Involvement of the GH38 Family Exoglycosidase α-Mannosidase in Strawberry Fruit Ripening
Abstract
1. Introduction
2. Results and Discussion
2.1. Computational Analysis
2.1.1. Candidate Genes of α-Man from F. × ananassa
2.1.2. Phylogenetic Classification of α-Man Protein Sequences
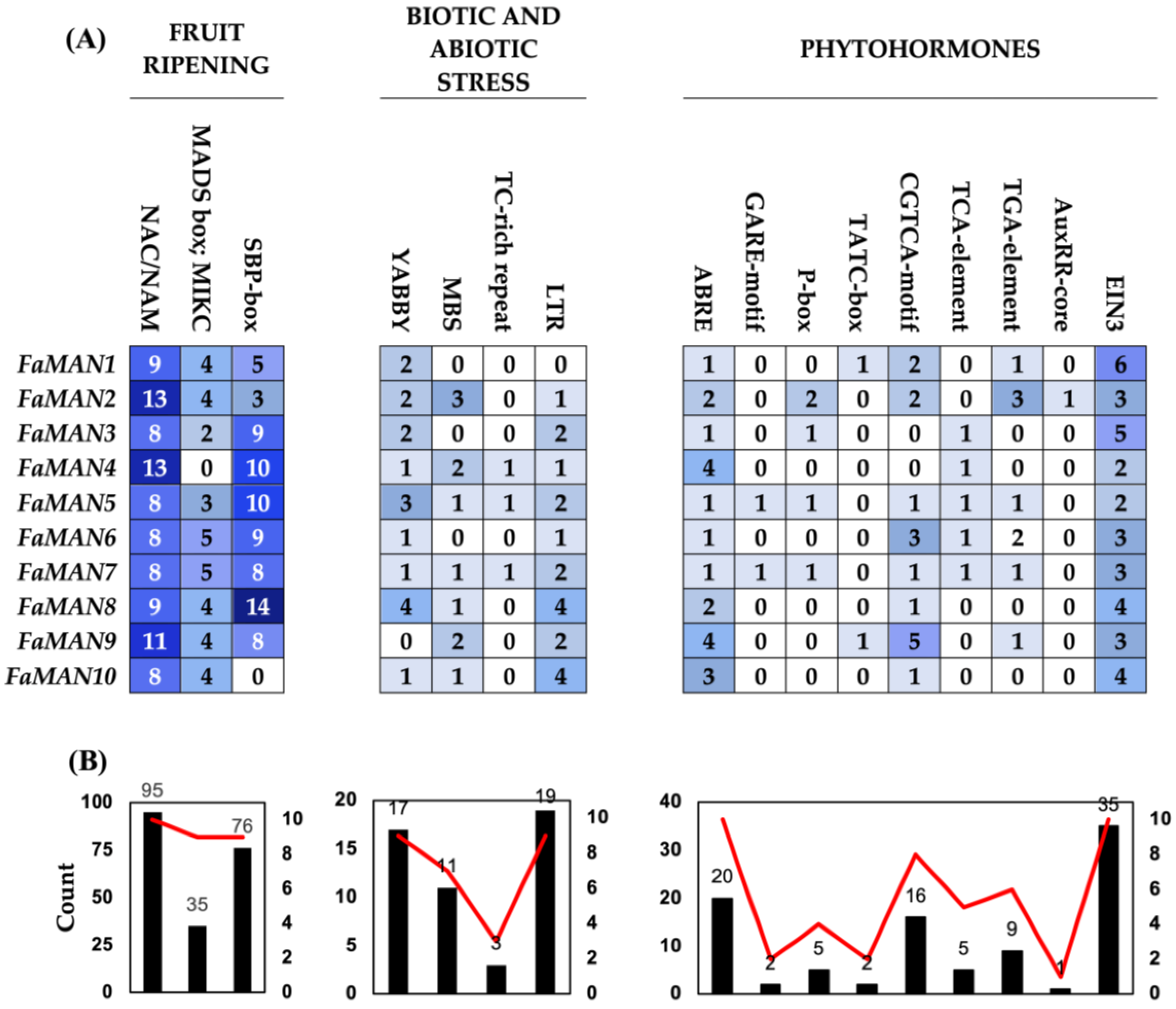
2.1.3. Promoter Analysis of FaMAN Genes
2.2. Molecular Assays
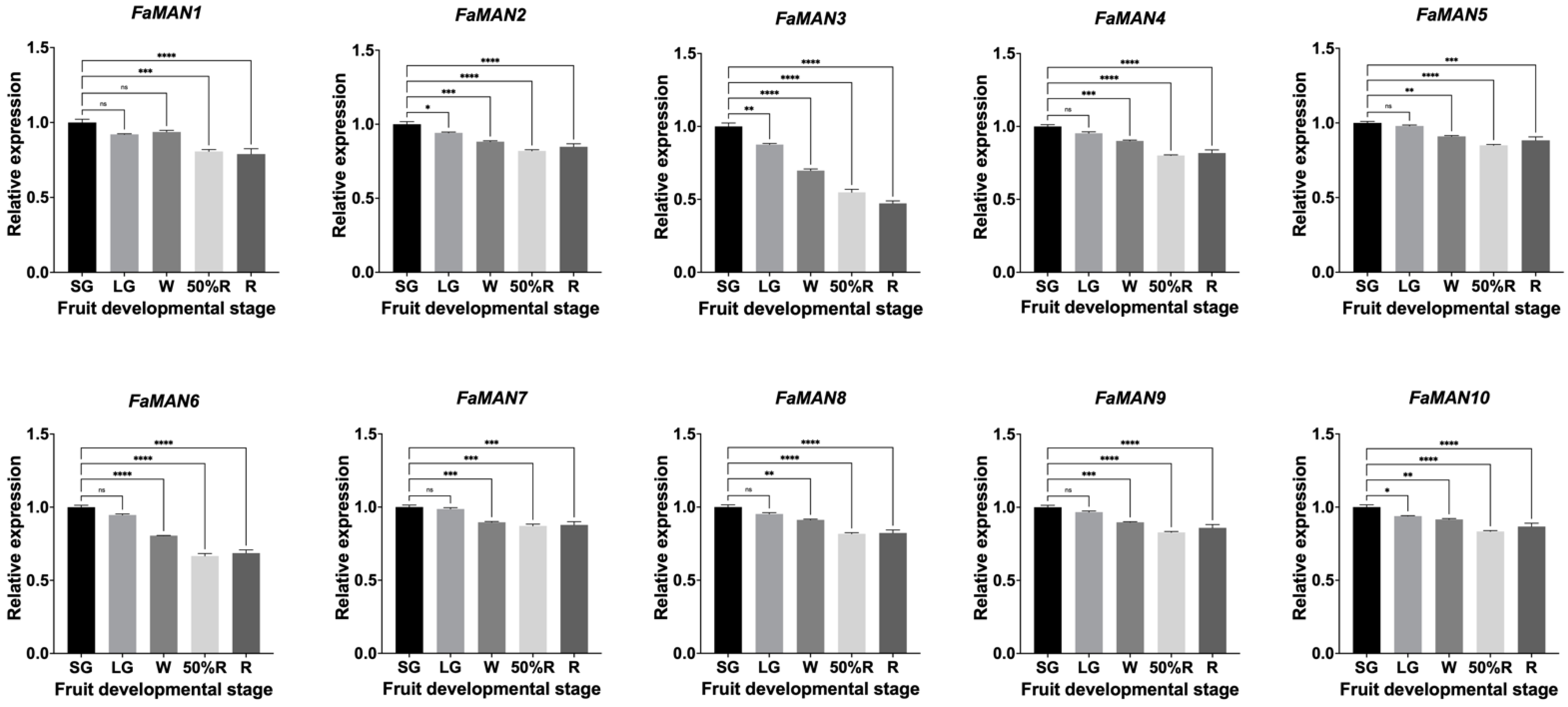
2.2.1. Relative Expression of FaMAN Genes
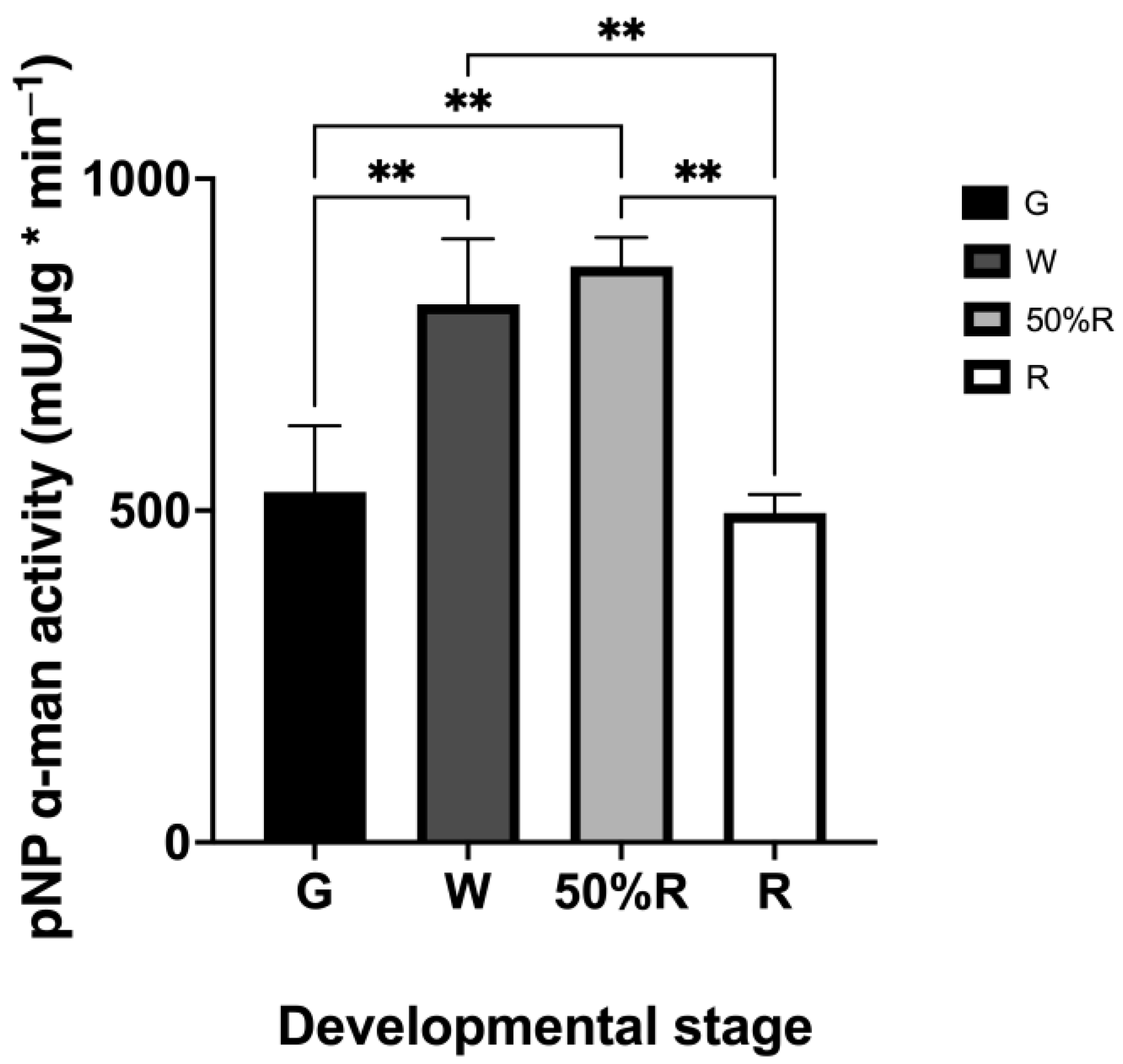
2.2.2. Total α-Man Enzyme Activity in Fruit Ripening
3. Materials and Methods
3.1. Identification of FaMAN Genes
3.2. In Silico Analysis of Promoter Sequences
3.3. Phylogenetic Analysis of the FaMAN Enzyme Family
3.4. Fragaria × ananassa Harvest in Orchard
3.5. RNA Extraction, cDNA Synthesis, and Real-Time qPCR (RT-qPCR) Assays
3.6. Total Protein Extraction and Enzymatic Activity Assay for α-Man
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Coninck, T.; Gistelink, K.; Van Rensburg, H.C.J.; Van den Ende, W.; Van Damme, E.J.M. Sweet modifications modulate plant development. Biomolecules 2021, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jia, X.-R.; Liu, L.; Voglmeir, J. Changes in protein N-glycosylation during the fruit development and ripening in melting-type peach. Food Mater. Res. 2021, 1, 2. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Vicré-Gibouin, M.; Gotté, M.; Plancot, B.; Lerouge, P.; Bardor, M.; Driouich, A. Cell wall O-glycoproteins and N-glycoproteins: Aspects of biosynthesis and function. Front. Plant Sci. 2014, 5, 499. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Van Damme, E.J.M. Review/N-glycans: The making of a varied toolbox. Plant Sci. 2015, 239, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R. Plant protein glycosylation. Glycobiology 2016, 26, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Lige, B.; Ma, S.; van Huystee, R.B. The Effects of the Site-Directed Removal of N-Glycosylation from Cationic Peanut Peroxidase on Its Function. Arch. Biochem. Biophys. 2001, 386, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liebminger, E.; Grass, J.; Altmann, F.; Mach, L.; Strasser, R. Characterizing the link between glycosylation state and enzymatic activity of the endo-β1,4-glucanase KORRIGAN1 from Arabidopsis thaliana. J. Biol. Chem. 2013, 288, 22270–22280. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Yañez, Á.; Beltrán, D.; Campano-Romero, C.; Molinett, S.; Herrera, R.; Moya-León, M.A.; Morales-Quintana, L. Glycosylation is important for FcXTH1 activity as judged by its structural and biochemical characterization. Plant Physiol. Biochem. 2017, 119, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Rosli, H.G.; Civello, P.M.; Martínez, G.A. Changes in cell wall composition of three Fragaria x ananassa cultivars with different softening rate during ripening. Plant Physiol. Biochem. 2004, 42, 823–831. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria × ananassa fruits. Sci. Hortic. 2010, 124, 454–462. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2018, 246, 557–562. [Google Scholar] [CrossRef]
- Priem, B.; Gross, K.C. Mannosyl- and Xylosyl-containing glycans promote tomato (Lycopersicon esculentum Mill.) fruit ripening. Plant Physiol. 1992, 98, 399–401. [Google Scholar] [CrossRef]
- Kobata, A. Exo- and endoglycosidases revisited. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Herscovics, A. Glycosidases of the Asparagine-linked oligosaccharide processing pathway. In Comprehensive Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 1999; Chapter 3; p. 21. [Google Scholar]
- Meli, V.S.; Ghosh, S.; Prabha, T.N.; Chakraborty, N.; Chakraborty, S.; Datta, A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 2413–2418. [Google Scholar] [CrossRef]
- Ghosh, S.; Meli, V.S.; Kumar, A.; Thakur, A.; Chakraborty, N.; Chakraborty, S.; Datta, A. The N-glycan processing enzymes α-mannosidase and β-D-N-acetylhexosaminidase are involved in ripening-associated softening in the non-climacteric fruits of capsicum. J. Exp. Bot. 2011, 62, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, B.H.; Prabha, T.N.; Srinivasan, K. Activities of β-hexosaminidase and α-mannosidase during development and ripening of bell capsicum (Capsicum annuum var. variata). Plant Sci. 2004, 167, 1263–1271. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Sherif, S.; Qubbaj, T.; Sullivan, A.J.; Jayasankar, S. Stimulated auxin levels enhance plum fruit ripening, but limit shelf-life characteristics. Postharvest Biol. Technol. 2016, 112, 215–223. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Labavitch, J.M. Cell Wall Metabolism in Ripening Fruit: II. Changes in carbohydrate-degrading enzymes in ripening ‘bartlett’ pears. Plant Physiol. 1980, 65, 1014–1016. [Google Scholar] [CrossRef]
- Fernandez-Bolaños, J.; Rodriguez, R.; Guillen, R.; Jimenez, A.; Heredia, A. Activity of cell wall-associated enzymes in ripening olive fruit. Physiol. Plant 1995, 93, 651–658. [Google Scholar] [CrossRef]
- Bose, S.K.; He, Y.; Howlader, P.; Wang, W.; Yin, H. The N-glycan processing enzymes β-D-N-acetylhexosaminidase are involved in ripening-associated softening in strawberry fruit. J. Food Sci. Technol. 2021, 58, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Parra-Palma, C.; Figueroa, C.R.; Zuñiga, P.E.; Valenzuela-Riffo, F.; Gonzalez, J.; Gaete-Eastman, C.; Morales-Quintana, L. Cell wall-related enzymatic activities and transcriptional profiles in four strawberry (Fragaria x ananassa) cultivars during fruit development and ripening. Sci. Hortic. 2018, 238, 325–332. [Google Scholar] [CrossRef]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; The AmiGO Hub; Web Presence Working Group. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakano, R.; Nakamura, K.; Hossain, T.; Kimura, Y. Molecular characterization of plant acidic α-mannosidase, a member of glycosylhydrolase family 38, involved in the turnover of N-glycans during tomato fruit ripening. J. Biochem. 2010, 148, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Ghosh, S.; Meli, V.S.; Kumar, A.; Kumar, V.; Chakraborty, N.; Chakraborty, S.; Datta, A. Fruit ripening regulation of α-mannosidase expression by the MADS Box transcription factor RIPENING INHIBITOR and Ethylene. Front. Plant Sci. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of gene duplication in plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Zhang, L.-K.; Zhang, K.; Chen, S.-M.; Hu, J.-B.; Cheng, F. The impact of tandem duplication on gene evolution in Solanaceae species. J. Integr. Agric. 2022, 21, 1004–1014. [Google Scholar] [CrossRef]
- Manning, K.; Tör, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006, 38, 948–952. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.; Hou, Z.; Luo, Z.; Wang, T.; Li, H.; Zhang, J.; Ye, Z. Members of the tomato FRUITFULL MADS-box family regulate style abscission and fruit ripening. J. Exp. Bot. 2014, 65, 3005–3014. [Google Scholar] [CrossRef]
- Carrasco-Orellana, C.; Stappung, Y.; Mendez-Yañez, A.; Allan, A.C.; Espley, R.V.; Plunkett, B.J.; Moya-Leon, M.A.; Herrera, R. Characterization of a ripening-related transcription factor FcNAC1 from Fragaria chiloensis fruit. Sci. Rep. 2018, 8, 10524. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, X.; Xu, J.; Liu, Y. Regulation of fruit ripening by MADS-box transcription factors. Sci. Hortic. 2023, 314, 111950. [Google Scholar] [CrossRef]
- Sánchez-Gómez, C.; Posé, D.; Martín-Pizarro, C. Insights into transcription factors controlling strawberry fruit development and ripening. Front. Plant Sci. 2022, 13, 1022369. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Wang, X.; Ye, B.; Jin, M.; Chen, W.; Wang, Y.; Zhou, Y.; Blanks, A.M.; Gu, M.; Zhang, P.; et al. Molecular and functional characterization of the SBP-box transcription factor SPL-CNR in tomato fruit ripening and cell death. J. Exp. Bot. 2020, 71, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Y.-Y.; Wu, C.-J.; Kuang, J.-F.; Chen, J.-Y.; Shan, W. MaSPL16 positively regulates fruit ripening in bananas via the direct transcriptional induction of MaNAC029. Hortic. Adv. 2023, 1, 10. [Google Scholar] [CrossRef]
- Song, H.; Zhao, K.; Jiang, G.; Sun, S.; Li, J.; Tu, M.; Wang, L.; Xie, H.; Chen, D. Genome-wide identification and expression analysis of the SBP-box gene family in loquat fruit development. Genes 2023, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.C.; Dunn, M.A.; Goddard, N.J.; Hughes, M.A. Identification of a novel low-temperature-response element in the promoter of the barley (Hordeum vulgare L) gene blt101.1. Planta 2001, 213, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Pati, P.K.; Pati, A.M.; Nagpal, A.K. In-silico analysis of cis-acting regulatory elements of pathogenesis-related proteins of Arabidopsis thaliana and Oryza sativa. PLoS ONE 2017, 12, e0184523. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, G.; Cai, M.; Priyadarshani, S.V.G.N.; Aslam, M.; Zhou, Q.; Huang, X.; Wang, X.; Liu, Y.; Qin, Y. Genome-wide analysis of the YABBY transcription factor family in pineapple and functional identification of AcYABBY4 involvement in salt stress. Int. J. Mol. Sci. 2019, 20, 5863. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, M.; Guo, Z.; Zhu, X.; Xia, Z. Identification of a 119-bp promoter of the maize sulfite oxidase gene (ZmSO) that confers high-level gene expression and ABA or drought inducibility in transgenic plants. Int. J. Mol. Sci. 2019, 20, 3326. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Li, D.; Liu, Y.; Yang, X. Roles of YABBY transcription factors in the modulation of morphogenesis, development, and phytohormone and stress responses in plants. J. Plant Res. 2020, 133, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.A.; Goddard, N.J.; Zhang, L.; Pearce, R.S.; Hughes, M.A. Low-temperature-responsive barley genes have different control mechanisms. Plant Mol. Biol. 1994, 24, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Ding, A.; Li, P.; Wang, J.; Cheng, T.; Bao, F.; Zhang, Q. Genome-wide identification and low-temperature expression analysis of bHLH genes in Prunus mume. Front. Genet. 2021, 12, 762135. [Google Scholar] [CrossRef] [PubMed]
- Edrisi Maryan, K.; Farrokhi, N.; Samizadeh Lahiji, H. Cold-responsive transcription factors in Arabidopsis and rice: A regulatory network analysis using array data and gene co-expression network. PLoS ONE 2023, 18, e0286324. [Google Scholar] [CrossRef] [PubMed]
- Perotti, M.F.; Posé, D.; Martín-Pizarro, C. Non-climacteric fruit development and ripening regulation: ‘The phytohormones show’. J. Exp. Bot. 2023, 74, 6237–6253. [Google Scholar] [CrossRef] [PubMed]
- Katel, S.; Mandal, H.R.; Kattel, S.; Yadav, S.P.S.; Lamshal, B.S. Impacts of plant growth regulators in strawberry plant: A review. Heliyon 2022, 8, e11959. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Medina-Puche, L.; Blanco-Portales, R.; Molina-Hidalgo, F.J.; Cumplido-Laso, G.; García-Caparrós, N.; Moyano-Cañete, E.; Caballero-Repullo, J.L.; Muñoz-Blanco, J.; Rodríguez-Franco, A. Extensive transcriptomic studies on the roles played by abscisic acid and auxins in the development and ripening of strawberry fruits. Funct. Integr. Genom. 2016, 16, 671–692. [Google Scholar] [CrossRef] [PubMed]
- Merchante, C.; Vallarino, J.G.; Osorio, S.; Aragüez, I.; Villarreal, N.; Ariza, M.T.; Martínez, G.A.; Medina-Escobar, N.; Civello, M.P.; Fernie, A.R.; et al. Ethylene is involved in strawberry fruit ripening in an organ-specific manner. J. Exp. Bot. 2013, 64, 4421–4439. [Google Scholar] [CrossRef]
- Hossain, A.; Nakamura, K.; Kimura, Y. α-mannosidase involved in turnover of plant complex type N-Glycans in tomato (Lycopersicum esculentum) Fruits. Biosci. Biotechnol. Biochem. 2009, 73, 140–146. [Google Scholar] [CrossRef]
- Dorairaj, D.; Puthusseri, B.; Shetty, N.P. Suppression of N-glycan processing enzymes by deoxynojirimycin in tomato (Solanum lycopersicum) fruit. 3 Biotech 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, T.; Cheng, C.-H.; Buble, K.; Zheng, P.; Yu, J.; Humann, J.; Ficklin, S.P.; Gasic, K.; Scott, K.; et al. 15 years of GDR: New data and functionality in the Genome Database for Rosaceae. Nucleic Acids Res. 2019, 47, D1137–D1145. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.N.; Lee, T.Y.; Hung, Y.C.; Li, G.Z.; Tseng, K.C.; Liu, Y.H.; Kuo, P.L.; Zheng, H.Q.; Chang, W.C. PlantPAN3.0: A new and updated resource for reconstructing transcriptional regulatory networks from ChIP-seq experiments in plants. Nucleic Acids Res. 2019, 47, D1155–D1163. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. The clustal omega multiple alignment package. Methods Mol. Biol. 2021, 2231, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45–e50. [Google Scholar] [CrossRef]
- Jagadeesh, B.H.; Prabha, T.N.; Srinivasan, K. Activities of glycosidases during fruit development and ripening of tomato (Lycopersicum esculantum L.): Implication in fruit ripening. Plant Sci. 2004, 166, 1451–1459. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Darvill, A.G.; Etzler, M.E.; Mohnen, D.; Perez, S.; Mortimer, J.C.; Pauly, M. Viridiplantae and Algae. In Essentials of Glycobiology; Edited by Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2022; p. 323. [Google Scholar]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Kaneko, T.; Nakamura, Y.; Kotani, H.; Kato, T.; Asamizu, E.; Miyajima, N.; Sasamoto, S.; Kimura, T.; Hosouchi, T.; et al. Sequence and analysis of chromosome 5 of the plant Arabidopsis thaliana. Nature 2000, 408, 6814. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kotani, H.; Nakamura, Y.; Kaneko, T.; Asamizu, E.; Fukami, M.; Miyajima, N.; Tabata, S. Structural analysis of Arabidopsis thaliana chromosome 5. I. Sequence features of the 1.6 Mb regions covered by twenty physically assigned P1 clones. DNA Res. 1997, 4, 215–219. [Google Scholar] [CrossRef] [PubMed]
- European Union Chromosome 3 Arabidopsis Genome Sequencing Consortium; The Institute for Genomic Research; Kazusa DNA Research Institute. Sequence and analysis of chromosome 3 of the plant Arabidopsis thaliana. Nature 2000, 408, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Chen, W.; Li, Y.; Bharti, A.K.; Saxena, R.K.; Schlueter, J.A.; Donoghue, M.T.; Azam, S.; Fan, G.; Whaley, A.M.; et al. Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat. Biotechnol. 2012, 30, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.; Cousido-Siah, A.; Lepage, M.L.; Schneider, J.P.; Bodlenner, A.; Mitschler, A.; Meli, A.; Izzo, I.; Alvarez, H.A.; Podjarny, A.; et al. Structural Basis of Outstanding Multivalent Effects in Jack Bean -Mannosidase Inhibition. Angew. Chem. Int. Ed. 2018, 57, 8002–8006. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.; Yeom, S.-I.; Kim, Y.-M.; Seo, E.; Kim, K.-T.; Kim, M.-S.; Lee, J.M.; Cheong, K.; Shin, H.-S.; et al. New reference genome sequences of hot pepper reveal the massive evolution of plant disease-resistance genes by retroduplication. Genome Biol. 2017, 18, 210. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Lee, T.; Baek, J.; Kim, J.H.; Kim, C.; Jeong, S.-C. Genome assembly of the popular Korean soybean cultivar Hwangkeum. G3 Genes|Genomes|Genet. 2021, 11, jkab272. [Google Scholar] [CrossRef] [PubMed]
- Nilssen, O.; Berg, T.; Riise, H.M.; Ramachandran, U.; Evjen, G.; Hansen, G.M.; Malm, D.; Tranebjaerg, L.; Tollersrud, O.K. α-Mannosidosis: Functional cloning of the lysosomal α-mannosidase cDNA and identification of a mutation in two affected siblings. Hum. Mol. Genet. 1997, 6, 717–726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rice Chromosomes 11 and 12 Sequencing Consortia. The sequence of rice chromosomes 11 and 12, rich in disease resistance genes and recent gene duplications. BMC Biol. 2005, 3, 20. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Lin, W.; Li, S.; Li, H.; Zhou, J.; Ni, P.; Dong, W.; Hu, S.; Zeng, C.; et al. The genomes of Oryza sativa: A history of duplications. PLoS Biol. 2005, 3, e38. [Google Scholar] [CrossRef] [PubMed]
- Verde, I.; The International Peach Genome Initiative; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Motamayor, J.C.; Mockaitis, K.; Schmutz, J.; Haiminen, N.; Iii, D.L.; Cornejo, O.; Findley, S.D.; Zheng, P.; Utro, F.; Royaert, S.; et al. The genome sequence of the most widely cultivated cacao type and its use to identify candidate genes regulating pod color. Genome Biol. 2013, 14, r53. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, Y.; Chen, J.; Shi, J.; Zhao, H.; Zhao, H.; Song, W.; Zhang, M.; Cui, Y.; Dong, X.; et al. Extensive intraspecific gene order and gene structural variations between Mo17 and other maize genomes. Nat. Genet. 2018, 50, 1289–1295. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Chromosomal Location | Strand | ORF Length (bp) | Protein Length (aa) | pI Value | MW (kDA) |
|---|---|---|---|---|---|---|---|
| FaMAN1 | FxaC_2g16900.t2 | Fvb1-2:7637155..7644785− | − | 3024 | 1008 | 5.68 | 112.94 |
| FaMAN2 | FxaC_1g12790.t1 | Fvb1-4:5491201..5498753− | − | 3024 | 1008 | 5.81 | 113.22 |
| FaMAN3 | FxaC_17g11250.t1 | Fvb5-1:5272631..5279568− | − | 3066 | 1022 | 6.28 | 115.27 |
| FaMAN4 | FxaC_17g11260.t1 | Fvb5-1:5280010..5286714− | − | 3033 | 1011 | 5.70 | 113.69 |
| FaMAN5 | FxaC_20g09800.t1 | Fvb5-2:5096401..5103273− | − | 3033 | 1011 | 5.66 | 113.92 |
| FaMAN6 | FxaC_19g09610.t1 | Fvb5-4:4739310..4746318− | − | 3024 | 1008 | 5.70 | 113.54 |
| FaMAN7 | FxaC_21g01480.t1 | Fvb6-1:668217..675394+ | + | 3048 | 1016 | 5.74 | 113.99 |
| FaMAN8 | FxaC_21g01481.t1 | Fvb6-1:675955..685254+ | + | 3069 | 1023 | 5.82 | 115.45 |
| FaMAN9 | FxaC_23g44241.t1 | Fvb6-2:27453374..27460546− | − | 3048 | 1016 | 5.84 | 113.84 |
| FaMAN10 | FxaC_23g44240.t1 | Fvb6-2:27444620..27452796− | − | 3069 | 1023 | 5.82 | 115.42 |
| Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| FaMAN1 | GGACGTTCCCTTCTCTCTCTATAA | CATTTCCACACATGAAACGACCA |
| FaMAN2 | CTTTGACGCAAGCACCACACAATG | CACACACTAAACGACCAAAAACTGAAGCA |
| FaMAN3 | GTTAACAACGAAATGCTGTAGCAC | ATGGAAACACAACTAGTACCATAAGAAGC |
| FaMAN4 | GTATCAATCAATTAATCGACAAAGACAACG | CAACTGCCATTGAAATGCAGGAG |
| FaMAN5 | GCTAGTATCAATTAATCGACATAGACAACG | ACGTCGTATTGTACTGTATGTACTTGG |
| FaMAN6 | ACCAATGCACACCTAGCTAGTAT | AGTAAGCCATTGAAATGCGTGAGT |
| FaMAN7 | TCCATTTCTTCGATCCTTCGTTTTTGG | CCATAGCTGAAAGCTTCACTGAAACT |
| FaMAN8 | AGAAGTCAGACAGAAAAAACAGTACAAG | AAGAAGAGCCAAGAAGAAGAGTAAACC |
| FaMAN9 | CTTCGATCCTTCGTTTTTGGCTTCTTATG | CAGTAACACTACCAGCAACAGCAACG |
| FaMAN10 | GCATTAGGAAGCGGAAGAAGAATG | CTTCTTAGCTACCTTATTGCTTTGCTT |
| FaGAPDH1 | TCCATCACTGCCACCCAGAAGACTG | AGCAGGCAGAACCTTTCCGACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Yáñez, A.; Sáez, D.; Rodríguez-Arriaza, F.; Letelier-Naritelli, C.; Valenzuela-Riffo, F.; Morales-Quintana, L. Involvement of the GH38 Family Exoglycosidase α-Mannosidase in Strawberry Fruit Ripening. Int. J. Mol. Sci. 2024, 25, 6581. https://doi.org/10.3390/ijms25126581
Méndez-Yáñez A, Sáez D, Rodríguez-Arriaza F, Letelier-Naritelli C, Valenzuela-Riffo F, Morales-Quintana L. Involvement of the GH38 Family Exoglycosidase α-Mannosidase in Strawberry Fruit Ripening. International Journal of Molecular Sciences. 2024; 25(12):6581. https://doi.org/10.3390/ijms25126581
Chicago/Turabian StyleMéndez-Yáñez, Angela, Darwin Sáez, Francisca Rodríguez-Arriaza, Claudio Letelier-Naritelli, Felipe Valenzuela-Riffo, and Luis Morales-Quintana. 2024. "Involvement of the GH38 Family Exoglycosidase α-Mannosidase in Strawberry Fruit Ripening" International Journal of Molecular Sciences 25, no. 12: 6581. https://doi.org/10.3390/ijms25126581
APA StyleMéndez-Yáñez, A., Sáez, D., Rodríguez-Arriaza, F., Letelier-Naritelli, C., Valenzuela-Riffo, F., & Morales-Quintana, L. (2024). Involvement of the GH38 Family Exoglycosidase α-Mannosidase in Strawberry Fruit Ripening. International Journal of Molecular Sciences, 25(12), 6581. https://doi.org/10.3390/ijms25126581







