hormone neurons. Nature 1989, 338, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.J.; Witkin, J.W.; Silverman, R.C.; Gibson, M.J. Modulation of gonadotropin-releasing hormone neuronal activity as evidenced by uptake of fluorogold from the vasculature. Synapse 1990, 6, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.J.; Livne, I.; Witkin, J.W. The gonadotropin-releasing hormone (GnRH) neuronal systems: Immunocytochemistry and in situ hybridization. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven: New York, NY, USA, 1994; pp. 1683–1709. [Google Scholar]
- Saitoh, Y.; Luchansky, L.L.; Claude, P.; Terasawa, E. Transplantation of the fetal olfactory placode restores reproductive cycles in female rhesus monkeys (Macaca mulatta) bearing lesions in the medial basal hypothalamus. Endocrinology 1995, 136, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Stern, K.; McClintock, M.K. Regulation of ovulation by human pheromones. Nature 1998, 392, 177–179. [Google Scholar] [CrossRef] [PubMed]
- McClintock, M.K.; Jacob, S.; Zelano, B.; Hayreh, D.J. Pheromones and vasanas: The functions of social chemosignals. Nebr. Symp. Motiv. 2001, 47, 75–112. [Google Scholar]
- Stowers, L.; Marton, T.F. What is a pheromone? Mammalian pheromones reconsidered. Neuron 2005, 46, 699–702. [Google Scholar] [CrossRef]
- Herde, M.K.; Iremonger, K.J.; Constantin, S.; Herbison, A.E. GnRH neurons elaborate a long-range projection with shared axonal and dendritic functions. J. Neurosci. 2013, 33, 12689–12697. [Google Scholar] [CrossRef] [PubMed]
- Jennes, L.; Stumpf, W.E.; Sheedy, M.E. Ultrastructural characterization of gonadotropin-releasing hormone (GnRH)-producing neurons. J. Comp. Neurol. 1985, 232, 534–547. [Google Scholar] [CrossRef]
- Martínez de la Escalera, G.; Choi, A.L.; Weiner, R.I. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: Intrinsic properties of the GT1–1 GnRH neuronal cell line. Proc. Natl. Acad. Sci. USA 1992, 89, 1852–1855. [Google Scholar] [CrossRef]
- Wetsel, W.C.; Valenca, M.M.; Merchenthaler, I.; Liposits, Z.; Lopez, F.J.; Weiner, R.I.; Mellon, P.L.; Negro-Vilar, A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc. Natl. Acad. Sci. USA 1992, 89, 4149–4153. [Google Scholar] [CrossRef]
- Witkin, J.W.; O’Sullivan, H.; Silverman, A.J. Novel associations among gonadotropin-releasing hormone neurons. Endocrinology 1995, 136, 4323–4330. [Google Scholar] [CrossRef]
- Chan, H.; Prescott, M.; Ong, Z.; Herde, M.K.; Herbison, A.E.; Campbell, R.E. Dendritic spine plasticity in gonadotropin-releasing hormone (GnRH) neurons activated at the time of the preovulatory surge. Endocrinology 2011, 152, 4906–4914. [Google Scholar] [CrossRef] [PubMed]
- Witkin, J.W.; O’Sullivan, H.; Ferin, M. Glial ensheathment of GnRH neurons in pubertal female rhesus macaques. J. Neuroendocrinol. 1995, 7, 665–671. [Google Scholar] [CrossRef]
- Kallo, I.; Vida, B.; Deli, L.; Molnár, C.S.; Hrabovszky, E.; Caraty, A.; Ciofi, P.; Coen, C.W.; Liposits, Z. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurons. J. Neuroendocrinol. 2012, 24, 464–476. [Google Scholar] [CrossRef]
- Farkas, I.; Bálint, F.; Farkas, E.; Vastagh, C.; Fekete, C.; Liposits, Z. Estradiol increases glutamate and GABA neurotransmission into GnRH neurons via retrograde NO-signaling in proestrous mice during the positive estradiol feedback period. Eneuro 2018, 5, 0057-18.2018. [Google Scholar] [CrossRef]
- Köves, K.; Molnár, J.; Kántor, O.; Lakatos, A.; Görcs, T.J.; Somogyvári-Vigh, A.; Fürst, Z.; Arimura, A. PACAP participates in the regulation of the hormonal events preceding the ovulation. Acta Biol. Sci. Hung. 1996, 47, 239–249. [Google Scholar]
- Kántor, O.; Molnár, J.; Heinzlmann, A.; Arimura, A.; Fürst, Z.; Köves, K. The inhibitory effect of PACAP38 on ovulation is mediated by CRF and endogenous opioids. Ann. N. Y. Acad. Sci. 2000, 921, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Köves, K.; Szabó, E.; Kántor, O.; Heinzlmann, A.; Szabó, F.; Csáki, Á. Current state of understanding of the role of PACAP in the hypothalamo-hypophyseal gonadotropin functions of mammals. Front. Endocrinol. 2020, 11, 506309. [Google Scholar] [CrossRef]
- Kántor, O.; Molnár, J.; Heinzlmann, A.; Arimura, A.; Fürst, Z.; Köves, K. Study on the hypothalamic factors mediating the inhibitory effect of PACAP38 on ovulation. Peptides 2001, 22, 2163–2168. [Google Scholar] [CrossRef]
- Smith, M.J.; Wise, P.M. Neurotensin gene expression increases during proestrus in the rostral medial preoptic nucleus: Potential for direct communication with gonadotropin-releasing hormone neurons. Endocrinology 2001, 142, 3006–3013. [Google Scholar] [CrossRef]
- Krsmanovic, L.Z.; Mores, N.; Navarro, C.E.; Arora, K.K.; Catt, K.J. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Herbison, A.E. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V). Brain Res. Rev. 2008, 57, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Krsmanovic, L.Z.; Hu, L.; Leung, P.K.; Feng, H.; Catt, K.J. The hypothalamic GnRH pulse generator: Multiple regulatory mechanisms. Trends Endocrinol. Metab. 2009, 20, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dungan, H.M.; Gottsch, M.L.; Zeng, H.; Gragerov, A.; Bergmann, J.E.; Vassilatis, D.K.; Clifton, D.K.; Steiner, R.A. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J. Neurosci. 2007, 27, 12088–12095. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef]
- Skrapits, K.; Borsay, B.; Herczeg, L.; Ciofi, P.; Liposits, Z.; Hrabovszky, E. Neuropeptide co-expression in hypothalamic kisspeptin neurons of laboratory animals and the human. Front. Neurosci. 2015, 9, 29. [Google Scholar] [CrossRef]
- Moore, A.M.; Coolen, L.M.; Porter, D.T.; Goodman, R.L.; Lehman, M.N. KNDy Cells Revisited. Endocrinology 2018, 159, 3219–3234. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; DeFazio, R.A.; Christian, C.A.; Milescu, L.S.; Schnell, S.; Moenter, S.M. Changes in both neuron intrinsic properties and neurotransmission are needed to drive the increase in GnRH neuron firing rate during estradiol-positive feedback. J. Neurosci. 2019, 39, 2091–2101. [Google Scholar] [CrossRef]
- Tsutsui, K.; Saigoh, E.; Ukena, K.; Teranishi, H.; Fujisawa, Y.; Kikuchi, M.; Ishii, S.; Sharp, P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000, 275, 661–667. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ubuka, T.; Bentley, G.E.; Kriegsfeld, L.J. Gonadotropin-inhibitory hormone (GnIH): Discovery, progress and prospect. Gen. Comp. Endocrinol. 2012, 177, 305–314. [Google Scholar] [CrossRef]
- McQuillan, H.J.; Clarkson, J.; Kauff, A.; Young Han, S.; Hoong Yip, S.; Cheong, I.; Porteous, R.; Heather, A.K.; Herbison, A.E. Definition of the estrogen negative feedback pathway controlling the GnRH pulse generator in female mice. Nat. Commun. 2022, 13, 1–15. [Google Scholar]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Chen, Z.; Li, X.; Cai, E.; Yang, H.; Chen, Y.; Wang, C.; Ju, L.; Deng, W.; Mu, L. Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction. Reprod. Biol. Endocrinol. 2022, 20, 81. [Google Scholar] [CrossRef]
- Barr, G.D.; Barraclough, C.A. Temporal changes in medial basal hypothalamic LH-RH correlated with plasma LH during the rat estrous cycle and following electrochemical stimulation of the medial preoptic area in pentobarbital-treated proestrous rats. Brain Res. 1978, 148, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.L.; Collins, W.E.; Fugo, N.W. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17 beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974, 94, 1704–1708. [Google Scholar] [CrossRef]
- Raven, P.H.; Johnson, G.B.; Mason, K.A.; Losos, J.B.; Duncan, T. Structure and function of the human female reproductive system. In Biology, 13th ed.; McGraw Hill: New York, NY, USA, 2023; pp. 1133–1151. [Google Scholar]
- de Kretser, D.M.; Hedger, M.P.; Loveland, K.L.; Phillips, D.J. Inhibins, activins and follistatin in reproduction. Hum. Reprod. Update 2002, 8, 529–541. [Google Scholar] [CrossRef]
- Chaffkin, L.M.; Luciano, A.A.; Peluso, J.J. Progesterone as an autocrine/paracrine regulator of human granulosa cell proliferation. J. Clin. Endocrinol. Metabol. 1992, 75, 1404–1408. [Google Scholar]
- Durlinger, A.L.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of Primordial Follicle Recruitment by Anti-Mullerian Hormone in the Mouse Ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef]
- Mittelman-Smith, M.A.; Krajewski-Hall, S.J.; McMullen, N.T.; Rance, N.E. Ablation of KNDy neurons results in hypogonadotropic hypogonadism and amplifies the steroid-induced LH surge in female rats. Endocrinology 2016, 157, 2015–2027. [Google Scholar] [CrossRef]
- Wang, L.; Vanacker, C.; Burger, L.L.; Barnes, T.; Shah, Y.M.; Myers, M.G.; Moenter, S.M. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 2019, 8, e43999. [Google Scholar] [CrossRef]
- Everett, J.W.; Sawyer, C.H. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 1950, 47, 198–218. [Google Scholar] [CrossRef] [PubMed]
- Scharrer, E. Photo-neuro-endocrine systems: General concepts. Ann. N. Y. Acad. Sci. 1964, 117, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.E.; Wagoner, N.; Cowan, W.M. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z. Zellforsch. Mikrosk. Anat. 1972, 135, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Lenn, N.J. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Ding, J.M.; Chen, D.; Fahrenkrug, J.; Larsen, P.J.; Gillette, M.U.; Mikkelsen, J.D. Pituitary Adenylate Cyclase-Activating Peptide (PACAP) in the retinohypothalamic tract: A potential daytime regulator of the biological clock. J. Neurosci. 1997, 7, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C.; Moore, R.Y.; Reppert, S. The Suprachiasmatic Nucleus: The Mind’s Clock; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Piet, R.; Fraissenon, A.; Boehm, U.; Herbison, A.E. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J. Neurosci. 2015, 35, 6881–6892. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.S.; Schwartz, N.B. The impact of constant light on the estrous cycle of the rat. Endocrinology 1980, 106, 1230–1238. [Google Scholar] [CrossRef]
- Bittman, E.L. Circadian function in multiple cell types is necessary for proper timing of the preovulatory LH surge. J. Biol. Rhythms. 2019, 34, 622–633. [Google Scholar] [CrossRef]
- Marsman, H.J. Women in Ugarit and Israel: Their Social and Religious Position in the Context of the Ancient near East; The Social Position of Women; Koninklijke Brill NV: Leiden, The Netherland, 2003; pp. 43–454. [Google Scholar]
- Johnston, D.R. The history of human infertility. Fertil. Steril. 1963, 14, 261–272. [Google Scholar] [CrossRef]
- Spallanzani, L. Dissertations Relative to the Natural History of Animals and Vegetables; Beddoes, T., Ed. and Translator; J. Murray: London, UK, 1784; Volume 2. [Google Scholar]
- Ombelet, W.; Van Robays, J. Artificial insemination history: Hurdles and milestones. Facts Views Vis. Obgyn. 2015, 7, 137–143. [Google Scholar]
- Marcus, M.D.; Loucks, T.L.; Berga, S.L. Psychological correlates of functional hypothalamic amenorrhea. Fertil. Steril. 2001, 76, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.E.; Berga, S.L. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: A controlled comparison. Fertil. Steril 1993, 60, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Berga, S.L.; Girton, L.G. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatr. Clin. N. Am. 1989, 12, 105–116. [Google Scholar] [CrossRef]
- Barbarino, A.; De Marinis, L.; Folli, G.; Tofani, A.; Della Casa, S.; D’Amico, C.; Mancini, A.; Corsello, S.M.; Sambo, P.; Barini, A. Corticotropin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism 1989, 38, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Oh, Y.; Yoon, B.K.; Choi, D. Prevalence of hyperprolactinemia in adolescents and young women with menstruation-related problems. Am. J. Obstet. Gynecol. 2012, 206, 213.E1–213.E5. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Hardy, K.; Lovelock, S.; Franks, S. Low-dose gonadotropin induction of ovulation in anovulatory women: Still needed in the age of IVF. Reproduction 2018, 156, F1–F10. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Dwivedy, I. Development of Estrogen Antagonists as Pharmaceutical Agents. Adv. Drug Res. 1997, 29, 171–270. [Google Scholar]
- Von Hofe, J.; Bates, G.W. Ovulation induction. Obstet. Gynecol. Clin. N. Am. 2015, 42, 27–37. [Google Scholar] [CrossRef]
- Adashi, E.Y. Clomiphene citrate: Mechanism(s) and site(s) of action--a hypothesis revisited. Fertil. Steril. 1984, 42, 331–344. [Google Scholar]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; DePaoli, A.M.; Karalis, A.; Mantzoros, C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Chamberland, J.P.; Liu, X.; Matarese, G.; Gao, C.; Stefanakis, R.; Brinkoetter, M.T.; Gong, H.; Arampatzi, K.; Mantzoros, C.S. Leptin is an effective treatment for hypothalamic amenorrhea. Proc. Natl. Acad. Sci. USA 2011, 108, 6585–6590. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 neurons are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Dhillo, W.S.; Chaudhri, O.B.; Patterson, M.; Thompson, E.L.; Murphy, K.G.; Badman, M.K.; McGowan, B.M.; Amber, V.; Patel, S.; Ghatei, M.A.; et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. Clin. Endocrinol. Metab. 2005, 90, 6609–6615. [Google Scholar] [CrossRef]
- Dhillo, W.S.; Chaudhri, O.B.; Thompson, E.L.; Murphy, K.G.; Patterson, M.; Ramachandran, R.; Nijher, G.K.; Amber, V.; Kokkinos, A.; Donaldson, M.; et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J. Clin. Endocrinol. Metab. 2007, 92, 3958–3966. [Google Scholar] [CrossRef]
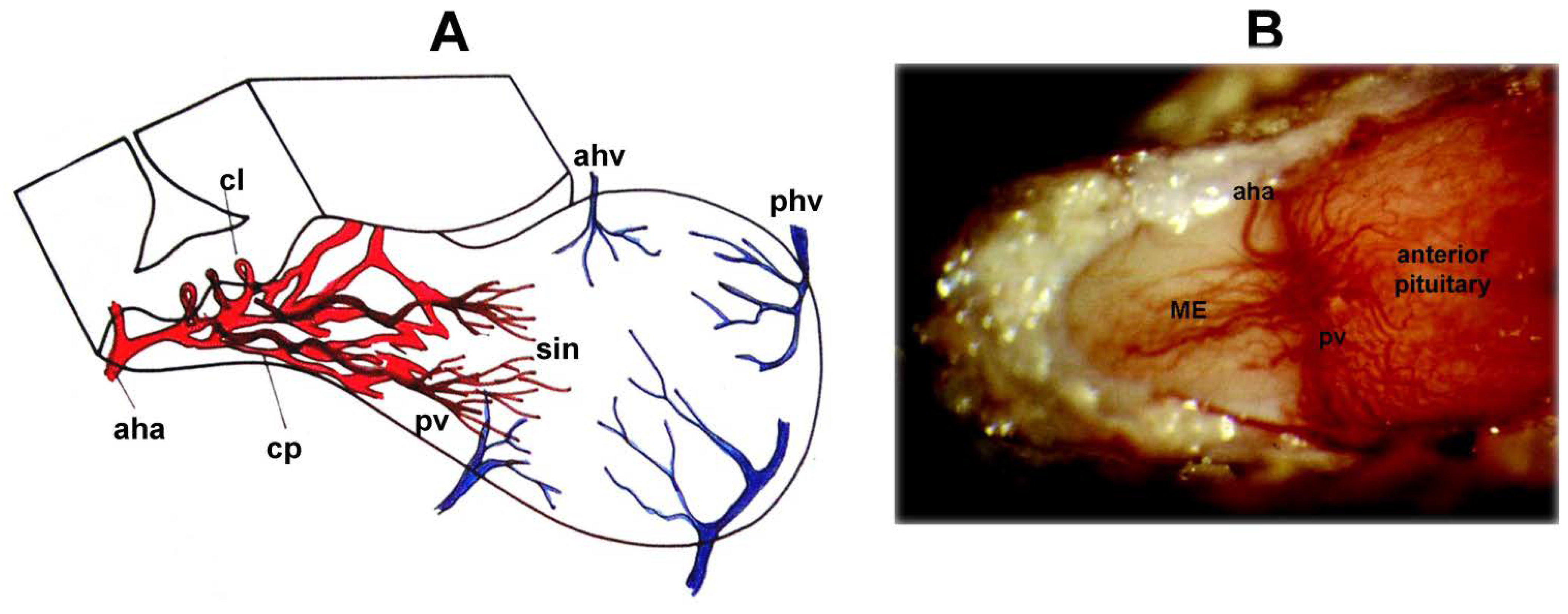
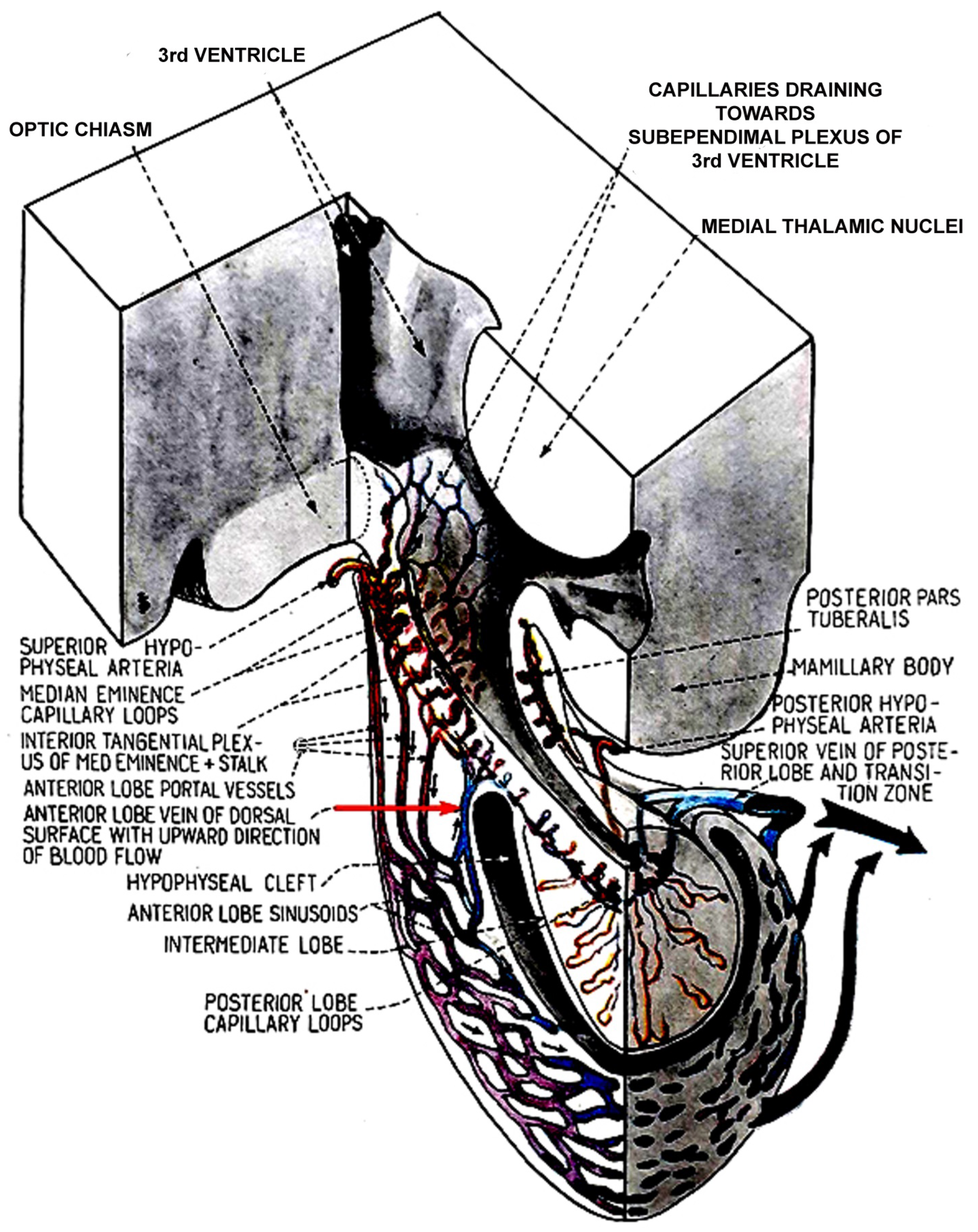
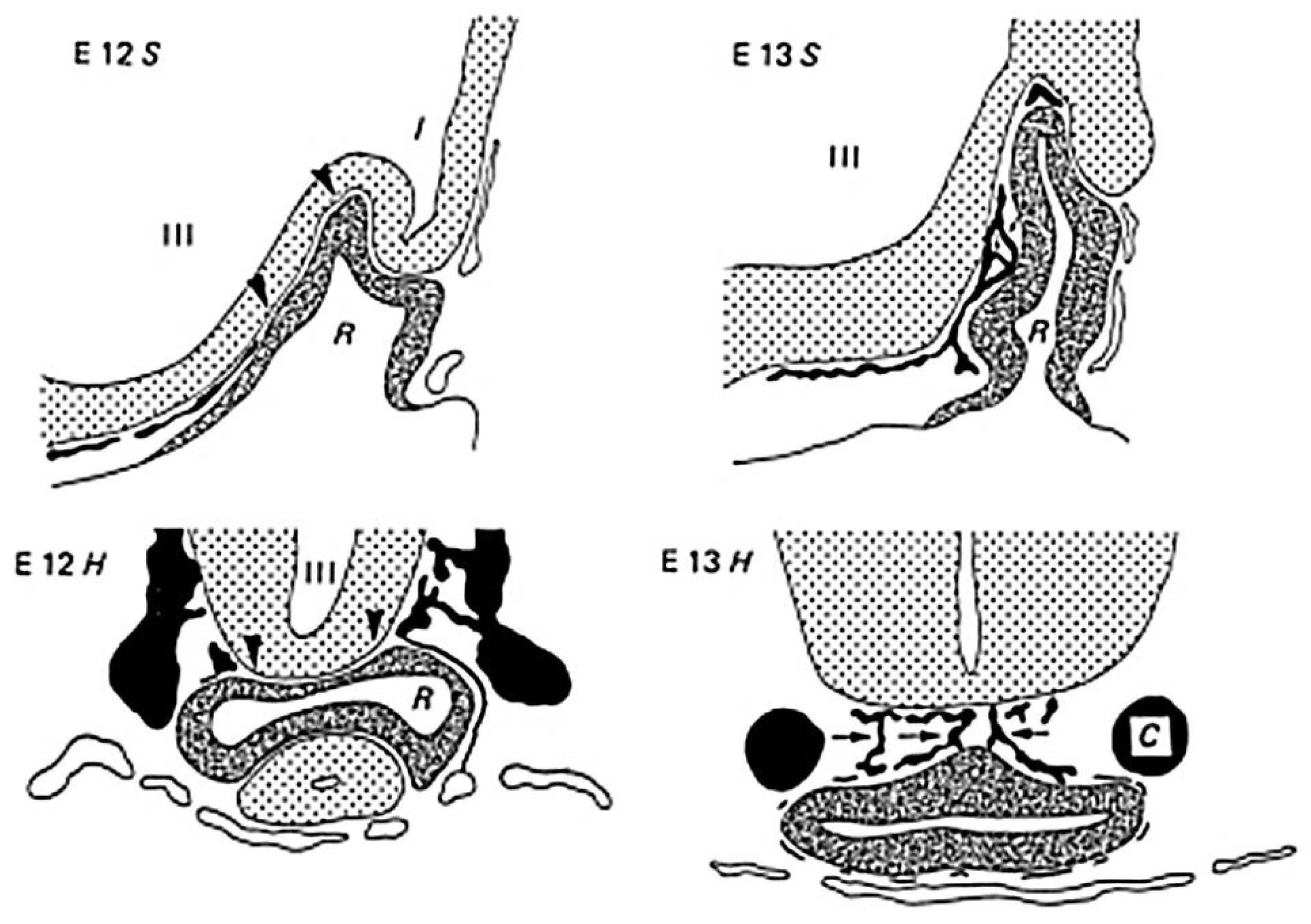

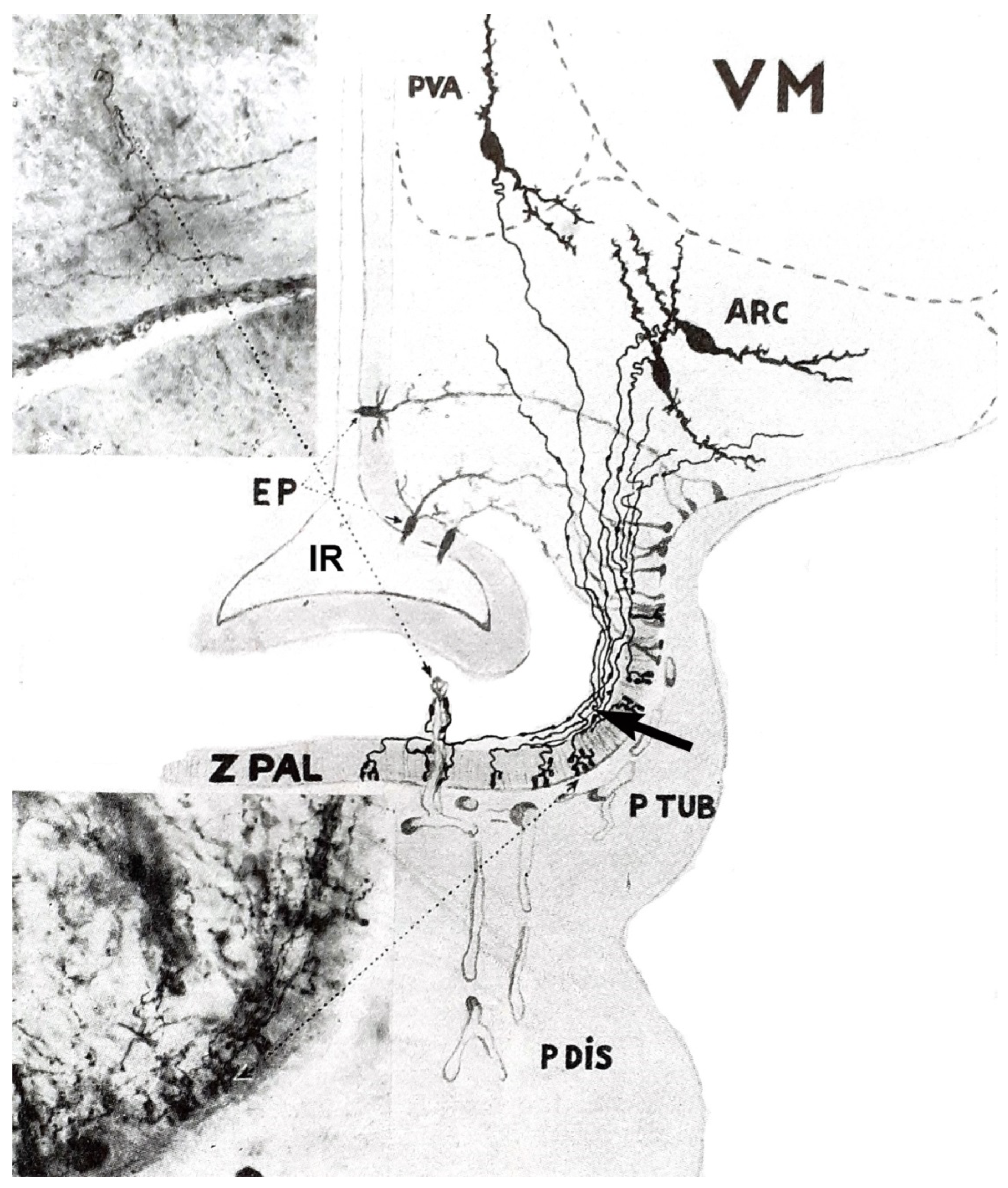

| Milestone | Year of Discovery | Author | Citation |
|---|---|---|---|
| Recognition of the portal system | 1742 | Lieutaud | [103] |
| Description of estrous cycle in rats | 1922 | Long and Evans | [134] |
| Discovery of estrogen | 1923 | Allen and Doisy | [74] |
| Discovery of progesterone | 1929 | Corner and Allen | [75] |
| Vascular ring between the pituitary and the hypothalamus | 1930 | Popa and Fielding | [19] |
| Effect of illumination on ovulation | 1934 | Marshall and Bowden | [18] |
| Importance of vaginal smears | 1941 | Papanicolau and Traut | [93] |
| Neural control of the pituitary gland | 1950 | Harris | [21] |
| Description of the magnocellular system | 1951 | Bargmann and Scharrer | [61] |
| Restitution of a pituitary graft in the median eminence | 1958 | Nikitovich-Winer and Everett | [125] |
| Radioimmunoassay | 1959 | Yallow and Berson | [94] |
| Reverse blood flow from pituitary to hypothalamus | 1962 | Török | [42] |
| Tuberoinfundibular parvicellular system | 1962 | Szentágotai | [22] |
| Description of hypophysiotropic area | 1965 | Halász and Papp | [124] |
| Discovery of estrogen receptor α | 1967 | Jensen | [77] |
| Chemical characterization of lactogenic hormone | 1969 | Li | [118] |
| Description of the structure of the median eminence | 1970 | Knigge and Scott | [66] |
| Chemical description of glycoprotein hormones | 1971 | Pierce | [120] |
| Isolation and properties of LH- and FSH-releasing hormones | 1971 | Schally | [145] |
| Radioimmunoassay of LHRH | 1973 | Arimura | [151] |
| Distribution of LHRH in the hypothalamus | 1974 | King | [156] |
| Pulsatility of GnRH in the portal blood | 1976 | Carmel | [165] |
| Characterization of GnRH receptor | 1981 | Clayton and Catt | [176] |
| Development of the pituitary gland | 1987 | Szabó | [60] |
| Origin of GnRH from the olfactory placode | 1989 | Schwanzel-Fukuda and Pfaff | [181] |
| Demonstration of hypophysiotropic GnRH neurons | 1989 | Merchenthaler | [170] |
| Discovery of neurosteroids | 1990 | Baulieu and Robel | [91] |
| Discovery of estrogen receptor β | 1996 | Kuiper | [80] |
| Molecular background of pituitary differentiation | 1998 | Treier | [104] |
| Estrogen α and β knockout models | 2000 | Dupont | [83] |
| Description of the surge generator | 2008 | Herbison | [203] |
| Description of the pulse generator | 2009 | Navarro | [206] |
| Description of the GnRH dendron | 2013 | Herde | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, F.; Köves, K.; Gál, L. History of the Development of Knowledge about the Neuroendocrine Control of Ovulation—Recent Knowledge on the Molecular Background. Int. J. Mol. Sci. 2024, 25, 6531. https://doi.org/10.3390/ijms25126531
Szabó F, Köves K, Gál L. History of the Development of Knowledge about the Neuroendocrine Control of Ovulation—Recent Knowledge on the Molecular Background. International Journal of Molecular Sciences. 2024; 25(12):6531. https://doi.org/10.3390/ijms25126531
Chicago/Turabian StyleSzabó, Flóra, Katalin Köves, and Levente Gál. 2024. "History of the Development of Knowledge about the Neuroendocrine Control of Ovulation—Recent Knowledge on the Molecular Background" International Journal of Molecular Sciences 25, no. 12: 6531. https://doi.org/10.3390/ijms25126531
APA StyleSzabó, F., Köves, K., & Gál, L. (2024). History of the Development of Knowledge about the Neuroendocrine Control of Ovulation—Recent Knowledge on the Molecular Background. International Journal of Molecular Sciences, 25(12), 6531. https://doi.org/10.3390/ijms25126531






