OV Modulators of the Paediatric Brain TIME: Current Status, Combination Strategies, Limitations and Future Directions
Abstract
:1. Introduction
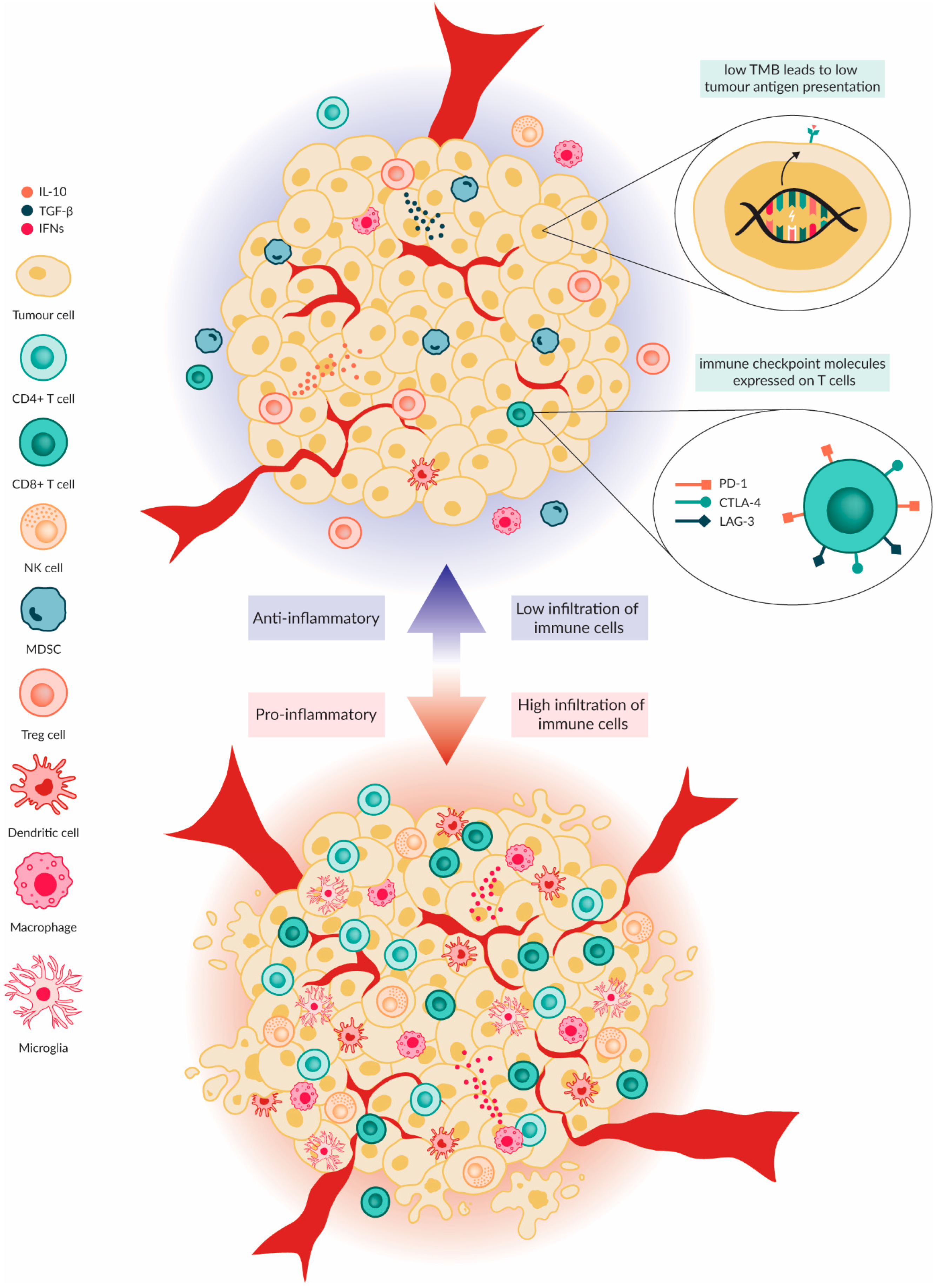
2. Paediatric Brain Tumour Immune Microenvironment
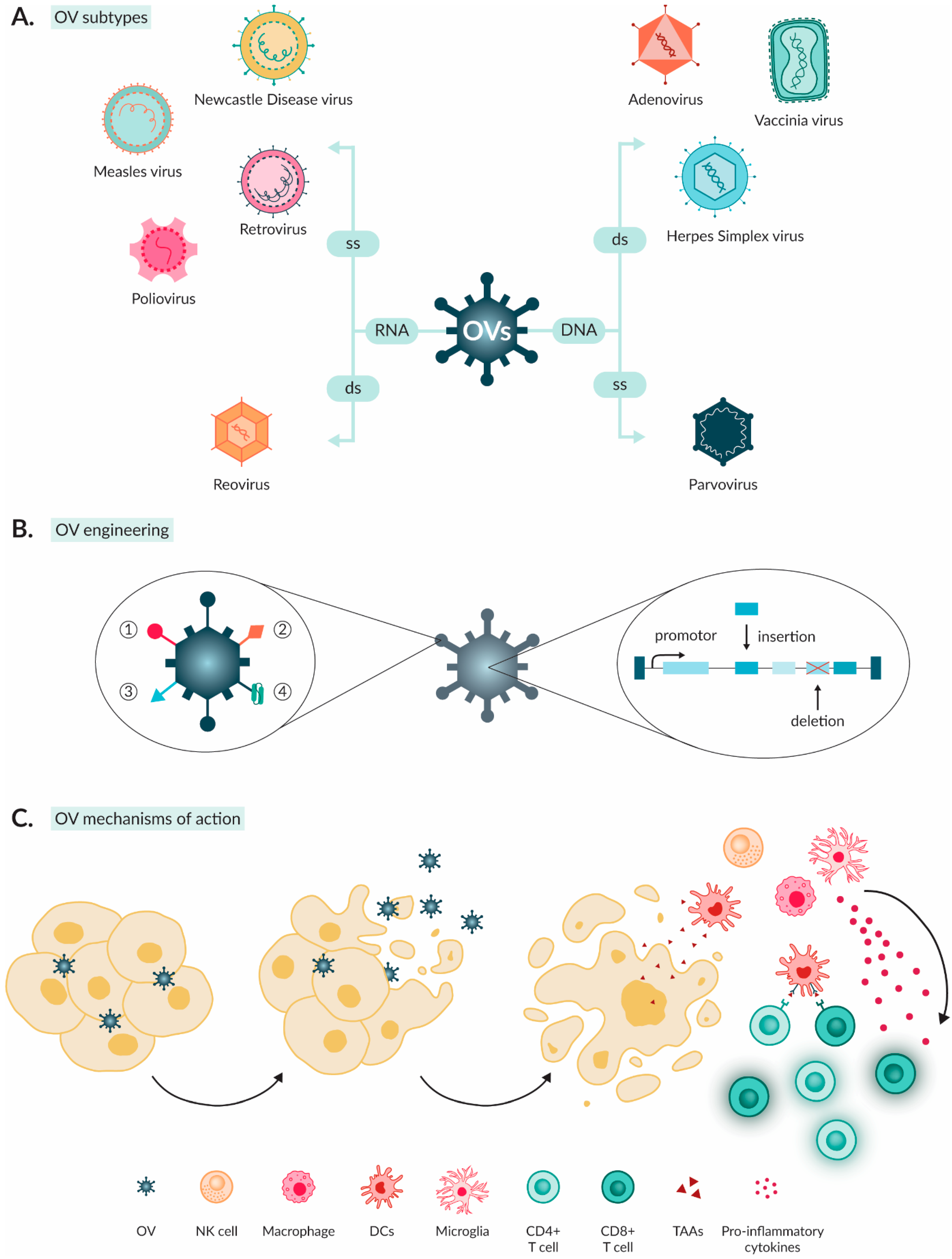
3. Oncolytic Viruses as Anti-Cancer Agents
3.1. Characteristics of OVs
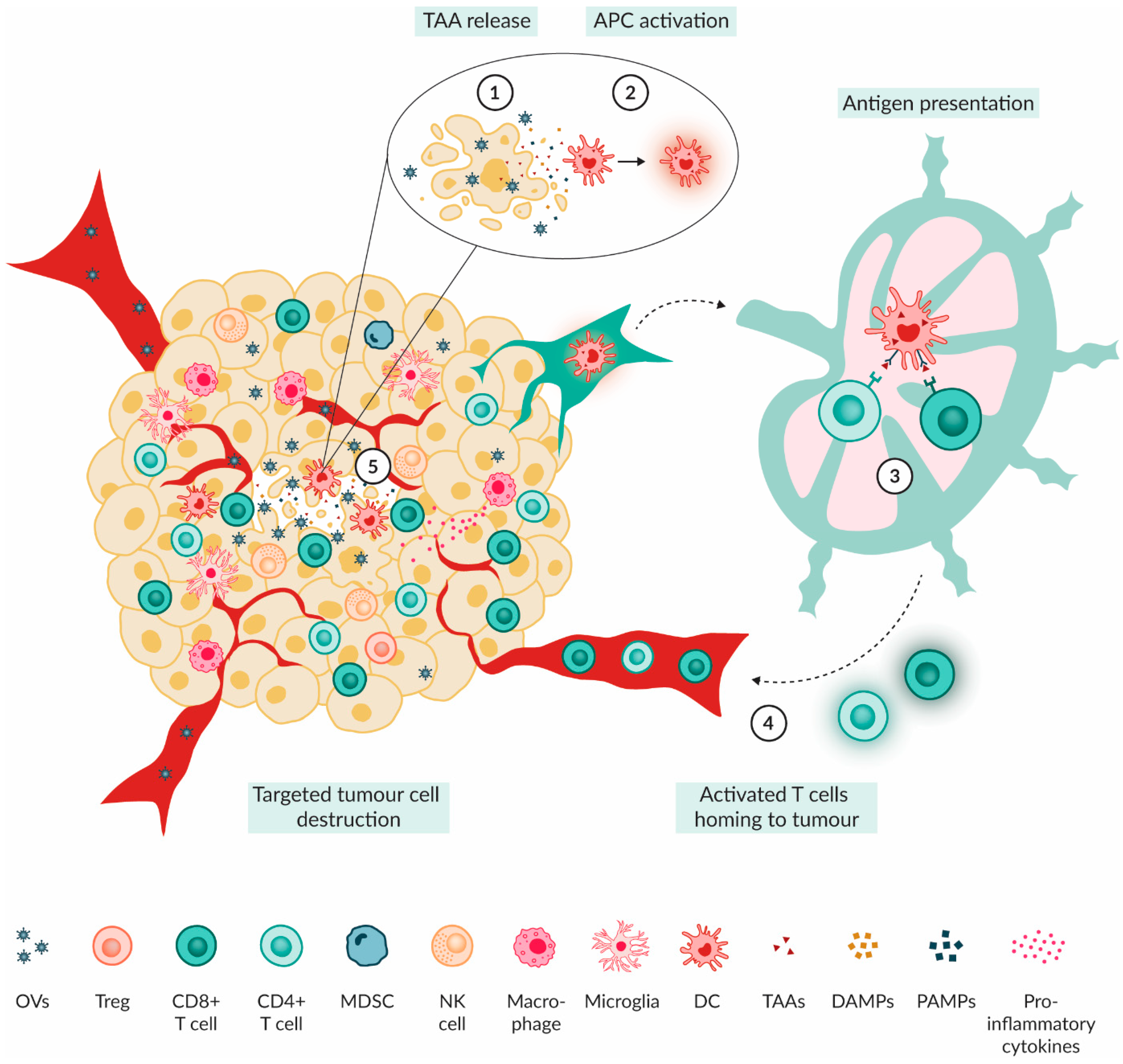
3.2. Mechanism of Action of OVs
4. Paediatric Brain Tumour Immune Microenvironment Modulation by OVs
4.1. Preclinical Evidence
| OV Type (Name) | OV Modifications | Tumour Type (Cell Line) | Animal Model | Survival | Immune Cell Type | Modulation | Year and Ref. |
|---|---|---|---|---|---|---|---|
| HSV (C134) | Deletion of both copies of the principal virulence gene γ134.5. Additionally has IRS1 gene under control by human cytomegalovirus immediate early promoter. | Mouse-MB (CMYC, MYCN) | Syngeneic C57BL/6 mice | Improved | CD4+ T cells | Increased influx in TME, Increased expression of PD-1 and LAG-3 | 2023 [51] |
| CD8+ T cells | Increased influx in TME, Increased surface expression of PD-1 and LAG-3 and increased expression of granzyme A & B genes | ||||||
| NK cells | Increased level in TME and increased expression of granzyme A, B and NKG7 genes | ||||||
| DCs | Increased influx in TME and increased expression of IFN and chemokine genes | ||||||
| Macrophages | Increased levels of M1 macrophages and increased expression of cytokine, chemokine and MHC I genes | ||||||
| Microglia | Increased expression of cytokine, chemokine and MHC I genes | ||||||
| Glioma (Neuroglial-Neuro2A, DBT mice glioma) | Syngeneic A/J mice and syngeneic Balb/C mice | Improved | CD8+ T cells | Increased influx in TME | 2018 [52] | ||
| Memory formation | Developed and capable of controlling rechallenge | ||||||
| AdV (Delta-24-RGD) | Insertion of RGD-4C peptide in the fibre knob. 24 bp deletion in E1A viral gene responsible for Rb-binding. | Glioma (Ham GSCs) | Syrian Hamster | Improved | CD4+ T cells | Increased influx in TME | 2021 [53] |
| CD8+ T cells | Increased influx in TME | ||||||
| DMG (TP54, TP80, NP53), HGG (CHLA-03-AA, PBT-24) | Balb/C mice | Improved | CD4+ T cells | Increased influx in TME | 2019 [59] | ||
| CD8+ T cells | Increased influx in TME | ||||||
| ATRT (CHLA-06), ETMR (PFSK-1) | CD34-humanized NSG-SGM3 mice | Improved | CD8+ T cells | Increased ratio within CD3+ population | 2021 [54] | ||
| Macrophages | Pronounced recruitment and activation at tumour margins | ||||||
| Microglia | Pronounced recruitment and activation at tumour margins |
4.2. Clinical Evidence
4.3. Armed OVs
4.3.1. Immunomodulatory Cytokines
4.3.2. Co-Stimulatory Molecules
4.3.3. Tumour Suppressor Genes
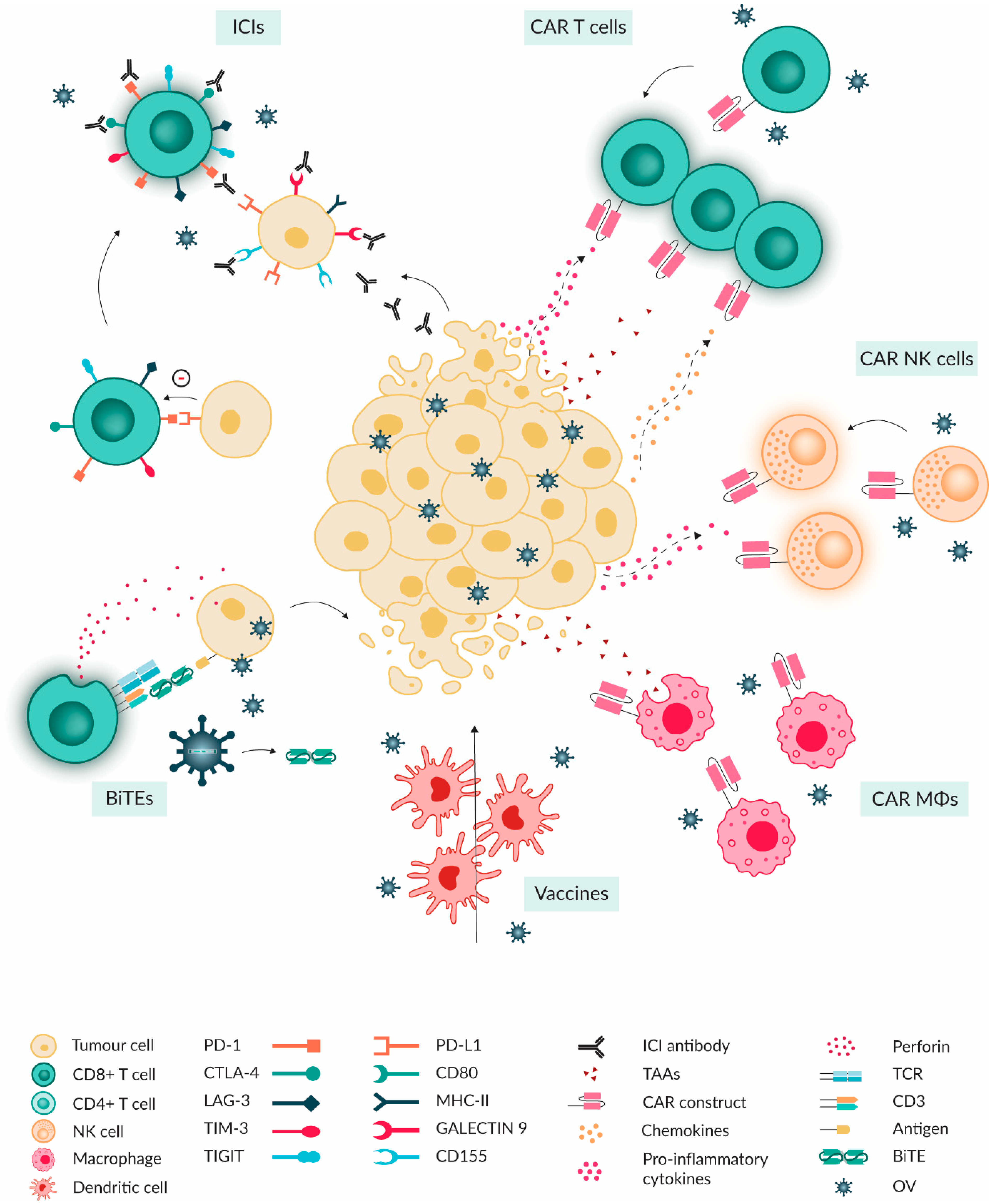
5. Combinational Treatment of OVs with Immunotherapies
5.1. Immune Checkpoint Inhibitors
5.2. Adoptive Cellular Therapy
5.2.1. CAR-T
5.2.2. NK and CAR-NK
5.2.3. CAR-Macrophages
5.3. Cancer Vaccines
5.4. Antibody Therapeutics
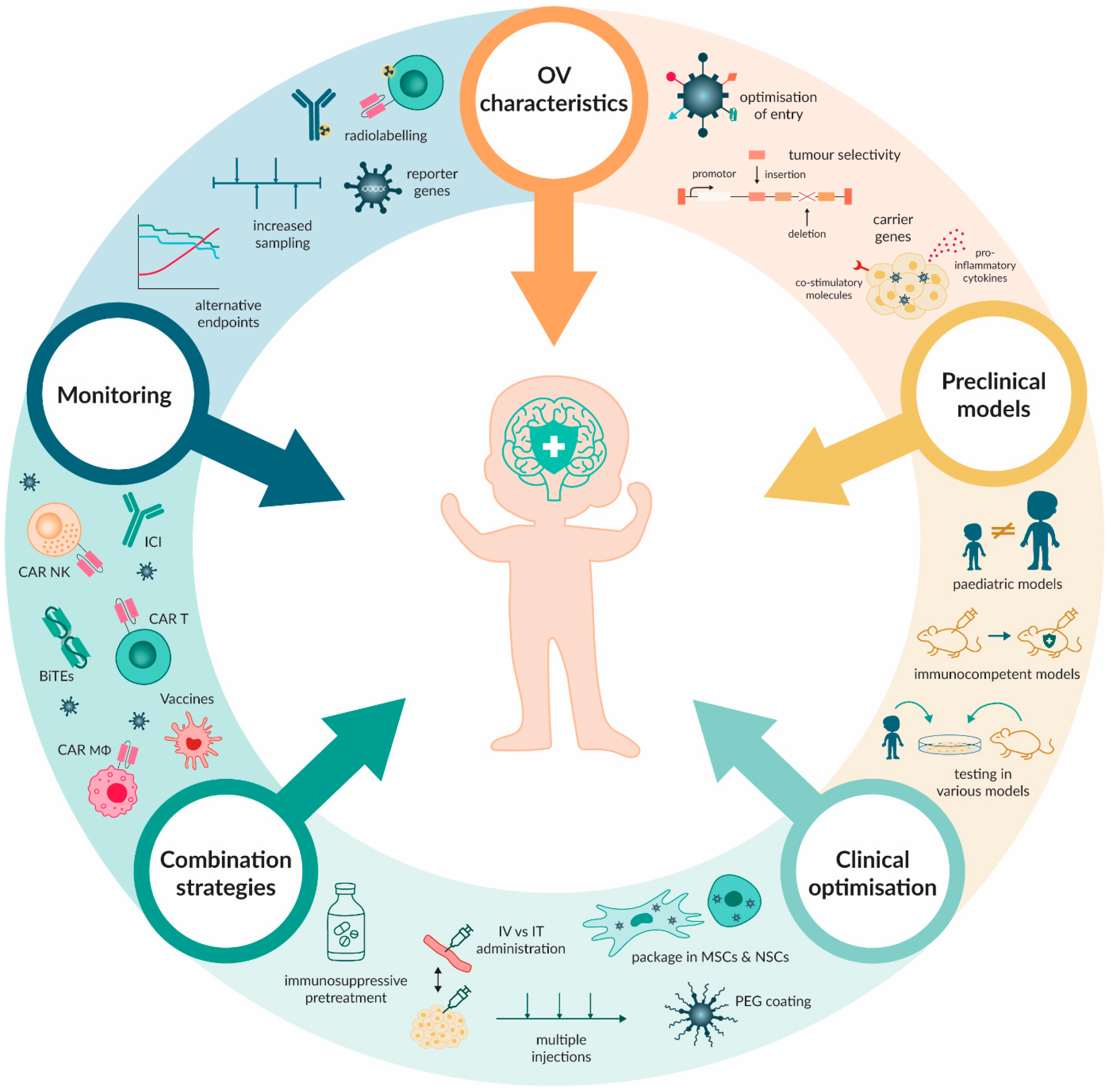
6. Overcoming Limitations and Obstacles of OV Therapy
6.1. Preclinical Barriers
6.2. Monitoring Barriers
6.3. The Interplay between Administration, Neutralising Antibodies and Anti-Viral Response
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
References
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.M.; Reyes-Múgica, M.; Chan, J.K.C.; Hasle, H.; Lazar, A.J.; Rossi, S.; Ferrari, A.; Jarzembowski, J.A.; Pritchard-Jones, K.; Hill, D.A.; et al. A Summary of the Inaugural WHO Classification of Pediatric Tumors: Transitioning from the Optical into the Molecular Era. Cancer Discov. 2022, 12, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, E.S.; van der Lugt, J.; Hulleman, E. Immunotherapy in pediatric brain tumors: Considerations, challenges and future directions. J. Mol. Clin. Med. 2019, 2, 79–91. [Google Scholar]
- Major, N.; Patel, N.A.; Bennett, J.; Novakovic, E.; Poloni, D.; Abraham, M.; Brown, N.J.; Gendreau, J.L.; Sahyouni, R.; Loya, J. The Current State of Radiotherapy for Pediatric Brain Tumors: An Overview of Post-Radiotherapy Neurocognitive Decline and Outcomes. J. Pers. Med. 2022, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; Merchant, T.E.; Gajjar, A.; Reddick, W.E.; Kun, L.E. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004, 5, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Sayour, E.J.; Mitchell, D.A. Immunotherapy for pediatric brain tumors. Brain Sci. 2017, 7, 137. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Ramkissoon, S.H.; Ross, J.; Weintraub, L. Tumor mutational burden and driver mutations: Characterizing the genomic landscape of pediatric brain tumors. Pediatr. Blood Cancer 2020, 67, e28338. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Power, E.A.; Rechberger, J.S.; Gupta, S.; Schwartz, J.D.; Daniels, D.J.; Khatua, S. Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors—An update. Adv. Drug Deliv. Rev. 2022, 185, 114303. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Delaidelli, A.; Vogel, H.; Sorensen, P.H. Pediatric Brain Tumours: Lessons from the Immune Microenvironment. Curr. Oncol. 2023, 30, 5024–5046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef] [PubMed]
- Lichty, B.D.; Breitbach, C.J.; Stojdl, D.F.; Bell, J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.-D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.R.; Gundelach, J.H.; Coffey, M.; West, E.; Scott, K.; Johnson, D.R.; Samson, A.; Melcher, A.; Vile, R.G.; Bram, R.J. Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors. Neurooncol. Adv. 2022, 4, vdac085. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Landi, D.; Brown, M.C.; Friedman, H.S.; McLendon, R.; Herndon, J.E.; Buckley, E.; Bolognesi, D.P.; Lipp, E.; Schroeder, K.; et al. Recombinant polio–rhinovirus immunotherapy for recurrent paediatric high-grade glioma: A phase 1b trial. Lancet Child. Amp Adolesc. Health 2023, 7, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang RM, A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Patel, D.; Foreman, P.; Nabors, B.; Riley, K.; Gillespie, Y.; Markert, J. Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients with Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum. Gene Ther. Clin. Dev. 2016, 27, 69–78. [Google Scholar] [CrossRef]
- Grabovska, Y.; Mackay, A.; O’Hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J.; et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Bockmayr, M.; Mohme, M.; Klauschen, F.; Winkler, B.; Budczies, J.; Rutkowski, S.; Schüller, U. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology 2018, 7, e1462430. [Google Scholar] [CrossRef] [PubMed]
- Melcher, V.; Kerl, K. The growing relevance of immunoregulation in pediatric brain tumors. Cancers 2021, 13, 5601. [Google Scholar] [CrossRef] [PubMed]
- du Chatinier, A.; Velilla, I.Q.; Meel, M.H.; Hoving, E.W.; Hulleman, E.; Metselaar, D.S. Microglia in pediatric brain tumors: The missing link to successful immunotherapy. Cell Rep. Med. 2023, 4, 101246. [Google Scholar] [CrossRef] [PubMed]
- Lieberman NA, P.; Degolier, K.; Kovar, H.M.; Davis, A.; Hoglund, V.; Stevens, J.; Winter, C.; Deutsch, G.; Furlan, S.N.; Vitanza, N.A.; et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: Implications for development of immunotherapy. Neuro Oncol. 2019, 21, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Cao, W.; Xi, X.; Ma, C.; Cui, L.; He, W. The NKG2D ligand ULBP4 binds to TCRγ9/δ2 and induces cytotoxicity to tumor cells through both TCRγδ and NKG2D. Blood 2009, 114, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-β. Front. Immunol. 2019, 10, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.I.; Sayour, E.J.; Flores, C.T.; Grant, G.; Wechsler-Reya, R.; Hoang-Minh, L.B.; Kieran, M.W.; Salcido, J.; Prins, R.M.; Figg, J.W.; et al. The current landscape of immunotherapy for pediatric brain tumors. Nat. Cancer 2022, 3, 11–24. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization of Tumor-Associated Macrophages in Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; Van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Guadagno, E.; Presta, I.; Maisano, D.; Donato, A.; Pirrone, C.K.; Cardillo, G.; Corrado, S.D.; Mignogna, C.; Mancuso, T.; Donato, G.; et al. Role of macrophages in brain tumor growth and progression. Int. J. Mol. Sci. 2018, 19, 1005. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.L.; Sauter, B.; Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLS. Nature 1998, 392, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gholamin, S.; Mitra, S.S.; Feroze, A.H.; Liu, J.; Kahn, S.A.; Zhang, M.; Esparza, R.; Richard, C.; Ramaswamy, V.; Remke, M.; et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 2017, 9, eaaf2968. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, X.; Yu, J.; Ge, S.; Fan, X. Oncolytic Virotherapy: From Bench to Bedside. Front. Cell Dev. Biol. 2021, 9, 790150. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Baxter, P.A.; Zhao, X.; Liu, Z.; Wadhwa, L.; Zhang, Y.; Su JM, F.; Tan, X.; Yang, J.; Adesina, A.; et al. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro Oncol. 2011, 13, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Xia, S.; Wang, Q.; Xu, W.; Huang, H.; Jiang, S.; Lu, L. Development of oncolytic virotherapy: From genetic modification to combination therapy. Front. Med. 2020, 14, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Long, X.; Liu, J.; Cheng, P. Glioblastoma microenvironment and its reprogramming by oncolytic virotherapy. Front. Cell Neurosci. 2022, 16, 819363. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Ordonez, D.; Chagoya, G.; Salehani, A.; Atchley, T.J.; Laskay, N.M.B.; Parr, M.S.; Elsayed, G.A.; Mahavadi, A.K.; Rahm, S.P.; Friedman, G.K.; et al. Immunovirotherapy for the Treatment of Glioblastoma and Other Malignant Gliomas. Neurosurg. Clin. N. Am. 2021, 32, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Tran, N.D.; Puduvalli, V.K.; Elder, J.B.; Fink, K.L.; Conrad, C.A.; Yung, W.K.A.; Penas-Prado, M.; Gomez-Manzano, C.; Peterkin, J.; et al. Phase 1b open-label randomized study of the oncolytic adenovirus DNX-2401 administered with or without interferon gamma for recurrent glioblastoma. J. Clin. Oncol. 2017, 35, 2002. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- van Putten EH, P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef]
- de Graaf, J.F.; de Vor, L.; Fouchier, R.A.M.; van den Hoogen, B.G. Armed oncolytic viruses: A kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 2018, 41, 28–39. [Google Scholar] [CrossRef]
- Hedberg, J.; Studebaker, A.; Smith, L.; Chen, C.Y.; Westfall, J.J.; Cam, M.; Gross, A.; Hernandez-Aguirre, I.; Martin, A.; Kim, D.; et al. Oncolytic virus-driven immune remodeling revealed in mouse medulloblastomas at single cell resolution. Mol. Ther. Oncolytics 2023, 30, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Ghonime, M.G.; Jackson, J.; Shah, A.; Roth, J.; Li, M.; Saunders, U.; Coleman, J.; Gillespie, G.Y.; Markert, J.M.; Cassady, K.A. Chimeric HCMV/HSV-1 and Δγ(1)34.5 oncolytic herpes simplex virus elicit immune mediated antigliomal effect and antitumor memory. Transl. Oncol. 2018, 11, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.M.; Li, S.; Gumin, J.; Daou, M.; Ledbetter, D.; Yang, J.; Singh, S.; Parker Kerrigan, B.C.; Hossain, A.; Yuan, Y.; et al. An immune-competent, replication-permissive Syrian Hamster glioma model for evaluating Delta-24-RGD oncolytic adenovirus. Neuro Oncol. 2021, 23, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moure, M.; Gonzalez-Huarriz, M.; Labiano, S.; Guruceaga, E.; Bandres, E.; Zalacain, M.; Marrodan, L.; de Andrea, C.; Villalba, M.; Martinez-Velez, N.; et al. Delta-24-RGD, an oncolytic adenovirus, increases survival and promotes proinflammatory immune landscape remodeling in models of AT/RT and CNS-PNET. Clin. Cancer Res. 2021, 27, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Denton, N.L.; Chen, C.Y.; Scott, T.R.; Cripe, T.P. Tumor-associated macrophages in oncolytic virotherapy: Friend or foe? Biomedicines 2016, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Meisen, W.H.; Wohleb, E.S.; Jaime-Ramirez, A.C.; Bolyard, C.; Yoo, J.Y.; Russell, L.; Hardcastle, J.; Dubin, S.; Muili, K.; Yu, J.; et al. The impact of macrophage- and microglia-secreted TNFα on oncolytic HSV-1 therapy in the glioblastoma tumor microenvironment. Clin. Cancer Res. 2015, 21, 3274–3285. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Piranlioglu, R.; Ye, F.; Shu, K.; Lei, T.; Nakashima, H. Immunosuppressive cells in oncolytic virotherapy for glioma: Challenges and solutions. Front. Cell. Infect. Microbiol. 2023, 13, 1141034. [Google Scholar] [CrossRef] [PubMed]
- Hofman, L.; Lawler, S.E.; Lamfers ML, M. The multifaceted role of macrophages in oncolytic virotherapy. Viruses 2021, 13, 1570. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vélez, N.; Garcia-Moure, M.; Marigil, M.; González-Huarriz, M.; Puigdelloses, M.; Gallego Pérez-Larraya, J.; Zalacaín, M.; Marrodán, L.; Varela-Guruceaga, M.; Laspidea, V.; et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat. Commun. 2019, 10, 2235. [Google Scholar] [CrossRef]
- Ghajar-Rahimi, G.; Kang, K.D.; Totsch, S.K.; Gary, S.; Rocco, A.; Blitz, S.; Kachurak, K.; Chambers, M.R.; Li, R.; Beierle, E.A.; et al. Clinical advances in oncolytic virotherapy for pediatric brain tumors. Pharmacol. Ther. 2022, 239, 108193. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Vicario, N.; Rong, L.; Valdes, P.A.; Choi, B.D.; Chen, J.A.; DiToro, D.; Osorio, D.S.; Kachurak, K.; Gessler, F.; et al. A novel in situ multiplex immunofluorescence panel for the assessment of tumor immunopathology and response to virotherapy in pediatric glioblastoma reveals a role for checkpoint protein inhibition. Oncoimmunology 2019, 8, e1678921. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, F.; Menotti, L.; Avitabile, E.; Appolloni, I.; Ceresa, D.; Marubbi, D.; Campadelli-Fiume, G.; Malatesta, P. Eradication of glioblastoma by immuno-virotherapy with a retargeted oncolytic HSV in a preclinical model. Oncogene 2019, 38, 4467–4479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, C.; Miao, J.; Wang, Z.; Wang, Z.; Cheng, Z.; Wang, P.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, Y. A Tumor-Targeted Replicating Oncolytic Adenovirus Ad-TD-nsIL12 as a Promising Therapeutic Agent for Human Esophageal Squamous Cell Carcinoma. Cells 2020, 9, 2438. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lu, T.; Li, Z.; Teng, K.Y.; Mansour, A.G.; Yu, M.; Tian, L.; Xu, B.; Ma, S.; Zhang, J.; et al. An Oncolytic Virus Expressing IL15/IL15Rα Combined with Off-the-Shelf EGFR-CAR NK Cells Targets Glioblastoma. Cancer Res. 2021, 81, 3635–3648. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Rivera-Molina, Y.; Gomez-Manzano, C.; Clise-Dwyer, K.; Bover, L.; Vence, L.M.; Yuan, Y.; Lang, F.F.; Toniatti, C.; Hossain, M.B.; et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017, 77, 3894–3907. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Swanner, J.; Jaime-Ramirez, A.C.; Wang, Y.; Sprague, A.; Banasavadi-Siddegowda, Y.; Yoo, J.Y.; Sizemore, G.M.; Kladney, R.; Zhang, J.; et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat. Commun. 2018, 9, 5006. [Google Scholar] [CrossRef] [PubMed]
- Sahu, U.; Mullarkey, M.P.; Pei, G.; Zhao, Z.; Hong, B.; Kaur, B. oHSV-P10 reduces glioma stem cell enrichment after oncolytic HSV therapy. Mol. Ther. Oncolytics 2023, 29, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Song, X.; Wang, Y.; Liu, F.; Wei, J. Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment. Front. Immunol. 2020, 11, 683. [Google Scholar] [CrossRef]
- Bartee, M.Y.; Dunlap, K.M.; Bartee, E. Tumor-localized secretion of soluble PD1 enhances oncolytic virotherapy. Cancer Res. 2017, 77, 2952–2963. [Google Scholar] [CrossRef]
- Xie, D.; Tian, Y.; Hu, D.; Wang, Y.; Yang, Y.; Zhou, B.; Zhang, R.; Ren, Z.; Liu, M.; Xu, J.; et al. Oncolytic adenoviruses expressing checkpoint inhibitors for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 436. [Google Scholar] [CrossRef]
- Engeland, C.E.; Grossardt, C.; Veinalde, R.; Bossow, S.; Lutz, D.; Kaufmann, J.K.; Shevchenko, I.; Umansky, V.; Nettelbeck, D.M.; Weichert, W.; et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014, 22, 1949–1959. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64. [Google Scholar] [CrossRef] [PubMed]
- Evgin, L.; Kottke, T.; Tonne, J.; Thompson, J.; Huff, A.L.; van Vloten, J.; Moore, M.; Michael, J.; Driscoll, C.; Pulido, J.; et al. Oncolytic virus–mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 2022, 14, 2231. [Google Scholar] [CrossRef]
- Boccalatte, F.; Mina, R.; Aroldi, A.; Leone, S.; Suryadevara, C.M.; Placantonakis, D.G.; Bruno, B. Advances and Hurdles in CAR T Cell Immune Therapy for Solid Tumors. Cancers 2022, 14, 5108. [Google Scholar] [CrossRef]
- Evgin, L.; Vile, R.G. Parking car t cells in tumours: Oncolytic viruses as valets or vandals? Cancers 2021, 13, 1106. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Diaconu, I.; Liu, H.; Cerullo, V.; Caruana, I.; Hoyos, V.; Bouchier-Hayes, L.; Savoldo, B.; Dotti, G. Armed Oncolytic Virus Enhances Immune Functions of Chimeric Antigen Receptor-Modified T Cells in Solid Tumors. Cancer Res. 2014, 74, 5195. [Google Scholar] [CrossRef]
- Rosewell Shaw, A.; Porter, C.E.; Watanabe, N.; Tanoue, K.; Sikora, A.; Gottschalk, S.; Brenner, M.K.; Suzuki, M. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells against Metastatic Head and Neck Cancer. Mol. Ther. 2017, 25, 2440–2451. [Google Scholar] [CrossRef]
- Aalipour, A.; Le Boeuf, F.; Tang, M.; Murty, S.; Simonetta, F.; Lozano, A.X.; Shaffer, T.M.; Bell, J.C.; Gambhir, S.S. Viral Delivery of CAR Targets to Solid Tumors Enables Effective Cell Therapy. Mol. Ther. Oncolytics 2020, 17, 232–240. [Google Scholar] [CrossRef]
- Evgin, L.; Huff, A.L.; Wongthida, P.; Thompson, J.; Kottke, T.; Tonne, J.; Schuelke, M.; Ayasoufi, K.; Driscoll, C.B.; Shim, K.G.; et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat. Commun. 2020, 11, 3187. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Pediatric Brain Tumor Consortium. HER2-Specific Chimeric Antigen Receptor (CAR) T Cells for Children with Ependymoma. NCT04903080. 2022. Available online: https://clinicaltrials.gov/study/NCT04903080 (accessed on 25 April 2024).
- St. Jude Children’s Research Hospital. B7-H3-Specific Chimeric Antigen Receptor Autologous T-Cell Therapy for Pediatric Patients with Solid Tumors (3CAR). NCT04897321. 2022. Available online: https://clinicaltrials.gov/study/NCT04897321 (accessed on 25 April 2024).
- Kulubya, E.S.; Kercher, M.J.; Phillips, H.W.; Antony, R.; Edwards, M.S.B. Advances in the Treatment of Pediatric Brain Tumors. Children 2023, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.M.; Khakoo, S.I.; Biron, C.A. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr. Opin. Virol. 2011, 1, 497–512. [Google Scholar] [CrossRef]
- Quixabeira, D.C.A.; Pakola, S.; Jirovec, E.; Havunen, R.; Basnet, S.; Santos, J.M.; Kudling, T.V.; Clubb, J.H.A.; Haybout, L.; Arias, V.; et al. Boosting cytotoxicity of adoptive allogeneic NK cell therapy with an oncolytic adenovirus encoding a human vIL-2 cytokine for the treatment of human ovarian cancer. Cancer Gene Ther. 2023, 30, 1679–1690. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, J.Y.; Lee, T.J.; Liu, J.; Yu, J.; Caligiuri, M.A.; Kaur, B.; Friedman, A. Complex role of NK cells in regulation of oncolytic virus–bortezomib therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Heipertz, E.L.; Zynda, E.R.; Stav-Noraas, T.E.; Hungler, A.D.; Boucher, S.E.; Kaur, N.; Vemuri, M.C. Current Perspectives on “Off-The-Shelf” Allogeneic NK and CAR-NK Cell Therapies. Front. Immunol. 2021, 12, 732135. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Li, Y.; He, C.; Wang, T.; Zheng, X.; Liu, H.; Wu, Z.; Zhang, J.; Liao, X.; Zhang, L. Anti-tumor efficacy of anti-GD2 CAR NK-92 cells in diffuse intrinsic pontine gliomas. Front. Immunol. 2023, 14, 1145706. [Google Scholar] [CrossRef]
- Chen, C.; Jing, W.; Chen, Y.; Wang, G.; Abdalla, M.; Gao, L.; Han, M.; Shi, C.; Li, A.; Sun, P.; et al. Intracavity generation of glioma stem cell–specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 2022, 14, eabn1128. [Google Scholar] [CrossRef]
- Moaven, O.; Mangieri, C.W.; Stauffer, J.A.; Anastasiadis, P.Z.; Borad, M.J. Evolving Role of Oncolytic Virotherapy: Challenges and Prospects in Clinical Practice. JCO Precis. Oncol. 2021, 5, 432–441. [Google Scholar] [CrossRef]
- Woller, N.; Knocke, S.; Mundt, B.; Gürlevik, E.; Strüver, N.; Kloos, A.; Boozari, B.; Schache, P.; Manns, M.P.; Malek, N.P.; et al. Virus-induced tumor inflammation facilitates effective DC cancer immunotherapy in a Treg-dependent manner in mice. J. Clin. Investig. 2011, 121, 2570–2582. [Google Scholar] [CrossRef]
- Slaney, C.Y.; Wang, P.; Darcy, P.K.; Kershaw, M.H. CARs versus BiTEs: A Comparison between T Cell–Redirection Strategies for Cancer Treatment. Cancer Discov. 2018, 8, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Lotze, M.T.; Zhu, Z.; Storkus, W.J.; Song, X.T. Bi- and Tri-specific T cell engager-armed oncolytic viruses: Next-generation cancer immunotherapy. Biomedicines 2020, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef]
- Yu, F.; Wang, X.; Guo, Z.S.; Bartlett, D.L.; Gottschalk, S.M.; Song, X.T. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol. Ther. 2014, 22, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Misuraca, K.L.; Cordero, F.J.; Becher, O.J. Pre-clinical models of diffuse intrinsic pontine glioma. Front. Oncol. 2015, 5, 172. [Google Scholar] [CrossRef]
- Du Chatinier, A.; Meel, M.H.; Das, A.I.; Metselaar, D.S.; Waranecki, P.; Bugiani, M.; Breur, M.; Simonds, E.F.; Lu, E.D.; Weiss, W.A.; et al. Generation of immunocompetent syngeneic allograft mouse models for pediatric diffuse midline glioma. Neurooncol Adv. 2022, 4, vdac079. [Google Scholar] [CrossRef]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva PM, A.; Bousbaa, H. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Baskar, G.; Palaniyandi, T.; Viswanathan, S.; Rajendran, B.K.; Ravi, M.; Sivaji, A. Development of patient derived organoids for cancer drug screening applications. Acta Histochem. 2022, 124, 151895. [Google Scholar] [CrossRef]
- Vazaios, K.; Stavrakaki, Ε.; Vogelezang, L.B.; Ju, J.; Waranecki, P.; Metselaar, D.S.; Meel, M.H.; Kemp, V.; van den Hoogen, B.G.; Hoeben, R.C.; et al. The heterogeneous sensitivity of pediatric brain tumors to different oncolytic viruses is predicted by unique gene expression profiles. Mol. Ther. Oncol. 2024, 32, 200804. [Google Scholar] [CrossRef]
- Stavrakaki, E.; van den Bossche, W.B.L.; Vogelezang, L.B.; Teodosio, C.; Mustafa, D.M.; van Dongen, J.J.M.; Dirven, C.M.F.; Balvers, R.K.; Lamfers, M.L. An autologous ex vivo model for exploring patient-specific responses to viro-immunotherapy in glioblastoma. Cell Rep. Methods 2024, 4, 100716. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Andtbacka RH, I.; Collichio, F.A.; Wolf, M.; Zhao, Z.; Shilkrut, M.; Puzanov, I.; Ross, M. Durable response rate as an endpoint in cancer immunotherapy: Insights from oncolytic virus clinical trials. J. Immunother. Cancer 2017, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Ratnasiri, K.; Wilk, A.J.; Lee, M.J.; Khatri, P.; Blish, C.A. Single-cell RNA-seq methods to interrogate virus-host interactions. Semin. Immunopathol. 2023, 45, 71–89. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Rojas, F.; Hernandez, S.; Lazcano, R.; Laberiano-Fernandez, C.; Parra, E.R. Multiplex Immunofluorescence and the Digital Image Analysis Workflow for Evaluation of the Tumor Immune Environment in Translational Research. Front. Oncol. 2022, 12, 889886. [Google Scholar] [CrossRef]
- Budhiraja, S.; Najem, H.; Tripathi, S.; Wadhawani, N.R.; Horbinski, C.; Mccord, M.; Lenzen, A.C.; Heimberger, A.B.; Decuypere, M. Immunobiology and Cytokine Modulation of the Pediatric Brain Tumor Microenvironment: A Scoping Review. Cancers 2023, 15, 3655. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Kim, S.I.; O’Leary, M.; Park, A.K.; Lu, J.; Kang, S.; Zhang, Z.; Yang, A.; Woo, Y.; Fong, Y.; et al. Toward comprehensive imaging of oncolytic viroimmunotherapy. Mol. Ther. Oncolytics 2021, 23, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Errington, F.; Diaz, R.M.; Pandha, H.S.; Harrington, K.J.; Melcher, A.A.; Vile, R.G. The Case of Oncolytic Viruses Versus the Immune System: Waiting on the Judgment of Solomon. Hum. Gene Ther. 2009, 20, 1119. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, H.; Kottke, T.; White, C.; Twigger, K.; Diaz, R.M.; Thompson, J.; Selby, P.; De Bono, J.; Melcher, A.; et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008, 14, 259–269. [Google Scholar] [CrossRef]
- Xu, F.; Lee, F.K.; Morrow, R.A.; Sternberg, M.R.; Luther, K.E.; Dubin, G.; Markowitz, L.E. Seroprevalence of Herpes Simplex Virus Type 1 in Children in the United States. J. Pediatr. 2007, 151, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Gumin, J.; Gao, F.; Hossain, A.; Shpall, E.J.; Kondo, A.; Parker Kerrigan, B.C.; Yang, J.; Ledbetter, D.; Fueyo, J.; et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J. Neurosurg. 2021, 136, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef]
- Cattaneo, R.; Miest, T.; Shashkova, E.V.; Barry, M.A. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat. Rev. Microbiol. 2008, 6, 529–540. [Google Scholar] [CrossRef] [PubMed]
| Virus Type | Name | Modifications | Clinical Trial (Administrative Route) | Paediatric or Adult | Patients (N) | Trial Status | Survival | Ref. |
|---|---|---|---|---|---|---|---|---|
| DNA viruses | ||||||||
| Adenovirus (AdV) | DNX-2401 | Insertion of RGD-4C peptide in the fiber knob. 24 bp deletion in E1A viral gene responsible for Rb-binding. | #NCT03178032 (IT) | Paediatric | 12 | Completed | MOS = 17.8 months/OSR = 50% at 18 moths | [15] |
| #NCT02197169 (IT) | Adult | 27 | Completed | OSR = 33% at 12 months, 22% at 18 months | [39] | |||
| #NCT00805376 (IT) | Adult | 37 | Completed | MOS = 12 months | [40] | |||
| #NCT01956734 (IT) | Adult | 31 | Completed | Not released yet | ||||
| #NCT01582516 (IT) | Adult | 20 | Completed | MOS = 4.3 months | [41] | |||
| #NCT02798406 (IT) | Adult | 49 | Completed | MOS = 12.5 months/OSR = 52.7% at 12 months | [19] | |||
| #NCT03896568 (IA) | Adult | 36 | Recruiting | - | ||||
| Ad-TD-nsIL12 | Deletion in E1A, E1B and E3gp-19k genes. Expresses a non-secretory form of IL-12 under E3gp-19k promoter. | #NCT05717712 (IT) | Paediatric | 18 | Recruiting | - | ||
| #NCT05717699 (IT) | Paediatric | 18 | Recruiting | - | ||||
| DNX-2440 | Insertion of RGD-4C peptide in the fibre knob. 24 bp deletion in E1A viral gene responsible for Rb-binding. Expresses the co-stimulatory OX40 ligand, replacing E3 region. | #NCT03714334 (IT) | Adult | 16 | Terminated | Not yet released | ||
| CRAd-S-pk7 | Deletion of native E1 promoter. E1A expression under control of an inserted human surviving promoter and additionally encodes pk7 polylysine. | #NCT05139056 (ICT) | Adult | 36 | Recruiting | MOS = 15.7 | ||
| #NCT06169280 (IT) | Adult | - | Not recruiting yet | - | ||||
| #NCT03072134 (IT) | Adult | 12 | Completed | MOS = 18.4 months | [42] | |||
| ONYX-015 | Deletion of E1B-55kD gene. | #NCT00006106 (IA) | Adult | 24 | Withdrawn | MOS = 6.2 months | [43] | |
| ICOVIR-5 | Insertion of RGD-4C peptide in the fibre knob. 24 bp deletion in E1A viral gene responsible for Rb-binding. In addition, the E1A promoter is replaced by E2F1-responsive elements. | #NCT04758533 (IV) | Paediatric | 12 | Recruiting | - | ||
| Herpes simplex virus (HSV) | C134 | Deletion of both copies of the principal virulence gene γ134.5. Additionally has IRS1 gene under control by human cytomegalovirus immediate early promoter. | #NCT03657576 (IT) | Adult | 24 | Recruiting | - | |
| G207 | Contains deletion of the diploid γ134.5 neurovirulence gene and has viral ribonucleotide reductase (UL39) disabled by insertion of Escherichia coli lacZ. | #NCT02457845 (IT) | Paediatric | 12 | Completed | MOS = 12.2 months | [16] | |
| #NCT03911388 (IT) | Paediatric | 24 | Active, not recruiting | - | ||||
| #NCT04482933 (IT) | Paediatric | 40 | Not yet recruiting | - | ||||
| M032 | Contains deletion of the diploid γ134.5 neurovirulence gene. Expresses the gene for IL-12. | #NCT02062827 (IT) | Adult | 29 | Active, not recruiting | - | [20] | |
| CAN-3110 | Deletion of one copy of the principal virulence genes γ134.5 and UL39. ICP-34.5 under control of human Nestin promoter. | #NCT03152318 (IT) | Adult | 62 | Recruiting | MOS = 11.6 months | [44] | |
| G47Delta | Deletion of both copies of the principal virulence gene γ134.5. Inactivation of the ICP6 gene. Deletion of the a47gene and US11 promoter. | #UMIN000015995 (IT) | Adult | 30 | Completed | MOS = 20.2 | [45] | |
| HSV1716 | 759bp deletion in both copies of the principal virulence gene γ134.5. | #NCT02031965 (IT) | Paediatric | 2 | Terminated | Not released yet | ||
| Vaccinia Virus (VV) | TG6002 | Deletion of viral thymidine kinase (TK) gene. Deletion of the viral ribonucleotide reductase (RR) gene. Addition of chimeric yeast FCU1 suicide gene in TK locus. | #NCT03294486 (IV) | Adult | 78 | Unknown | Not yet released | |
| Parvovirus (PVV) | ParvOryx | Unmodified. | #NCT01301430 (IT/IV) | Adult | 18 | Completed | MOS = 15 months | [46] |
| RNA viruses | ||||||||
| Reovirus (RV) | Pelareorep | Unmodified. | #NCT02444546 (IV) | Paediatric | 6 | Completed | MOS = 11.7 months | [17] |
| #NCT01166542 (IV) | Adult | 167 | Completed | Not released yet | ||||
| Newcastle disease virus (NDV) | NDV-HJU | Unmodified. | #NCT01174537 (IV) | Paediatric and Adult | 30 | Withdrawn | Not released yet | [47] |
| Measles virus (MV) | MV-NIS | Expresses human thyroidal NIS. | #NCT02962167 (IT/LB) | Paediatric | 34 | Completed | Not yet released | |
| MV-CEA | Secretes the extracellular domain of human CEA. | #NCT00390299 (IT) | Adult | 23 | Completed | MOS = 11.6/OSR = 45.5% at 12 months | [45] | |
| Poliovirus (PV) | PVSRIPO | Live-attenuated poliovirus vaccine carrying a heterologous internal ribosomal entry site (IRES) of human rhinovirus type 2 (HRV2). | #NCT03043391 (IT) | Paediatric | 8 | Completed | MOS = 4.1 months | [18] |
| #NCT02986178 (IT) | Adult | 122 | Active, not recruiting | MOS = 12.5 months/OSR = 21% at 24 and 36 months | [48] | |||
| Immune Cell Type | Modulation | OV Type (Name) | Tumour Type | Ref. |
|---|---|---|---|---|
| CD4+ T cells | Increased influx in TME | AdV (DNX-2401) | DMG | [15] |
| Durable increased influx in TME and adjacent + distant sites | HSV (HSV-1 G207) | HGG | [16] | |
| CD8+ T cells | Increased influx in TME | AdV (DNX-2401), PV (PVSRIPO) | DMG, HGG | [15,18] |
| Durable increased influx in TME and adjacent + distant sites | HSV (HSV-1 G207) | HGG | [16] | |
| Tregs | Absent after OV treatment | AdV (DNX-2401) | DMG | [15] |
| B cells | Increased levels in TME | HSV (HSV-1 G207) | HGG | [16] |
| Plasma cells | Increased levels in TME | HSV (HSV-1 G207) | HGG | [16] |
| DCs | Increased levels of monocytes | RV (Pelareorep) | GBM, DMG, MB | [17] |
| Macrophages | Up-regulation of immune response terms | AdV (DNX-2401) | DMG | [15] |
| Increased levels of monocytes | RV (Pelareorep) | GBM, DMG, MB | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazaios, K.; van Berkum, R.E.; Calkoen, F.G.; van der Lugt, J.; Hulleman, E. OV Modulators of the Paediatric Brain TIME: Current Status, Combination Strategies, Limitations and Future Directions. Int. J. Mol. Sci. 2024, 25, 5007. https://doi.org/10.3390/ijms25095007
Vazaios K, van Berkum RE, Calkoen FG, van der Lugt J, Hulleman E. OV Modulators of the Paediatric Brain TIME: Current Status, Combination Strategies, Limitations and Future Directions. International Journal of Molecular Sciences. 2024; 25(9):5007. https://doi.org/10.3390/ijms25095007
Chicago/Turabian StyleVazaios, Konstantinos, Ronja E. van Berkum, Friso G. Calkoen, Jasper van der Lugt, and Esther Hulleman. 2024. "OV Modulators of the Paediatric Brain TIME: Current Status, Combination Strategies, Limitations and Future Directions" International Journal of Molecular Sciences 25, no. 9: 5007. https://doi.org/10.3390/ijms25095007
APA StyleVazaios, K., van Berkum, R. E., Calkoen, F. G., van der Lugt, J., & Hulleman, E. (2024). OV Modulators of the Paediatric Brain TIME: Current Status, Combination Strategies, Limitations and Future Directions. International Journal of Molecular Sciences, 25(9), 5007. https://doi.org/10.3390/ijms25095007






