Gastric-Type Expression Signature in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Parameters
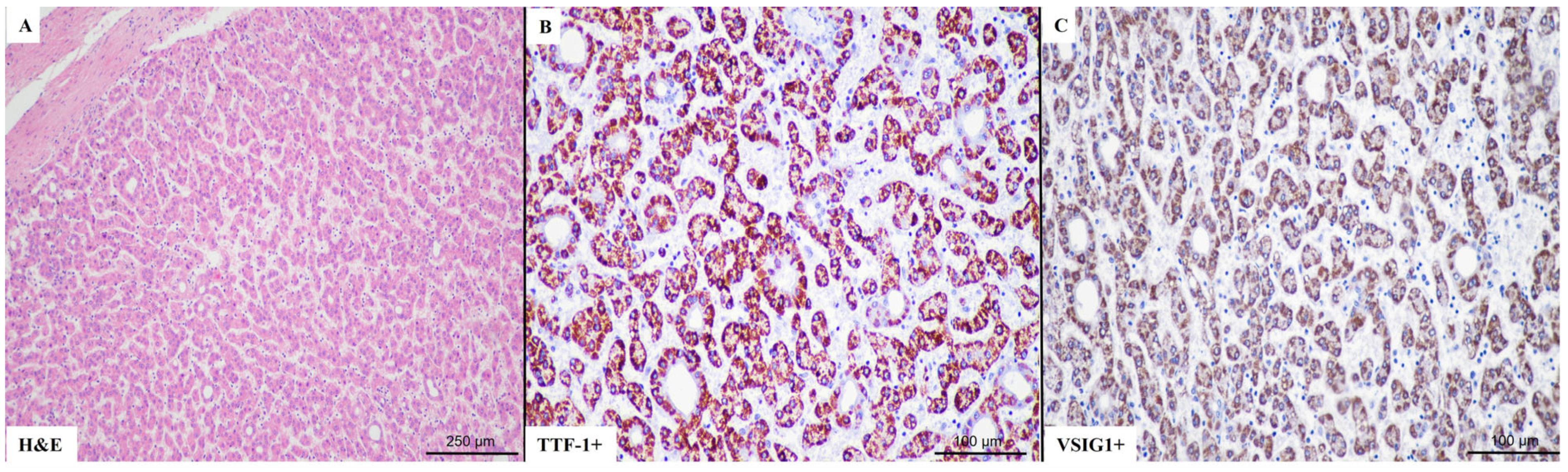
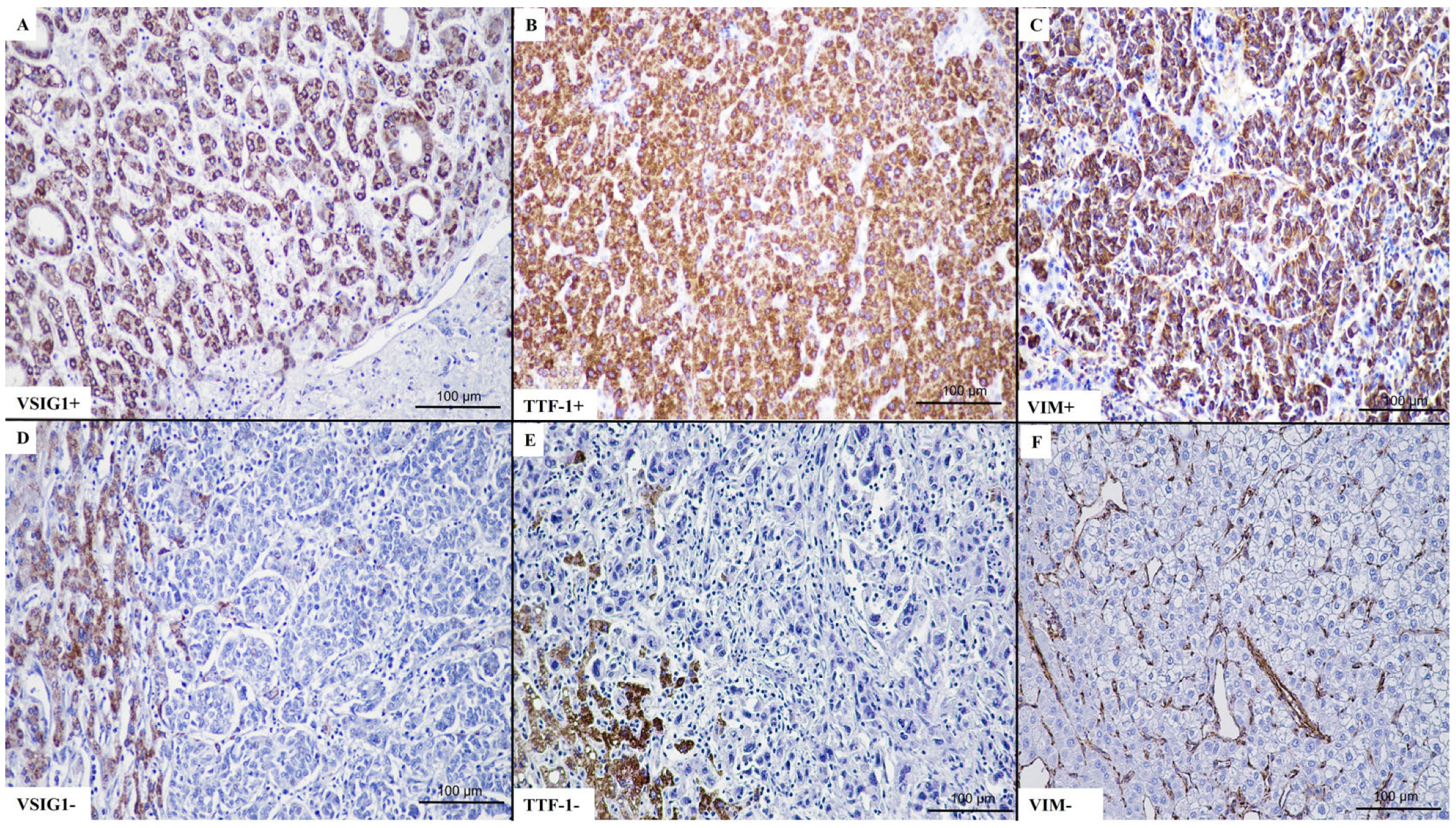
2.2. VSIG1 Correlation with Clinicopathological Parameters
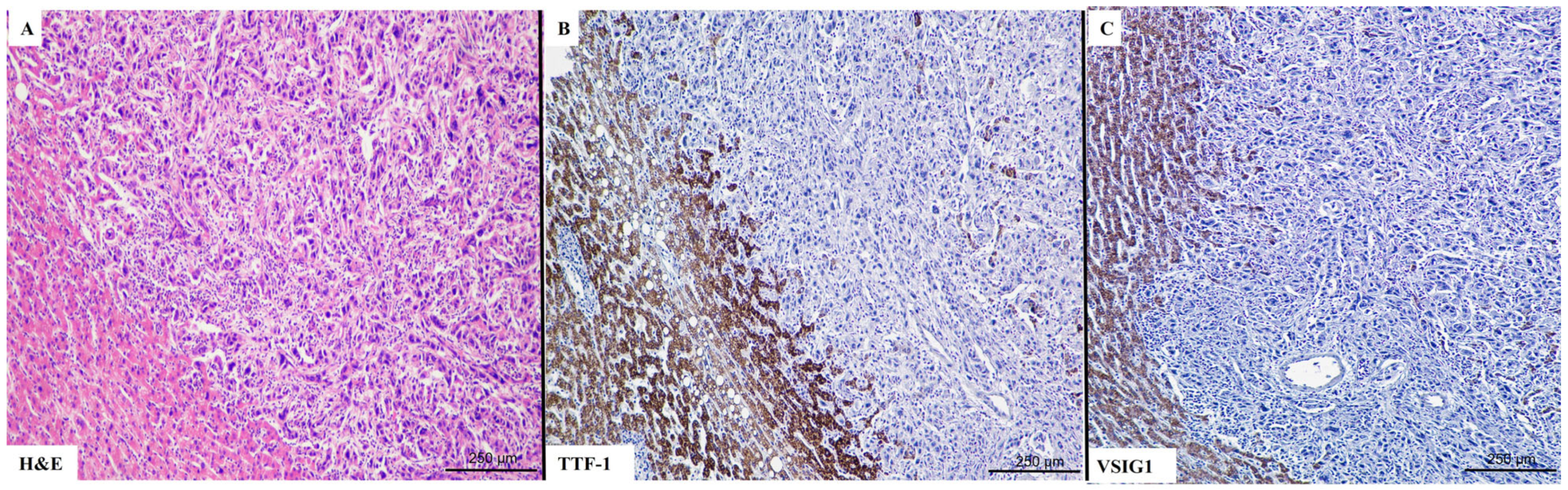
2.3. TTF-1 Correlation with Clinicopathological Parameters
2.4. Vimentin Correlation with Clinicopathological Parameters
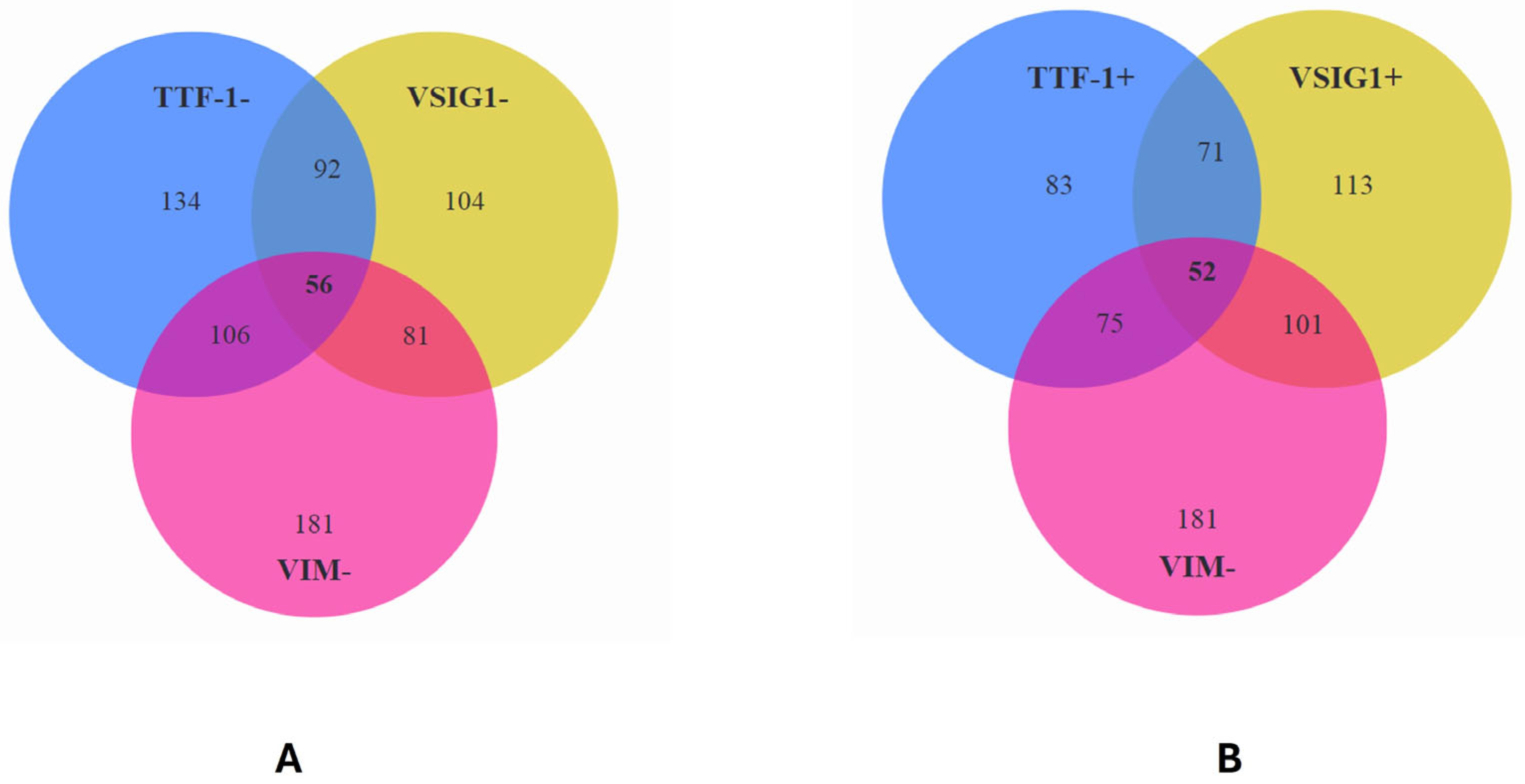
2.5. Correlation between VSIG1, TTF-1, VIM, and Clinicopathological Parameters
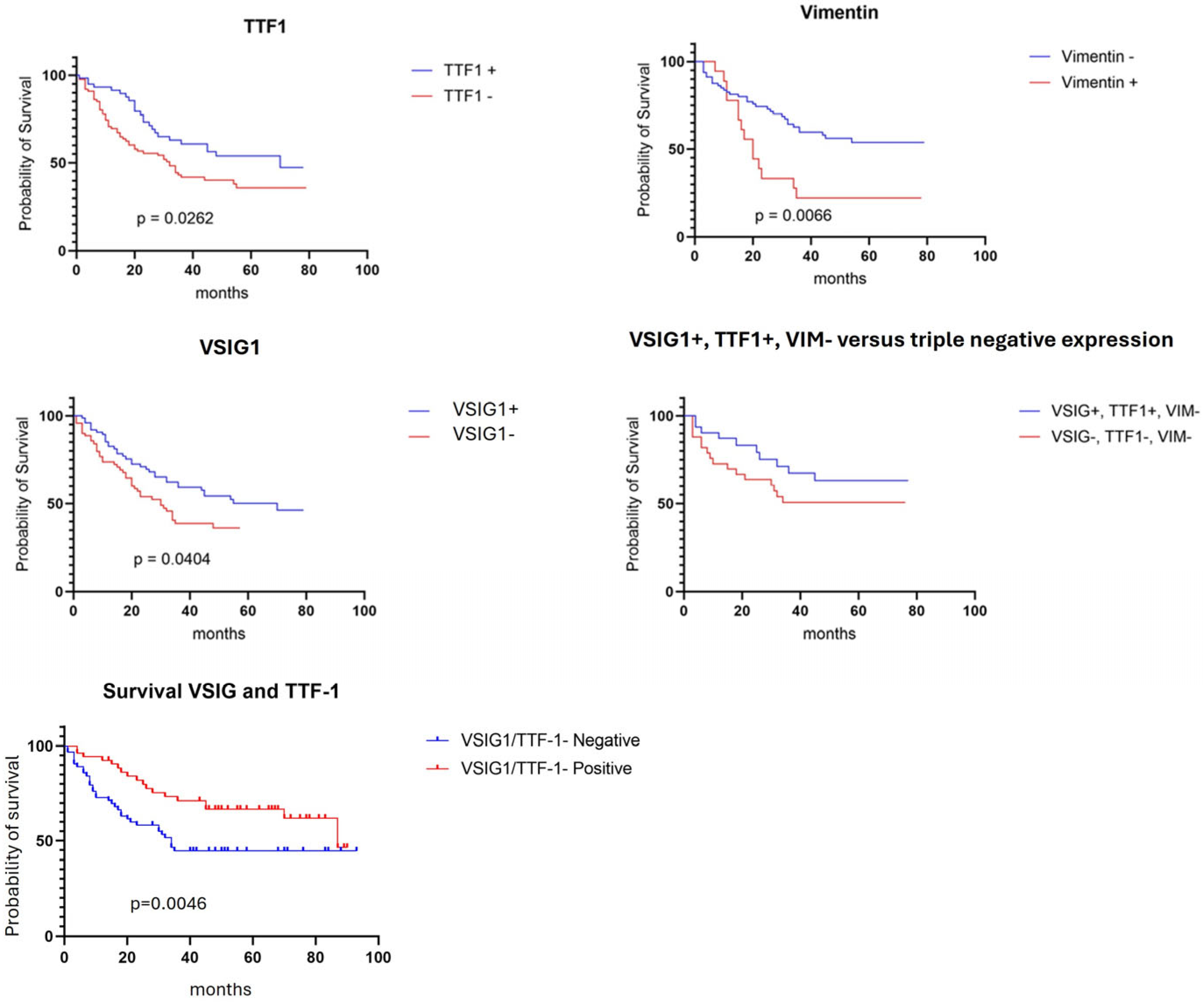
2.6. Survival Rate
3. Discussion
4. Materials and Methods
4.1. Selection of HCC Cases
4.2. Immunohistochemistry
4.3. Interpretation of IHC Stains
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurzu, S.; Sugimura, H.; Szederjesi, J.; Szodorai, R.; Braicu, C.; Kobori, L.; Fodor, D.; Jung, I. Interaction between Cadherins, Vimentin, and V-Set and Immunoglobulin Domain Containing 1 in Gastric-Type Hepatocellular Carcinoma. Histochem. Cell Biol. 2021, 156, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P. Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, M.; Fu, Z.; Wang, P.; Zhang, Y.; Gao, Y.; Yue, M.; Ning, S.; Li, D. Immunoglobulin Superfamily Genes Are Novel Prognostic Biomarkers for Breast Cancer. Oncotarget 2017, 8, 2444–2456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oidovsambuu, O.; Nyamsuren, G.; Liu, S.; Göring, W.; Engel, W.; Adham, I.M. Adhesion Protein VSIG1 Is Required for the Proper Differentiation of Glandular Gastric Epithelia. PLoS ONE 2011, 6, e25908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Khan, S.; Huang, D.; Li, L. V-Set and Immunoglobulin Domain Containing (VSIG) Proteins as Emerging Immune Checkpoint Targets for Cancer Immunotherapy. Front. Immunol. 2022, 13, 938470. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Bang, H.; Kim, Y.-H.; Park, N.-E.; Park, Y.-H.; Park, C.; Lee, S.-R.; Lee, J.-W.; Song, B.-S.; Kim, J.-S.; et al. V-Set and Immunoglobulin Domain-Containing 1 (VSIG1), Predominantly Expressed in Testicular Germ Cells, Is Dispensable for Spermatogenesis and Male Fertility in Mice. Animals 2021, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.-Y.; Bourne, P.A.; diSant’Agnese, P.A.; Huang, J. Cytoplasmic Staining of TTF-1 in the Differential Diagnosis of Hepatocellular Carcinoma vs. Cholangiocarcinoma and Metastatic Carcinoma of the Liver. Am. J. Clin. Pathol. 2006, 125, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, T.J.; Pinkus, J.L.; Glickman, J.N.; Pinkus, G.S. Comparison of Thyroid Transcription Factor-1 and Hepatocyte Antigen Immunohistochemical Analysis in the Differential Diagnosis of Hepatocellular Carcinoma, Metastatic Adenocarcinoma, Renal Cell Carcinoma, and Adrenal Cortical Carcinoma. Am. J. Clin. Pathol. 2002, 118, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-C.; Chen, P.C.-H.; Tsay, S.-H.; Chiang, H. Cytoplasmic Immunoreactivity for Thyroid Transcription Factor-1 in Hepatocellular Carcinoma: A Comparative Immunohistochemical Analysis of Four Commercial Antibodies Using a Tissue Array Technique. Am. J. Clin. Pathol. 2004, 121, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Satala, C.B.; Jung, I.; Kobori, L.; Kovacs, Z.; Fodor, D.; Szodorai, R.; Gurzu, S. Benefits of the 8th American Joint Committee on Cancer System for Hepatocellular Carcinoma Staging. J. Gastrointest. Cancer 2021, 52, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, M.J.; Ritter, G.; Yin, B.W.T.; Williams, C.; Cohen, L.S.; Coplan, K.A.; Fortunato, S.R.; Frosina, D.; Lee, S.-Y.; Murray, A.E.; et al. Glycoprotein A34, a Novel Target for Antibody-Based Cancer Immunotherapy. Cancer Immun. 2006, 6, 2. [Google Scholar] [PubMed]
- Inoue, Y.; Matsuura, S.; Yoshimura, K.; Iwashita, Y.; Kahyo, T.; Kawase, A.; Tanahashi, M.; Maeda, M.; Ogawa, H.; Inui, N.; et al. Characterization of V-Set and Immunoglobulin Domain Containing 1 Exerting a Tumor Suppressor Function in Gastric, Lung, and Esophageal Cancer Cells. Cancer Sci. 2017, 108, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Satala, C.B.; Jung, I.; Kovacs, Z.; Stefan-Van Staden, R.I.; Molnar, C.; Bara, T.; Patrichi, A.I.; Gurzu, S. V-set and immunoglobulin domain containing 1 (VSIG1) as an emerging target for epithelial-mesenchymal transition of gastric cancer. Sci. Rep. 2022, 12, 16241. [Google Scholar] [CrossRef] [PubMed]
- Gherman, C.; Braicu, O.L.; Zanoaga, O.; Jurj, A.; Pileczki, V.; Maralani, M.; Drigla, F.; Braicu, C.; Budisan, L.; Achimas-Cadariu, P.; et al. Caffeic Acid Phenethyl Ester Activates Pro-Apoptotic and Epithelial-Mesenchymal Transition-Related Genes in Ovarian Cancer Cells A2780 and A2780cis. Mol. Cell. Biochem. 2016, 413, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gurzu, S.; Kobori, L.; Fodor, D.; Jung, I. Epithelial Mesenchymal and Endothelial Mesenchymal Transitions in Hepatocellular Carcinoma: A Review. Biomed. Res. Int. 2019, 2019, 2962580. [Google Scholar] [CrossRef] [PubMed]
- Straub, B.K.; Rickelt, S.; Zimbelmann, R.; Grund, C.; Kuhn, C.; Iken, M.; Ott, M.; Schirmacher, P.; Franke, W.W. E-N-Cadherin Heterodimers Define Novel Adherens Junctions Connecting Endoderm-Derived Cells. J. Cell. Biol. 2011, 195, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, X.; Li, T.; Qiu, W.; Peng, C.; Shen, B.; Zhu, Z. NR1D2 Accelerates Hepatocellular Carcinoma Progression by Driving the Epithelial-to-Mesenchymal Transition. OncoTargets Ther. 2020, 13, 3931–3942. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhu, X.; Dai, C.; Zhang, X.; Gao, X.; Wei, J.; Sheng, Y.; Zheng, Y.; Yu, J.; Xie, L.; et al. Osteopontin Promotes Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma through Regulating Vimentin. Oncotarget 2016, 7, 12997–13012. [Google Scholar] [CrossRef] [PubMed]

| Parameters | N = 217 |
|---|---|
| Age (years), median (range) | 65.48 (31–91 years) |
| Gender (male/female) | 151/66 |
| Tumor architecture (uni-/multifocal) | 93/124 |
| Tumor size—the diameter of the larger tumor (mm) | 43.89 (7–140) |
| pT stage—8th AJCC edition (1/2/3) | 97/89/31 |
| Histologic type (trabecular/solid-acinar/solid-clear cells/solid-scirrhous) | 90/47/56/24 |
| Grade of differentiation (G1/G2/G3/G4) | 13/79/81/44 |
| Vascular invasion (present/absent) | 95/122 |
| Liver cirrhosis (present/absent) | 143/74 |
| Hepatitis (yes/no) | 105/112 |
| VSIG1 (0/1+/2+/3+) | 104/23/54/36 |
| Vimentin (negative/positive) | 181/36 |
| TTF-1 (negative/positive) | 134/83 |
| Parameter | VSIG1 (n = 217) | p Value | |
|---|---|---|---|
| Negative (n = 104, 47.92%) | Positive (n = 113, 52.07%) | ||
| Age (yrs.), median (range) | 65.14 (31–82 years) | 64.07 (39–91 years) | 0.86 |
| Gender | 0.91 | ||
| Male (n = 151) | 72 (69.23%) | 79 (69.91%) | |
| Female (n = 66) | 32 (30.76%) | 34 (30.08%) | |
| Tumor architecture | 0.49 | ||
| Unifocal (n = 93) | 42 (40.38%) | 51 (45.13%) | |
| Multicentric (n = 124) | 62 (59.61%) | 62 (54.86%) | |
| pT stage—8th AJCC edition | 0.26 | ||
| pT1 (n = 98) | 49 (47.11%) | 49 (43.36%) | |
| pT2 (n = 88) | 37 (35.57%) | 51 (45.13%) | |
| pT3 (n = 31) | 18 (17.30%) | 13 (11.50%) | |
| Histologic type | <0.0001 | ||
| Trabecular (n = 90) | 33 (31.73%) | 57 (50.44%) | |
| Solid-acinar (n = 47) | 24 (23.07%) | 23 (20.35%) | |
| Solid-scirrhous (n = 24) | 21 (20.19%) | 3 (2.65%) | |
| Solid-clear cells (n = 56) | 26 (25%) | 30 (26.54%) | |
| Grade of differentiation | <0.0001 | ||
| G1 + G2 (n = 92) | 23 (22.11%) | 69 (61.06%) | |
| G3 + G4 (n = 125) | 81 (77.88%) | 44 (38.93%) | |
| Vascular invasion | 0.49 | ||
| Present (n = 96) | 49 (47.11%) | 47 (41.59%) | |
| Absent (n = 121) | 55 (52.88%) | 66 (58.40%) | |
| Liver cirrhosis | 0.88 | ||
| Present (n = 143) | 68 (65.38%) | 75 (66.37%) | |
| Absent (n = 74) | 36 (34.61%) | 38 (33.62%) | |
| Hepatitis B or C history | 0.68 | ||
| Yes (n = 98) | 45 (43.26%) | 53 (46.90%) | |
| No (n = 119) | 59 (56.73%) | 60 (53.09%) | |
| Vimentin—tumor cells | 0.02 | ||
| Negative (n = 182) | 81 (77.88%) | 101 (89.38%) | |
| Positive (n = 35) | 23 (22.11%) | 12 (10.61%) | |
| TTF-1—tumor cells | <0.0001 | ||
| Negative (n = 134) | 92 (88.46%) | 42 (37.16%) | |
| Positive (n = 83) | 12 (11.53%) | 71 (62.83%) | |
| Parameter | TTF-1 (n = 217) | p Value | |
|---|---|---|---|
| Negative (n = 134, 61.75%) | Positive (n = 83, 38.24%) | ||
| Age (yrs.), median (range) | 64.54 (31–91 years) | 64.39 (39–81) | 0.84 |
| Gender | 0.87 | ||
| Male (n = 151) | 94 (70.14%) | 57 (68.67%) | |
| Female (n = 66) | 40 (29.85%) | 26 (31.32%) | |
| Tumor architecture | 0.40 | ||
| Unifocal (n = 108) | 70 (52.23%) | 38 (45.78%) | |
| Multicentric (n = 109) | 64 (47.76%) | 45 (54.21%) | |
| pT stage—8th AJCC edition | 0.26 | ||
| pT1 (n = 96) | 59 (44.02%) | 37 (44.57%) | |
| pT2 (n = 90) | 52 (38.80%) | 38 (45.78%) | |
| pT3 (n = 31) | 23 (17.16%) | 8 (9.63%) | |
| Histologic type | 0.07 | ||
| Trabecular (n = 90) | 51 (38.05%) | 39 (46.98%) | |
| solid-acinar (n = 47) | 30 (22.38%) | 17 (20.48%) | |
| solid-clear cells (n = 56) | 33 (24.62%) | 23 (27.71%) | |
| solid-scirrhous (n = 24) | 20 (14.92%) | 4 (4.81%) | |
| Grade of differentiation | <0.0001 | ||
| G1 + G2 (n = 92) | 43 (32.08%) | 49 (59.03%) | |
| G3 + G4 (n = 125) | 91 (67.91%) | 34 (40.96%) | |
| Vascular invasion | 0.88 | ||
| present (n = 96) | 60 (44.77%) | 36 (43.37%) | |
| absent (n = 121) | 74 (55.22%) | 47 (56.62%) | |
| Liver cirrhosis | 0.76 | ||
| Present (n = 142) | 89 (66.41%) | 53 (63.85%) | |
| Absent (n = 75) | 45 (33.58%) | 30 (36.14%) | |
| Hepatitis B or C history | 0.26 | ||
| yes (n = 105) | 69 (51.49%) | 36 (43.37%) | |
| no (n = 112) | 65 (48.50%) | 47 (56.62%) | |
| Vimentin—tumor cells | 0.03 | ||
| negative (n = 181) | 106 (79.10%) | 75 (90.36%) | |
| positive (n = 36) | 28 (20.89%) | 8 (9.63%) | |
| VSIG1—tumor cells | <0.0001 | ||
| negative (n = 134) | 92 (68.65%) | 12 (14.45%) | |
| positive (n = 83) | 42 (31.34%) | 71 (85.54%) | |
| 0/1+/2+/3+ | (92/13/21/8) | (12/10/33/28) | |
| Parameter | VIM (n = 217) | p Value | |
|---|---|---|---|
| Negative (n = 181, 83.41%) | Positive (n = 36, 16.58%) | ||
| Age (yrs.), median (range) | 65.37 (39–84) | 67.04 (59–91) | 0.54 |
| Gender | 0.87 | ||
| Male (n = 149) | 125 (69.06%) | 24 (66.66%) | |
| Female (n = 68) | 56 (30.93%) | 12 (33.33%) | |
| Tumor architecture | 0.18 | ||
| Unifocal (n = 127) | 110 (60.77%) | 17 (47.22%) | |
| Multicentric (n = 90) | 71 (39.22%) | 19 (52.77%) | |
| pT stage—8th AJCC edition | 0.35 | ||
| pT1 (n = 96) | 84 (46.40%) | 12 (33.33%) | |
| pT2 (n = 95) | 76 (41.98%) | 19 (52.77%) | |
| pT3 (n = 26) | 21 (11.60%) | 5 (13.88%) | |
| Histologic type | 0.05 | ||
| Trabecular (n = 92) | 80 (44.19%) | 12 (33.33%) | |
| Solid-acinar (n = 35) | 30 (16.57%) | 5 (13.88%) | |
| Solid-clear cells (n = 72) | 60 (33.14%) | 12 (33.33%) | |
| Solid-scirrhous (n = 18) | 11 (6.07%) | 7 (19.44%) | |
| Grade of differentiation | 0.46 | ||
| G1 + G2 (n = 94) | 76 (41.98%) | 18 (50%) | |
| G3 + G4 (n = 123) | 105 (58.01%) | 18 (50%) | |
| Vascular invasion | 0.34 | ||
| Present (n = 96) | 84 (46.40%) | 23 (63.88%) | |
| Absent (n = 121) | 97 (53.59%) | 13 (36.11%) | |
| Liver cirrhosis | 0.08 | ||
| Present (n = 133) | 116 (64.08%) | 17 (47.22%) | |
| Absent (n = 84) | 65 (35.91%) | 19 (52.77%) | |
| Hepatitis B or C history | 0.73 | ||
| Yes (n = 93) | 79 (43.64%) | 14 (38.88%) | |
| No (n = 112) | 102 (56.35%) | 22 (61.11%) | |
| TTF-1—tumor cells | 0.04 | ||
| Negative (n = 134) | 106 (58.56%) | 28 (77.77%) | |
| Positive (n = 83) | 75 (41.43%) | 8 (22.22%) | |
| VSIG1—tumor cells | 0.03 | ||
| Negative (n = 105) | 81 (44.75%) | 24 (66.66%) | |
| Positive (n = 112) | 100 (55.24%) | 12 (33.33%) | |
| Parameters | VSIG1+ TTF-1+ = 71 | VSIG1− TTF-1− = 92 | p Value |
|---|---|---|---|
| Age (yrs.), median (range) | 64 (39–81 years) | 65.5 (31–91 years) | 0.87 |
| Gender | 0.47 | ||
| Male (n = 112) | 49 (69.01%) | 63 (68.47%) | |
| Female (n = 51) | 22 (30.98%) | 29 (31.52%) | |
| Tumor architecture | 0.32 | ||
| Unifocal (n = 78) | 32 (45.07%) | 46 (50%) | |
| Multicentric (n = 85) | 39 (54.92%) | 46 (50%) | |
| pT stage—8th AJCC edition | 0.38 | ||
| pT1 (n = 75) | 32 (45.07%) | 43 (46.73%) | |
| pT2 (n = 66) | 32 (45.07%) | 34 (36.95%) | |
| pT3 (n = 22) | 7 (9.85%) | 15 (16.30%) | |
| Histologic type | 0.006 | ||
| Trabecular (n = 64) | 35 (49.29%) | 29 (31.52%) | |
| Solid-acinar (n = 35) | 14 (19.71%) | 21 (22.82%) | |
| Solid-clear cells (n = 41) | 19 (26.76%) | 22 (23.91%) | |
| Solid-scirrhous (n = 23) | 3 (4.22%) | 20 (21.73%) | |
| Grade of differentiation | <0.0001 | ||
| G1 + G2 (n = 62) | 44 (61.97%) | 18 (19.56%) | |
| G3 + G4 (n = 101) | 27 (38.02%) | 74 (80.43%) | |
| Vascular invasion | 0.34 | ||
| Present (n = 73) | 30 (42.25%) | 43 (46.73%) | |
| Absent (n = 100) | 41 (57.74%) | 49 (53.26%) | |
| Liver cirrhosis | 0.52 | ||
| Present (n = 104) | 45 (63.38%) | 59 (64.13%) | |
| Absent (n = 59) | 26 (36.61%) | 33 (35.86%) | |
| Hepatitis | 0.18 | ||
| Yes (n = 81) | 32 (45.07%) | 49 (53.26%) | |
| No (n = 82) | 39 (54.92%) | 43 (46.73%) |
| Parameters | VSIG1+ TTF-1+ VIM− = 52 | VSIG1− TTF-1− VIM− = 56 | p Value |
|---|---|---|---|
| Age (yrs.), median (range) | 65.20 (39–91) | 64.8 (30–82) | 0.87 |
| Gender | 0.40 | ||
| Male | 34 (65.38%) | 41 (73.21%) | |
| Female | 18 (34.61%) | 15 (26.78%) | |
| Tumor architecture | 0.43 | ||
| Unifocal | 28 (53.85%) | 35 (62.5%) | |
| Multicentric | 24 (46.15%) | 21 (37.5%) | |
| pT stage—8th AJCC edition | 0.75 | ||
| pT1 (n = 49) | 23 (44.23%) | 26 (46.42%) | |
| pT2 (n = 46) | 23 (44.23%) | 23 (41.07%) | |
| pT3 (n = 13) | 6 (11.53%) | 7 (12.05%) | |
| Histologic type | 0.02 | ||
| Trabecular (n = 47) | 27 (51.92%) | 20 (35.71%) | |
| Solid-acinar (n = 18) | 7 (13.46%) | 11 (19.64%) | |
| Solid-clear cells (n = 35) | 18 (34.61%) | 17 (30.35%) | |
| Solid-scirrhous (n = 8) | 0 (0.00%) | 8 (14.28%) | |
| Grade of differentiation | <0.0001 | ||
| G1 + G2 (n = 43) | 31 (59.61%) | 12 (21.42%) | |
| G3 + G4 (n = 65) | 21 (40.38%) | 44 (78.57%) | |
| Vascular invasion | 0.70 | ||
| Present | 24 (46.15%) | 28 (50%) | |
| Absent | 28 (53.85%) | 28 (50%) | |
| Liver cirrhosis | 0.32 | ||
| Present | 28 (53.85%) | 36 (64.28%) | |
| Absent | 24 (46.15%) | 20 (35.71%) | |
| Hepatitis | 0.33 | ||
| Yes | 20 (38.46%) | 27 (48.21%) | |
| No | 32 (61.53%) | 29 (51.78%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szodorai, R.; Banias, L.; Kovalszky, I.; Dezső, K.; Kovács, Z.; Gurzu, S. Gastric-Type Expression Signature in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 6588. https://doi.org/10.3390/ijms25126588
Szodorai R, Banias L, Kovalszky I, Dezső K, Kovács Z, Gurzu S. Gastric-Type Expression Signature in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2024; 25(12):6588. https://doi.org/10.3390/ijms25126588
Chicago/Turabian StyleSzodorai, Rita, Laura Banias, Ilona Kovalszky, Katalin Dezső, Zsolt Kovács, and Simona Gurzu. 2024. "Gastric-Type Expression Signature in Hepatocellular Carcinoma" International Journal of Molecular Sciences 25, no. 12: 6588. https://doi.org/10.3390/ijms25126588
APA StyleSzodorai, R., Banias, L., Kovalszky, I., Dezső, K., Kovács, Z., & Gurzu, S. (2024). Gastric-Type Expression Signature in Hepatocellular Carcinoma. International Journal of Molecular Sciences, 25(12), 6588. https://doi.org/10.3390/ijms25126588









