Dynamic Alterations in Acetylation and Modulation of Histone Deacetylase Expression Evident in the Dentine–Pulp Complex during Dentinogenesis
Abstract
:1. Introduction
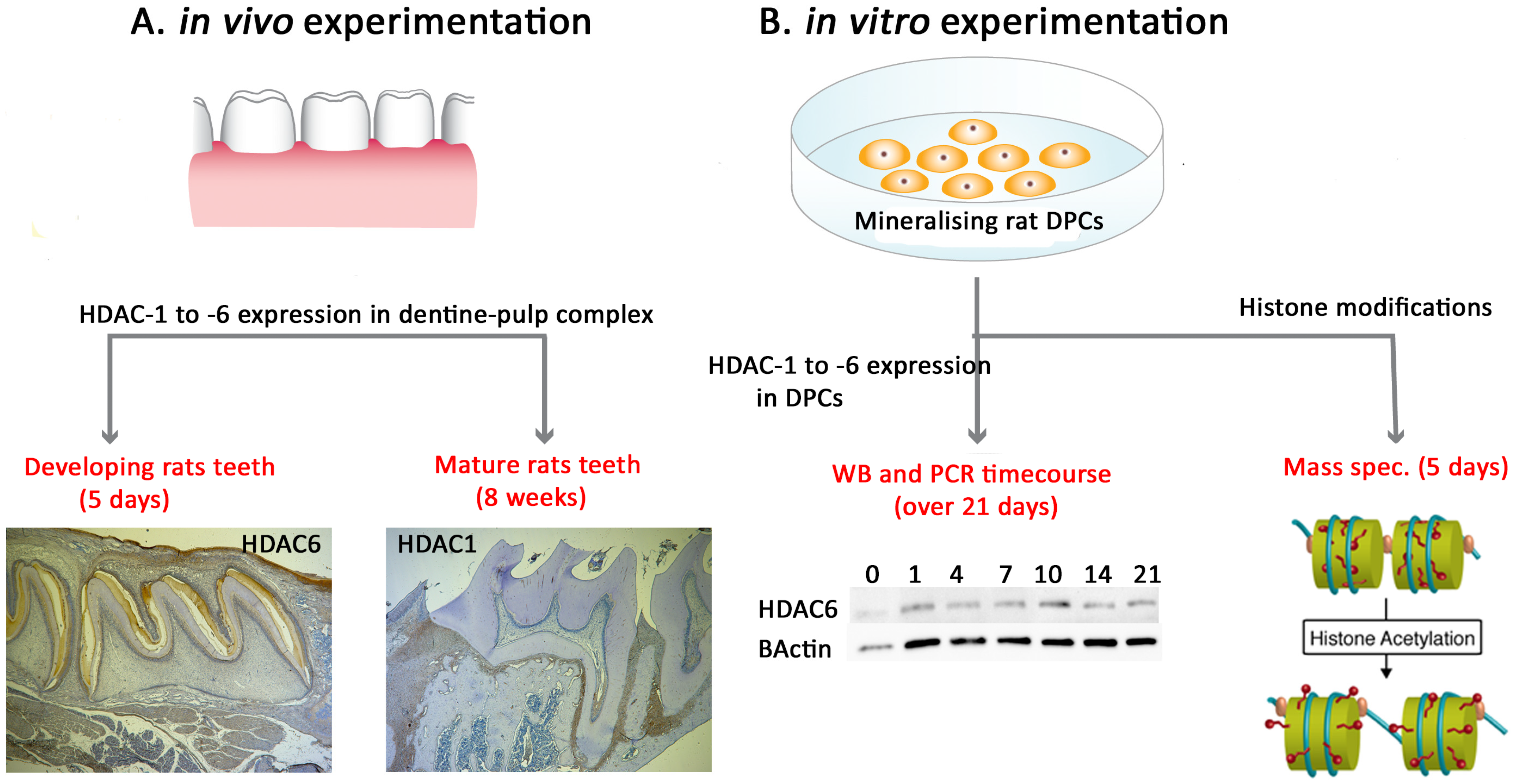
2. Results
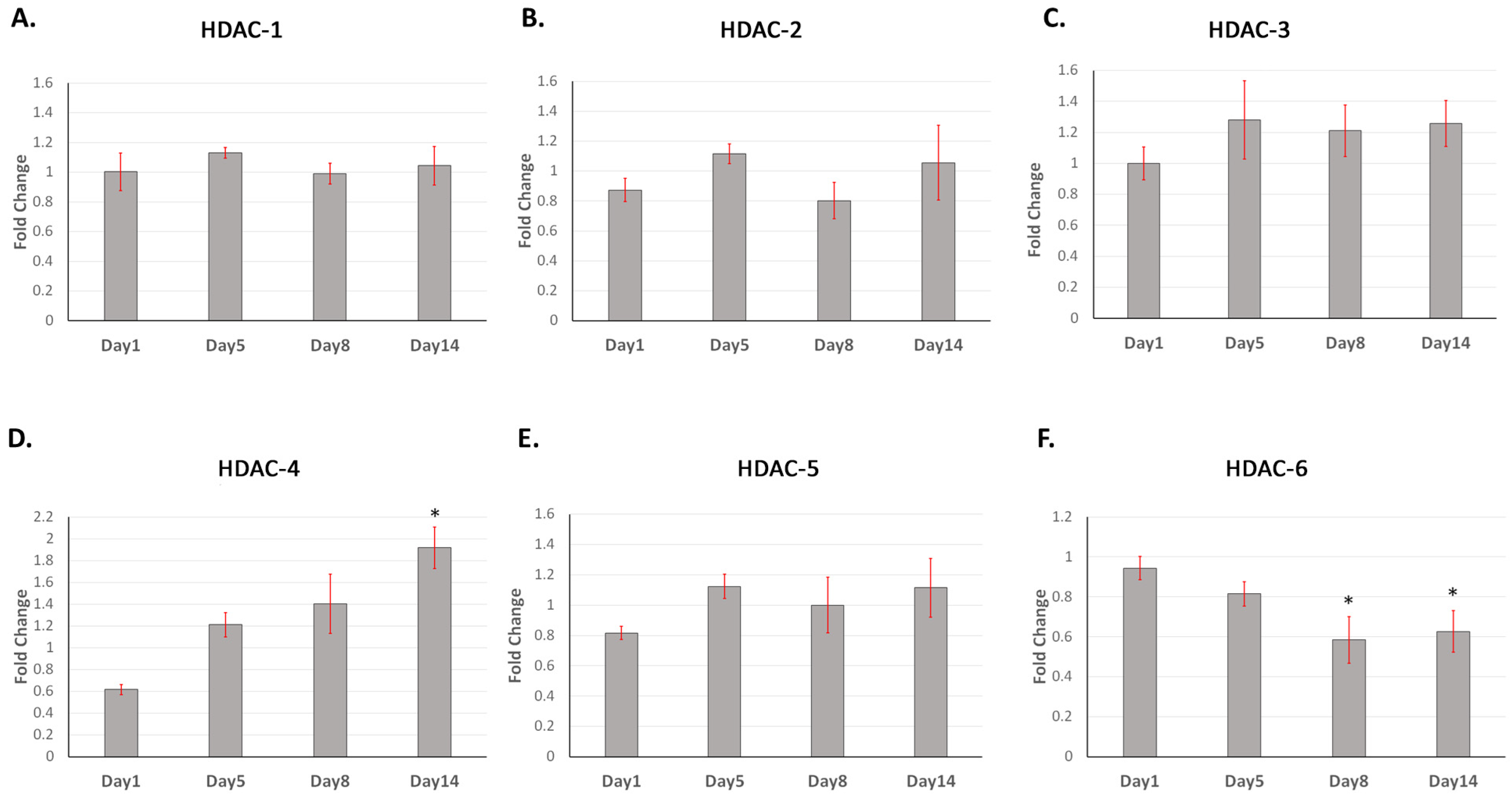
2.1. Class II HDAC-4 Showed Increased Expression during DPC Mineralisation In Vitro with HDAC-6 Showing Decreased Gene Expression
2.2. HDAC-1 Isoforms Exhibit Altered Expression in Postnatal and Adult Molar Teeth
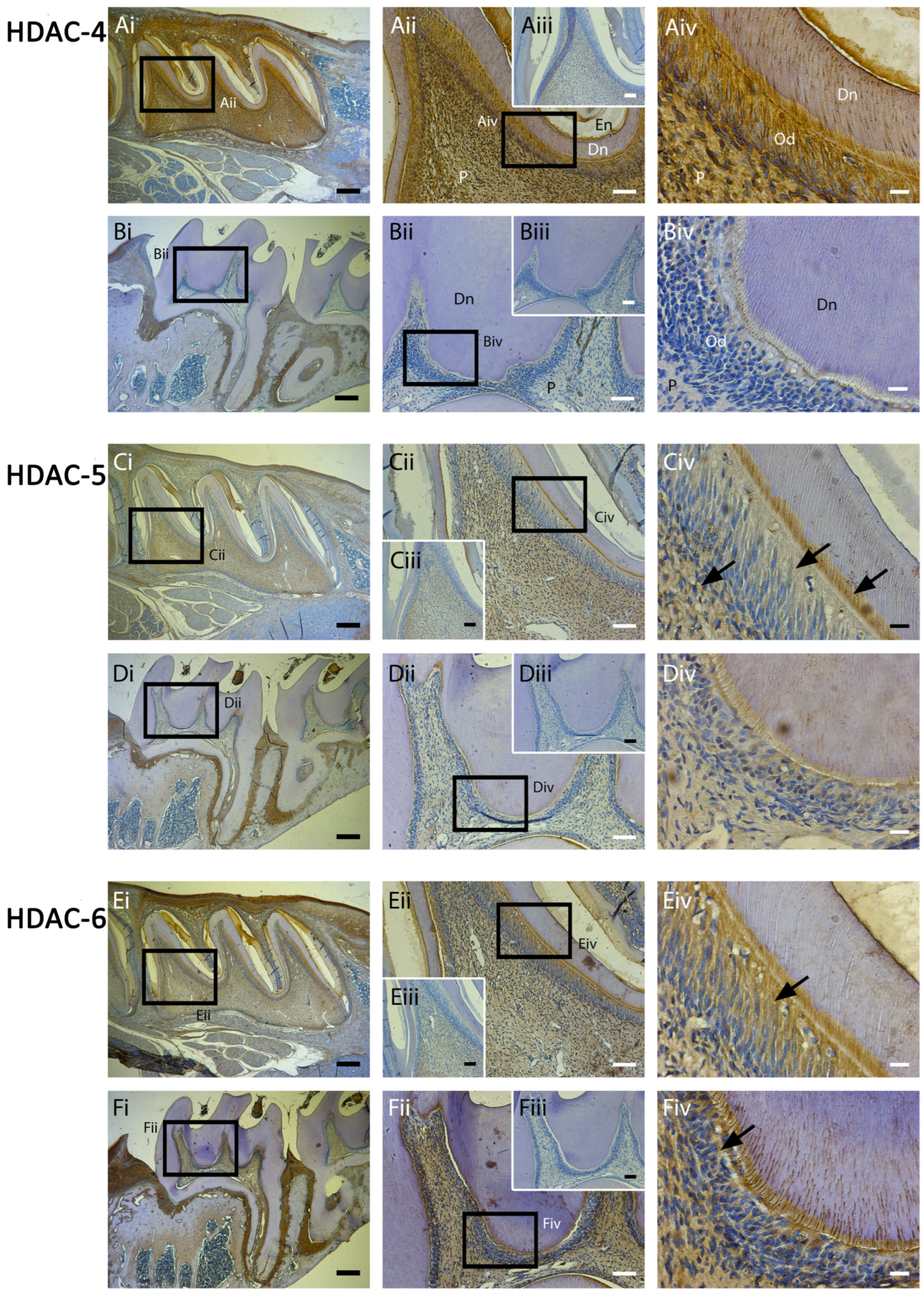
2.3. Class II HDAC-4 and -6 Showed High Expression during Tooth Development with HDAC-4 Reducing in Mature Teeth
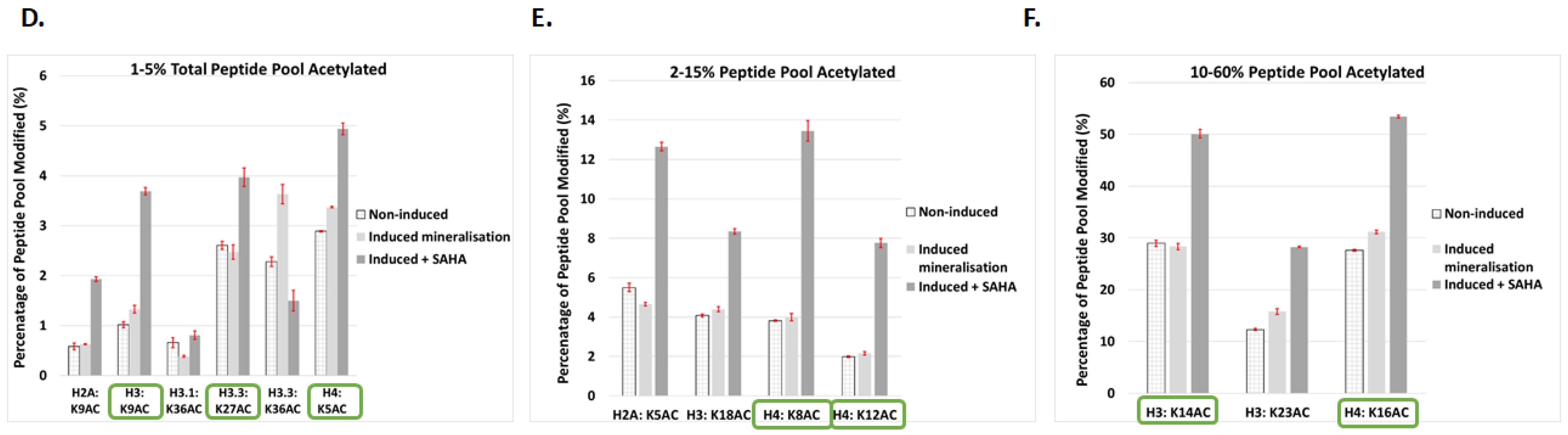
2.4. Post-Translational Histone Modification Alters Significantly during Mineralisation
3. Discussion
4. Materials and Methods
4.1. Animals
In Vivo Model and Immunohistochemical (IHC) Staining
4.2. Dental Pulp Cell Studies
4.2.1. Primary Cell Isolation and Culture
4.2.2. Histone Deacetylase Inhibitor Preparation
4.2.3. Mineralisation Assay
4.2.4. Western Blotting
4.2.5. RNA Isolation and cDNA Synthesis
4.2.6. Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
4.2.7. Mass Spectrometric Analysis of Post-Translational Modifications in Mineralising DPCs
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duncan, H.F.; Kobayashi, Y.; Kearney, M.; Shimizu, E. Epigenetic therapeutics in dental pulp treatment: Hopes, challenges and concerns for the development of next-generation biomaterials. Bioact. Mater. 2023, 27, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Cooper, P.R.; Friedlander, L.T.; Rizwan, S.; Seo, B.; Rich, A.M.; Hussaini, H.M. Potential application of immunotherapy for modulation of pulp inflammation: Opportunities for vital pulp treatment. Int. Endod. J. 2021, 54, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Yamada, M.; Deyama, Y.; Suzuki, K.; Kaga, M.; Yawaka, Y.; Ogawa, T. Effect of N-acetylcysteine on rat dental pulp cells cultured on mineral trioxide aggregate. J. Endod. 2011, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Varalakshmi, P.R.; Kavitha, M.; Govindan, R.; Narasimhan, S. Effect of statins with α-tricalcium phosphate on proliferation, differentiation, and mineralization of human dental pulp cells. J. Endod. 2013, 39, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.; Park, H.J.; Baek, K.; Lee, H.L.; Park, J.C.; Woo, K.M.; Ryoo, H.M.; Baek, J.H. Suberoylanilide hydroxamic acid enhances odontoblast differentiation. J. Dent. Res. 2012, 91, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Q.; Rao, L.; Yi, B.; Xu, Q. Effect of 5-Aza-deoxycytidine on odontogenic differentiation of human dental pulp cells. J. Endod. 2015, 41, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Smith, A.J.; Fleming, G.J.; Partridge, N.C.; Shimizu, E.; Moran, G.P.; Cooper, P.R. The histone-deacetylase-inhibitor suberoylanilide hydroxamic acid promotes dental pulp repair mechanisms through modulation of matrix metalloproteinase-13 activity. J. Cell. Physiol. 2016, 231, 798–816. [Google Scholar] [CrossRef]
- Kornberg, R.D.; Lorch, Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 1999, 98, 285–294. [Google Scholar] [CrossRef]
- Margueron, R.; Trojer, P.; Reinberg, D. The key to development: Interpreting the histone code? Curr. Opin. Genet. Dev. 2005, 15, 63–76. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Gregoretti, I.V.; Lee, Y.M.; Goodson, H.V. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J. Mol. Biol. 2004, 338, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.W.; Carpio, L.R.; van Wijnen, A.J.; McGee-Lawrence, M.E.; Westendorf, J.J. Histone deacetylases in bone development and skeletal disorders. Physiol. Rev. 2015, 95, 1359–1381. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Davis, C.A.; Potthoff, M.J.; Haberland, M.; Fielitz, J.; Qi, X.; Hill, J.A.; Richardson, J.A.; Olson, E.N. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007, 21, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Ricarte, F.; Nakatani, T.; Partridge, N. PTH Signaling and epigenetic control of bone remodeling. Curr. Mol. Biol. Rep. 2016, 2, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Westendorf, J.J.; Zaidi, S.K.; Cascino, J.E.; Kahler, R.; van Wijnen, A.J.; Lian, J.B.; Yoshida, M.; Stein, G.S.; Li, X. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol. Cell. Biol. 2002, 22, 7982–7992. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Chen, T.; Partridge, N.C. MMP-13 is one of the critical mediators of the effect of HDAC4 deletion on the skeleton. Bone 2016, 90, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.M.; Kahler, R.A.; Li, X.; Westendorf, J.J. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J. Biol. Chem. 2004, 279, 41998–42007. [Google Scholar] [CrossRef] [PubMed]
- Klinz, F.J.; Korkmaz, Y.; Bloch, W.; Raab, W.H.; Addicks, K. Histone deacetylases 2 and 9 are coexpressed and nuclear localized in human molar odontoblasts in vivo. Histochem. Cell Biol. 2012, 137, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of histone deacetylases enhances the osteogenic differentiation of human periodontal ligament cells. J. Cell. Biochem. 2016, 117, 1384–1395. [Google Scholar] [CrossRef]
- Gopinathan, G.; Kolokythas, A.; Luan, X.; Diekwisch, T.G. Epigenetic marks define the lineage and differentiation potential of two distinct neural crest-derived intermediate odontogenic progenitor populations. Stem Cells Dev. 2013, 22, 1763–1778. [Google Scholar] [CrossRef]
- Duncan, H.F.; Smith, A.J.; Fleming, G.J.; Cooper, P.R. Histone deacetylase inhibitors induced differentiation and accelerated mineralization of pulp-derived cells. J. Endod. 2012, 38, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Verner, E.; Buggy, J.J. Isoform-specific histone deacetylase inhibitors: The next step? Cancer Lett. 2009, 280, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, E.; Altamura, S.; Branca, D.; Cecchetti, O.; Ferrigno, F.; Orsale, M.V.; Palumbi, M.C.; Rowley, M.; Scarpelli, R.; Steinkühler, C.; et al. 2-Trifluoroacetylthiophene oxadiazoles as potent and selective class II human histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 6083–6087. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.D.; Li, J.; Wong, J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol. Cell. Biol. 2005, 25, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Halsall, J.A.; Turan, N.; Wiersma, M.; Turner, B.M. Cells adapt to the epigenomic disruption caused by histone deacetylase inhibitors through a coordinated, chromatin-mediated transcriptional response. Epigenet. Chromatin 2015, 8, 29. [Google Scholar] [CrossRef]
- Yan, C.; Boyd, D.D. Histone H3 acetylation and H3 K4 methylation define distinct chromatin regions permissive for transgene expression. Mol. Cell. Biol. 2006, 26, 6357–6371. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.A.R.; Stein, J.L.; Westendorf, J.J.; van Wijnen, A.J. Chromatin modifiers and histone modifications in bone formation, regeneration, and therapeutic intervention for bone-related disease. Bone 2015, 81, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Paino, F.; La Noce, M.; Tirino, V.; Naddeo, P.; Desiderio, V.; Pirozzi, G.; De Rosa, A.L.; Laino, L.; Altucci, L.; Papaccio, G. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: Evidence for HDAC2 involvement. Stem Cells 2014, 32, 279–289. [Google Scholar] [CrossRef]
- Jin, H.; Park, J.-Y.; Choi, H.; Choung, P.-H. HDAC inhibitor trichostatin A promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng. Part A 2013, 19, 613–624. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, T.; Han, Q.; Chen, M.; You, J.; Fang, F.; Peng, L.; Wu, B. HDAC inhibitor LMK-235 promotes the odontoblast differentiation of dental pulp cells. Mol. Med. Rep. 2018, 17, 1445–1452. [Google Scholar] [CrossRef]
- Man, K.; Lawlor, L.; Jiang, L.H.; Yang, X.B. The Selective Histone Deacetylase Inhibitor MI192 Enhances the Osteogenic Differentiation Efficacy of Human Dental Pulp Stromal Cells. Int. J. Mol. Sci. 2021, 22, 5224. [Google Scholar] [CrossRef] [PubMed]
- Ono, W.; Sakagami, N.; Nishimori, S.; Ono, N.; Kronenberg, H.M. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat Commun. 2016, 7, 11277. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Lasagni, L.; Mazzinghi, B.; Lazzeri, E.; Romagnani, S. Pharmacological modulation of stem cell function. Curr. Med. Chem. 2007, 14, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.R.; Seo, M.S.; Roh, K.H.; Park, S.B.; Hwang, J.W.; Sun, B.; Seo, K.; Lee, Y.; Kang, S.; et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009, 42, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Jamaladdin, S.; Kelly, R.D.; O’Regan, L.; Dovey, O.M.; Hodson, G.E.; Millard, C.J.; Portolano, N.; Fry, A.M.; Schwabe, J.W.R.; Cowley, S.M. Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 9840–9845. [Google Scholar] [CrossRef] [PubMed]
- Noer, A.; Lindeman, L.C.; Collas, P. Histone H3 modifications associated with differentiation and long-term culture of mesenchymal adipose stem cells. Stem Cells Dev. 2009, 18, 725–736. [Google Scholar] [CrossRef]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G. Differentiation of neural-crest-derived intermediate pluripotent progenitors into committed periodontal populations involves unique molecular signature changes, cohort shifts, and epigenetic modifications. Stem Cells Dev. 2011, 20, 39–52. [Google Scholar] [CrossRef]
- Patel, A.B.; He, Y.; Radhakrishnan, I. Histone acetylation and deacetylation—Mechanistic insights from structural biology. Gene 2024, 890, 147798. [Google Scholar] [CrossRef]
- Wilkins, B.J.; Hahn, L.E.; Heitmüller, S.; Frauendorf, H.; Valerius, O.; Braus, G.H.; Neumann, H. Genetically encoding lysine modifications on histone H4. ACS Chem. Biol. 2015, 10, 939–944. [Google Scholar] [CrossRef]
- Nair, P.N.; Duncan, H.F.; Pitt Ford, T.R.; Luder, H.U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar] [CrossRef]
- Garcia, B.A.; Mollah, S.; Ueberheide, B.M.; Busby, S.A.; Muratore, T.L.; Shabanowitz, J.; Hunt, D.F. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat. Protoc. 2007, 2, 933–938. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Guccione, E.; Bassi, C.; Casadio, F.; Martinato, F.; Cesaroni, M.; Schuchlautz, H.; Lüscher, B.; Amati, B. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature 2007, 449, 933–937. [Google Scholar] [CrossRef] [PubMed]



| Technique | Experimental Teeth Used | ||
|---|---|---|---|
| Developmental In Vivo | Adult In Vivo | Mineralisation Assay In Vitro | |
| Histology | 5-day-old Wistar Hannover rats, maxillary 1st molar teeth | 8-week-old Wistar Hannover rats, maxillary 1st and 3rd molar teeth | |
| DPC Culture | Male Wistar Hannover rats aged 25–30 days and weighing between 120 and 140 g, maxillary and mandibular incisor teeth | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, Y.; Shimizu, E.; Duncan, H.F. Dynamic Alterations in Acetylation and Modulation of Histone Deacetylase Expression Evident in the Dentine–Pulp Complex during Dentinogenesis. Int. J. Mol. Sci. 2024, 25, 6569. https://doi.org/10.3390/ijms25126569
Yamauchi Y, Shimizu E, Duncan HF. Dynamic Alterations in Acetylation and Modulation of Histone Deacetylase Expression Evident in the Dentine–Pulp Complex during Dentinogenesis. International Journal of Molecular Sciences. 2024; 25(12):6569. https://doi.org/10.3390/ijms25126569
Chicago/Turabian StyleYamauchi, Yukako, Emi Shimizu, and Henry F. Duncan. 2024. "Dynamic Alterations in Acetylation and Modulation of Histone Deacetylase Expression Evident in the Dentine–Pulp Complex during Dentinogenesis" International Journal of Molecular Sciences 25, no. 12: 6569. https://doi.org/10.3390/ijms25126569
APA StyleYamauchi, Y., Shimizu, E., & Duncan, H. F. (2024). Dynamic Alterations in Acetylation and Modulation of Histone Deacetylase Expression Evident in the Dentine–Pulp Complex during Dentinogenesis. International Journal of Molecular Sciences, 25(12), 6569. https://doi.org/10.3390/ijms25126569







