Quantitative Virus-Associated RNA Detection to Monitor Oncolytic Adenovirus Replication
Abstract
:1. Introduction
2. Results
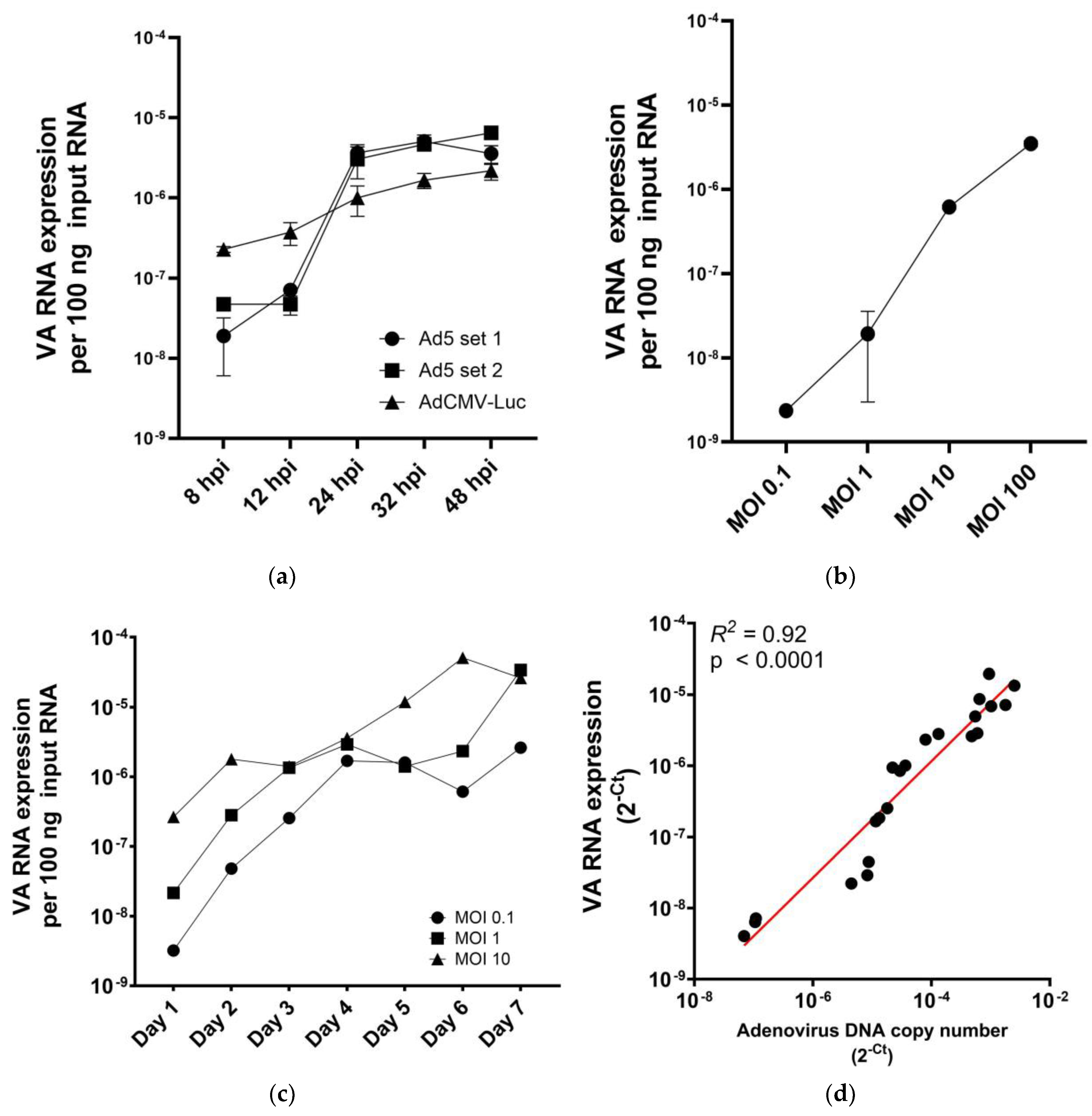
2.1. VA RNA I Levels in Adenovirus-Infected Cells Are Replication and Dose Dependent
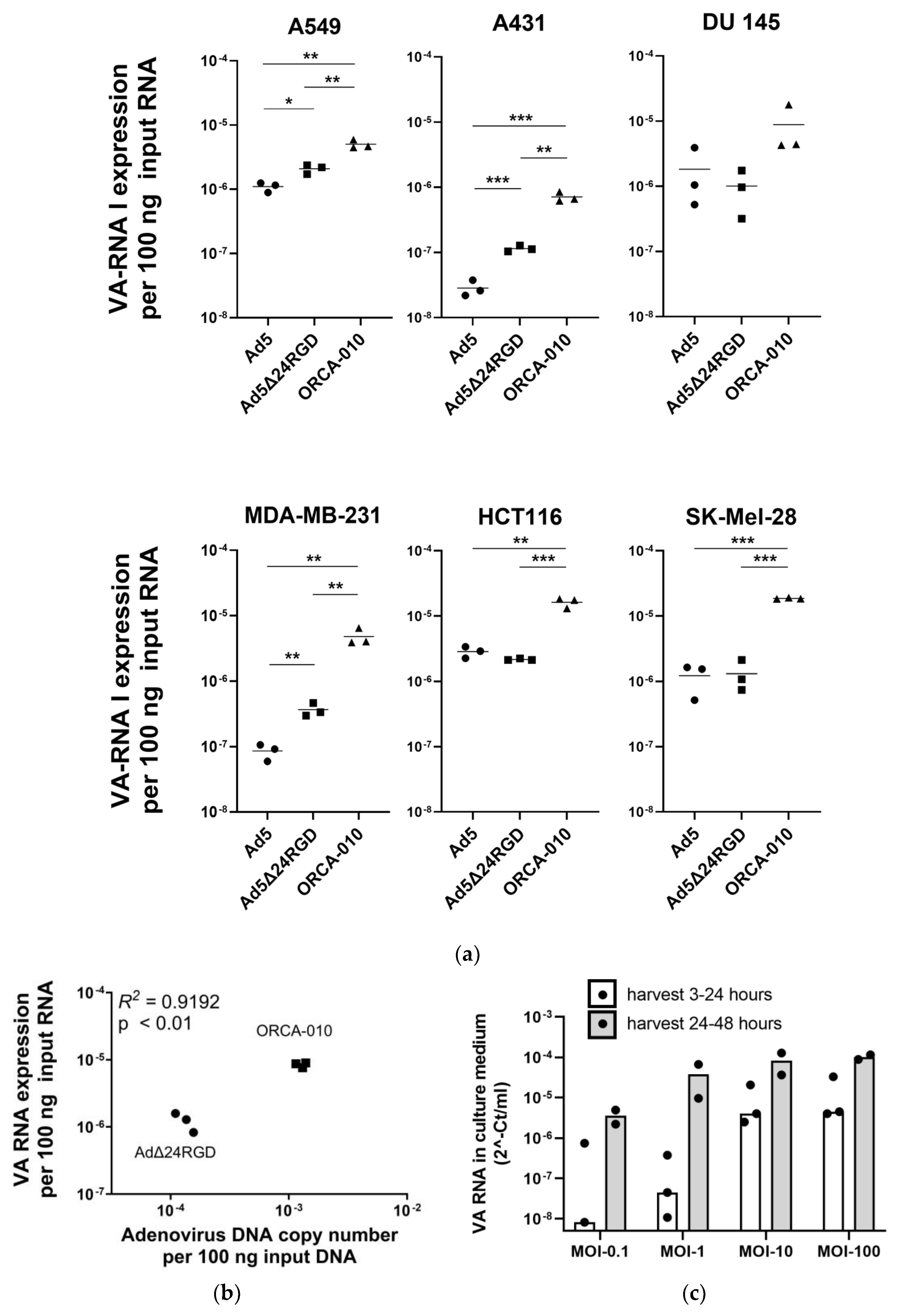
2.2. Measuring VA RNA Expression Levels to Compare Adenovirus Replication Efficiencies in Cancer Cell Lines
2.3. Detection of VA RNA in Extracellular Vesicles Produced by Adenovirus-Infected Cancer Cells
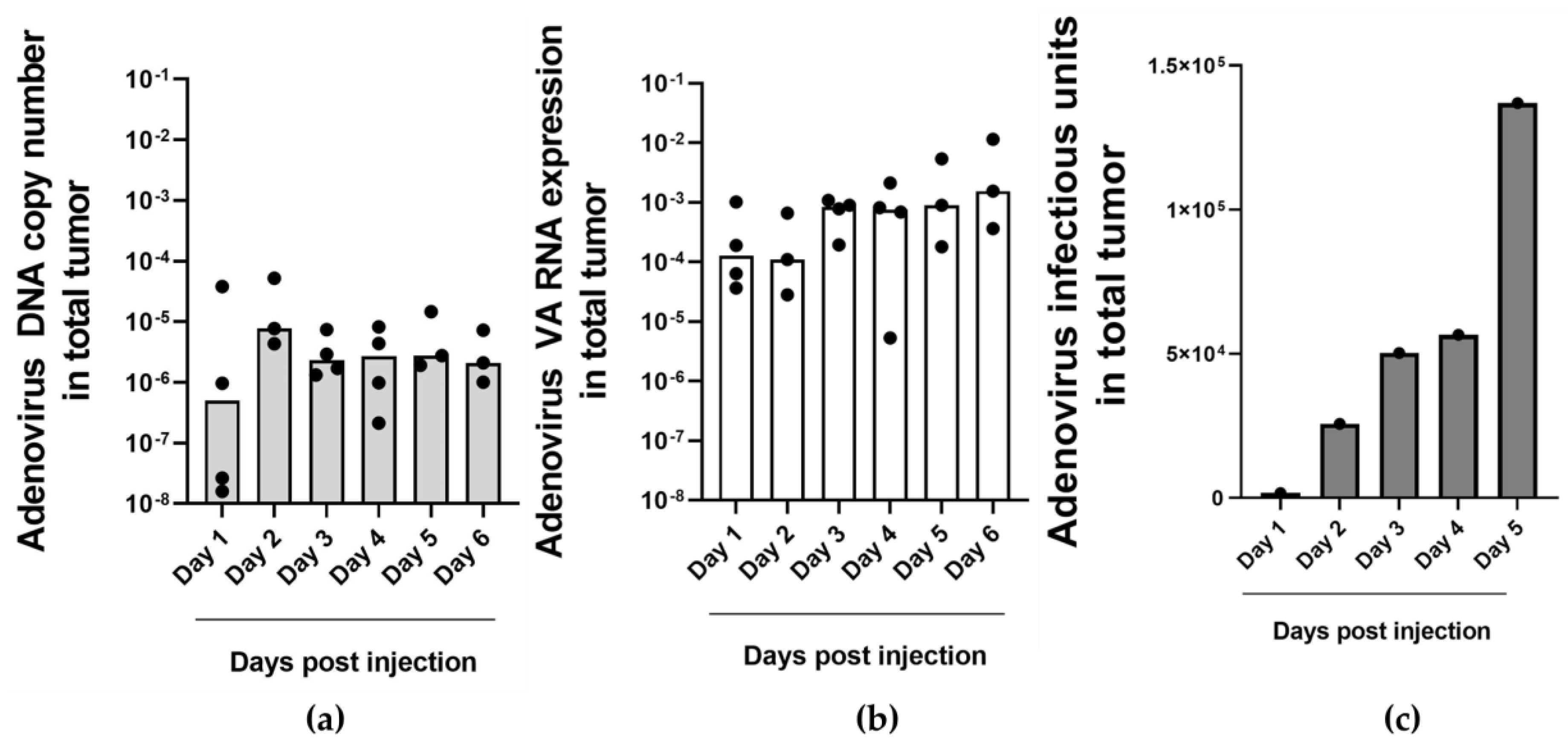
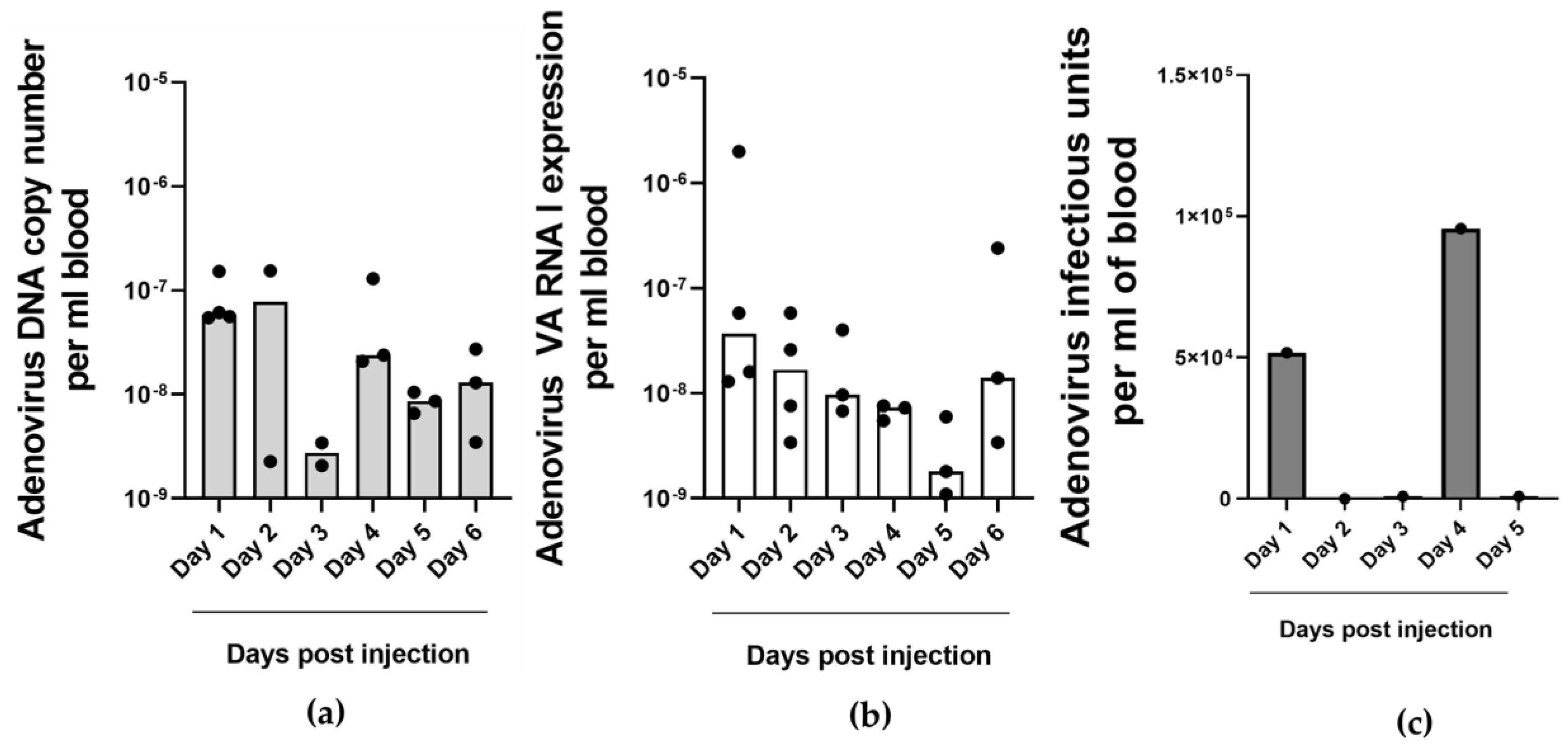
2.4. Detection of Adenovirus DNA and VA RNA Copy Numbers in Oncolytic Virus-Injected Xenograft Tumors and Blood In Vivo
2.5. Detection of Adenovirus VA RNA in Cerebrospinal Fluid of Patients with Glioblastoma Multiforme Treated with Oncolytic Adenovirus Ad5Δ24RGD
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Adenoviruses
4.3. Extracellular Vesicles Isolation and Analysis
4.4. VA RNA and miR-1 Expression Analysis Using RT-qPCR
4.5. Analysis of Adenovirus Genome Copy Number Using qPCR
4.6. In Vivo Experiments in a NSCLC-CAM Tumor Model
4.7. Cerebrospinal Fluid from Patients with Glioblasoma Multiforme Who Were Injected with Oncolytic Adenovirus
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef] [PubMed]
- Farrera-Sal, M.; Moya-Borrego, L.; Bazan-Peregrino, M.; Alemany, R. Evolving Status of Clinical Immunotherapy with Oncolytic Adenovirus. Clin. Cancer Res. 2021, 27, 2979–2988. [Google Scholar] [CrossRef]
- DeWeese, T.L.; van der Poel, H.; Li, S.; Mikhak, B.; Drew, R.; Goemann, M.; Hamper, U.; DeJong, R.; Detorie, N.; Rodriguez, R.; et al. A Phase I Trial of CV706, a Replication-Competent, PSA Selective Oncolytic Adenovirus, for the Treatment of Locally Recurrent Prostate Cancer Following Radiation Therapy. Cancer Res. 2001, 61, 7464–7472. [Google Scholar] [PubMed]
- Reid, T.; Galanis, E.; Abbruzzese, J.; Sze, D.; Wein, L.M.; Andrews, J.; Randlev, B.; Heise, C.; Uprichard, M.; Hatfield, M.; et al. Hepatic Arterial Infusion of a Replication-Selective Oncolytic Adenovirus (Dl1520): Phase II Viral, Immunologic, and Clinical Endpoints. Cancer Res. 2002, 62, 6070–6079. [Google Scholar]
- Machiels, J.-P.; Salazar, R.; Rottey, S.; Duran, I.; Dirix, L.; Geboes, K.; Wilkinson-Blanc, C.; Pover, G.; Alvis, S.; Champion, B.; et al. A Phase 1 Dose Escalation Study of the Oncolytic Adenovirus Enadenotucirev, Administered Intravenously to Patients with Epithelial Solid Tumors (EVOLVE). J. Immunother. Cancer 2019, 7, 20. [Google Scholar] [CrossRef]
- Hodzic, J.; Sie, D.; Vermeulen, A.; van Beusechem, V.W. Functional Screening Identifies Human MiRNAs That Modulate Adenovirus Propagation in Prostate Cancer Cells. Hum. Gene Ther. 2017, 28, 766–780. [Google Scholar] [CrossRef]
- Punga, T.; Darweesh, M.; Akusjärvi, G. Synthesis, Structure, and Function of Human Adenovirus Small Non-Coding RNAs. Viruses 2020, 12, 1182. [Google Scholar] [CrossRef]
- Aparicio, O.; Razquin, N.; Zaratiegui, M.; Narvaiza, I.; Fortes, P. Adenovirus Virus-Associated RNA Is Processed to Functional Interfering RNAs Involved in Virus Production. J. Virol. 2006, 80, 1376–1384. [Google Scholar] [CrossRef]
- Kamel, W.; Segerman, B.; Öberg, D.; Punga, T.; Akusjärvi, G. The Adenovirus VA RNA-Derived MiRNAs Are Not Essential for Lytic Virus Growth in Tissue Culture Cells. Nucleic Acids Res. 2013, 41, 4802–4812. [Google Scholar] [CrossRef]
- Thimmappaya, B.; Weinberger, C.; Schneider, R.J.; Shenk, T. Adenovirus VAI RNA Is Required for Efficient Translation of Viral MRNAs at Late Times after Infection. Cell 1982, 31, 543–551. [Google Scholar] [CrossRef]
- O’Malley, R.P.; Mariano, T.M.; Siekierka, J.; Mathews, M.B. A Mechanism for the Control of Protein Synthesis by Adenovirus VA RNAI. Cell 1986, 44, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Vachon, V.K.; Conn, G.L. Adenovirus VA RNA: An Essential pro-Viral Non-Coding RNA. Virus Res. 2016, 212, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Machitani, M.; Tachibana, M.; Sakurai, F.; Mizuguchi, H. A MicroRNA Derived from Adenovirus Virus-Associated RNAII Promotes Virus Infection via Posttranscriptional Gene Silencing. J. Virol. 2019, 93, e01265-18. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, O.; Carnero, E.; Abad, X.; Razquin, N.; Guruceaga, E.; Segura, V.; Fortes, P. Adenovirus VA RNA-Derived MiRNAs Target Cellular Genes Involved in Cell Growth, Gene Expression and DNA Repair. Nucleic Acids Res. 2010, 38, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Segerman, B.; Zhou, X.; Akusjarvi, G. Adenovirus Virus-Associated RNAII-Derived Small RNAs Are Efficiently Incorporated into the RNA-Induced Silencing Complex and Associate with Polyribosomes. J. Virol. 2007, 81, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.R.; Forget, B.G.; Weissman, S.M.; Rose, J.A. RNA of Low Molecular Weight in KB Cells Infected with Adenovirus Type 2. J. Mol. Biol. 1966, 17, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, H.; Pettersson, U.; Vennström, B.; Philipson, L.; Mathews, M.B. A New Species of Virus-Coded Low Molecular Weight RNA from Cells Infected with Adenovirus Type 2. Cell 1976, 7, 585–593. [Google Scholar] [CrossRef]
- Sehulster, L.M.; Varricchio, F.; Raska, K. Synthesis of Transfer Ribonucleic Acid in KB Cells Infected with Adenovirus Type 2. J. Gen. Virol. 1978, 40, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- Kamel, W.; Segerman, B.; Punga, T.; Akusjärvi, G. Small RNA Sequence Analysis of Adenovirus VA RNA-Derived MiRNAs Reveals an Unexpected Serotype-Specific Difference in Structure and Abundance. PLoS ONE 2014, 9, e105746. [Google Scholar] [CrossRef]
- Chen, C. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Brachtlova, T.; van Ginkel, J.-W.; Luinenburg, M.J.; de Menezes, R.X.; Koppers-Lalic, D.; Pegtel, D.M.; Dong, W.; de Gruijl, T.D.; van Beusechem, V.W. Expression of Oncolytic Adenovirus-Encoded RNAi Molecules Is Most Effective in a Pri-MiRNA Precursor Format. Mol. Ther. Oncolytics 2020, 19, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Machitani, M.; Yamaguchi, T.; Shimizu, K.; Sakurai, F.; Katayama, K.; Kawabata, K.; Mizuguchi, H. Adenovirus Vector-Derived VA-RNA-Mediated Innate Immune Responses. Pharmaceutics 2011, 3, 338–353. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawabata, K.; Kouyama, E.; Ishii, K.J.; Katayama, K.; Suzuki, T.; Kurachi, S.; Sakurai, F.; Akira, S.; Mizuguchi, H. Induction of Type I Interferon by Adenovirus-Encoded Small RNAs. Proc. Natl. Acad. Sci. USA 2010, 107, 17286–17291. [Google Scholar] [CrossRef]
- Herz, J.; Gerard, R.D. Adenovirus-Mediated Transfer of Low Density Lipoprotein Receptor Gene Acutely Accelerates Cholesterol Clearance in Normal Mice. Proc. Natl. Acad. Sci. USA 1993, 90, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Fueyo, J.; Krasnykh, V.; Reynolds, P.N.; Curiel, D.T.; Alemany, R. A Conditionally Replicative Adenovirus with Enhanced Infectivity Shows Improved Oncolytic Potency. Clin. Cancer Res. 2001, 7, 120–126. [Google Scholar] [CrossRef]
- Dong, W.; van Ginkel, J.-W.H.; Au, K.Y.; Alemany, R.; Meulenberg, J.J.M.; van Beusechem, V.W. ORCA-010, a Novel Potency-Enhanced Oncolytic Adenovirus, Exerts Strong Antitumor Activity in Preclinical Models. Hum. Gene Ther. 2014, 25, 897–904. [Google Scholar] [CrossRef]
- Van Beusechem, V.W.; van den Doel, P.B.; Grill, J.; Pinedo, H.M.; Gerritsen, W.R. Conditionally Replicative Adenovirus Expressing P53 Exhibits Enhanced Oncolytic Potency. Cancer Res. 2002, 62, 6165–6171. [Google Scholar] [CrossRef]
- Clancy, J.W.; D’Souza-Schorey, C. Tumor-Derived Extracellular Vesicles: Multifunctional Entities in the Tumor Microenvironment. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 205–229. [Google Scholar] [CrossRef]
- Van Eijndhoven, M.A.J.; Baglio, S.R.; Pegtel, D.M. Packaging RNA Drugs into Extracellular Vesicles. Nat. Biomed. Eng. 2020, 4, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Pratama, M.Y.; Vo, N.V.T.; Masadah, R.; Tiribelli, C. The Crosstalk between Tumor Cells and the Microenvironment in Hepatocellular Carcinoma: The Role of Exosomal MicroRNAs and Their Clinical Implications. Cancers 2020, 12, 823. [Google Scholar] [CrossRef]
- Van Eijndhoven, M.A.J.; Zijlstra, J.M.; Groenewegen, N.J.; Drees, E.E.E.; van Niele, S.; Baglio, S.R.; Koppers-Lalic, D.; van der Voorn, H.; Libregts, S.F.W.M.; Wauben, M.H.M.; et al. Plasma Vesicle MiRNAs for Therapy Response Monitoring in Hodgkin Lymphoma Patients. JCI Insight 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step Isolation of Extracellular Vesicles by Size-exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- De Vrij, J.; Maas, S.L.; van Nispen, M.; Sena-Esteves, M.; Limpens, R.W.; Koster, A.J.; Leenstra, S.; Lamfers, M.L.; Broekman, M.L. Quantification of Nanosized Extracellular Membrane Vesicles with Scanning Ion Occlusion Sensing. Nanomedicine 2013, 8, 1443–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brachtlova, T.; van der Meulen-Muileman, I.H.; Kleerebezem, S.; Liu, C.; Li, P.; van Beusechem, V.W. Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 15425. [Google Scholar] [CrossRef]
- Van Putten, E.H.P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Martínez-Quintanilla, J.; Puig, C.; Guedan, S.; Molleví, D.G.; Alemany, R.; Cascallo, M. Bioselection of a Gain of Function Mutation That Enhances Adenovirus 5 Release and Improves Its Antitumoral Potency. Cancer Res. 2008, 68, 8928–8937. [Google Scholar] [CrossRef]
- Nadeau, I.; Kamen, A. Production of Adenovirus Vector for Gene Therapy. Biotechnol. Adv. 2003, 20, 475–489. [Google Scholar] [CrossRef]
- Hoeben, R.C.; Uil, T.G. Adenovirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a013003. [Google Scholar] [CrossRef]

| EV RNA Target | Control | AdΔ24E3-U6 | AdΔ24E3- U6.pri-miR-1 | |
|---|---|---|---|---|
| Size exclusion (0.86 mL medium) | VA-RNAI-5p | Below detection level | 2.47 × 10−8 | 1.29 × 10−8 |
| miR-1 | 3.06 × 10−11 | 2.40 × 10−11 | 9.41 × 10−10 | |
| Iodixanol gradient (21.43 mL medium) | VA-RNAI-5p | Below detection level | 9.11 × 10−8 | 5.48 × 10−9 |
| miR-1 | 8.74 × 10−11 | 1.12 × 10−10 | 3.03 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brachtlova, T.; Li, J.; van der Meulen-Muileman, I.H.; Sluiter, F.; von Meijenfeldt, W.; Witte, I.; Massaar, S.; van den Oever, R.; de Vrij, J.; van Beusechem, V.W. Quantitative Virus-Associated RNA Detection to Monitor Oncolytic Adenovirus Replication. Int. J. Mol. Sci. 2024, 25, 6551. https://doi.org/10.3390/ijms25126551
Brachtlova T, Li J, van der Meulen-Muileman IH, Sluiter F, von Meijenfeldt W, Witte I, Massaar S, van den Oever R, de Vrij J, van Beusechem VW. Quantitative Virus-Associated RNA Detection to Monitor Oncolytic Adenovirus Replication. International Journal of Molecular Sciences. 2024; 25(12):6551. https://doi.org/10.3390/ijms25126551
Chicago/Turabian StyleBrachtlova, Tereza, Jing Li, Ida H. van der Meulen-Muileman, Femke Sluiter, Willem von Meijenfeldt, Isabella Witte, Sanne Massaar, Ruben van den Oever, Jeroen de Vrij, and Victor W. van Beusechem. 2024. "Quantitative Virus-Associated RNA Detection to Monitor Oncolytic Adenovirus Replication" International Journal of Molecular Sciences 25, no. 12: 6551. https://doi.org/10.3390/ijms25126551





