Precision Medicine for Blood Glutamate Grabbing in Ischemic Stroke
Abstract
:1. Introduction
2. Results
2.1. Sample Description
2.2. Demographic and Clinical Features Associated with Poor Functional Outcome at 3 Months
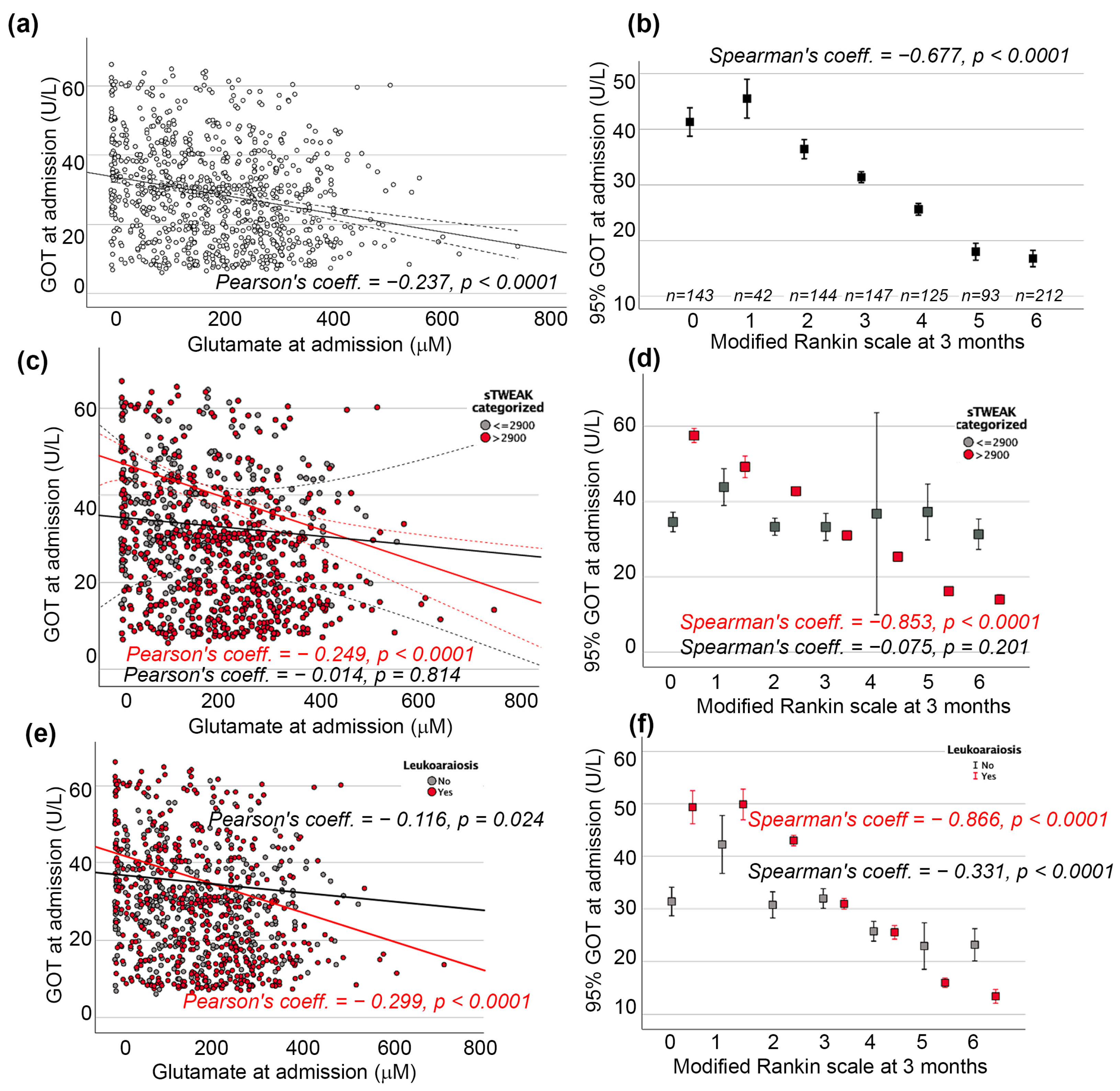
2.3. GOT and sTWEAK Association with Poor Functional Outcome at 3 Months
2.4. GOT and Leukoaraiosis Association with Poor Functional Outcome at 3 Months
3. Discussion
4. Materials and Methods
4.1. Standard Protocol Approval and Patient Consent
4.2. Study Design
4.3. Inclusion and Exclusion Criteria
4.4. Biochemical and Hematological Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing Populations: The Challenges Ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- World Health Organization Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 22 January 2024).
- Rochmah, T.N.; Rahmawati, I.T.; Dahlui, M.; Budiarto, W.; Bilqis, N. Economic Burden of Stroke Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7552. [Google Scholar] [CrossRef]
- Rudberg, A.-S.; Berge, E.; Gustavsson, A.; Näsman, P.; Lundström, E. Long-Term Health-Related Quality of Life, Survival and Costs by Different Levels of Functional Outcome Six Months after Stroke. Eur. Stroke J. 2018, 3, 157–164. [Google Scholar] [CrossRef]
- Langhorne, P.; Ramachandra, S.; Stroke Unit Trialists’ Collaboration. Organised Inpatient (Stroke Unit) Care for Stroke: Network Meta-Analysis. Cochrane Database Syst. Rev. 2020, 4, CD000197. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Campbell, B. Thrombolysis and Thrombectomy for Acute Ischemic Stroke: Strengths and Synergies. Semin. Thromb. Hemost. 2016, 43, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Symons, S.P.; Hopyan, J.; Aviv, R.I. Factors Influencing Clinically Meaningful Recanalization after IV-RtPA in Acute Ischemic Stroke. Am. J. Neuroradiol. 2013, 34, 146–152. [Google Scholar] [CrossRef]
- El Amki, M.; Wegener, S. Improving Cerebral Blood Flow after Arterial Recanalization: A Novel Therapeutic Strategy in Stroke. Int. J. Mol. Sci. 2017, 18, 2669. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Rey, R.; Rodríguez-Yáñez, M.; Rodríguez-Castro, E.; Pumar, J.M.; Arias, S.; Santamaría, M.; López-Dequidt, I.; Hervella, P.; Correa-Paz, C.; Sobrino, T.; et al. Worse Outcome in Stroke Patients Treated with Rt-PA without Early Reperfusion: Associated Factors. Transl. Stroke Res. 2018, 9, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hervella, P.; Rodríguez-Castro, E.; Rodríguez-Yáñez, M.; Arias, S.; Santamaría-Cadavid, M.; López-Dequidt, I.; Estany-Gestal, A.; Maqueda, E.; López-Loureiro, I.; Sobrino, T.; et al. Intra- and Extra-Hospital Improvement in Ischemic Stroke Patients: Influence of Reperfusion Therapy and Molecular Mechanisms. Sci. Rep. 2020, 10, 3513. [Google Scholar] [CrossRef] [PubMed]
- Hervella, P.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Sobrino, T.; Campos, F.; Castillo, J.; da Silva-Candal, A.; Iglesias-Rey, R. STWEAK as Predictor of Stroke Recurrence in Ischemic Stroke Patients Treated with Reperfusion Therapies. Front. Neurol. 2021, 12, 652867. [Google Scholar] [CrossRef] [PubMed]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 870141. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Fisher, M. Future of Neuroprotection for Acute Stroke: In the Aftermath of the SAINT Trials. Ann. Neurol. 2007, 61, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Black, S.E.; Hakim, A.M. Toward Wisdom From Failure. Stroke 2002, 33, 2123–2136. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.H.; Sheth, K.N.; Jha, R.M. Precision Medicine in Neurocritical Care for Cerebrovascular Disease Cases. Stroke 2023, 54, 1392–1402. [Google Scholar] [CrossRef]
- Hinman, J.D.; Rost, N.S.; Leung, T.W.; Montaner, J.; Muir, K.W.; Brown, S.; Arenillas, J.F.; Feldmann, E.; Liebeskind, D.S. Principles of Precision Medicine in Stroke. J. Neurol. Neurosurg. Psychiatry 2017, 88, 54–61. [Google Scholar] [CrossRef]
- Castellanos, M.; Sobrino, T.; Pedraza, S.; Moldes, O.; Pumar, J.M.; Silva, Y.; Serena, J.; García-Gil, M.; Castillo, J.; Dávalos, A.; et al. High Plasma Glutamate Concentrations Are Associated with Infarct Growth in Acute Ischemic Stroke. Neurology 2008, 71, 1862–1868. [Google Scholar] [CrossRef]
- Meng, X.; Li, N.; Guo, D.-Z.; Pan, S.-Y.; Li, H.; Yang, C. High Plasma Glutamate Levels Are Associated with Poor Functional Outcome in Acute Ischemic Stroke. Cell Mol. Neurobiol. 2015, 35, 159–165. [Google Scholar] [CrossRef]
- Castillo, J.; Dávalos, A.; Naveiro, J.; Noya, M. Neuroexcitatory Amino Acids and Their Relation to Infarct Size and Neurological Deficit in Ischemic Stroke. Stroke 1996, 27, 1060–1065. [Google Scholar] [CrossRef]
- Castillo, J.; Dávalos, A.; Noya, M. Progression of Ischaemic Stroke and Excitotoxic Aminoacids. Lancet 1997, 349, 79–83. [Google Scholar] [CrossRef]
- Dávalos, A.; Castillo, J.; Serena, J.; Noya, M.; Dávalos, A.; Castillo, J.; Serena, J.; Noya, M. Duration of Glutamate Release after Acute Ischemic Stroke. Stroke 1997, 28, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Castillo, J.; Loza, M.I.; Mirelman, D.; Brea, J.; Blanco, M.; Sobrino, T.; Campos, F. A Novel Mechanism of Neuroprotection: Blood Glutamate Grabber. J. Cereb. Blood Flow Metab. 2016, 36, 292–301. [Google Scholar] [CrossRef]
- Zlotnik, A.; Sinelnikov, I.; Gruenbaum, B.F.; Gruenbaum, S.E.; Dubilet, M.; Dubilet, E.; Leibowitz, A.; Ohayon, S.; Regev, A.; Boyko, M.; et al. Effect of Glutamate and Blood Glutamate Scavengers Oxaloacetate and Pyruvate on Neurological Outcome and Pathohistology of the Hippocampus after Traumatic Brain Injury in Rats. Anesthesiology 2012, 116, 73–83. [Google Scholar] [CrossRef]
- da Silva-Candal, A.; Pérez-Díaz, A.; Santamaría, M.; Correa-Paz, C.; Rodríguez-Yáñez, M.; Ardá, A.; Pérez-Mato, M.; Iglesias-Rey, R.; Brea, J.; Azuaje, J.; et al. Clinical Validation of Blood/Brain Glutamate Grabbing in Acute Ischemic Stroke. Ann. Neurol. 2018, 84, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Teichberg, V.I.; Cohen-Kashi-Malina, K.; Cooper, I.; Zlotnik, A. Homeostasis of Glutamate in Brain Fluids: An Accelerated Brain-to-Blood Efflux of Excess Glutamate Is Produced by Blood Glutamate Scavenging and Offers Protection from Neuropathologies. Neuroscience 2009, 158, 301–308. [Google Scholar] [CrossRef]
- Rajeev, V.; Fann, D.Y.; Dinh, Q.N.; Kim, H.A.; De Silva, T.M.; Lai, M.K.P.; Chen, C.L.-H.; Drummond, G.R.; Sobey, C.G.; Arumugam, T.V. Pathophysiology of Blood Brain Barrier Dysfunction during Chronic Cerebral Hypoperfusion in Vascular Cognitive Impairment. Theranostics 2022, 12, 1639–1658. [Google Scholar] [CrossRef]
- Yepes, M. TWEAK and Fn14 in the Neurovascular Unit. Front. Immunol. 2013, 4, 367. [Google Scholar] [CrossRef]
- O’Sullivan, M. Leukoaraiosis. Pract. Neurol. 2008, 8, 26–38. [Google Scholar] [CrossRef]
- da Silva-Candal, A.; Custodia, A.; López-Dequidt, I.; Rodríguez-Yáñez, M.; Alonso-Alonso, M.L.; Ávila-Gómez, P.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Campos, F.; et al. STWEAK Is a Leukoaraiosis Biomarker Associated with Neurovascular Angiopathy. Ann. Clin. Transl. Neurol. 2022, 9, 171–180. [Google Scholar] [CrossRef]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Castillo, J. Oxaloacetate: A Novel Neuroprotective for Acute Ischemic Stroke. Int. J. Biochem. Cell Biol. 2012, 44, 262–265. [Google Scholar] [CrossRef]
- Dopico-López, A.; Pérez-Mato, M.; da Silva-Candal, A.; Iglesias-Rey, R.; Rabinkov, A.; Bugallo-Casal, A.; Sobrino, T.; Mirelman, D.; Castillo, J.; Campos, F. Inhibition of Endogenous Blood Glutamate Oxaloacetate Transaminase Enhances the Ischemic Damage. Transl. Res. 2021, 230, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Argibay, B.; Agulla, J.; Pérez-Mato, M.; Rodríguez-González, R.; Brea, D.; Castillo, J. Neuroprotection by Glutamate Oxaloacetate Transaminase in Ischemic Stroke: An Experimental Study. J. Cereb. Blood Flow Metab. 2011, 31, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Castellanos, M.; Blanco, M.; Rodríguez-Yáñez, M.; Serena, J.; Leira, R.; Castillo, J. High Blood Glutamate Oxaloacetate Transaminase Levels Are Associated with Good Functional Outcome in Acute Ischemic Stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Rodríguez-Yáñez, M.; Castellanos, M.; Arias, S.; Pérez-Mato, M.; Sobrino, T.; Blanco, M.; Serena, J.; Castillo, J. Blood Levels of Glutamate Oxaloacetate Transaminase Are More Strongly Associated with Good Outcome in Acute Ischaemic Stroke than Glutamate Pyruvate Transaminase Levels. Clin. Sci. 2011, 121, 11–17. [Google Scholar] [CrossRef]
- The Promise of Precision Medicine. Available online: https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/promise-precision-medicine (accessed on 29 May 2024).
- Akhoon, N. Precision Medicine: A New Paradigm in Therapeutics. Int. J. Prev. Med. 2021, 12, 12. [Google Scholar] [CrossRef]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision Medicine: Concept and Tools. Med. J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kamtchum-Tatuene, J.; Jickling, G.C. Blood Biomarkers for Stroke Diagnosis and Management. Neuromol. Med. 2019, 21, 344–368. [Google Scholar] [CrossRef]
- Simpkins, A.N.; Janowski, M.; Oz, H.S.; Roberts, J.; Bix, G.; Doré, S.; Stowe, A.M. Biomarker Application for Precision Medicine in Stroke. Transl. Stroke Res. 2020, 11, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Winkles, J.A. The TWEAK–Fn14 Cytokine–Receptor Axis: Discovery, Biology and Therapeutic Targeting. Nat. Rev. Drug Discov. 2008, 7, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, R.; Gongora, M.C.; Winkles, J.A.; Yepes, M. Tumor Necrosis Factor-Like Weak Inducer of Apoptosis Increases the Permeability of the Neurovascular Unit through Nuclear Factor-ΚB Pathway Activation. J. Neurosci. 2005, 25, 10094–10100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Winkles, J.A.; Gongora, M.C.; Polavarapu, R.; Michaelson, J.S.; Hahm, K.; Burkly, L.; Friedman, M.; Li, X.-J.; Yepes, M. TWEAK—Fn14 Pathway Inhibition Protects the Integrity of the Neurovascular Unit during Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2007, 27, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.; Sbai, O.; Wen, J.; Couraud, P.-O.; Putterman, C.; Khrestchatisky, M.; Desplat-Jégo, S. TWEAK/Fn14 Pathway Modulates Properties of a Human Microvascular Endothelial Cell Model of Blood Brain Barrier. J. Neuroinflamm. 2013, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Candal, A.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Ávila-Gómez, P.; Sobrino, T.; Campos, F.; Castillo, J.; Hervella, P.; et al. The Presence of Leukoaraiosis Enhances the Association between STWEAK and Hemorrhagic Transformation. Ann. Clin. Transl. Neurol. 2020, 7, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-X.; Cai, J.-Y.; Sun, J.; Lin, Q.; Yu, Z.-Q. Serum Soluble Tumor Necrosis Factor-like Weak Inducer of Apoptosis Is a Potential Biomarker for Outcome Prediction of Patients with Aneurysmal Subarachnoid Hemorrhage. Clin. Chim. Acta 2020, 510, 354–359. [Google Scholar] [CrossRef]
- da Silva-Candal, A.; López-Dequidt, I.; Rodriguez-Yañez, M.; Ávila-Gómez, P.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Campos, F.; Iglesias-Rey, R.; Hervella, P. STWEAK Is a Marker of Early Haematoma Growth and Leukoaraiosis in Intracerebral Haemorrhage. Stroke Vasc. Neurol. 2021, 6, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhong, Z.; Qiu, Z.; Wu, H.-P.; Hu, J.-Y.; Ma, J.-P.; Wu, J.-P. Serum Soluble TWEAK Levels in Severe Traumatic Brain Injury and Its Prognostic Significance. Clin. Chim. Acta 2019, 495, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Comertpay, E.; Vural, S.; Eroğlu, O.; Dindar Badem, N.; Bilgili, Y.; Coskun, F. The Diagnostic Value of STWEAK in Acute Ischemic Stroke. Balkan Med. J. 2020, 37, 336–340. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Feng, C.; Huang, X.; Wong, L.; Liu, X.; Nie, Z.; Xi, G. Blood-Brain Barrier Damage as the Starting Point of Leukoaraiosis Caused by Cerebral Chronic Hypoperfusion and Its Involved Mechanisms: Effect of Agrin and Aquaporin-4. Biomed Res. Int. 2018, 2018, 2321797. [Google Scholar] [CrossRef]
- Lin, J.; Wang, D.; Lan, L.; Fan, Y. Multiple Factors Involved in the Pathogenesis of White Matter Lesions. Biomed Res. Int. 2017, 2017, 9372050. [Google Scholar] [CrossRef]
- Simpson, J.E.; Wharton, S.B.; Cooper, J.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Shaw, P.J.; Savva, G.; Matthews, F.E.; Brayne, C.; et al. Alterations of the Blood–Brain Barrier in Cerebral White Matter Lesions in the Ageing Brain. Neurosci. Lett. 2010, 486, 246–251. [Google Scholar] [CrossRef]
- Zhang, J.; Puri, A.S.; Khan, M.A.; Goddeau, R.P.; Henninger, N. Leukoaraiosis Predicts a Poor 90-Day Outcome after Endovascular Stroke Therapy. Am. J. Neuroradiol. 2014, 35, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.-S.; Woo, S.-H.; Schellingerhout, D.; Jang, M.U.; Park, K.-J.; Hong, K.-S.; Jeong, S.-W.; Na, J.-Y.; Cho, K.-H.; Kim, J.-T.; et al. Stroke Outcomes Are Worse with Larger Leukoaraiosis Volumes. Brain 2017, 140, 158–170. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 594672. [Google Scholar] [CrossRef]
- Hawkins, R.; Viña, J. How Glutamate Is Managed by the Blood–Brain Barrier. Biology 2016, 5, 37. [Google Scholar] [CrossRef]
- Boyko, M.; Zlotnik, A.; Gruenbaum, B.F.; Gruenbaum, S.E.; Ohayon, S.; Kuts, R.; Melamed, I.; Regev, A.; Shapira, Y.; Teichberg, V.I. Pyruvate’s Blood Glutamate Scavenging Activity Contributes to the Spectrum of Its Neuroprotective Mechanisms in a Rat Model of Stroke. Eur. J. Neurosci. 2011, 34, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Miguel, P.; Albalate, M.; Durán-Laforet, V.; Peña-Martínez, C.; de Sequera, P.; Bouarich, H.; Peña-Esparragoza, K.; López-Ongil, S.; Lizasoain, I.; Sánchez-Prieto, J.; et al. Effective Glutamate Clearance from the Systemic Circulation by Hemodialysis: Potential Relevance for Cerebral Ischemia Management. Artif. Organs 2021, 45, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Rogachev, B.; Tsesis, S.; Gruenbaum, B.F.; Gruenbaum, S.E.; Boyko, M.; Klein, M.; Shapira, Y.; Vorobiev, M.; Zlotnik, A. The Effects of Peritoneal Dialysis on Blood Glutamate Levels. J. Neurosurg. Anesthesiol. 2013, 25, 262–266. [Google Scholar] [CrossRef]
- Montaner, J.; Alvarez-Sabín, J. NIH Stroke Scale and Its Adaptation to Spanish. Neurologia 2006, 21, 192–202. [Google Scholar]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.J.; Cvoro, V.; MacLullich, A.M.J.; Shenkin, S.D.; Sandercock, P.A.G.; Sakka, E.; Wardlaw, J.M. Visual Rating Scales of White Matter Hyperintensities and Atrophy: Comparison of Computed Tomography and Magnetic Resonance Imaging. J. Stroke Cerebrovasc. Dis. 2018, 27, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Hart, R.J.; Fry, J.C. An Evaluation of the Waters Pico-Tag System for the Amino-Acid Analysis of Food Materials. J. Automat. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef] [PubMed]
| Good Outcome n = 329 | Poor Outcome n = 577 | p | |
|---|---|---|---|
| Latency time | 277.8 ± 214.5 | 269.9 ± 180.7 | 0.002 |
| Age, years | 68.2 ± 13.2 | 76.1 ± 12.1 | 0.003 |
| Female gender, % | 38.9 | 54.2 | <0.0001 |
| Wake-up stroke, % | 12.5 | 14.6 | 0.423 |
| Pre-morbid mRS [IQR] | 0 [0, 0] | 0 [0, 1] | 0.055 |
| Arterial hypertension, % | 60.8 | 63.8 | 0.392 |
| Diabetes mellitus, % | 30.4 | 24.4 | 0.060 |
| Smoking, % | 20.4 | 12.0 | <0.0001 |
| Alcohol consumption, % | 9.7 | 16.6 | 0.004 |
| Hyperlipidemia, % | 30.4 | 32.9 | 0.459 |
| Ischemic heart disease, % | 10.0 | 13.3 | 0.169 |
| Atrial fibrillation, % | 12.5 | 31.9 | <0.0001 |
| Heart failure, % | 2.7 | 5.4 | 0.066 |
| Carotid disease, % | 0.9 | 0.9 | 0.604 |
| Temperature at admission, °C | 37.1 ± 0.7 | 37.3 ± 0.8 | 0.029 |
| Glucose at admission, mg/dL | 130.7 ± 54.0 | 154.6 ± 77.7 | <0.0001 |
| Leukocytes at admission, ×103/mL | 8.9 ± 2.8 | 10.3 ± 3.7 | <0.0001 |
| Fibrinogen at admission, mg/dL | 443.9 ± 103.0 | 478.2 ± 104.8 | 0.459 |
| LDL cholesterol, mg/dL | 119.9 ± 53.2 | 113.3 ± 47.6 | 0.203 |
| Sedimentation rate, mm/1st hr | 25.5 ± 23.8 | 33.0 ± 25.1 | 0.310 |
| GOT at admission, U/L | 39.7 ± 13.3 | 22.6 ± 10.3 | <0.0001 |
| Intravenous fibrinolysis, % | 14.9 | 14.2 | 0.769 |
| Thrombectomy, % | 5.8 | 5.2 | 0.761 |
| Leukoaraiosis | <0.0001 | ||
| No, % | 50.2 | 36.2 | |
| Grade I, % | 31.0 | 26.5 | |
| Grade II, % | 14.9 | 18.2 | |
| Grade III, % | 4.0 | 19.1 | |
| TOAST | <0.0001 | ||
| Atherothrombotic, % | 20.1 | 20.8 | |
| Cardioembolic, % | 15.5 | 49.4 | |
| Lacunar, % | 23.4 | 1.4 | |
| Indeterminate, % | 38.6 | 26.9 | |
| Other, % | 2.4 | 1.6 | |
| NIHSS at admission | 5 [3, 11] | 17 [11, 22] | <0.0001 |
| mRs at 3 months | 1 [0, 2] | 5 [3, 6] | <0.0001 |
| Glutamate at admission, μM | 134.2 ± 112.5 | 227.1 ± 123.8 | 0.036 |
| sTWEAK at admission, pg/mL | 3143.2 ± 2677.1 | 5828.6 ± 3248.9 | 0.002 |
| IL-6, pg/mL | 20.9 ± 17.8 | 30.9 ± 18.3 | 0.743 |
| sTWEAK ≤ 2900 pg/mL | ||||||
| Not Adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Latency time | 1.00 | 0.99–1.01 | 0.973 | 1.00 | 1.00–1.05 | 0.061 |
| Age | 1.06 | 1.03–1.08 | <0.0001 | 1.01 | 0.97–1.06 | 0.601 |
| Women | 1.86 | 1.08–3.19 | 0.025 | 1.71 | 0.26–2.91 | 0.494 |
| Smoking | 0.36 | 0.16–0.84 | 0.018 | 0.86 | 0.38–9.02 | 0.443 |
| Alcoholism | 1.12 | 0.46–2.71 | 0.803 | 1.15 | 0.21–6.39 | 0.870 |
| Atrial fibrillation | 4.36 | 2.32–8.18 | <0.0001 | 1.12 | 0.34–3.61 | 0.854 |
| Temperature at admission | 1.03 | 1.00–1.44 | 0.047 | 1.24 | 0.16–5.61 | 0.433 |
| Glucose at admission | 1.00 | 0.99–1.01 | 0.178 | 1.00 | 0.99–1.05 | 0.146 |
| Leukocytes at admission | 1.12 | 1.02–1.22 | 0.013 | 1.21 | 1.00–1.47 | 0.042 |
| Cardioembolic | 5.22 | 3.05–6.18 | <0.0001 | 1.37 | 1.11–4.16 | 0.035 |
| Lacunar | 0.64 | 0.34–0.93 | <0.0001 | 0.85 | 0.47–1.03 | 0.126 |
| GOT at admission | 0.98 | 0.96–1.01 | 0.170 | 0.98 | 0.94–1.03 | 0.514 |
| NIHSS at admission | 1.18 | 1.13–1.24 | <0.0001 | 1.31 | 1.12–1.44 | <0.0001 |
| sTWEAK > 2900 pg/mL | ||||||
| Not Adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Latency time | 1.00 | 0.99–1.00 | 0.688 | 1.01 | 0.99–1.01 | 0.387 |
| Age | 1.04 | 1.02–1.06 | <0.0001 | 0.95 | 0.87–1.04 | 0.292 |
| Women | 1.60 | 1.39–2.92 | 0.019 | 2.35 | 0.32–17.35 | 0.401 |
| Smoking | 0.89 | 0.48–1.65 | 0.703 | 0.83 | 0.03–21.34 | 0.908 |
| Alcoholism | 1.34 | 1.15–1.76 | 0.009 | 3.39 | 0.17–68.08 | 0.425 |
| Atrial fibrillation | 2.88 | 1.59–5.21 | <0.0001 | 1.51 | 0.04–6.29 | 0.600 |
| Temperature at admission | 3.18 | 2.31–4.38 | <0.0001 | 1.72 | 0.11–4.72 | 0.735 |
| Glucose at admission | 1.00 | 1.00–1.01 | 0.010 | 0.98 | 0.97–1.00 | 0.093 |
| Leukocytes at admission | 1.14 | 1.06–1.22 | <0.0001 | 1.46 | 0.99–2.14 | 0.055 |
| Cardioembolic | 3.18 | 2.17–5.39 | <0.0001 | 2.57 | 1.19–5.63 | 0.048 |
| Lacunar | 0.81 | 0.63–0.99 | <0.0001 | 0.89 | 0.70–1.09 | 0.084 |
| GOT at admission | 0.64 | 0.56–0.71 | <0.0001 | 0.41 | 0.28– 0.68 | <0.0001 |
| NIHSS at admission | 1.27 | 1.21–1.33 | <0.0001 | 1.14 | 1.00–1.31 | 0.006 |
| No Leukoaraiosis | ||||||
| Not Adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Latency time | 1.00 | 0.99–1.00 | 0.899 | 1.00 | 1.00–1.00 | 0.061 |
| Age | 1.04 | 1.02–1.06 | <0.0001 | 1.02 | 0.98–1.04 | 0.295 |
| Women | 1.66 | 1.43–1.99 | 0.045 | 1.62 | 0.32–2.20 | 0.154 |
| Smoking | 0.46 | 0.27–0.79 | 0.005 | 0.55 | 0.22–1.35 | 0.189 |
| Alcoholism | 1.68 | 0.87–3.23 | 0.123 | 1.60 | 0.58–4.31 | 0.362 |
| Atrial fibrillation | 1.69 | 1.23–2.69 | <0.0001 | 1.55 | 0.69–3.50 | 0.290 |
| Temperature at admission | 1.06 | 0.81–1.40 | 0.654 | 1.68 | 0.43–3.07 | 0.097 |
| Glucose at admission | 1.00 | 1.00–1.01 | 0.023 | 1.00 | 0.99–1.00 | 0.316 |
| Leukocytes at admission | 1.10 | 1.02–1.18 | 0.006 | 1.05 | 0.95–1.16 | 0.335 |
| Cardioembolic | 4.72 | 2.71–7.23 | <0.0001 | 3.77 | 1.62–5.23 | 0.019 |
| Lacunar | 0.71 | 0.50–0.97 | <0.0001 | 0.77 | 0.52–0.94 | 0.048 |
| GOT at admission | 0.95 | 0.93–0.97 | <0.0001 | 0.95 | 0.92–1.02 | 0.105 |
| NIHSS at admission | 1.15 | 1.11–1.19 | <0.0001 | 1.17 | 1.10–1.23 | <0.0001 |
| Leukoaraiosis | ||||||
| Not Adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Latency time | 1.00 | 0.99–1.00 | 0.407 | 0.99 | 0.99–1.00 | 0.556 |
| Age | 1.05 | 1.04–1.07 | <0.0001 | 1.06 | 1.00–1.12 | 0.036 |
| Women | 1.46 | 1.31–1.67 | <0.0001 | 1.23 | 0.36–4.22 | 0.075 |
| Smoking | 0.65 | 0.39–1.10 | 0.111 | 0.32 | 0.06–1.78 | 0.193 |
| Alcoholism | 1.89 | 1.07–3.34 | 0.028 | 1.16 | 0.21–6.35 | 0.864 |
| Atrial fibrillation | 3.97 | 2.35–6.71 | <0.0001 | 4.42 | 0.85–6.37 | 0.270 |
| Temperature at admission | 2.84 | 2.17–3.75 | <0.0001 | 2.49 | 0.92–6.72 | 0.071 |
| Glucose at admission | 1.00 | 1.00–1.01 | <0.0001 | 1.00 | 0.99–1.00 | 0.711 |
| Leukocytes at admission | 1.18 | 1.11–1.26 | <0.0001 | 1.26 | 1.01–1.56 | 0.039 |
| Cardioembolic | 3.29 | 1.58–6.03 | <0.0001 | 3.58 | 1.23–6.19 | 0.038 |
| Lacunar | 0.58 | 0.39–0.91 | <0.0001 | 0.69 | 0.43–0.90 | 0.024 |
| GOT at admission | 0.77 | 0.74–0.82 | <0.0001 | 0.75 | 0.69–0.82 | <0.0001 |
| NIHSS at admission | 1.27 | 1.21–1.32 | <0.0001 | 1.14 | 1.03–1.25 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hervella, P.; Sampedro-Viana, A.; Fernández-Rodicio, S.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Mosqueira, A.J.; Bazarra-Barreiros, M.; Abengoza-Bello, M.T.; Ortega-Espina, S.; et al. Precision Medicine for Blood Glutamate Grabbing in Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 6554. https://doi.org/10.3390/ijms25126554
Hervella P, Sampedro-Viana A, Fernández-Rodicio S, Rodríguez-Yáñez M, López-Dequidt I, Pumar JM, Mosqueira AJ, Bazarra-Barreiros M, Abengoza-Bello MT, Ortega-Espina S, et al. Precision Medicine for Blood Glutamate Grabbing in Ischemic Stroke. International Journal of Molecular Sciences. 2024; 25(12):6554. https://doi.org/10.3390/ijms25126554
Chicago/Turabian StyleHervella, Pablo, Ana Sampedro-Viana, Sabela Fernández-Rodicio, Manuel Rodríguez-Yáñez, Iria López-Dequidt, José M. Pumar, Antonio J. Mosqueira, Marcos Bazarra-Barreiros, María Teresa Abengoza-Bello, Sara Ortega-Espina, and et al. 2024. "Precision Medicine for Blood Glutamate Grabbing in Ischemic Stroke" International Journal of Molecular Sciences 25, no. 12: 6554. https://doi.org/10.3390/ijms25126554








