Absent in Melanoma 2 Mediates Inflammasome Signaling Activation against Clostridium perfringens Infection
Abstract
:1. Introduction
2. Results
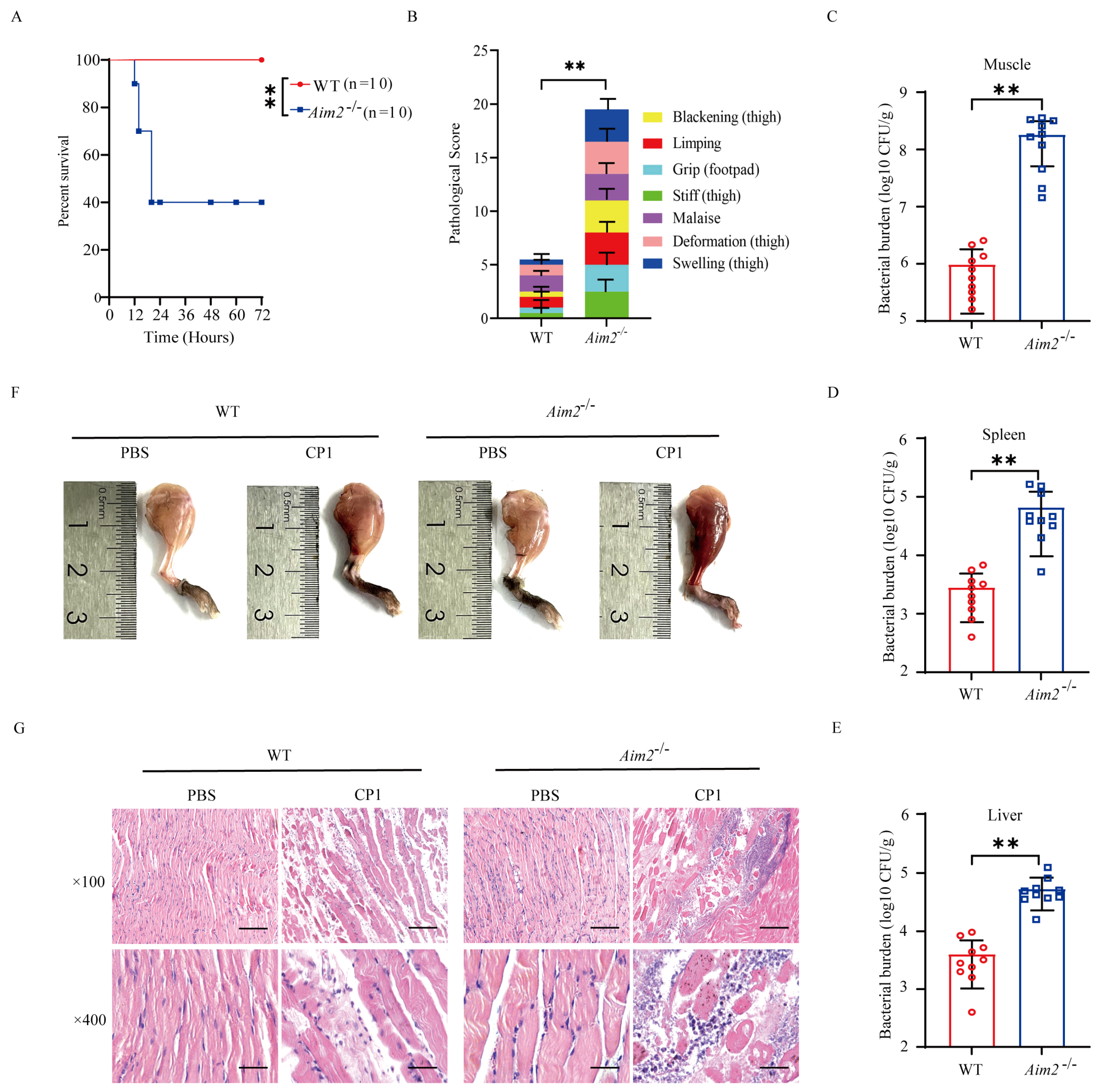
2.1. AIM2 Contributes to Host Protection against Pathogenic C. perfringens Gas Gangrene
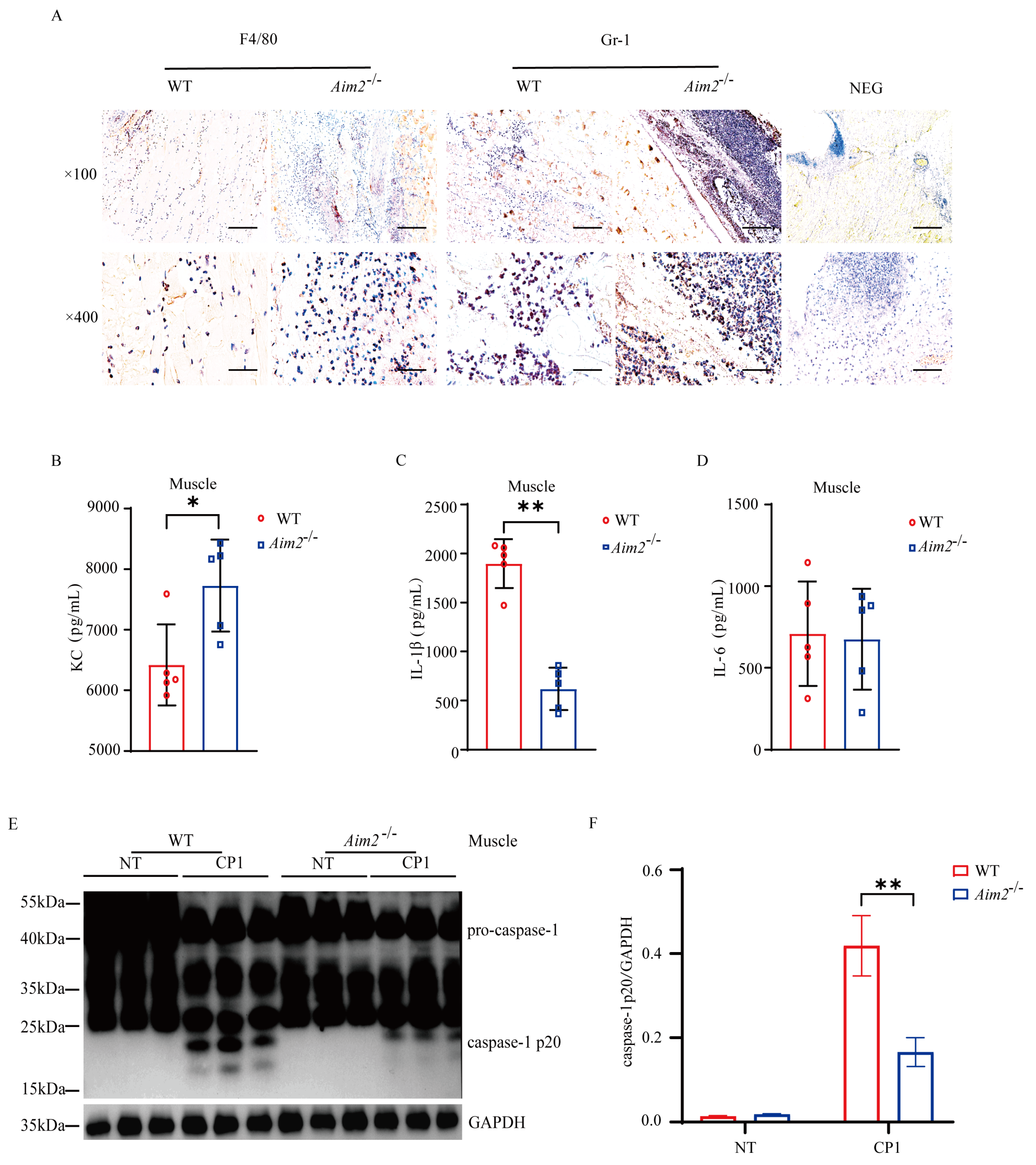
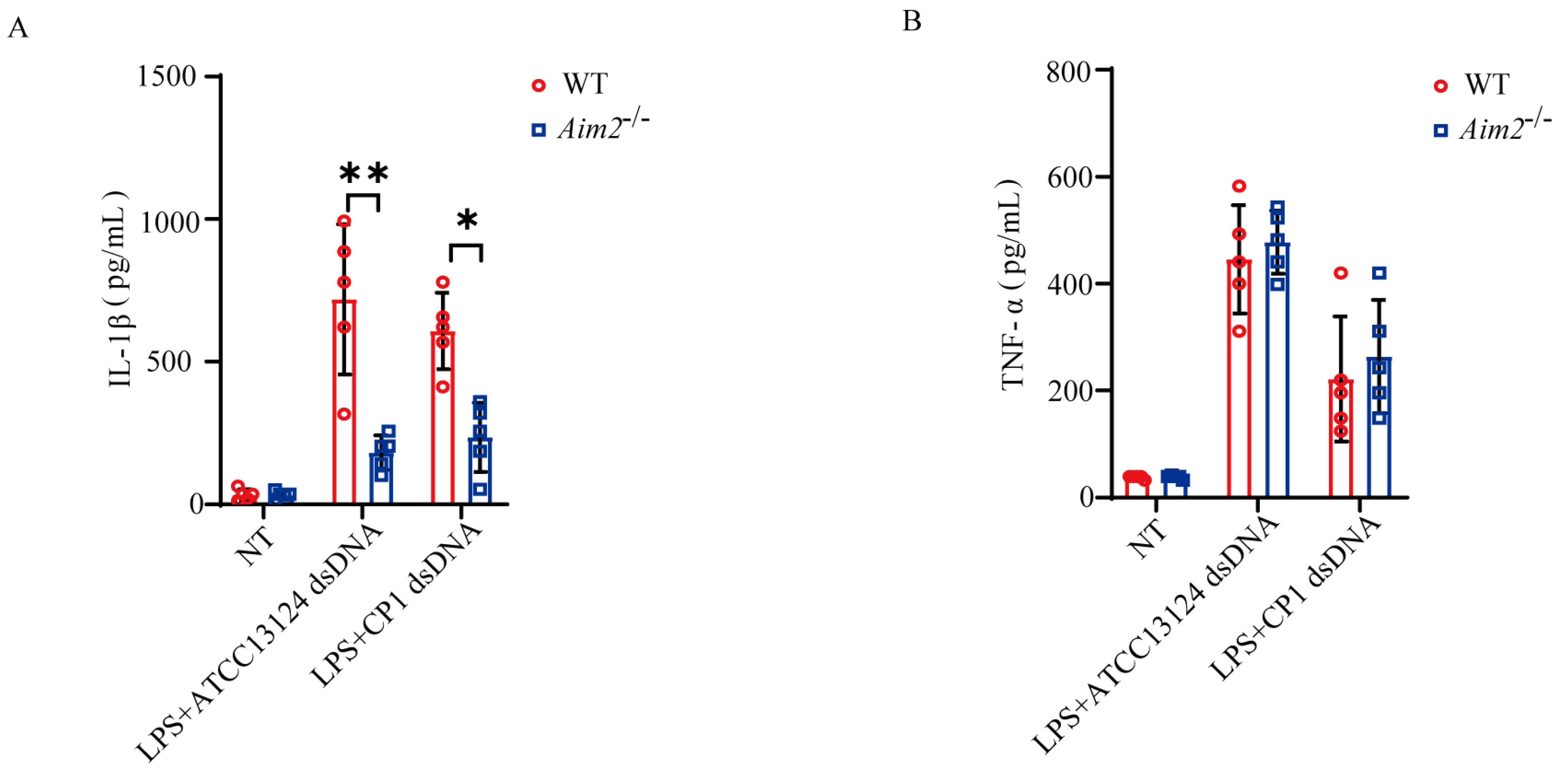
2.2. Aim2 Deficiency Leads to Impaired Inflammasome Signaling Activation
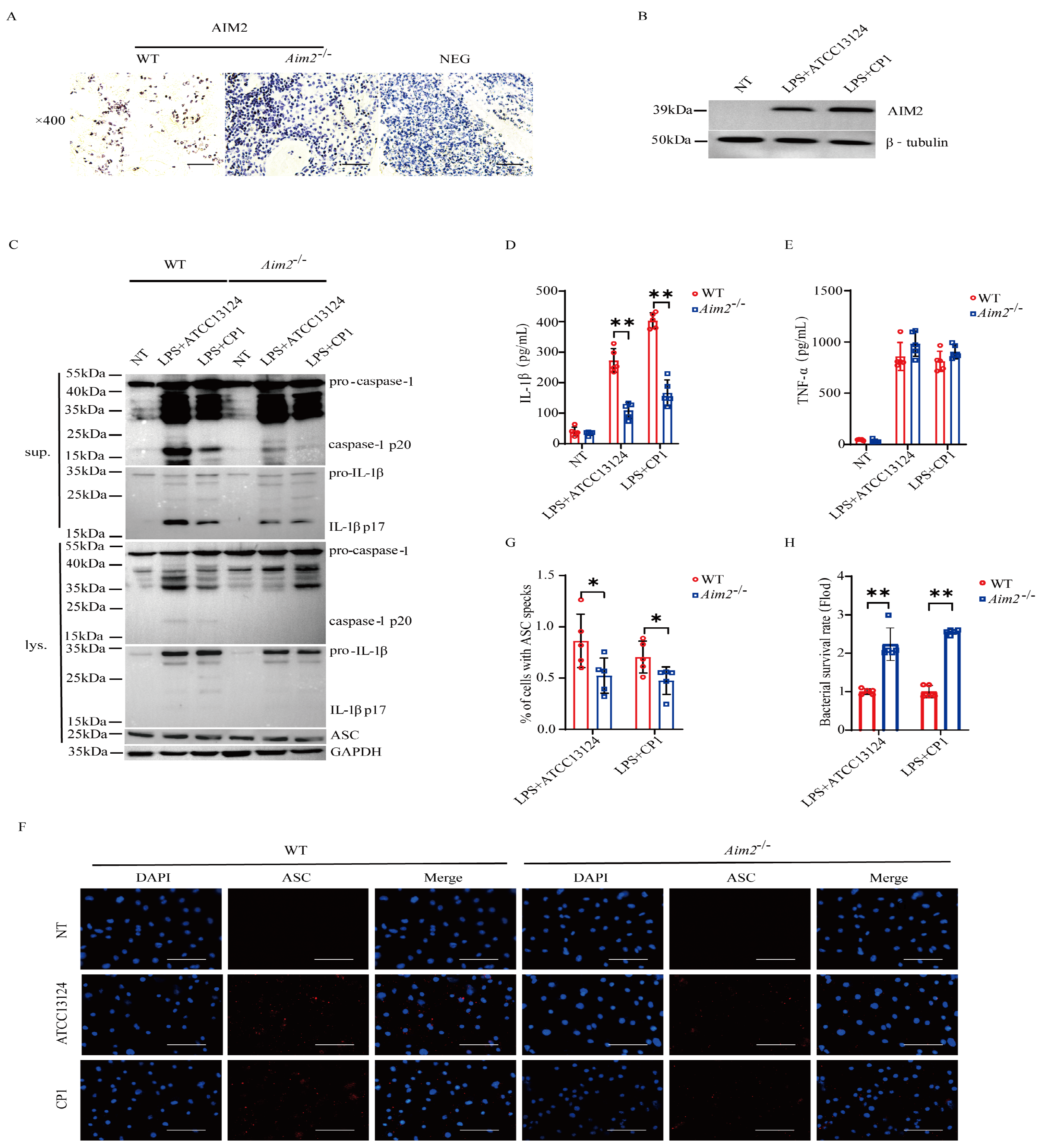
2.3. AIM2 Promotes Inflammasome Signaling Activation in Macrophages
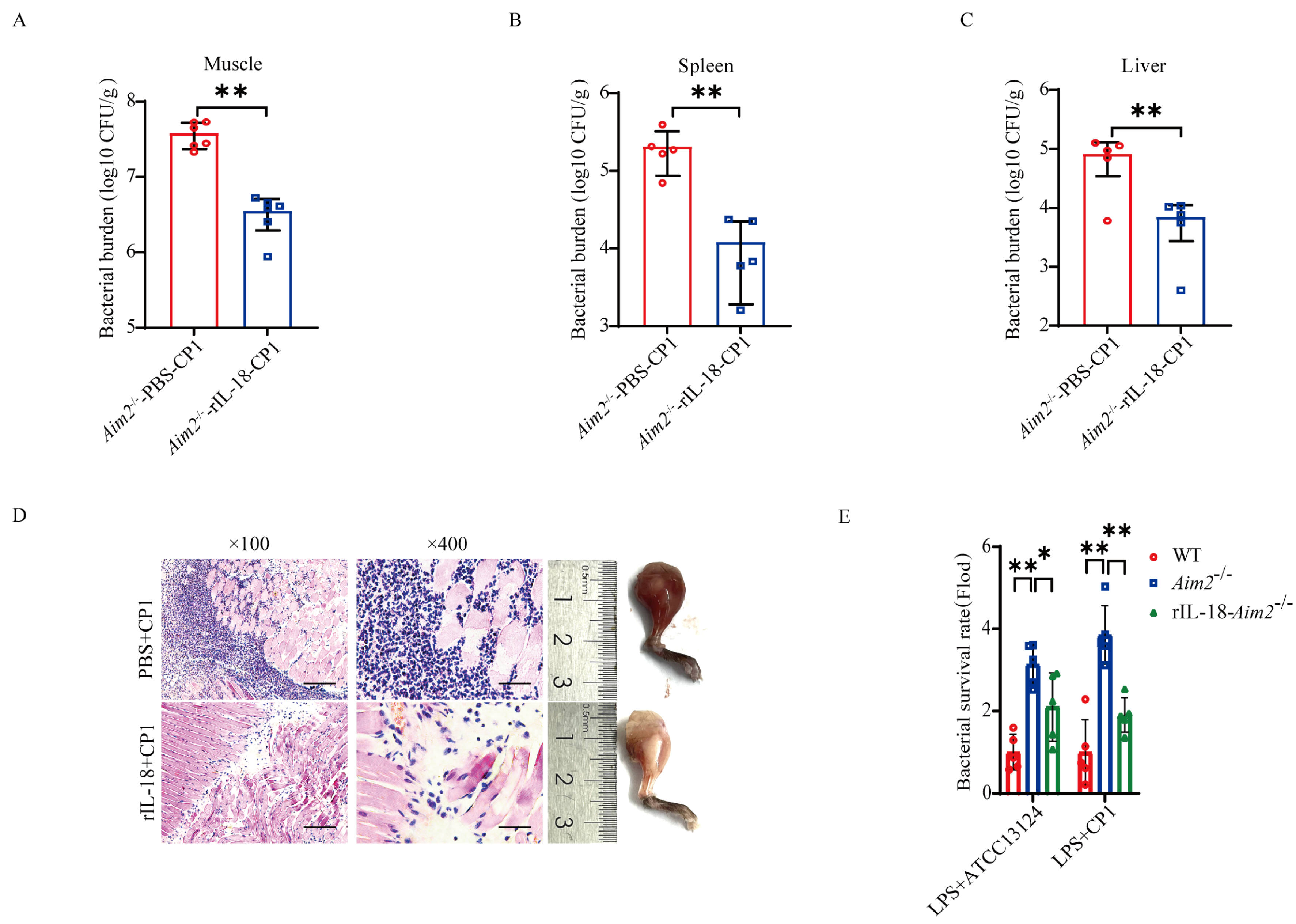
2.4. AIM2-Mediated Inflammasome Signaling Facilitates Bacterial Killing and Clearance
2.5. Pathogenic C. perfringens-Derived Genomic DNA Mediates Activation of AIM2 Inflammasome Signaling
3. Discussion
4. Materials and Methods
4.1. Mice and Cell
4.2. Chemical
4.3. In Vivo Infection
4.4. Bacterial Burden and Cytokine Measurements
4.5. Tissue Histology and Immunofluorescence Staining
4.6. Inflammasome Activation Assay
4.7. Bacterial Killing Analysis
4.8. Administration of Exogenous rIL-18
4.9. Immunoblotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sathish, S.; Swaminathan, K. Genetic diversity among toxigenic clostridia isolated from soil, water, meat and associated polluted sites in South India. Indian J. Med. Microbiol. 2009, 27, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Zhu, L.W.; Wang, H.F.; Zhao, N.; Luo, J.; Wang, C.M.; Wang, Y.T.; Liu, Y.H.; Zhou, W.; et al. Identification, Isolation, and Phylogenetic Analysis of Clostridium perfringens Type A and Type C from Wild Boar (Sus scrofa) in the People’s Republic of China. J. Wildl. Dis. 2017, 53, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Balch, H.H.; Ganley, O.H. Observations on the pathogenesis of Clostridium welchii myonecrosis. Ann. Surg. 1957, 146, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.E.; Bayer, C.R.; Aldape, M.J.; Wallace, R.J.; Titball, R.W.; Stevens, D.L. Clostridium perfringens phospholipase C-induced platelet/leukocyte interactions impede neutrophil diapedesis. J. Med. Microbiol. 2006, 55, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Su, W.; Wen, C.; Li, W.; Zhang, Y.; Gong, T.; Du, S.; Wang, X.; Lu, Z.; Jin, M.; et al. Effect of Porcine Clostridium perfringens on Intestinal Barrier, Immunity, and Quantitative Analysis of Intestinal Bacterial Communities in Mice. Front. Vet. Sci. 2022, 9, 881878. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, S.; Timmons, J.; Fitz-coy, S.; Parveen, S. Characterization of Clostridium perfringens recovered from broiler chicken affected by necrotic enteritis. Poult. Sci. 2019, 98, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; May, A.; Obremskey, W.T. Necrotizing Soft-tissue Infections: An Orthopaedic Emergency. J. Am. Acad. Orthop. Surg. 2019, 27, E199–E206. [Google Scholar] [CrossRef]
- Lien, E.; Ingalls, R.R. Toll-like receptors. Crit. Care Med. 2002, 30, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, L.H.; Li, F.X.; Wang, N.; Ma, Y.Z.; Li, J.W.; Wu, Y.J.; Liang, J.; Lei, Y.X.; Wang, X.Y.; et al. Mixed lineage kinase-like protein protects against Clostridium perfringens infection by enhancing NLRP3 inflammasome-extracellular traps axis (vol 25, 105121, 2022). Iscience 2023, 26, 106149. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, Y.X.; Li, J.W.; Ma, Y.Z.; Wang, X.Y.; Meng, F.H.; Wu, Y.J.; Wang, N.; Liang, J.; Zhao, C.Q.; et al. G Protein-Coupled Receptor 120 Mediates Host Defense against Clostridium perfringens Infection through Regulating NOD-like Receptor Family Pyrin Domain-Containing 3 Inflammasome Activation. J. Agric. Food Chem. 2023, 71, 7119–7130. [Google Scholar] [CrossRef] [PubMed]
- Takehara, M.; Kobayashi, K.; Nagahama, M. Toll-Like Receptor 4 Protects Against Clostridium perfringens Infection in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 633440. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, K.L.; Ray, M.E.; Su, Y.A.; Anzick, S.L.; Johnstone, R.W.; Trapani, J.A.; Meltzer, P.S.; Trent, J.M. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 1997, 15, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Cridland, J.A.; Curley, E.Z.; Wykes, M.N.; Schroder, K.; Sweet, M.J.; Roberts, T.L.; Ragan, M.A.; Kassahn, K.S.; Stacey, K.J. The mammalian PYHIN gene family: Phylogeny, evolution and expression. BMC Evol. Biol. 2012, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.D.; Hara, H.; Sakai, S.; Hernandez-Cuellar, E.; Mitsuyama, M.; Kawamura, I.; Tsuchiya, K. Type I Interferon Signaling Regulates Activation of the Absent in Melanoma 2 Inflammasome during Streptococcus pneumoniae Infection. Infect. Immun. 2014, 82, 2310–2317. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Jiang, Z.Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–403. [Google Scholar] [CrossRef]
- Park, E.; Na, H.S.; Song, Y.R.; Shin, S.Y.; Kim, Y.M.; Chung, J. Activation of NLRP3 and AIM2 Inflammasomes by Porphyromonas gingivalis Infection. Infect. Immun. 2014, 82, 112–123. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Malireddi, R.K.S.; Gurung, P.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.-D. Concerted Activation of the AIM2 and NLRP3 Inflammasomes Orchestrates Host Protection against Aspergillus Infection. Cell Host Microbe 2015, 17, 357–368. [Google Scholar] [CrossRef]
- Hu, G.Q.; Song, P.X.; Li, N.; Chen, W.; Lei, Q.Q.; Yu, S.X.; Zhang, X.J.; Du, C.T.; Deng, X.M.; Han, W.Y.; et al. AIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infection. Mucosal Immunol. 2016, 9, 1330–1339. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, U514–U516. [Google Scholar] [CrossRef] [PubMed]
- Knodler, L.A.; Crowley, S.M.; Sham, H.P.; Yang, H.J.; Wrande, M.; Ma, C.X.; Ernst, R.K.; Steele-Mortimer, O.; Celli, J.; Vallance, B.A. Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens. Cell Host Microbe 2014, 16, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.; Feriotti, C.; McGinley, A.; Carlile, S.R.; Jiang, Z.Z.; Calderon-Gonzalez, R.; Dumigan, A.; Sá-Pessoa, J.; Sutton, C.E.; Kearney, J.; et al. PYHIN protein IFI207 regulates cytokine transcription and IRF7 and contributes to the establishment of K. pneumoniae infection. Cell Rep. 2023, 42, 112341. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.; Ma, Z.; Miller, J.; Yu, J.; Malik, M.; Bakshi, C.S. Comparative analysis of absent in melanoma 2-inflammasome activation in Francisella tularensis and Francisella novicida. Front. Microbiol. 2023, 14, 1188112. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.E.; Deswaerte, V.; West, A.C.; Sun, E.K.M.; Wray-McCann, G.; Livis, T.; Kumar, B.; Rodriguez, E.; Gabay, C.; Ferrero, R.L.; et al. The cytosolic DNA sensor AIM2 promotes Helicobacter-induced gastric pathology via the inflammasome. Immunol. Cell Biol. 2023, 101, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Tall, A.R. AIMing 2 treat atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X.; Li, W.; Wang, H.; Yan, L.; Zhao, L.; Zhang, X.; Wang, N.; An, W.; Liu, T.; et al. Clostridium perfringens alpha toxin damages the immune function, antioxidant capacity and intestinal health and induces PLCgamma1/AMPK/mTOR pathway-mediated autophagy in broiler chickens. Heliyon 2024, 10, e26114. [Google Scholar] [CrossRef] [PubMed]
- García-Vela, S.; Guay, L.D.; Rahman, M.R.T.; Biron, E.; Torres, C.; Fliss, I. Antimicrobial Activity of Synthetic Enterocins A, B, P, SEK4, and L50, Alone and in Combinations, against Clostridium perfringens. Int. J. Mol. Sci. 2024, 25, 1597. [Google Scholar] [CrossRef]
- Briken, V.; Ahlbrand, S.E.; Shah, S. Mycobacterium tuberculosis and the host cell inflannmasome: A complex relationship. Front. Cell. Infect. Microbiol. 2013, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Aldrich, A.; Kielian, T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J. Neurochem. 2014, 129, 704–711. [Google Scholar] [CrossRef]
- Xia, S.Y.; Zhang, Z.B.; Magupalli, V.G.; Pablo, J.L.; Dong, Y.; Vora, S.M.; Wang, L.F.; Fu, T.M.; Jacobson, M.P.; Greka, A.; et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 2021, 593, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, M.; Kawakami, Y.; Salzer, S.; Kreuter, A.; Dombrowski, Y.; Koglin, S.; Kresse, S.; Ruzicka, T.; Schauber, J. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch. Dermatol. Res. 2013, 305, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Kabaleeswaran, V.; Fu, T.M.; Magupalli, V.G.; Wu, H. Crystal Structure of the F27G AIM2 PYD Mutant and Similarities of Its Self-Association to DED/DED Interactions. J. Mol. Biol. 2014, 426, 1420–1427. [Google Scholar] [CrossRef]
- Martinon, F.; Tschopp, J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004, 117, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Saiga, H.; Kitada, S.; Shimada, Y.; Kamiyama, N.; Okuyama, M.; Makino, M.; Yamamoto, M.; Takeda, K. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int. Immunol. 2012, 24, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, X.; Kouadir, M.; Shi, F.; Ding, T.; Liu, C.; Liu, J.; Wang, M.; Yang, L.; Yin, X.; et al. the AIM2 inflammasome is involved in macrophage activation during infection with virulent Mycobacterium bovis strain. J. Infect. Dis. 2013, 208, 1849–1858. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Hara, H.; Kawamura, I.; Nomura, T.; Yamamoto, T.; Daim, S.; Dewamitta, S.R.; Shen, Y.N.; Fang, R.D.; Mitsuyama, M. Involvement of Absent in Melanoma 2 in Inflammasome Activation in Macrophages Infected with Listeria monocytogenes. J. Immunol. 2010, 185, 1186–1195. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.W.; Juliana, C.; Solorzano, L.; Kang, S.; Wu, J.H.; Datta, P.; McCormick, M.; Huang, L.; McDermott, E.; et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 2010, 11, 385–394. [Google Scholar] [CrossRef]
- Yu, S.X.; Du, C.T.; Chen, W.; Lei, Q.Q.; Li, N.; Qi, S.; Zhang, X.J.; Hu, G.Q.; Deng, X.M.; Han, W.Y.; et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 17935. [Google Scholar] [CrossRef]
- Awad, M.M.; Ellemor, D.M.; Boyd, R.L.; Emmins, J.J.; Rood, J.I. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 2001, 69, 7904–7910. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Lou, Y.; Wang, N.; Zhao, Y.; Zhang, S.; Zhang, M.; Li, J.; Xu, Q.; He, A.; Yu, S. Absent in Melanoma 2 Mediates Inflammasome Signaling Activation against Clostridium perfringens Infection. Int. J. Mol. Sci. 2024, 25, 6571. https://doi.org/10.3390/ijms25126571
Ma Z, Lou Y, Wang N, Zhao Y, Zhang S, Zhang M, Li J, Xu Q, He A, Yu S. Absent in Melanoma 2 Mediates Inflammasome Signaling Activation against Clostridium perfringens Infection. International Journal of Molecular Sciences. 2024; 25(12):6571. https://doi.org/10.3390/ijms25126571
Chicago/Turabian StyleMa, Zhaoguo, Yanan Lou, Na Wang, Yi Zhao, Shuxin Zhang, Mingyue Zhang, Jiaqi Li, Qian Xu, Aobo He, and Shuixing Yu. 2024. "Absent in Melanoma 2 Mediates Inflammasome Signaling Activation against Clostridium perfringens Infection" International Journal of Molecular Sciences 25, no. 12: 6571. https://doi.org/10.3390/ijms25126571




