Versatile Self-Assembly of Triblock Peptides into Stable Collagen Mimetic Heterotrimers
Abstract
1. Introduction
2. Results
2.1. Rational Design of Triblock Peptides
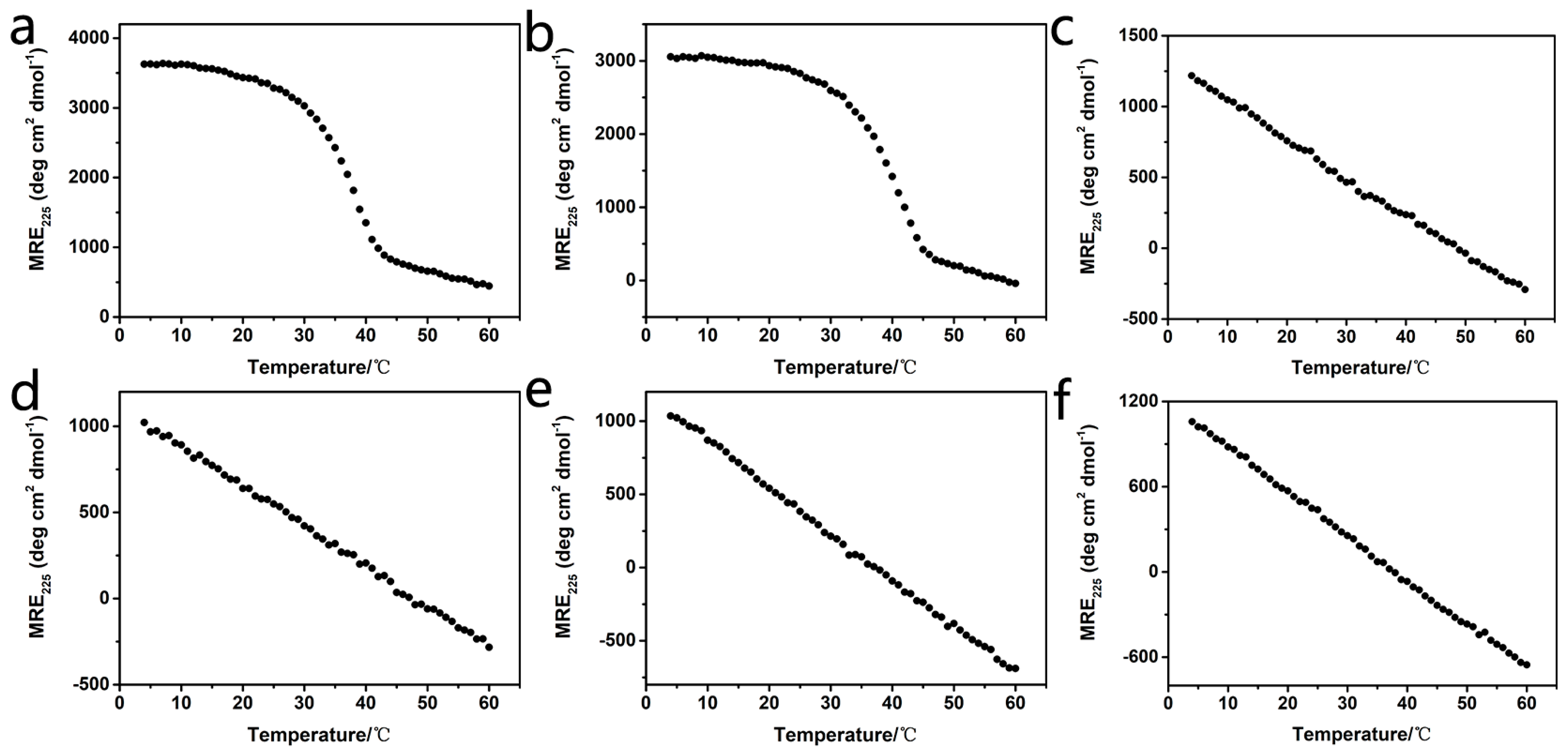
2.2. Stability of Homotrimers
2.3. Stability of Heterotrimers
2.4. Mimicking the Natural Composition of Type I Collagen
2.5. Composition Analysis of Collagen Mimetic Heterotrimers
2.6. Structural Features of Collagen Mimetic Heterotrimers
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Circular Dichroism Spectroscopy
4.3. Solution-State Nuclear Magnetic Resonance Spectroscopy
4.4. Molecular Dynamics Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhadi, S.A.; Restuccia, A.; Sorrentino, A.; Cruz-Sanchez, A.; Hudalla, G.A. Heterogeneous protein co-assemblies with tunable functional domain stoichiometry. Mol. Syst. Des. Eng. 2022, 7, 44–57. [Google Scholar] [CrossRef]
- Rudashevskaya, E.L.; Sacco, R.; Kratochwill, K.; Huber, M.L.; Gstaiger, M.; Superti-Furga, G.; Bennett, K.L. A method to resolve the composition of heterogeneous affinity-purified protein complexes assembled around a common protein by chemical cross-linking, gel electrophoresis and mass spectrometry. Nat. Protoc. 2013, 8, 75–97. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Mager, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Ottl, J.; Gabriel, D.; Murphy, G.; Knäuper, V.; Tominaga, Y.; Nagase, H.; Kröger, M.; Tschesche, H.; Bode, W.; Moroder, L. Recognition and catabolism of synthetic heterotrimeric collagen peptides by matrix metalloproteinases. Chem. Biol. 2000, 7, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Renner, C.; Sacca, B.; Moroder, L. Synthetic heterotrimeric collagen peptides as mimics of cell adhesion sites of the basement membrane. Biopolymers 2004, 76, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Sacca, B.; Fiori, S.; Moroder, L. Studies of the local conformational properties of the cell-adhesion domain of collagen type IV in synthetic heterotrimeric peptides. Biochemistry 2003, 42, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Sacca, B.; Moroder, L. Synthesis of heterotrimeric collagen peptides containing the alpha1beta1 integrin recognition site of collagen type IV. J. Pept. Sci. 2002, 8, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Sacca, B.; Renner, C.; Moroder, L. The chain register in heterotrimeric collagen peptides affects triple helix stability and folding kinetics. J. Mol. Biol. 2002, 324, 309–318. [Google Scholar] [CrossRef]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef]
- Yang, L.; van der Werf, K.O.; Fitie, C.F.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of native and cross-linked type I collagen fibrils. Biophys. J. 2008, 94, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.R.; San Antonio, J.D.; Mayne, R.; McQuillan, D.J.; Sarris, G.; Santoro, S.A.; Iozzo, R.V. Decorin binds near the C terminus of type I collagen. J. Biol. Chem. 2000, 275, 21801–21804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Kapyla, J.; Puranen, J.S.; Knight, C.G.; Tiger, C.F.; Pentikainen, O.T.; Johnson, M.S.; Farndale, R.W.; Heino, J.; Gullberg, D. alpha 11beta 1 integrin recognizes the GFOGER sequence in interstitial collagens. J. Biol. Chem. 2003, 278, 7270–7277. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Niyibizi, C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem. Cells 2007, 25, 3183–3193. [Google Scholar] [CrossRef]
- Castillo-Briceno, P.; Bihan, D.; Nilges, M.; Hamaia, S.; Meseguer, J.; Garcia-Ayala, A.; Farndale, R.W.; Mulero, V. A role for specific collagen motifs during wound healing and inflammatory response of fibroblasts in the teleost fish gilthead seabream. Mol. Immunol. 2011, 48, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Hulmes, D.J. Fibrillar Collagens. Subcell. Biochem. 2017, 82, 457–490. [Google Scholar] [PubMed]
- Fiori, S.; Saccà, B.; Moroder, L. Structural properties of a collagenous heterotrimer that mimics the collagenase cleavage site of collagen type I. J. Mol. Biol. 2002, 319, 1235–1242. [Google Scholar] [CrossRef]
- Jalan, A.A.; Sammon, D.; Hartgerink, J.D.; Brear, P.; Stott, K.; Hamaia, S.W.; Hunter, E.J.; Walker, D.R.; Leitinger, B.; Farndale, R.W. Chain alignment of collagen I deciphered using computationally designed heterotrimers. Nat. Chem. Biol. 2020, 16, 423–429. [Google Scholar] [CrossRef]
- Luo, T.; Kiick, K.L. Collagen-Like Peptide Bioconjugates. Bioconjug. Chem. 2017, 28, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.; Chmielewski, J. Advances in the design and higher-order assembly of collagen mimetic peptides for regenerative medicine. Curr. Opin. Biotechnol. 2017, 46, 34–41. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, L.E.; Fallas, J.A.; Hartgerink, J.D. Positive and negative design leads to compositional control in AAB collagen heterotrimers. J. Am. Chem. Soc. 2011, 133, 5432–5443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lu, C.; Lan, J.; Fan, S.; Nanda, V.; Xu, F. How electrostatic networks modulate specificity and stability of collagen. Proc. Natl. Acad. Sci. USA 2018, 115, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.A.; Raines, R.T. Stereoelectronic and steric effects in the collagen triple helix: Toward a code for strand association. J. Am. Chem. Soc. 2005, 127, 15923–15932. [Google Scholar] [CrossRef] [PubMed]
- Hentzen, N.B.; Islami, V.; Kohler, M.; Zenobi, R.; Wennemers, H. A Lateral Salt Bridge for the Specific Assembly of an ABC-Type Collagen Heterotrimer. J. Am. Chem. Soc. 2020, 142, 2208–2212. [Google Scholar] [CrossRef] [PubMed]
- Gauba, V.; Hartgerink, J.D. Self-assembled heterotrimeric collagen triple helices directed through electrostatic interactions. J. Am. Chem. Soc. 2007, 129, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Jalan, A.A.; Demeler, B.; Hartgerink, J.D. Hydroxyproline-Free Single Composition ABC Collagen Heterotrimer. J. Am. Chem. Soc. 2013, 135, 6014–6017. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.G.; Morton, L.F.; Onley, D.J.; Peachey, A.R.; Messent, A.J.; Smethurst, P.A.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. J. Biol. Chem. 1998, 273, 33287–33294. [Google Scholar] [CrossRef]
- Malcor, J.D.; Bax, D.; Hamaia, S.W.; Davidenko, N.; Best, S.M.; Cameron, R.E.; Farndale, R.W.; Bihan, D. The synthesis and coupling of photoreactive collagen-based peptides to restore integrin reactivity to an inert substrate, chemically-crosslinked collagen. Biomaterials 2016, 85, 65–77. [Google Scholar] [CrossRef]
- Siljander, P.R.; Hamaia, S.; Peachey, A.R.; Slatter, D.A.; Smethurst, P.A.; Ouwehand, W.H.; Knight, C.G.; Farndale, R.W. Integrin activation state determines selectivity for novel recognition sites in fibrillar collagens. J. Biol. Chem. 2004, 279, 47763–47772. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, W.; Yu, J.; Luo, L.; Wang, J.; Xiao, J. Ln3+-Triggered self-assembly of a heterotrimer collagen mimetic peptide into luminescent nanofibers. Chem. Commun. 2020, 56, 15141–15144. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Soliakov, A.; Lewis, R.J. In vitro fibrillogenesis of collagen type I in varying ionic and pH conditions. Micron 2013, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, M.; Wang, L.; Luo, L.; Wang, J.; Xiao, J. Luminescent Biofunctional Collagen Mimetic Nanofibers. ACS Omega 2019, 4, 16270–16279. [Google Scholar] [CrossRef]
- Rele, S.; Song, Y.; Apkarian, R.P.; Qu, Z.; Conticello, V.P.; Chaikof, E.L. D-periodic collagen-mimetic microfibers. J. Am. Chem. Soc. 2007, 129, 14780–14787. [Google Scholar] [CrossRef]
- O’Leary, L.E.; Fallas, J.A.; Bakota, E.L.; Kang, M.K.; Hartgerink, J.D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 2011, 3, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Rainey, J.K.; Goh, M.C. An interactive triple-helical collagen builder. Bioinformatics 2004, 20, 2458–2459. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N·Log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]





| Peptide Name | Peptide Sequence | Tm/°C |
|---|---|---|
| α1 | (GPO)3GFOGER(GPO)3 | 38.0 |
| α2 | (GPO)3GPOGES(GPO)3 | 41.0 |
| K-α1-D | K5(GPO)3GFOGER(GPO)3D5 | <4.0 |
| D-α1-K | D5(GPO)3GFOGER(GPO)3K5 | <4.0 |
| K-α2-D | K5(GPO)3GPOGES(GPO)3D5 | <4.0 |
| D-α2-K | D5(GPO)3GPOGES(GPO)3K5 | <4.0 |
| K-α1*-D | K5(GPO)3GFOG*ER(GPO)3D5 | - |
| D-α2*-K | D5(GPO)3GPOG*ES(GPO)3K5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Ling, B.; Zhao, S.; Yu, F.; Liu, H.; Wang, S.; Xiao, J. Versatile Self-Assembly of Triblock Peptides into Stable Collagen Mimetic Heterotrimers. Int. J. Mol. Sci. 2024, 25, 6550. https://doi.org/10.3390/ijms25126550
Yao L, Ling B, Zhao S, Yu F, Liu H, Wang S, Xiao J. Versatile Self-Assembly of Triblock Peptides into Stable Collagen Mimetic Heterotrimers. International Journal of Molecular Sciences. 2024; 25(12):6550. https://doi.org/10.3390/ijms25126550
Chicago/Turabian StyleYao, Linyan, Biyang Ling, Sha Zhao, Fansen Yu, Huanxiang Liu, Shenlin Wang, and Jianxi Xiao. 2024. "Versatile Self-Assembly of Triblock Peptides into Stable Collagen Mimetic Heterotrimers" International Journal of Molecular Sciences 25, no. 12: 6550. https://doi.org/10.3390/ijms25126550
APA StyleYao, L., Ling, B., Zhao, S., Yu, F., Liu, H., Wang, S., & Xiao, J. (2024). Versatile Self-Assembly of Triblock Peptides into Stable Collagen Mimetic Heterotrimers. International Journal of Molecular Sciences, 25(12), 6550. https://doi.org/10.3390/ijms25126550






