Biological Activity of Complexes Involving Nitro-Containing Ligands and Crystallographic-Theoretical Description of 3,5-DNB Complexes
Abstract
:1. Introduction
2. Nitrobenzoic Acid (NBA)
3. Biological Activity of NBA Derivative Complexes
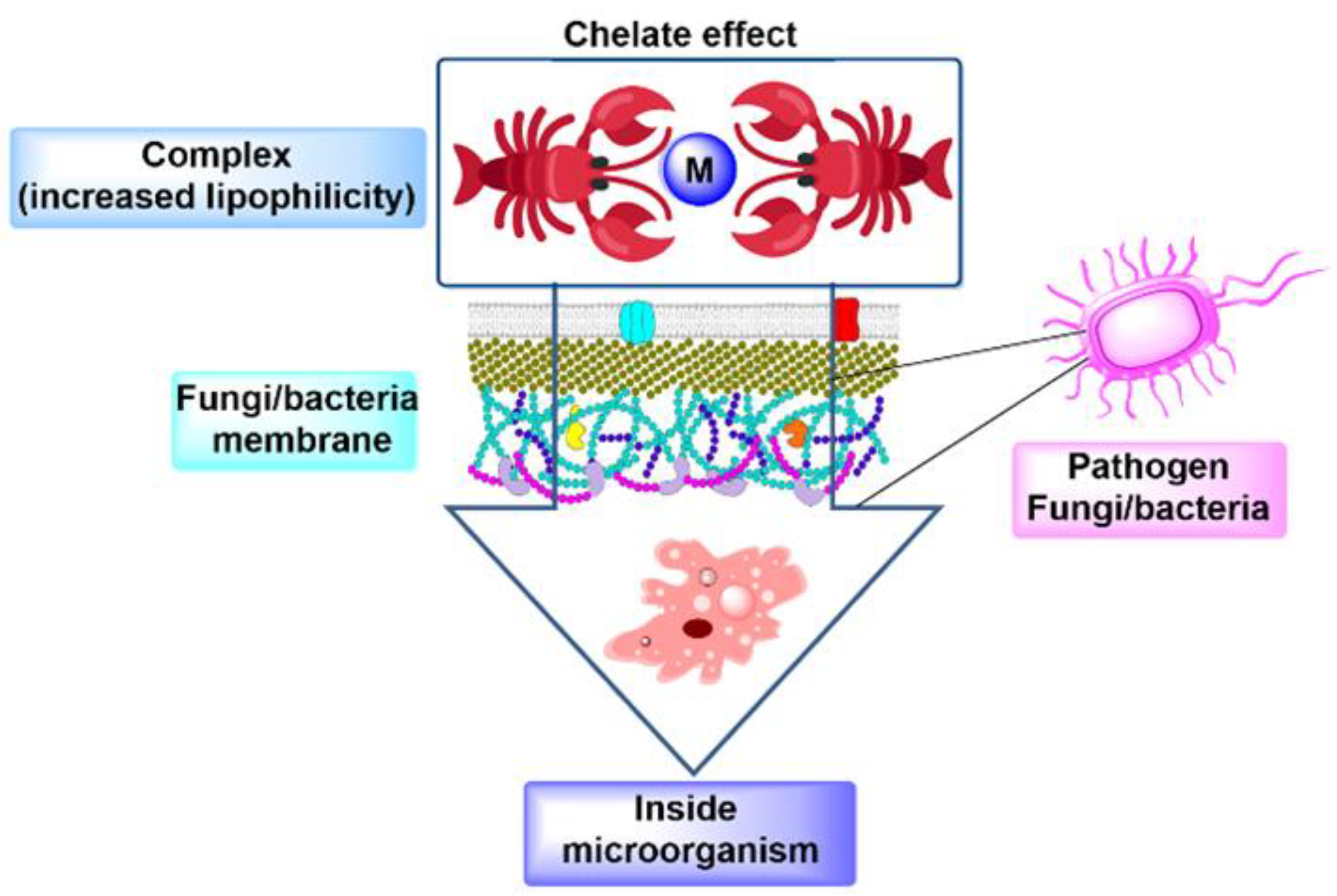
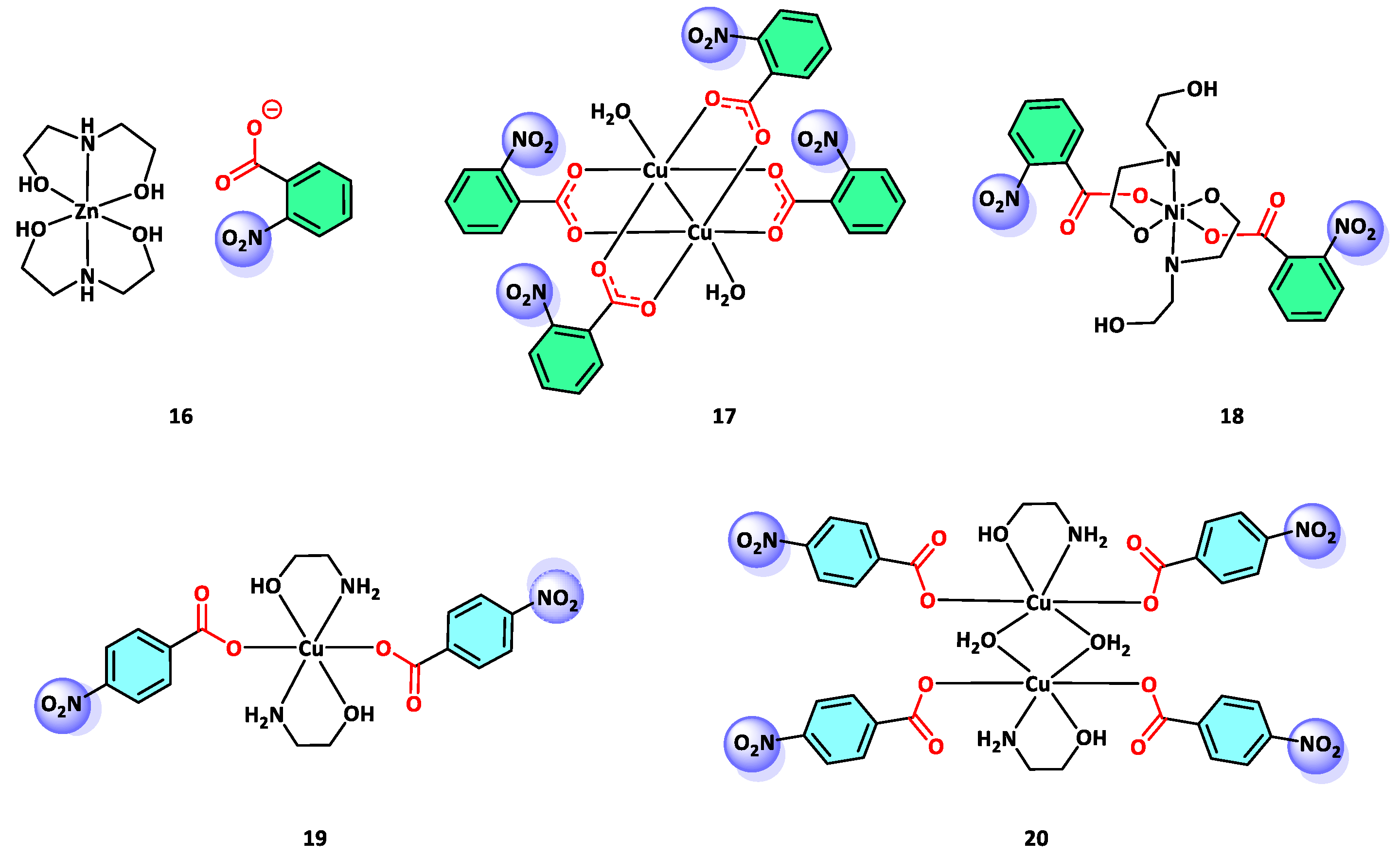
3.1. Antifungal and Antibacterial Activity of NBA Derivative Complexes
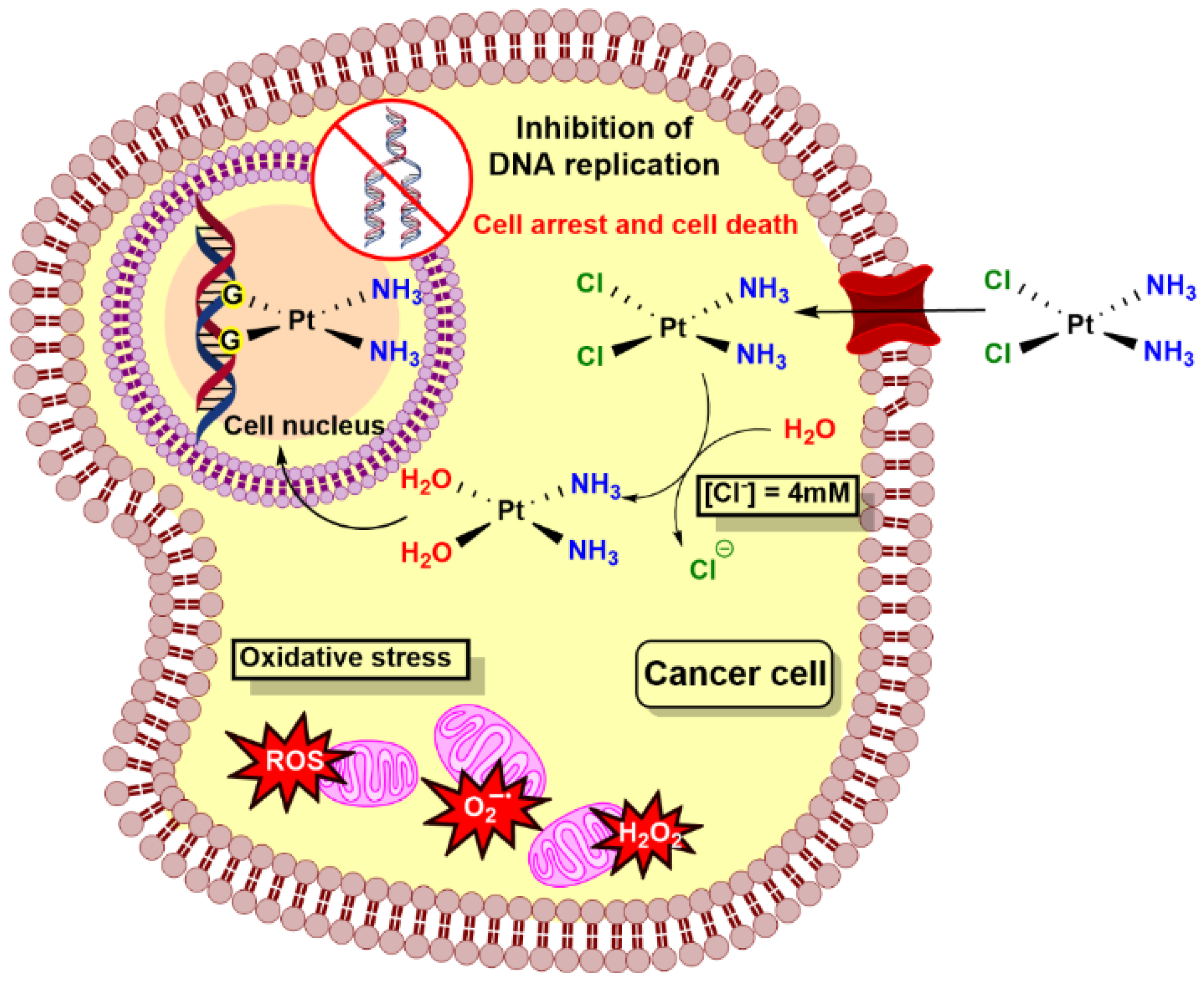
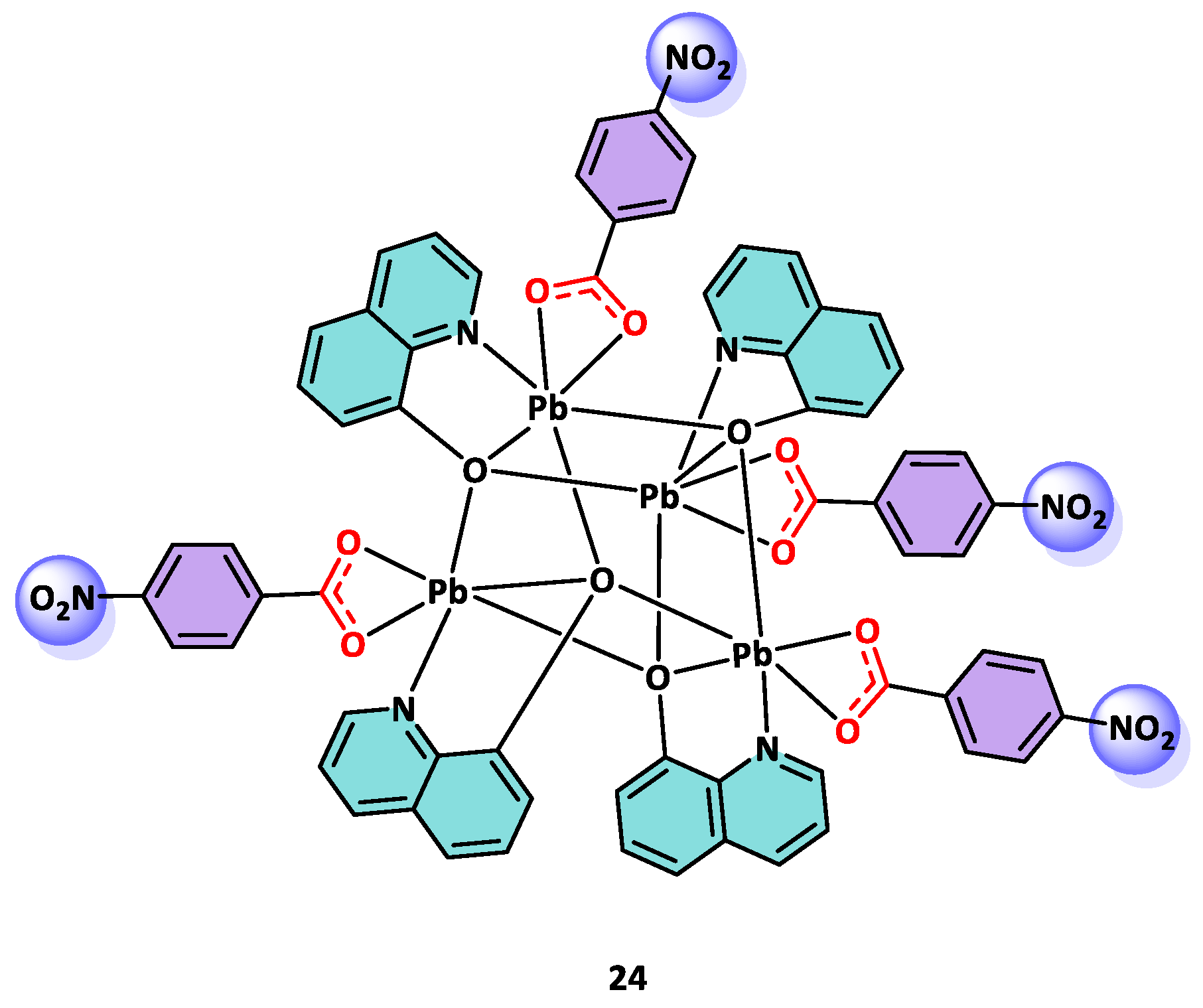
3.2. Anticancer Activity of NBA Derivative Complexes
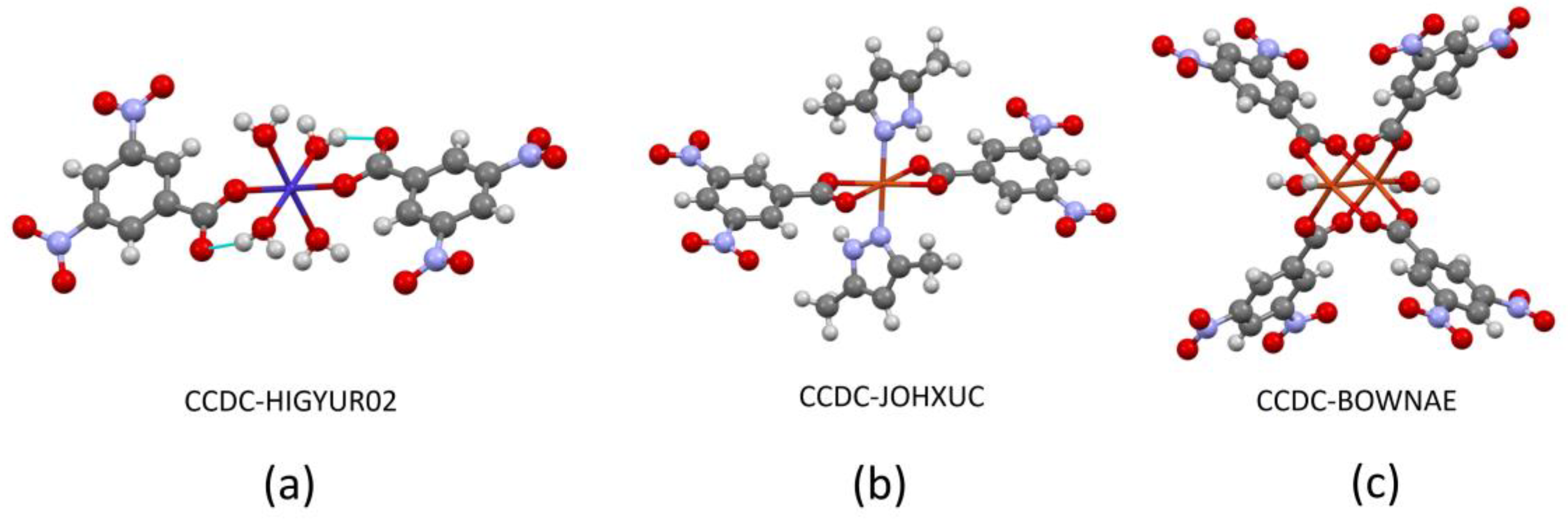
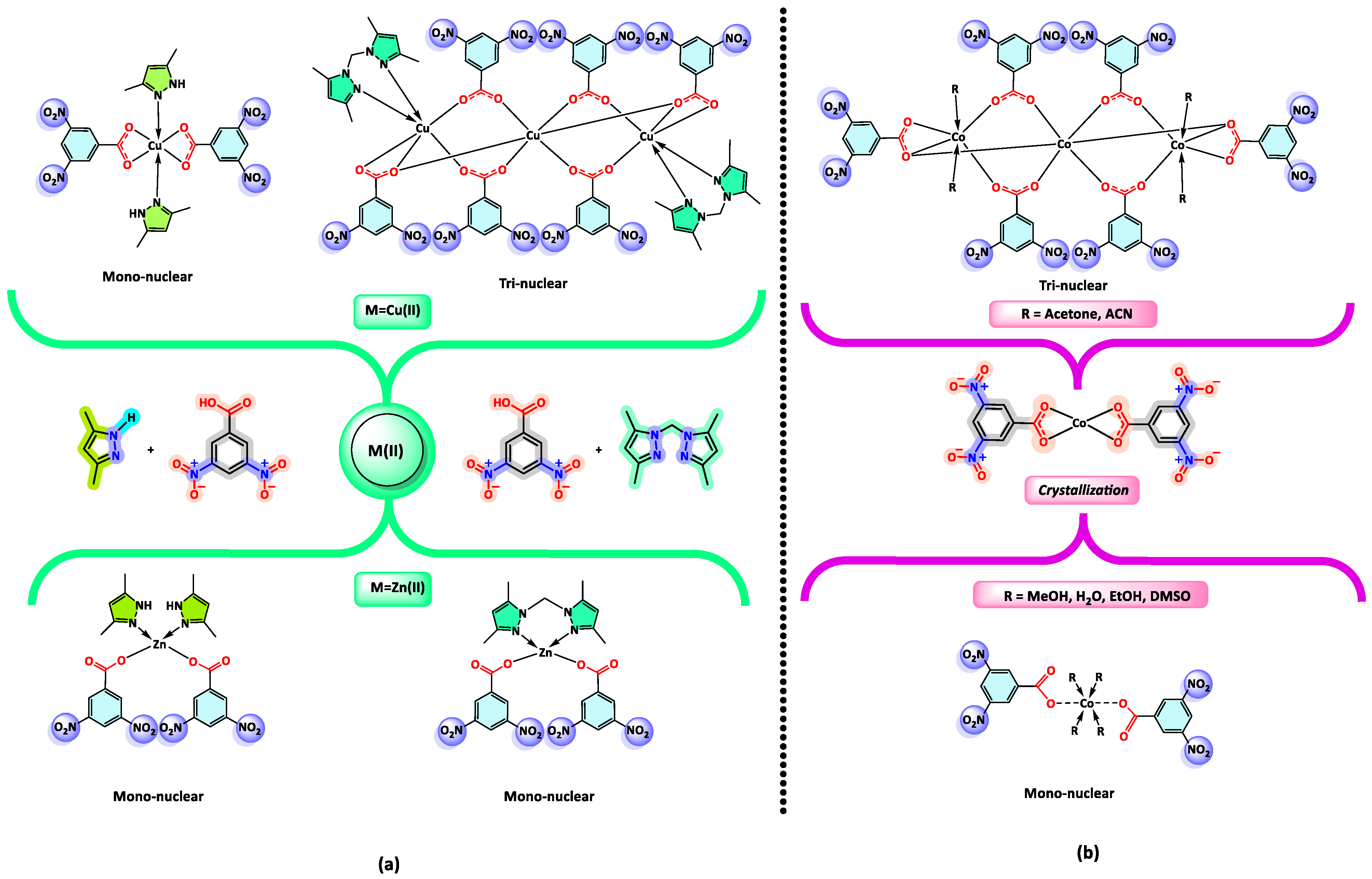
4. Coordination Behavior of the 3,5-DNB Ligand and Crystallization Methods of the Complexes: A Perspective from the Crystal Structures in the CCDC Database
5. Theoretical Study of 3,5- and 2,4-Dinitrobenzoate Metal Complexes
6. Conclusions
7. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raman, N.; Sakthivel, A.; Rajasekaran, K. Synthesis and Spectral Characterization of Antifungal Sensitive Schiff Base Transition Metal Complexes. Mycobiology 2007, 35, 150–153. [Google Scholar] [CrossRef]
- Fonseca, D.; Pérez-Torres, A.F.; Cobo, J.; Zapata-Rivera, J.; Hurtado, J.J.; Macías, M.A. Influence of the Lewis Basicity Hardness of Crystallization Solvents on the Coordination Sphere of the Complex [Co(3,5-Dinitrobenzoate-O,O′)2]: Crystallographic and Theoretical Analysis. CrystEngComm 2022, 24, 2982–2991. [Google Scholar] [CrossRef]
- Kani, I.; Atlier, Ö.; Güven, K. Mn(II) Complexes with Bipyridine, Phenanthroline and Benzoic Acid: Biological and Catalase-like Activity. J. Chem. Sci. 2016, 128, 523–536. [Google Scholar] [CrossRef]
- Jin, Y.; Che, Y.X.; Zheng, J.M. Unusual Supramolecularly Assembled Hexadecameric Water Cluster in Complex of Co(II). Inorganica Chim. Acta 2008, 361, 2799–2802. [Google Scholar] [CrossRef]
- Torres, J.F.; Macías, M.A.; Franco-Ulloa, S.; Miscione, G.P.; Cobo, J.; Hurtado, J.J. Cu(II) and Zn(II) Complexes with Dinitrobenzoates and Pyrazolyl Ligands: Structural and Thermal Stability Influence of N–H Moiety. Cryst. Growth Des. 2019, 19, 3348–3357. [Google Scholar] [CrossRef]
- Jassal, A.K.; Sharma, S.; Hundal, G.; Hundal, M.S. Structural Diversity, Thermal Studies, and Luminescent Properties of Metal Complexes of Dinitrobenzoates: A Single Crystal to Single Crystal Transformation from Dimeric to Polymeric Complex of Copper(II). Cryst. Growth Des. 2015, 15, 79–93. [Google Scholar] [CrossRef]
- Wang, X.-L.; Le, M.; Lin, H.-Y.; Luan, J.; Liu, G.-C.; Shao, J.-Y. Syntheses, Structures and Properties of Five Cu(II) Coordination Polymers Derived from Semi-Rigid Bis-Pyridyl-Bis-Amide and Nitro-Substituted Aromatic Carboxylates. Polyhedron 2015, 101, 290–298. [Google Scholar] [CrossRef]
- Yang, Q.; Ge, J.; Gong, Q.; Song, X.; Zhao, J.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Two Energetic Complexes Incorporating 3,5-Dinitrobenzoic Acid and Azole Ligands: Microwave-Assisted Synthesis, Favorable Detonation Properties, Insensitivity and Effects on the Thermal Decomposition of RDX. New J. Chem. 2016, 40, 7779–7786. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y.; Zhang, Y.; Liu, L.; Zhang, S. Synthesis, Crystal Structure, and Properties of Energetic Copper(II) Complex Based on 3,5-Dinitrobenzoic Acid and 1,5-Diaminotetrazole. Z. Anorg. Allg. Chem. 2017, 643, 647–652. [Google Scholar] [CrossRef]
- Yang, G.; Weng Ng, S. Polymeric Metal-Organic Octupolar NLO Materials: Lithium(I) and Sodium(I) 3,5-Dinitrobenzoates. Cryst. Res. Technol. 2007, 42, 201–206. [Google Scholar] [CrossRef]
- Jones, H.P.; Gillon, A.L.; Davey, R.J. Sodium 3,5-Di nitro benzoate. Acta Cryst. E 2005, 61, m1131–m1132. [Google Scholar] [CrossRef]
- Smith, G. Poly[Μ3-Aqua-Aqua(Μ3-3,5-Dinitro benzoato-κO1:O3:O5)Caesium]. Acta Cryst. E 2012, 68, m1241–m1242. [Google Scholar] [CrossRef]
- Askarinejad, A.; Morsali, A. Thermal and Structural Studies of Two New TlI Three-Dimensional Coordination Polymers, [Tl2(μ-CSB)]n and [Tl2(μ-ADC)]n, CSB2− = 4-[(4-Carboxyphenyl)Sulfonyl]-1-Benzenecarboxylate and ADC2− = acetylendicarboxylate. J. Coord. Chem. 2007, 60, 1903–1912. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, X.; Dai, T.; Wang, L.; Shi, H.; Wang, H.; Chen, Z. An AIE Photosensitizer with Unquenched Fluorescence Based on Nitrobenzoic Acid for Tumor-Targeting and Image-Guided Photodynamic Therapy. Biomater. Sci. 2022, 10, 4866–4875. [Google Scholar] [CrossRef] [PubMed]
- Hadi, Z.; Ghanbari, K. A Novel Electrochemical Sensor for Determination of Uric Acid in the Presence of Ascorbic Acid and Dopamine Based on a Carbon Paste Electrode Modified with an Electrochemically Reduced Para -Nitrobenzoic Acid/Graphene Oxide Nanocomposite. New J. Chem. 2022, 46, 12941–12951. [Google Scholar] [CrossRef]
- Li, H.; Mirzayans, P.M.; Butler, M.S.; Lacey, A.E.; Vuong, D.; Chen, R.; Kalaitzis, J.A.; Moggach, S.A.; Lacey, E.; Piggott, A.M.; et al. Discovery of Brevijanazines from Aspergillus brevijanus Reveals the Molecular Basis for p-Nitrobenzoic Acid in Fungi. Chem. Commun. 2022, 58, 6296–6299. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, M.; Sinha, L.; Prasad, O.; Asiri, A.M.; Cinar, M.; Shukla, V.K. FT-IR, FT-Raman, NMR, UV and Quantum Chemical Studies on Monomeric and Dimeric Conformations of 3,5-Dimethyl-4-Methoxybenzoic Acid. Spectrochim. Acta A 2014, 123, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Błaziak, K.; Danikiewicz, W.; Mąkosza, M. How Does Nucleophilic Aromatic Substitution Really Proceed in Nitroarenes? Computational Prediction and Experimental Verification. J. Am. Chem. Soc. 2016, 138, 7276–7281. [Google Scholar] [CrossRef] [PubMed]
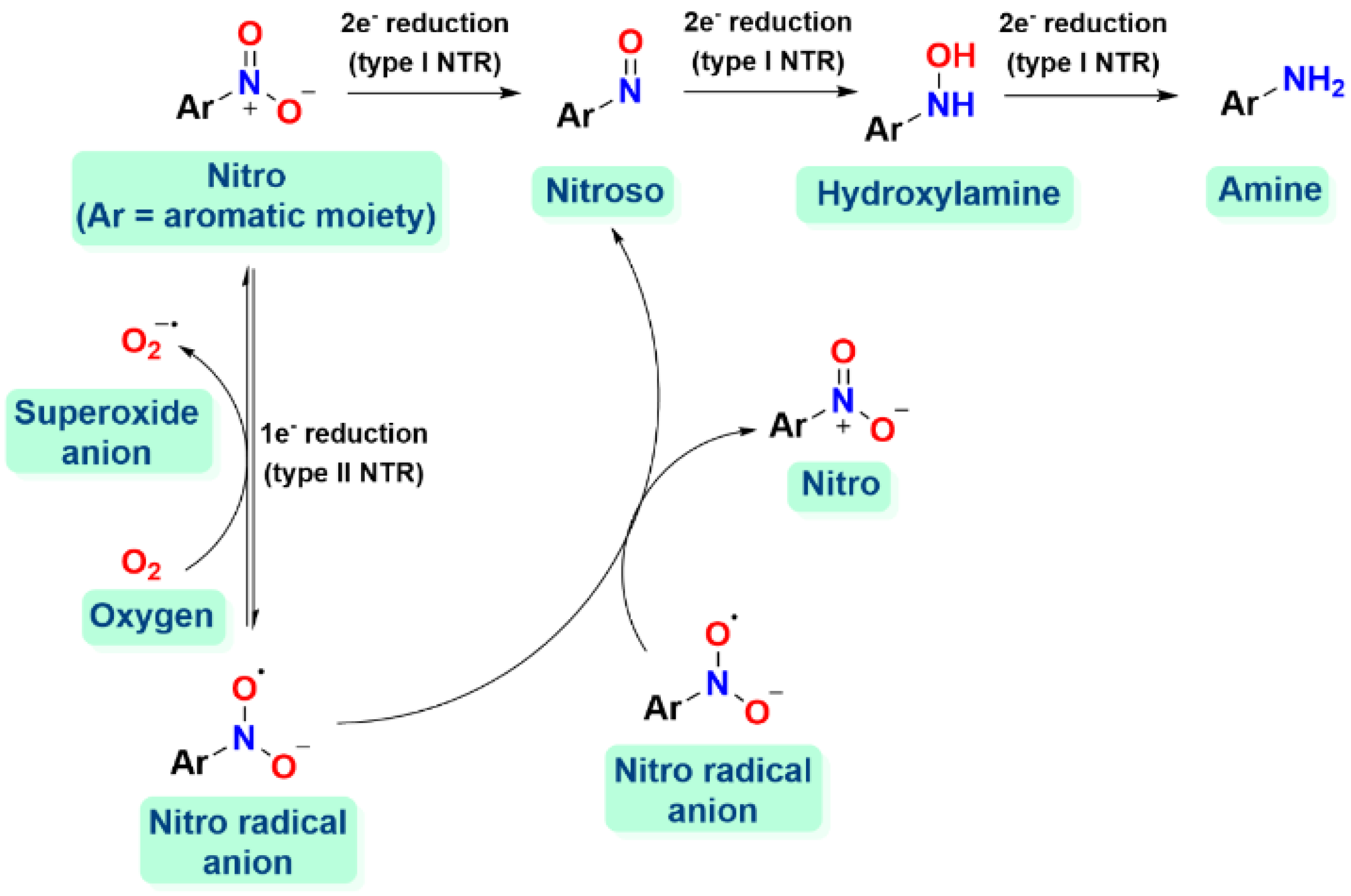
- Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines. Org. Process Res. Dev. 2018, 22, 430–445. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Mąkosza, M. Reactions of Nucleophiles with Nitroarenes: Multifacial and Versatile Electrophiles. Chem. Eur. J. 2014, 20, 5536–5545. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Yuan, Y.; Jiao, N.; Liu, L. Nitrogen-Rich Ligands Directed Transition Metal (Co/Ni/Zn) 3,5-Dinitrobenzoic Acid Energetic Complexes: Syntheses, Crystal Structures and Properties. ChemistrySelect 2018, 3, 10298–10304. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef]
- Maya, J.D.; Cassels, B.K.; Iturriaga-Vásquez, P.; Ferreira, J.; Faúndez, M.; Galanti, N.; Ferreira, A.; Morello, A. Mode of Action of Natural and Synthetic Drugs against Trypanosoma Cruzi and Their Interaction with the Mammalian Host. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 601–620. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Bot, C.; Kelly, J.M.; Hall, B.S. Trypanocidal Activity of Nitroaromatic Prodrugs: Current Treatments and Future Perspectives. Curr. Top. Med. Chem. 2011, 11, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Wyllie, S. Nitro Drugs for the Treatment of Trypanosomatid Diseases: Past, Present, and Future Prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bello-Vieda, N.J.; Pastrana, H.F.; Garavito, M.F.; Ávila, A.G.; Celis, A.M.; Muñoz-Castro, A.; Restrepo, S.; Hurtado, J.J. Antibacterial Activities of Azole Complexes Combined with Silver Nanoparticles. Molecules 2018, 23, 361. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Ward, M. Antimicrobial Resistance in Humans, Livestock and the Wider Environment|Philosophical Transactions of the Royal Society B: Biological Sciences. Phil. Trans. R. Soc. B 2014, 1670, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.F.; Bello-Vieda, N.J.; Nuñez-Dallos, N.G.; Pastrana, H.F.; Celis, A.M.; Restrepo, S.; Hurtado, J.J.; Ávila, A.J. Metal Complex Derivatives of Azole: A Study on Their Synthesis, Characterization, and Antibacterial and Antifungal Activities. J. Braz. Chem. Soc. 2016, 27, 2334–2347. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Psomas, G.; Raptopoulou, C.P.; Kessissoglou, D.P. Copper(II) Complexes with Phenoxyalkanoic Acids and Nitrogen Donor Heterocyclic Ligands: Structure and Bioactivity. J. Inorg. Biochem. 2001, 83, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Sadeek, S.A.; Zordok, W.A.; El-Shwiniy, W.H. Synthesis, Characterization, DFT Modeling and Antimicrobial Studies on the Ti(IV), Y(III) and Ce(IV) Ofloxacin Solid Complexes. J. Korean Chem. Soc. 2013, 57, 574–590. [Google Scholar] [CrossRef]
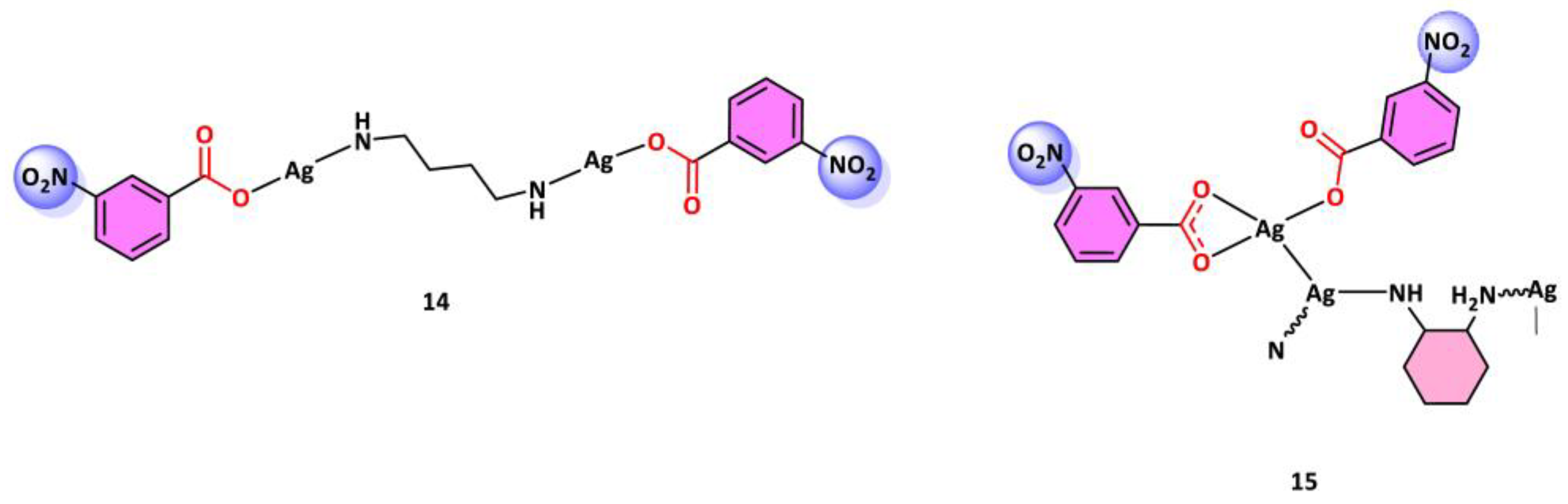
- Han, Y.J.; Wang, L.; Li, Q.B.; Xue, L.W. Synthesis, Crystal Structure, and Antimicrobial Activity of Silver(I) Complexes Derived from 3-Nitrobenzoic Acid and Diamines. Russ J. Coord. Chem. 2017, 43, 457–462. [Google Scholar] [CrossRef]
- Murcia, R.; Leal, S.; Roa, M.; Nagles, E.; Muñoz-Castro, A.; Hurtado, J. Development of Antibacterial and Antifungal Triazole Chromium(III) and Cobalt(II) Complexes: Synthesis and Biological Activity Evaluations. Molecules 2018, 23, 2013. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Mayer, F.L.; Wilson, D.; Hube, B.; Mayer, F.L.; Wilson, D.; Hube, B. Candida Albicans Pathogenicity Mechanisms. Virulence 2013, 15, 119–128. [Google Scholar] [CrossRef] [PubMed]
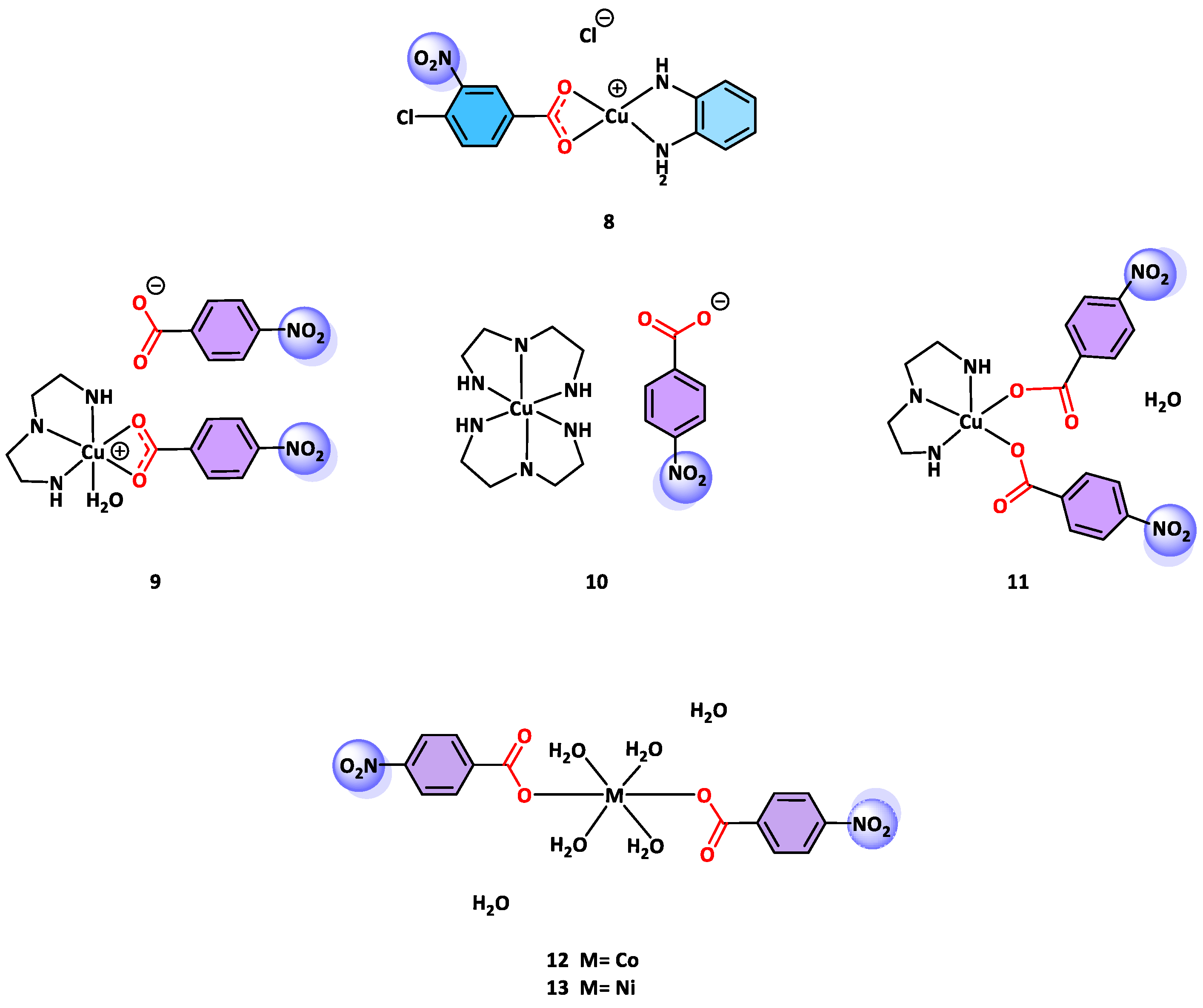
- Kabbani, A.T.; Hammud, H.H.; Ghannoum, A.M. Preparation and Antibacterial Activity of Copper and Cobalt Complexes of 4-Chloro-3-Nitrobenzoate with a Nitrogen Donor Ligand. Chem. Pharm. Bull. 2007, 55, 446–450. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Jannakoudakis, P.D.; Kessissoglou, D.P.; Manoussakis, G.E.; Mentzafos, D.; Terzis, A. Copper(II) Complexes with Anti-Inflammatory Drugs as Ligands. Solution Behaviour and Electrochemistry of Mono- and Bi-Nuclear Complexes. J. Chem. Soc. Dalton Trans. 1992, 22, 3259. [Google Scholar] [CrossRef]
- Kumar, S.; Pal Sharma, R.; Venugopalan, P.; Singh Gondil, V.; Chhibber, S.; Aree, T.; Witwicki, M.; Ferretti, V. Hybrid Inorganic-Organic Complexes: Synthesis, Spectroscopic Characterization, Single Crystal X-ray Structure Determination and Antimicrobial Activities of Three Copper(II)-Diethylenetriamine-p-Nitrobenzoate Complexes. Inorganica Chim. Acta 2018, 469, 288–297. [Google Scholar] [CrossRef]
- Roy, S.M.; Sudarsanakumar, M.R.; Dhanya, V.S.; Suma, S.; Kurup, M.R.P. Crystal Growth, Spectral, Magnetic, Antibacterial and Antifungal Studies of Co(II) and Ni(II) Complexes of 4-Nitrobenzoic Acid. J. Korean Chem. Soc. 2014, 58, 258–266. [Google Scholar] [CrossRef]
- Ibragimov, A.B.; Ashurov, J.M.; Ibragimov, B.T.; Zakirov, B.S. Preparation, Structures and Antimicrobial Activity of Four Different Type Metal Complexes on the Base of Diethanolamine and o-Nitrobenzoic Acid. J. Mol. Struct. 2017, 1128, 307–316. [Google Scholar] [CrossRef]
- Ibragimov, A.B.; Ashurov, Z.M.; Ibragimov, A.B.; Tashpulatov, Z.Z. Synthesis, Structure, and Fungicidal Activity of Mono-and Binuclear Mixed-Ligand Copper Complex with p-Nitrobenzoic Acid and Monoethanolamine. Russ. J. Coord. Chem. 2017, 43, 1070–3284. [Google Scholar] [CrossRef]
- Ibragimov, A.B.; Ashurov, J.M.; Ibragimov, A.B.; Eshimbetov, A.G. Synthesis of the Three Mixed-Ligand Metal Complexes and One Organic Salt of 3,5-Dinitrobenzoic Acid for Biopharmaceutical Optimization through Monoethanolamine: Structures and DFT Studies of Complexes. J. Chem. Crystallogr. 2021, 51, 405–417. [Google Scholar] [CrossRef]
- Lee, C.K.; Park, K.K.; Lim, S.S.; Park, J.H.Y.; Chung, W.Y. Effects of the Licorice Extract against Tumor Growth and Cisplatin-Induced Toxicity in a Mouse Xenograft Model of Colon Cancer. Biol. Pharm. Bull. 2007, 30, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Ghosh, M.K.; Mandal, S.; Rane, V.; Kadam, R.; Chatterjee, A.; Bhattacharyya, A.; Chattopadhyay, S. The First Examples of Multiply Bonded Dirhenium(iii, ii) Paramagnetic Complexes Containing Nitrobenzoate Ligands: Spectroscopic, Structural, Cytotoxicity and Computational Studies. Dalton Trans. 2017, 46, 5670–5679. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.-J.; Meng, B.; Su, J.-Q.; Peng, T.-T.; Qi, Z.-Z.; Jia, B.; Feng, Y.-H.; Zhu, M.-C. Structure, DNA Bonding, and Biological Activity of a Novel Pb(II) Complex of 1,1-Bis(5-(Pyrazin-2-Yl)-1,2,4-Triazol-3-Yl) Methane. J. Struct. Chem. 2017, 58, 1560–1566. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Yadav, H.R.; Roy, A.; Duraipandian, N.; Kiran, M.S.; Ghosh, R. Synthesis and Crystal Structures of Pyridine-2-Carboxaldehyde Thiosemicarbazone, Its Mononuclear and Cytotoxic Cu(II) and Polynuclear Pb(II) Complexes: Effect of Size of Metal Ion on Nucleation of the Complexes. Indian J. Chem. 2017, 56, 616–620. [Google Scholar]
- El-Megharbel, S.M.; Hamza, R.Z.; Refat, M.S. Preparation, Spectroscopic, Thermal, Antihepatotoxicity, Hematological Parameters and Liver Antioxidant Capacity Characterizations of Cd(II), Hg(II), and Pb(II) Mononuclear Complexes of Paracetamol Anti-Inflammatory Drug. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 131, 534–544. [Google Scholar] [CrossRef]
- Lv, L.-L.; Xia, W.-M.; Cheng, Y.-Z.; Zhang, L.-P.; Wang, X.-D. Synthesis, Structure, DNA Binding and Anticancer Activity of a New Tetranuclear Pb(II) Complex Constructed by 8-Hydroxyquinolinate and 4-Nitrobenzoate Ligands. Main Group Met. Chem. 2019, 42, 60–66. [Google Scholar] [CrossRef]
- Zeng, Z.-F.; Huang, Q.-P.; Cai, J.-H.; Zheng, G.-J.; Huang, Q.-C.; Liu, Z.-L.; Chen, Z.-L.; Wei, Y.-H. Synthesis, Characterization, DNA/HSA Interactions, and Anticancer Activity of Two Novel Copper(II) Complexes with 4-Chloro-3-Nitrobenzoic Acid Ligand. Molecules 2021, 26, 4028. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Taiwaikuli, M.; Mei, H.; Wang, T.; Huang, X. Solubility Measurement, Thermodynamic Correlation, and Comprehensive Analysis of 3,5-Dinitrobenzoic Acid in 13 Pure Solvents. J. Chem. Eng. Data 2022, 67, 2833–2844. [Google Scholar] [CrossRef]
- Kuznetsova, O.V.; Egorochkin, A.N.; Khamaletdinova, N.M.; Domratcheva-Lvova, L.G. Bond Lengths in Organometallic and Coordination Complexes: Substituent Effects. J. Organomet. Chem. 2013, 745–746, 34–41. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Su, Z.; Chen, S.; Xie, G.; Wei, Q.; Gao, S. 3D High-Energy-Density and Low Sensitivity Materials: Synthesis, Structure and Physicochemical Properties of an Azide–Cu(II) Complex with 3,5-Dinitrobenzoic Acid. RSC Adv. 2014, 4, 16087–16093. [Google Scholar] [CrossRef]
- Jassal, A.K.; Sran, B.S.; Suffren, Y.; Bernot, K.; Pointillart, F.; Cador, O.; Hundal, G. Structural Diversity and Photo-Physical and Magnetic Properties of Dimeric to 1D Polymeric Coordination Polymers of Lighter Lanthanide(III) Dinitrobenzoates. Dalton Trans. 2018, 47, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Koroteev, P.S.; Ilyukhin, A.B.; Babeshkin, K.A.; Efimov, N.N. Charge Transfer Complexes of Lanthanide 3,5-Dinitrobenzoates and 1,2-Phenylenediamine. J. Mol. Struct. 2020, 1207, 127800. [Google Scholar] [CrossRef]
- Hazra, D.K.; Sen, R.; Koner, S.; Helliwell, M.; Mukherjee, M. Self-Assembly of 1D Coordination Polymers of Two Rare-Earth Complexes with Carboxylate Linkages: Synthesis, Crystal Structure and DFT Studies. Polyhedron 2011, 30, 2195–2202. [Google Scholar] [CrossRef]
- Torres, J.F.; Bello-Vieda, N.J.; Macías, M.A.; Rabelo, R.; Lloret, F.; Muñoz-Castro, A.; Hurtado, J.J. Intermolecular Interaction Energies and Magnetic Properties of Spin-Isolated Multinuclear CuII Complexes. Acta Crystallogr. B 2020, 76, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.-X.; Chen, P.-K.; Luo, F.; Xue, L.; Che, Y.; Zheng, J.-M. Three Ni/Co(II) Complexes with 4,4′-Bipyridine and Benzoic Acid Derivatives as Mixed Coligands: Hydrothermal Syntheses, Structures, and Magnetisms. Inorganica Chim. Acta 2007, 360, 4077–4084. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, W.; Zhang, J.; Lu, Q.; Luan, J.; Liu, G.; Lin, H.; Tian, A. Three Isomeric Copper(II) Coordination Polymers Based on a Bis-Triazole-Bis-Amide Ligand: Assembly, Structures, and Luminescent Properties. J. Coord. Chem. 2013, 66, 3561–3571. [Google Scholar] [CrossRef]
- Lin, H.; Le, M.; Liu, D.; Liu, G.; Wang, X.; Duan, S. Four Cu(II)/Co(II) Coordination Polymers Based on N,N′-Di(3-Pyridyl) Sebacicdiamide: Influence of Different Carboxylate Ancillary Ligands on Structures and Properties. J. Coord. Chem. 2016, 69, 934–946. [Google Scholar] [CrossRef]
- Wang, X.-L.; Yang, S.; Liu, G.-C.; Hou, L.; Lin, H.-Y.; Tian, A.-X. Cobalt(II) Coordination Polymers Based on 3,5-Dinitrobenzoate and Flexible Bis(Benzimidazole) Derivatives Bearing Different Spacer Lengths and Substituents. Transit. Met. Chem. 2011, 36, 891–896. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Jiang, Y.-Y.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Syntheses, Structures and Photoluminescence of Zinc(II) and Silver(I) Coordination Polymers Based on 1,1′-(1,4-Butanediyl)Bis(2-Methylbenzimidazole) and Different Carboxylate Ligands. CrystEngComm 2011, 13, 6118–6129. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhao, W.; Zhang, J.-W.; Lu, Q.-L. Three Tetranuclear Copper(II) Cluster-Based Complexes Constructed from 4-Amino-1,2,4-Triazole and Different Aromatic Carboxylates: Assembly, Structures, Electrochemical and Magnetic Properties. J. Solid State Chem. 2013, 198, 162–168. [Google Scholar] [CrossRef]
- Kamath, A.; Pilet, G.; Tamang, A.; Sinha, B. [{Diaquo(3,5-Dinitrobenzoato-κ1O1)(1,10-Phenanthroline-κ2N1:N10)}copper(II)] 3,5-Dinitrobenzoate: Hydrothermal Synthesis, Crystal Structure and Magnetic Properties. J. Mol. Struct. 2020, 1199, 126933. [Google Scholar] [CrossRef]
- Wang, X.-L.; Hou, L.-L.; Zhang, J.-W.; Liu, G.-C.; Lin, H.-Y. Cobalt(II) Coordination Polymers Based on a Semi-Rigid Bis(Benzimidazole) Ligand: From Linear Chain to 3D 3-Fold Interpenetrated Diamond Framework Tuned by the Auxiliary Carboxylates. Polyhedron 2013, 61, 65–72. [Google Scholar] [CrossRef]
- Wang, X.-L.; Hou, L.-L.; Zhang, J.-W.; Gong, C.-H.; Liu, G.-C. Bis(Benzimidazole)-Based Ligands-Directed the Various Dimensionality of Metal–Organic Complexes Based on Carboxylates Co-Ligands: Syntheses, Structures and Properties. Inorganica Chim. Acta 2013, 405, 58–64. [Google Scholar] [CrossRef]
- Guo, H.-X.; Chen, J.-X.; Wang, Q.-H.; You, X.-L. Catena-Poly[[(2,2′-Bipyridyl-κ2N,N′)Manganese(II)]-Di-μ-3,5-Dinitro benzoato-κ4O:O′]. Acta Cryst. E 2007, 63, m915–m917. [Google Scholar] [CrossRef]
- Liu, L.-L.; Huang, J.-J.; Wang, X.-L.; Liu, G.-C.; Yang, S.; Lin, H.-Y. Ligand-Controlled Assembly of Cobalt(II) Metal–Organic Complexes from Different Semirigid Bis(Imidazole) Derivatives and Aromatic Monocarboxylates: Electrochemical Behaviors and Fluorescent Properties. Inorganica Chim. Acta 2013, 394, 715–722. [Google Scholar] [CrossRef]
- Lu, Q.-L.; Zhao, W.; Zhang, J.-W.; Wang, X.-L.; Luan, J. Two pH-Dependent 1D Copper(II) Coordination Polymers Based on a Bis-Triazole-Bis-Amide Ligand: Syntheses, Structures, and Properties. Z. Anorg. Allg. Chem. 2013, 639, 587–591. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, H.-G.; Zhang, L.-Z.; Cai, Z.-G.; Chen, X.-M. Synthesis, Structure and Second Harmonic Effect of Bis(Mu2-3,5-Dinitrobenzoato-O,O′)Zinc(II) and Tetraaquabis(3,5-Dinitrobenzoato-O)Cobalt(II) Tetrahydrate. Aust. J. Chem. 2000, 53, 601–605. [Google Scholar] [CrossRef]
- Fonseca, D.; Leal-Pinto, S.M.; Roa-Cordero, M.V.; Vargas, J.D.; Moreno-Moreno, E.M.; Macías, M.A.; Suescun, L.; Muñoz-Castro, Á.; Hurtado, J.J. Inhibition of C. albicans Dimorphic Switch by Cobalt(II) Complexes with Ligands Derived from Pyrazoles and Dinitrobenzoate: Synthesis, Characterization and Biological Activity. Int. J. Mol. Sci. 2019, 20, 3237. [Google Scholar] [CrossRef]
- Zhang, C.-X.; Zhang, Y.-Y.; Sun, Y.-Q. Synthesis, Structures and Magnetic Properties of Copper(II) and Cobalt(II) Complexes Containing Pyridyl-Substituted Nitronyl Nitroxide and 3,5-Dinitrobenzoate. Polyhedron 2010, 29, 1387–1392. [Google Scholar] [CrossRef]
- Wu, S.; Lin, G.; Ge, Y.; Yang, Z.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.; Gao, S.; Lu, J.Y. A New Cu(II)-Based Energetic Complex Constructed Using Mixed Building Blocks: Synthesis, Structure and Standard Molar Enthalpy of Formation. Polyhedron 2019, 157, 18–24. [Google Scholar] [CrossRef]
- Vasková, Z.; Padělková, Z.; Mazur, M.; Valigura, D.; Moncol, J. Synthesis, Properties and Crystal Structures of Nitrobenzoatocopper(II) Complexes with Pyrazinecarboxamide. Transit. Met. Chem. 2011, 36, 883–889. [Google Scholar] [CrossRef]
- Vasková, Z.; Moncol, J.; Korabik, M.; Medvecká, J.; Švorec, J.; Padělková, Z.; Valko, M.; Valigura, D. 2D-Supramolecular Layers Built of Antiferromagnetically Coupled [Cu(x-O2Nbz)2(Denia)1(H2O)1]n 1D-Polymers with R22(10) and R22(12) Supramolecular Synthons. Polyhedron 2011, 30, 86–92. [Google Scholar] [CrossRef]
- Lv, L.; Zhong, Y.; Kong, L.; Huang, H.; Zhang, T.; Li, Z. Syntheses, Crystal Structures, and Thermal Properties of Energetic Semicarbazide Salt, Manganese(II) Salt, and Coordination Compound of 3,5-Dinitrobenzoic Acid. Z. Anorg. Allg. Chem. 2019, 645, 975–980. [Google Scholar] [CrossRef]
- Lephoto, M.L.; Nakano, K.; Appavoo, D.; Owaga, B.O.; Nozaki, K.; Darkwa, J. Pyrazole Supported Zinc(II) Benzoates as Catalysts for the Ring Opening Copolymerization of Cyclohexene Oxide and Carbon Dioxide. Catalysts 2016, 6, 17. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Shen, X.-Q.; Wan, X.-S.; Zhang, H.-Y.; Wang, H.; Yang, R.; Niu, C.-Y. Syntheses and Crystal Structures of Two Zn(II) Complexes, [Zn(Fura)2(2,2′-Bipy)(H2O)] and [Zn(μ-Dnbc)2] (Fura=Furoic Acid, 2,2′-Bpy=2,2′-Bipyridine, Dnba=3,5-Dinitrobenzoic Acid). J. Coord. Chem. 2007, 60, 1317–1325. [Google Scholar] [CrossRef]
- Roy, S.; Bauza, A.; Frontera, A.; Schaper, F.; Banik, R.; Purkayastha, A.; Reddy, B.M.; Sridhar, B.; Drew, M.G.B.; Das, S.K.; et al. Structural Diversity and Non-Covalent Interactions in Cd(II) and Zn(II) Complexes Derived from 3,5-Dinitrobenzoic Acid and Pyridine: Experimental and Theoretical Aspects. Inorganica Chim. Acta 2016, 440, 38–47. [Google Scholar] [CrossRef]
- Roy, S.; Oyarzabal, I.; Vallejo, J.; Cano, J.; Colacio, E.; Bauza, A.; Frontera, A.; Kirillov, A.M.; Drew, M.G.B.; Das, S. Two Polymorphic Forms of a Six-Coordinate Mononuclear Cobalt(II) Complex with Easy-Plane Anisotropy: Structural Features, Theoretical Calculations, and Field-Induced Slow Relaxation of the Magnetization. Inorg. Chem. 2016, 55, 8502–8513. [Google Scholar] [CrossRef]
- Pedireddi, V.R.; Varughese, S. Solvent-Dependent Coordination Polymers: Cobalt Complexes of 3,5-Dinitrobenzoic Acid and 3,5-Dinitro-4-Methylbenzoic Acid with 4,4′-Bipyrdine. Inorg. Chem. 2004, 43, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Fonari, A.; Leonova, E.S.; Antipin, M.Y. On Justification of Cu(II) Environment in Mononuclear Complexes: Joint X-Ray and AIM Studies. Polyhedron 2011, 30, 1710–1717. [Google Scholar] [CrossRef]
- Torres, J.F.; Bello-Vieda, N.J.; Macías, M.A.; Muñoz-Castro, A.; Rojas-Dotti, C.; Martínez-Lillo, J.; Hurtado, J. Water Dissociation of a Dinuclear Bis(3,5-Dimethylpyrazolyl)Methane Copper(II) Complex: X-ray Diffraction Structure, Magnetic Properties, and Characteristic Absorption of the (CuN2Cl2)2 Core. Eur. J. Inorg. Chem. 2018, 2018, 3644–3651. [Google Scholar] [CrossRef]
- Wei, W.; Xie, R.-K.; Du, S.-W.; Tian, C.-B.; Chai, G.-L. Synthesis, Structure, Magnetocaloric Effect and DFT Calculations of a MnII Cluster-Based Inorganic Coordination Polymer. J. Alloys Compd. 2021, 878, 160353. [Google Scholar] [CrossRef]
- Nuñez-Dallos, N.; Lopez-Barbosa, N.; Muñoz-Castro, A.; Mac-Leod Carey, D.; De Nisi, A.; Monari, M.; Osma, J.F.; Hurtado, J. A New Copper(I) Coordination Polymer from 2,6-Bis(1H-Benzotriazol-1-Ylmethyl)Pyridine: Synthesis, Characterization, and Use as Additive in Transparent Submicron UV Filters. J. Coord. Chem. 2017, 70, 3363–3378. [Google Scholar] [CrossRef]
- Kariem, M.; Yawer, M.; Kumar, M.; Sheikh, H.N.; Sood, P.; Frontera, A.; Ortega-Castro, J. Supramolecular Coordination Polymers of La(III), Ce(III), Sm(III), Gd(III) and Eu(III) Decorated with Rigid 5-Hydroxy-1,3-Benzenedicarboxylate and Flexible Hexane-1,6-Dicarboxylate Linkers: Syntheses, Structures, DFT Study, Luminescence and Magnetic Properties. Polyhedron 2017, 134, 153–165. [Google Scholar] [CrossRef]
- Hurtado, J.; Ibarra, L.; Yepes, D.; García-Huertas, P.; Macías, M.A.; Triana-Chavez, O.; Nagles, E.; Suescun, L.; Muñoz-Castro, A. Synthesis, Crystal Structure, Catalytic and Anti-Trypanosoma Cruzi Activity of a New Chromium(III) Complex Containing Bis(3,5-Dimethylpyrazol-1-Yl)Methane. J. Mol. Struct. 2017, 1146, 365–372. [Google Scholar] [CrossRef]
- Hurtado, J.; Ugarte, J.; Rojas, R.; Valderrama, M.; Mac-Leod Carey, D.; Muñoz-Castro, A.; Arratia-Pérez, R.; Fröhlich, R. New Bis(Azolylcarbonyl)Pyridine Chromium(III) Complexes as Initiators for Ethylene Polymerization. Inorganica Chim. Acta 2011, 378, 218–223. [Google Scholar] [CrossRef]
- Nuñez-Dallos, N.; Muñoz-Castro, A.; Fuentealba, M.; Pérez, E.G.; Hurtado, J.J. Facile Synthesis of a Luminescent Copper(I) Coordination Polymer Containing a Flexible Benzotriazole-Based Ligand: An Effective Catalyst for Three-Component Azide-Alkyne Cycloaddition. Inorganica Chim. Acta 2019, 498, 119136. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Liu, X.; Qu, X.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. High-Energy Metal–Organic Frameworks (HE-MOFs): Synthesis, Structure and Energetic Performance. Coord. Chem. Rev. 2016, 307, 292–312. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Jayamani, N.; Mathammal, R.; Parasuraman, K. Density Functional Theory Calculations and Vibrational Spectra of 2-Bromo-4-Chloro Phenol and 2-Chloro-4-Nitro Phenol. J. Raman Spectrosc. 2009, 40, 1551–1556. [Google Scholar] [CrossRef]
- Jones, C.L.; Skelton, J.M.; Parker, S.C.; Raithby, P.R.; Walsh, A.; Wilson, C.C.; Thomas, L.H. Living in the Salt-Cocrystal Continuum: Indecisive Organic Complexes with Thermochromic Behaviour. CrystEngComm 2019, 21, 1626–1634. [Google Scholar] [CrossRef]
- Mudsainiyan, R.K.; Pandey, S.K. A Combined Theoretical Calculation and Hirshfeld Surface Analysis of Cooperative Non-Covalent Interactions in the Crystal Packing in [Cu(L1)2(EDA)]. Z. Anorg. Allg. Chem. 2017, 643, 1245–1252. [Google Scholar] [CrossRef]
- Amalanathan, M.; Rastogi, V.K.; Hubert Joe, I.; Palafox, M.A.; Tomar, R. Density Functional Theory Calculations and Vibrational Spectral Analysis of 3,5-(Dinitrobenzoic Acid). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Shmelev, M.A.; Chistyakov, A.S.; Razgonyaeva, G.A.; Kovalev, V.V.; Voronina, J.K.; Dolgushin, F.M.; Gogoleva, N.V.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Effect of Non-Covalent Interactions on the 2,4- and 3,5-Dinitrobenzoate Eu-Cd Complex Structures. Crystals 2022, 12, 508. [Google Scholar] [CrossRef]
- Bello-Vieda, N.J.; Murcia, R.A.; Munõz-Castro, A.; MacIás, M.A.; Hurtado, J.J. Coordination Polymers Containing 1,3-Phenylenebis-((1H-1,2,4-Triazol-1-Yl)Methanone) Ligand: Synthesis and ″-Caprolactone Polymerization Behavior. Molecules 2017, 22, 1860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.-F.; Tian, X.-M.; Ji, J.; Luo, H.; Yao, S.-L.; Liu, S.-J.; Tang, B.-E.; Zhao, Y.-J.; Mao, J.; Zhao, Q.; et al. Two Gd2 Cluster Complexes with Monocarboxylate Ligands Displaying Significant Magnetic Entropy Changes. J. Mol. Struct. 2020, 1200, 127094. [Google Scholar] [CrossRef]
- Dong, C.-H.; Zhang, D.-H.; Ren, N.; Xi, G.-Q.; Hao, J.-J. Bis(μ-3,5-Dinitro benzoato-κ2O1:O1′)bis (μ-3,5-Dinitro benzoato)-κ3O1,O1′:O1;κ3O1:O1,O1′-bis [(3,5-Dinitro benzoato-κ2O1,O1′)(1,10-Phenanthroline-κ2N,N)Dysprosium(III)]. Acta Cryst E 2011, 67, m102. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Xiao, H.-P.; Wang, S.; Li, X.-H. Crystal structure of di(μ-3,5-dinitrobenzoato-O,O′)di(μ-3,5-dinitrobenzoato-O,O:O′)di(3,5-dinitrobenzoato-O,O′)di(1,10-phenanthroline-N,N′)-diterbium(III), Tb2(C12H8N2)2(C7H3N2O6)6. Z. Kristallogr. NCS 2003, 218, 491–493. [Google Scholar] [CrossRef]
- Chen, Z.F. Syntheses and Crystal Structures of Two Samarium(III) Complexes with Bio-Relevant Ligands. J. Coord. Chem. 2007, 60, 1317–1325. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Ilyukhin, A.B.; Efimov, N.N.; Minin, V.V.; Tyurin, A.V.; Dobrokhotova, Z.V.; Novotortsev, V.M. Charge Transfer Adducts of Binuclear Rare Earth 3,5-Dinitrobenzoates with N,N-Dimethylaniline and Toluene. Polyhedron 2015, 89, 238–249. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Ilyukhin, A.B.; Efimov, N.N.; Ugolkova, E.A.; Dobrokhotova, Z.V.; Minin, V.V.; Novotortsev, V.M. Charge Transfer Adducts of Rare Earth 3,5-Dinitrobenzoates with N,N,N,N-Tetramethyl-p-Phenylenediamine. Inorganica Chim. Acta 2016, 442, 86–96. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Structure, DFT Calculations, and Magnetic Characterization of Coordination Polymers of Bridged Dicyanamido-Metal(II) Complexes. Magnetochemistry 2019, 5, 41. [Google Scholar] [CrossRef]
- Meza-Morales, P.J.; Santana-Vargas, A.; Curet-Arana, M.C. DFT Analysis of Coordination Polymer Ligands: Unraveling the Electrostatic Properties and Their Effect on CO2 Interaction. Adsorption 2015, 21, 533–546. [Google Scholar] [CrossRef]




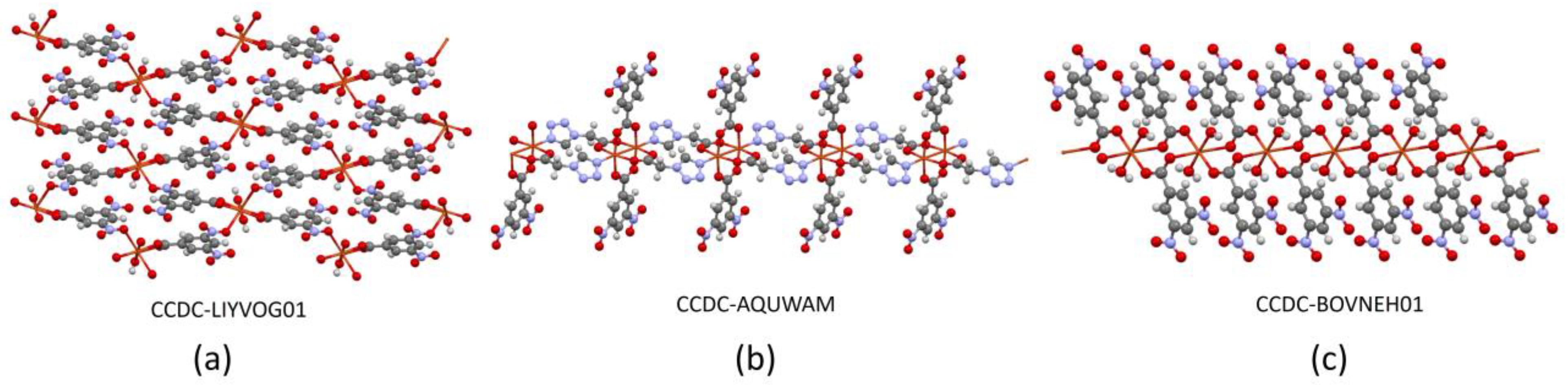
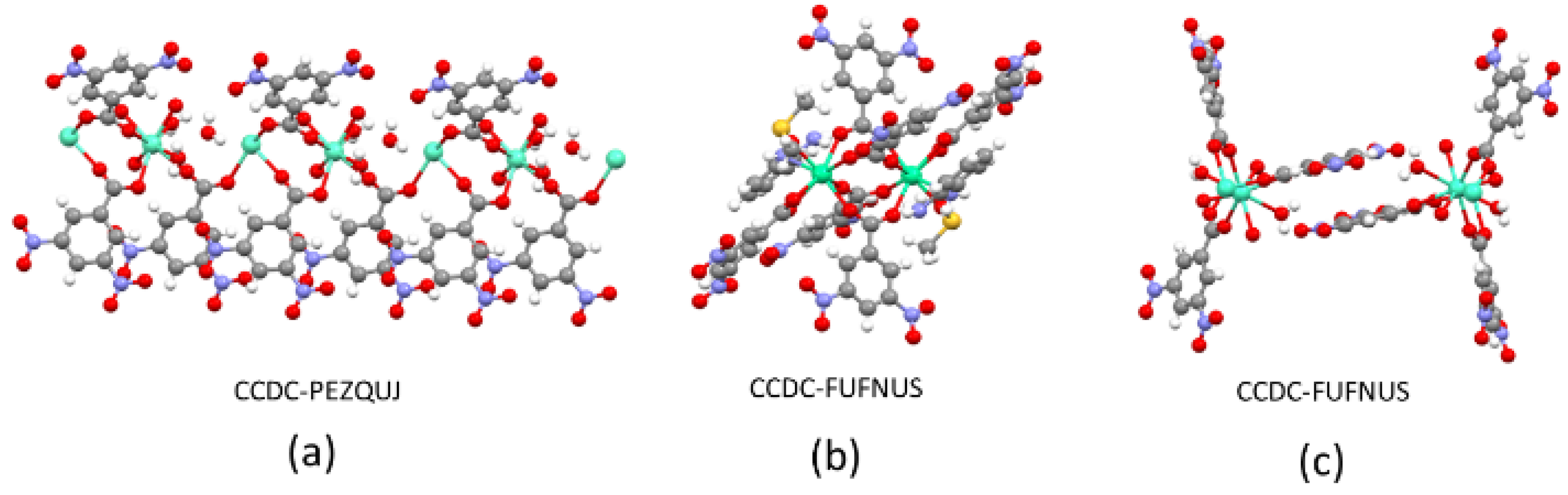
| Ln Element | CCDC Code |
|---|---|
| La | ADIQAG, EXATUT, RAHSAW |
| Ce | EREQIC, HIHGOU |
| Pr | MAKZEE, XOKSIZ |
| Nd | FUFNEC, PEZQET, RAHRUP |
| Sm | SUVXIQ |
| Eu | PEZQUJ, VOTRUT, VOTSUU, VOTTAB, VOVFIX |
| Gd | EXAVAB, FUFNIG, QAWNUX, VOTSOO, VOVFAP |
| Tb | PUZLON, VOTSEE, VOTTIJ, VOVFET |
| Dy | BOSDIY, FAGJOP, FUFPEE, LAVDOF, VOTRIH |
| Ho | FUFNUS, PUZMEE |
| Er | FUFPOO, PUZMII, PUZZER |
| Tm | GEWZEQ |
| Yb | GEWZIU |
| Compound Formula | Temp. of Synthesis (°C) | Days of Synthesis | Yield (%) | Teflon Reactor Volume (mL) | # Ref. |
|---|---|---|---|---|---|
| [Cu(3,5-DNB)(µ2-OH)] | 120 | 4 | 18 | 25 | [7] |
| [Ni2(3,5-DNB)4(bpy)2(H2O)] a | 140 | 3 | 60 | 23 | [60] |
| [Co2(3,5-DNB)2(bpy)2] a | 140 | 3 | 50 | 23 | [60] |
| [Cu(dtcd)(3,5-DNB)2]n b | 120 | 3 | - | 25 | [61] |
| [Cu(L)(3,5-DNB)2] c | 120 | 4 | 34 | 25 | [62] |
| [Co(L)(3,5-DNB)2] c | 120 | 4 | 40 | 25 | [62] |
| [Co2(3,5-DNB)2(pbdmbm)] d | 150 | 3 | 30 | 25 | [63] |
| [Co2(3,5-DNB)2(pbbm)2] e | 150 | 3 | 35 | 25 | [63] |
| [Zn(3,5-DNB)2(bbmi)] f | 140 | 3 | 43 | 10 | [64] |
| [Cu4(µ3-OH)2(atrz)2(3,5-DNB)6] g | 120 | 3 | 50 | 25 | [65] |
| [Cu(3,5-DNB)(phen)(H2O)2]·(3,5-DNB) | 150 | 2 | 70 | 10 | [66] |
| [Co2(bbix)3(3,5-DNB)4(H2O)] · 0.5(bbix) h | 150 | 3 | 68 | 25 | [67] |
| [Ni(bbmmb)2(3,5-DNB)2(H2O)2] i | 150 | 3 | 46 | 25 | [68] |
| [Ni(bbbm)1.5(3,5-DNB)2(H2O)] 0.5(bbbm)(H2O) j | 150 | 3 | 52 | 25 | [68] |
| [Mn(3,5-DNB)2(bpy)] k | 170 | 3 | 62 | 25 | [69] |
| [Co(bix)(3,5-DNB)2] l | 150 | 3 | 54 | 25 | [70] |
| Compound Formula | Temp. of Synthesis (°C) | Yield (%) | pH | # Ref. |
|---|---|---|---|---|
| [Co(3,5-DNB)2(H2O)4] · 4H2O | RT | 80 | 5 | [72] |
| [Zn(3,5-DNB)2]n | RT | 80 | 5 | [72] |
| [Ni(3,5-DNB)2(H2O)4] · 4H2O | 70 | 70 | - | [23] |
| [Co(SCZ)2(3,5-DNB)2] a | 70 | 70 | - | [23] |
| [Ni(SCZ)2(3,5-DNB)2] a | 70 | 68 | - | [23] |
| [Zn(SCZ)2(3,5-DNB)2] a | 70 | 72 | - | [23] |
| [Co(DAT)2(3,5-DNB)2(H2O)2] b | 70 | 72 | - | [23] |
| [Ni(DAT)2(3,5-DNB)2(H2O)2] b | 70 | 69 | - | [23] |
| [Co(3,5-DNB)2] | RT | 98 | - | [73] |
| [Cu(NIT3Py)2(3,5-DNB)2] c | RT | - | - | [74] |
| [Cu(TO)2(3,5-DNBA)2(3,5-DNB)2] d | 60 | 42 | 6 | [75] |
| [Cu(3,5-DNB)2(H2O)2] | 70 | 86 | 6–7 | [9] |
| [Cu(DAT)2(3,5-DNB)2 | 70 | 82 | 6–7 | [9] |
| [Cu(3,5-DNB)(pca)(H2O2] e | RT | 56 | - | [76] |
| [Cu(3,5-DNB)2(µ-denia)(H2O)]n f | RT | - | - | [77] |
| [Cu(3,5-DNB)2(L1)2] g | RT | 72 | - | [5] |
| [Zn(3,5-DNB)2(L1)2] g | RT | 64 | - | [5] |
| [Mn(3,5-DNB)2(H2O)2]n | 60 | 72 | 6–7 | [78] |
| [Mn(3,5-DNB)2(SCZ)2] h | 60 | 62 | 6–7 | [78] |
| [Zn(L1)(3,5-DNB)2] i | RT | 66 | - | [79] |
| [Zn(µ-3,5-DNB)2] | 60 | 42 | 7 | [80] |
| [Zn(3,5-DNB)2(py)2] | RT | 43 | - | [81] |
| Compound a | Method | HOMO-LUMO Gap | # Ref. | |
|---|---|---|---|---|
| kJ·mol−1 | eV | |||
| 3,5-DNBA | B3LYP/6-31G(d,p) | 498.93 | 5.171 | This work |
| 2,4-DNBA | B3LYP/6-31G(d,p) | 467.47 | 4.845 | This work |
| 3,5-DNBA | B3LYP/6-31G(d) | 1279.30 | 13.26 | [96] |
| 3,5-DNBA | B3LYP/6-31G | 330.95 | 3.430 | [6] |
| 2,4-DNBA | B3LYP/6-31G | 320.14 | 3.318 | [6] |
| [La(3,5-DNB)3(H2O)2] | PBE/DZP | 253.76 | 2.63 | [58] |
| [Gd(3,5-DNB)3(H2O)2] | PBE/DZP | 179.46 | 1.86 | [58] |
| [Eu2(NO3)2Cd2(phen)2(2,4-DNB)8]n·2n MeCN | PBE/def2-TZVP | – – – | – – – | [97] |
| [Eu2(MeCN)2Cd2(phen)2(3,5-DNB)10] | PBE/def2-TZVP | – – – | – – – | [97] |
| [Cu(2,4-DNB)2(EDA)] | M06-2X/6-31G** | 659.284 | 6.833 | [95] |
| [Cd2(µ-3,5-DNB)2(3,5-DNB)2(py)4] | BP86-D3/def2-TZVP | – – – | – – – | [82] |
| [Zn(3,5-DNB)2(py)2] | BP86-D3/def2-TZVP | – – – | – – – | [82] |
| [Co(3,5-DNB)2] | B3LYP-DKH-D3BJ/def2-TZVP | – – – | – – – | [2] |
| [Cu(3,5-DNB)2(MEA)2] | B3LYP/6-311G(d,p) | 409.10 | 4.24 b | [42] |
| [Ni(3,5-DNB)2(MEA)2] | B3LYP/6-311G(d,p) | 380.15 | 3.94 b | [42] |
| [Co(3,5-DNB)2(MEA)2] | B3LYP/6-311G(d,p) | 364.71 | 3.78 b | [42] |
| [Cu(II) trans-(pz)2(3,5-DNB)2] | M06/6-31+G* | – – – | – – – | [5] |
| [Zn(II) cis-(pz)2(3,5-DNB)2] | M06/6-31+G* | – – – | – – – | [5] |
| [Cu(II)(pz-CH2-pz)2(3,5-DNB)6] | M06/6-31+G* | – – – | – – – | [5] |
| [Zn(II)(pz-CH2-pz)(3,5-DNB)2] | M06/6-31+G* | – – – | – – – | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca-López, D.; Lozano, J.D.; Macías, M.A.; Muñoz-Castro, Á.; MacLeod-Carey, D.; Nagles, E.; Hurtado, J. Biological Activity of Complexes Involving Nitro-Containing Ligands and Crystallographic-Theoretical Description of 3,5-DNB Complexes. Int. J. Mol. Sci. 2024, 25, 6536. https://doi.org/10.3390/ijms25126536
Fonseca-López D, Lozano JD, Macías MA, Muñoz-Castro Á, MacLeod-Carey D, Nagles E, Hurtado J. Biological Activity of Complexes Involving Nitro-Containing Ligands and Crystallographic-Theoretical Description of 3,5-DNB Complexes. International Journal of Molecular Sciences. 2024; 25(12):6536. https://doi.org/10.3390/ijms25126536
Chicago/Turabian StyleFonseca-López, Daniela, Johan D. Lozano, Mario A. Macías, Álvaro Muñoz-Castro, Desmond MacLeod-Carey, Edgar Nagles, and John Hurtado. 2024. "Biological Activity of Complexes Involving Nitro-Containing Ligands and Crystallographic-Theoretical Description of 3,5-DNB Complexes" International Journal of Molecular Sciences 25, no. 12: 6536. https://doi.org/10.3390/ijms25126536






